Abstract
Purpose
To compare the peripapillary retinal nerve fiber layer (RNFL) thickness of normal patients and those with various glaucoma diseases by time domain (Stratus) and spectral domain (Spectralis) optical coherence tomography (OCT).
Methods
The RNFL thickness as measured by the Stratus and Spectral OCT was compared (paired t-test). The relationship and agreement of RNFL thickness between the two OCT modalities were evaluated by Pearson correlation, Bland-Altman plot, and area under the receiver operating characteristic curve.
Results
Two-hundred seventeen eyes of 217 patients, including twenty-four normal eyes, ninety-one glaucoma suspects, seventy-six normal tension glaucoma cases, and twenty-six primary open angle glaucoma cases (POAG) were analyzed. The peripapillary RNFL thicknesses as measured by Stratus OCT were significantly greater than those measured by Spectralis OCT. However, in quadrant comparisons, the temporal RNFL thickness obtained using Stratus OCT were significantly less than those obtained using Spectralis OCT. Correlations between RNFL parameters were strong (Pearson correlation coefficient for mean RNFL thickness = 0.88); a high degree of correlation was found in the POAG group. Bland-Altman plotting demonstrated that agreement in the temporal quadrant was greater than any other quadrant.
Conclusions
Both OCT systems were highly correlated and demonstrated strong agreement. However, absolute measurements of peripapillary RNFL thickness differed between Stratus OCT and Spectralis OCT. Thus, measurements with these instruments should not be considered interchangeable. The temporal quadrant was the only sector where RNFL thickness as measured by Spectralis OCT was greater than by Stratus OCT; this demonstrated greater agreement than other sectors.
Keywords: Retinal nerve fiber layer thickness, Spectral domain optical coherence tomography, Time domain optical coherence tomography
Glaucoma is an optic neuropathy characterized by specific and progressive injury to the optic nerve and retinal nerve fiber layer (RNFL). Glaucoma results in field defects and irreversible vision loss. As interventions are available that halt or retard the natural progression of the disease, resulting in eventual blindness, early detection and diagnosis is very important. Change in the RNFL thickness is one of the most important findings for the early diagnosis and determination of glaucoma progression [1]. Optical coherence tomography (OCT) is a non-invasive, cross-sectional imaging technique that makes routine measurement of RNFL thickness possible [2]. OCT has been shown to be a highly reproducible imaging modality [3,4] that correlates with ex vivo histologic measurements of the retina [5,6].
Time domain (TD) OCT is a third-generation modality that has a resolution of 8 µm to 10 µm and is capable of differentiating between healthy and glaucomatous eyes [7,8]. However, the speed of TD-OCT scanning is limited by the temporal coherence properties of the light source, as well as the need for a movable reference mirror. In contrast to TD-OCT, the newer spectral-domain (SD) OCT technology has a much higher scan speed than TD-OCT. SD-OCT provides better scan resolution and allows for a greater number of scans acquired at a faster rate than TD-OCT technology [9,10]. These improvements have the potential to provide clinicians with enhanced tools for the diagnosis and treatment of glaucoma.
However, before a new diagnostic instrument can be accepted for use in clinical practice, it is important to determine if measurement from previous generation OCT technologies are compatible with new generation technologies, as well as if these measurements are comparable with each other for determining pathologic changes or disease progression. The aim of this study was to determine the relationship between peripapillary RNFL thickness measurements from TD-OCT (Stratus) and SD-OCT (Spectralis) modalities in normal controls and patients with glaucoma to identify any systemic differences.
