Abstract
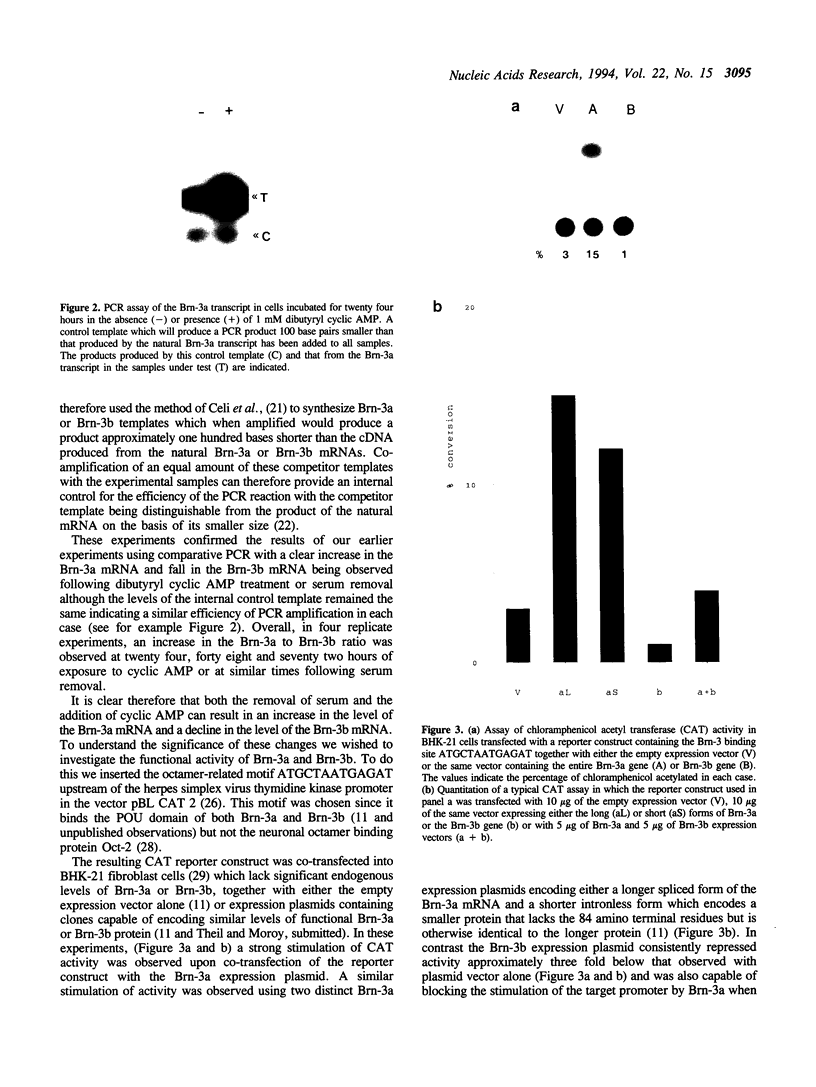
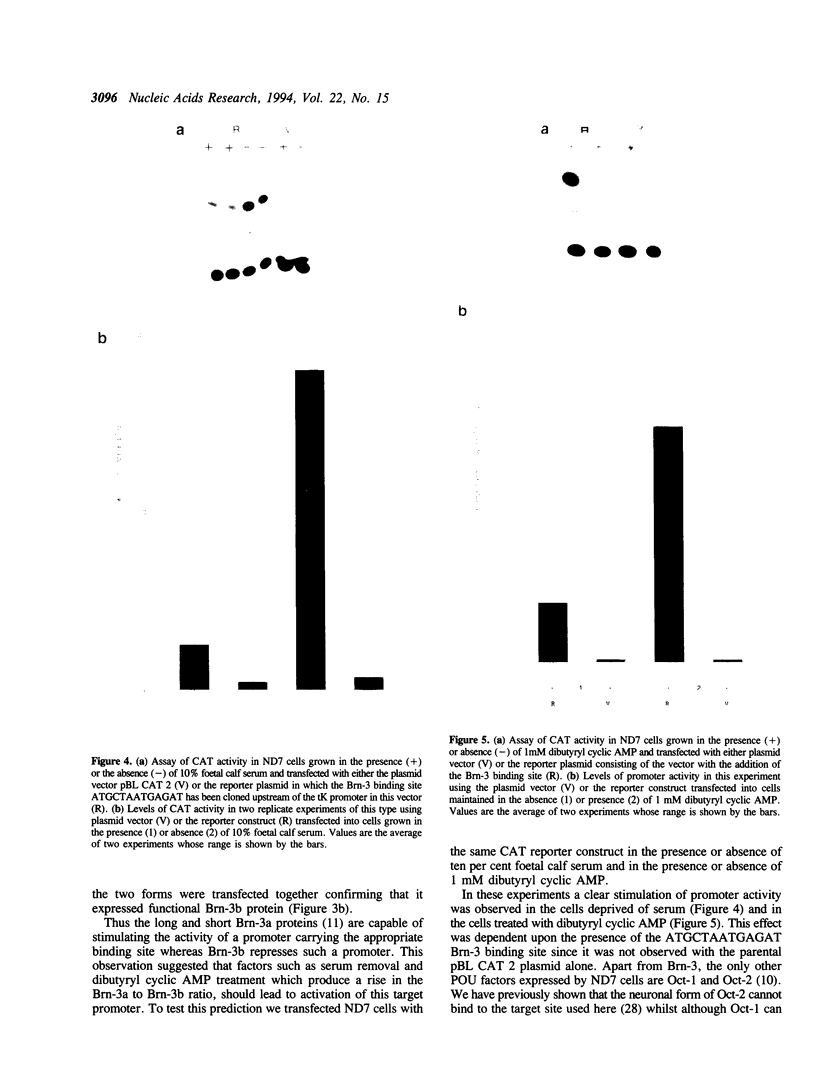
The POU factors Brn-3a and Brn-3b are closely related transcription factors which are expressed in neuronal cells. The levels of the transcripts encoding these factors are regulated in opposite directions in neuronal cells by specific cellular signalling pathways with dibutyryl cyclic AMP treatment and serum removal enhancing the level of Brn-3a and reducing the level of Brn-3b expression. This opposite expression pattern is paralleled by the ability of Brn-3a to specifically transactivate a target promoter bearing its DNA binding site whereas this promoter is repressed by Brn-3b. As predicted from these observations this target promoter is strongly activated by serum removal or addition of dibutyryl cyclic AMP. Therefore changes in Brn-3a and b expression can have a functional effect on promoter activity indicating that Brn-3a and Brn-3b can regulate gene expression via a specific binding site in response to the activation of specific cellular signalling pathways. The reasons for the differences in activity between these two related factors and their role in regulating gene activity in the nervous system are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abken H., Reifenrath B. A procedure to standardize CAT reporter gene assay. Nucleic Acids Res. 1992 Jul 11;20(13):3527–3527. doi: 10.1093/nar/20.13.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Celi F. S., Zenilman M. E., Shuldiner A. R. A rapid and versatile method to synthesize internal standards for competitive PCR. Nucleic Acids Res. 1993 Feb 25;21(4):1047–1047. doi: 10.1093/nar/21.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collum R. G., Fisher P. E., Datta M., Mellis S., Thiele C., Huebner K., Croce C. M., Israel M. A., Theil T., Moroy T. A novel POU homeodomain gene specifically expressed in cells of the developing mammalian nervous system. Nucleic Acids Res. 1992 Sep 25;20(18):4919–4925. doi: 10.1093/nar/20.18.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Dent C. L., McIndoe G. A., Latchman D. S. The constitutively expressed octamer binding protein OTF-1 and a novel octamer binding protein expressed specifically in cervical cells bind to an octamer-related sequence in the human papillomavirus 16 enhancer. Nucleic Acids Res. 1991 Aug 25;19(16):4531–4535. doi: 10.1093/nar/19.16.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C., Garriga G., McIntire S. L., Horvitz H. R. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature. 1988 Dec 15;336(6200):638–646. doi: 10.1038/336638a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finney M., Ruvkun G., Horvitz H. R. The C. elegans cell lineage and differentiation gene unc-86 encodes a protein with a homeodomain and extended similarity to transcription factors. Cell. 1988 Dec 2;55(5):757–769. doi: 10.1016/0092-8674(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Gerrero M. R., McEvilly R. J., Turner E., Lin C. R., O'Connell S., Jenne K. J., Hobbs M. V., Rosenfeld M. G. Brn-3.0: a POU-domain protein expressed in the sensory, immune, and endocrine systems that functions on elements distinct from known octamer motifs. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10841–10845. doi: 10.1073/pnas.90.22.10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Treacy M. N., Simmons D. M., Ingraham H. A., Swanson L. W., Rosenfeld M. G. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989 Jul 6;340(6228):35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Monuki E. S., Lemke G. The gene encoding the transcription factor SCIP has features of an expressed retroposon. Mol Cell Biol. 1991 Sep;11(9):4642–4650. doi: 10.1128/mcb.11.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J. S., Cleary M. A., Herr W. A single amino acid exchange transfers VP16-induced positive control from the Oct-1 to the Oct-2 homeo domain. Genes Dev. 1992 Nov;6(11):2058–2065. doi: 10.1101/gad.6.11.2058. [DOI] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
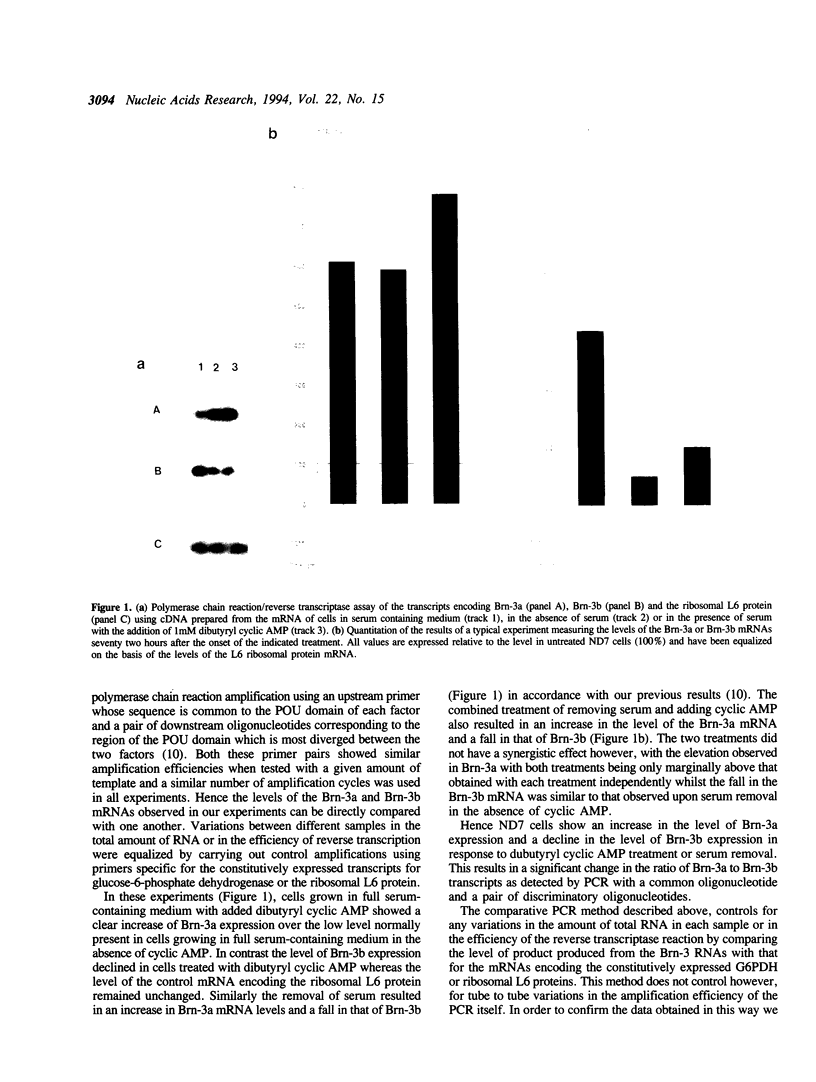
- Lillycrop K. A., Budrahan V. S., Lakin N. D., Terrenghi G., Wood J. N., Polak J. M., Latchman D. S. A novel POU family transcription factor is closely related to Brn-3 but has a distinct expression pattern in neuronal cells. Nucleic Acids Res. 1992 Oct 11;20(19):5093–5096. doi: 10.1093/nar/20.19.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- McCormick A., Brady H., Theill L. E., Karin M. Regulation of the pituitary-specific homeobox gene GHF1 by cell-autonomous and environmental cues. Nature. 1990 Jun 28;345(6278):829–832. doi: 10.1038/345829a0. [DOI] [PubMed] [Google Scholar]