Materials and Methods
After excluding nineteen patients with unreliable test results, 217 consecutive patients were identified as eligible participants. The study was conducted between December 2008 and May 2009 at the glaucoma clinic of the Department of Ophthalmology, Konkuk University Hospital. This study was approved by the Ethical Committee of Konkuk College of Medicine. Four groups of participants were enrolled in this study: normal controls, glaucoma suspects, patients with normal tension glaucoma (NTG), and those with primary open angle glaucoma (POAG). Primary angle closure glaucoma (PACG) was excluded because of too few PACG patients (nine patients) in this study. Each participant underwent a complete ophthalmic evaluation including visual acuity testing, intraocular pressure determination as measured by Goldman applanation tonometry, biomicroscopy, and optic nerve evaluation, as well as review of previously acquired Humphrey Visual Field (Carl Zeiss Meditec, Dublin, CA, USA) examination.
To be included in the study, participants had to have a best-corrected visual acuity (BCVA) better than or equal to 20 / 40, spherical refraction within ±5.0 diopters (D), and cylinder correlation within ±3.0 D. Inclusion criteria for normal participants was an intraocular pressure (IOP) under 21 mmHg, a normal visual field, a normal appearing optic nerve head without asymmetry, hemorrhages, or notches, and absence of any ophthalmic disease, except for mild cataracts. Those suspected of having glaucoma had one or more risk factors that may lead to glaucoma, but these participants did not have definite glaucomatous optic nerve damage or visual field defects. A normal visual field was defined as a mean deviation and pattern deviation within a 95% confidence interval and a glaucoma hemifield test result, "within normal limits". Patients with glaucoma are characterized by glaucomatous optic nerve damage and visual field defects demonstrated in at least two consecutive, reliable examinations. Patients with glaucoma who had an open angle detected by gonioscopy and a statistically normal IOP were defined as NTG. Patients with glaucoma who had an open angle and an IOP equal to or greater than 21 mmHg were defined as POAG.
Exclusion criteria included patients who presented with a BCVA worse than 20 / 40, other intraocular eye diseases (including secondary glaucoma, diabetic retinopathy, age-related macular degeneration, acute anterior segment diseases, etc.), optic nerve diseases (including non-glaucomatous optic neuropathy, other disease affecting the visual field), or a history of intraocular surgery (except uncomplicated cataract surgeries). This study also excluded those with an unreliable visual field or low quality, poor signal strength OCT. (Stratus OCT, signal strength < 6; Spectralis OCT, image quality score < 16)
Optical coherence tomography technique
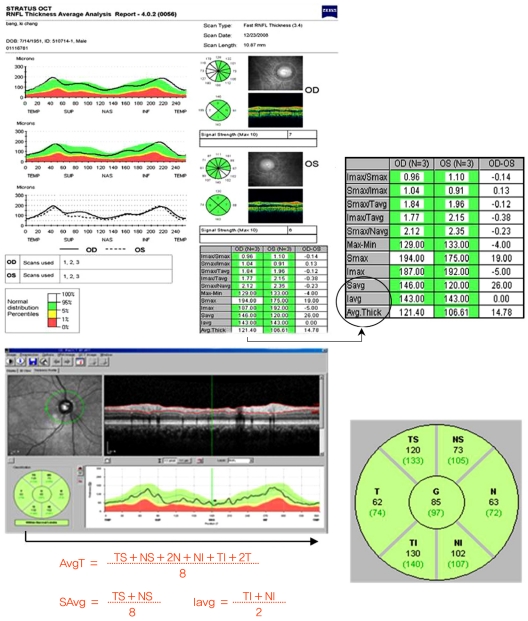
Following pupil dilatation with instillation of Mydrin-P ophthalmic solution (Santen Pharmaceutical, Osaka, Japan) and fixation instruction in one randomly selected eye per participant, patients were scanned in a single session, by a single operator, with both OCT systems, on the same day. Peripapillary RNFL thickness measurements of average (AvgT), superior quadrant (Savg), inferior quadrant (Iavg), nasal quadrant (Navg), and the temporal quadrant (Tavg) were analyzed. TD-OCT was performed using a Stratus OCT (Carl Zeiss Meditec) and SD-OCT was performed using a Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany). In Stratus OCT, the fast RNFL thickness acquisition protocol (software ver. 4.0.2) was used. Three scans were consecutively acquired using a circle with a standardized diameter of 3.4 mm. An automated computer algorithm delineated the anterior and posterior margins of the RNFL. A scan-circle was positioned around the disc by an experienced operator and the image was acquired and saved. With Spectral OCT, RNFL thickness was measured around the disc with 16 averaged consecutive circular B-scans (diameter of 3.5 mm, 768 A-scans); an online tracking system was used to compensate for eye movement. The RNFL thickness (from Internal limiting membrane inner margin to RNFL layer outer margin) was automatically segmented using the Spectralis software ver. 4.0.0.0). Six cases in which the RNFL upper and lower borders were poorly identified, and required manual correction were excluded (Fig. 1).