- Monuki E. S., Weinmaster G., Kuhn R., Lemke G. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron. 1989 Dec;3(6):783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Morris P. J., Ring C. J., Lillycrop K. A., Latchman D. S. Transactivation of the human papilloma virus 16 octamer motif by the octamer binding protein Oct-2 requires both the N and C terminal activation domains. Nucleic Acids Res. 1993 Sep 25;21(19):4506–4510. doi: 10.1093/nar/21.19.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkina N. N., Stevens G. E., Wood J. N., Richardson W. D. A novel Brn3-like POU transcription factor expressed in subsets of rat sensory and spinal cord neurons. Nucleic Acids Res. 1993 Jul 11;21(14):3175–3182. doi: 10.1093/nar/21.14.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Radovick S., Nations M., Du Y., Berg L. A., Weintraub B. D., Wondisford F. E. A mutation in the POU-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science. 1992 Aug 21;257(5073):1115–1118. doi: 10.1126/science.257.5073.1115. [DOI] [PubMed] [Google Scholar]
- Ring C. J., Latchman D. S. The human Brn-3b POU transcription factor shows only limited homology to the Brn-3a/RDC-1 factor outside the conserved POU domain. Nucleic Acids Res. 1993 Jun 25;21(12):2946–2946. doi: 10.1093/nar/21.12.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. Competitive PCR. Nature. 1992 Oct 8;359(6395):557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- Steinfelder H. J., Radovick S., Wondisford F. E. Hormonal regulation of the thyrotropin beta-subunit gene by phosphorylation of the pituitary-specific transcription factor Pit-1. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5942–5945. doi: 10.1073/pnas.89.13.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suburo A. M., Wheatley S. C., Horn D. A., Gibson S. J., Jahn R., Fischer-Colbrie R., Wood J. N., Latchman D. S., Polak J. M. Intracellular redistribution of neuropeptides and secretory proteins during differentiation of neuronal cell lines. Neuroscience. 