Fig. 1.
Example Stratus time domain-optical coherence tomography (OCT) and Spectralis spectral-domain-OCT scan image and retinal nerve fiber layer thickness map display in the eye of a healthy participant. Imax = inferior maximum; Smax = superior maximum; Tavg = temporal quadrant average thickness; Navg = nasal quadrant average thickness; Savg = superior quadrant average thickness; Iavg = inferior quadrant average thickness; Avg.Thick = thickness measurements of average; TS = temporal superior thickness; NS = nasal superior thickness; N = nasal thickness; NI = nasal inferior thickness; TI = temporal inferior thickness; T = temporal thickness; G = global thickness.
Statistical analysis
Statistical analysis was performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Results are expressed as the mean ± standard deviation. The paired t-test was used to compare RNFL thicknesses between machines. The Pearson correlation was used to assess the relationship between Stratus and Spectralis OCT. A Bland-Altman plot of the mean-paired difference was performed to assess agreement between OCT instruments. Diagnostic performance between instruments was compared based on the analysis obtained from the area under the receiver operating characteristic curve (AUC) of RNFL thickness in normal control and glaucoma patients (NTG and POAG groups). Statistical significance was defined at values of p ≤ 0.05.
Results
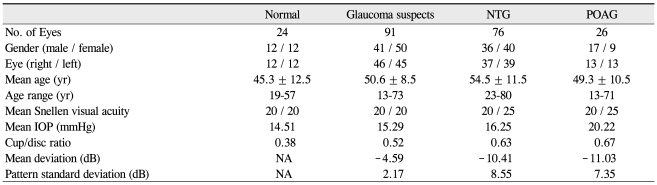
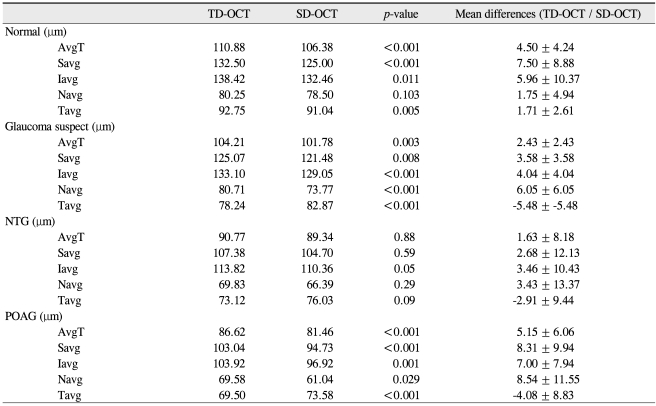
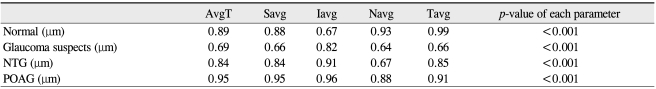
Two-hundred seventeen eyes of 217 patients, including ninety-one glaucoma suspects, seventy-six NTG, twenty-six POAG, and twenty-four normal patients, were included in this study. There were 111 women and 106 men; all were Asian. Table 1 summarizes the demographic and clinical characteristics of the study participants. The average age of those in the glaucoma groups was greater than of those in the normal and glaucoma suspect groups. For all diagnoses, the mean peripapillary RNFL thickness as measured by Stratus was significantly greater than those measured by Spectralis. In comparisons of quadrants, the temporal RNFL thickness obtained using Stratus was significantly less than that obtained using Spectralis (Table 2). The average RNFL thickness for normal patients as measured by Stratus was 110.88 ± 9.61 µm, whereas the average RNFL thickness as measured by Spectralis was 106.38 ± 9.01 µm. The average RNFL thickness for glaucoma suspects was 104.21 ± 10.13 µm using Stratus and 101.78 ± 8.74 µm using Spectralis. Using the ]Stratus method, the mean RNFL thicknesses in NTG and POAG groups were 