1992;46(4):881–889. doi: 10.1016/0306-4522(92)90191-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Theil T., McLean-Hunter S., Zörnig M., Möröy T. Mouse Brn-3 family of POU transcription factors: a new aminoterminal domain is crucial for the oncogenic activity of Brn-3a. Nucleic Acids Res. 1993 Dec 25;21(25):5921–5929. doi: 10.1093/nar/21.25.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treacy M. N., He X., Rosenfeld M. G. I-POU: a POU-domain protein that inhibits neuron-specific gene activation. Nature. 1991 Apr 18;350(6319):577–584. doi: 10.1038/350577a0. [DOI] [PubMed] [Google Scholar]
- Treacy M. N., Neilson L. I., Turner E. E., He X., Rosenfeld M. G. Twin of I-POU: a two amino acid difference in the I-POU homeodomain distinguishes an activator from an inhibitor of transcription. Cell. 1992 Feb 7;68(3):491–505. doi: 10.1016/0092-8674(92)90186-g. [DOI] [PubMed] [Google Scholar]
- Turner E. E., Jenne K. J., Rosenfeld M. G. Brn-3.2: a Brn-3-related transcription factor with distinctive central nervous system expression and regulation by retinoic acid. Neuron. 1994 Jan;12(1):205–218. doi: 10.1016/0896-6273(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Van der Vliet P. C. POU domain transcription factors. Biochim Biophys Acta. 1993 Apr 29;1173(1):1–21. doi: 10.1016/0167-4781(93)90237-8. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., van Oosterhout J. A., van der Vliet P. C. The Oct-1 POU domain mediates interactions between Oct-1 and other POU proteins. Mol Cell Biol. 1992 Feb;12(2):542–551. doi: 10.1128/mcb.12.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M., Drolet D. W., Rosenfeld M. G. POU-domain proteins: structure and function of developmental regulators. Curr Opin Cell Biol. 1993 Jun;5(3):488–498. doi: 10.1016/0955-0674(93)90015-i. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Bevan S. J., Coote P. R., Dunn P. M., Harmar A., Hogan P., Latchman D. S., Morrison C., Rougon G., Theveniau M. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990 Sep 22;241(1302):187–194. doi: 10.1098/rspb.1990.0084. [DOI] [PubMed] [Google Scholar]
- Xiang M., Zhou L., Peng Y. W., Eddy R. L., Shows T. B., Nathans J. Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron. 1993 Oct;11(4):689–701. doi: 10.1016/0896-6273(93)90079-7. [DOI] [PubMed] [Google Scholar]