90.77 ± 14.82 µm, 86.62 ± 19.58 µm, respectively (mean ± standard deviation). When using the Spectralis method, mean RNFL thickness in NTG and POAG groups were 89.34 ± 13.6 µm and 81.46 ± 19.73 µm, respectively (mean ± standard deviation). When comparing Stratus versus Spectralis methods between the various groups, p-values by paired t-test were as follows: normal controls Stratus > Spectralis, p < 0.001; suspect cases Stratus > Spectralis, p = 0.003; NTG Stratus > Spectralis, p = 0.88; POAG Stratus > Spectralis, p < 0.001. RNFL measurement differences may vary with the specific disease diagnosis. NTG groups demonstrated a statistical difference only in Iavg. However, both glaucoma suspects and the POAG group demonstrated statistical significance in all measurements (Table 3).
Table 1.
Demographic data from 217 eyes of the 217 individuals included in the analysis
NTG = normal tension glaucoma; POAG = primary open angle glaucoma; IOP = intraocular pressure; dB = decibel; NA = not applicable.
Table 2.
Summary of retinal nerve fiber layer thickness of total volunteers for each quadrant
TD = time domain; OCT = optical coherence tomography; SD = spectral-domain; AvgT = thickness measurements of average; Savg = superior quadrant; Iavg = inferior quadrant; Navg = nasal quadrant; Tavg = temporal quadrant.
Table 3.
Summary of mean paired RNFL thickness and differences for each disease diagnosis group
TD = time domain; OCT = optical coherence tomography; SD = spectral-domain; AvgT = thickness measurements of average; Savg = superior quadrant; Iavg = inferior quadrant; Navg = nasal quadrant; Tavg = temporal quadrant; NTG = normal tension glaucoma; POAG = primary open angle glaucoma.
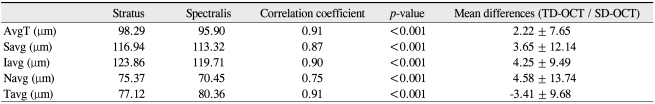
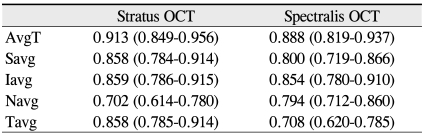
The Pearson correlation coefficient for the mean RNFL thicknesses between the two instruments was 0.91 (p < 0.001). The RNFL thickness as measured by Stratus OCT and Spectralis OCT were correlated in all sectors, including the average RNFL thickness. However, these correlations were weaker in the nasal quadrant. The glaucoma suspect group demonstrated a relatively low correlation coefficient when compared to the other groups. However, a high degree of correlation was found in the POAG group (Table 4).
Table 4.
Pearson correlation coefficient of retinal nerve fiber layer thickness between Stratus OCT and Spectralis OCT
OCT = optical coherence tomography; AvgT = thickness measurements of average; Savg = superior quadrant; Iavg = inferior quadrant; Navg = nasal quadrant; Tavg = temporal quadrant; NTG = normal tension glaucoma; POAG = primary open angle glaucoma.
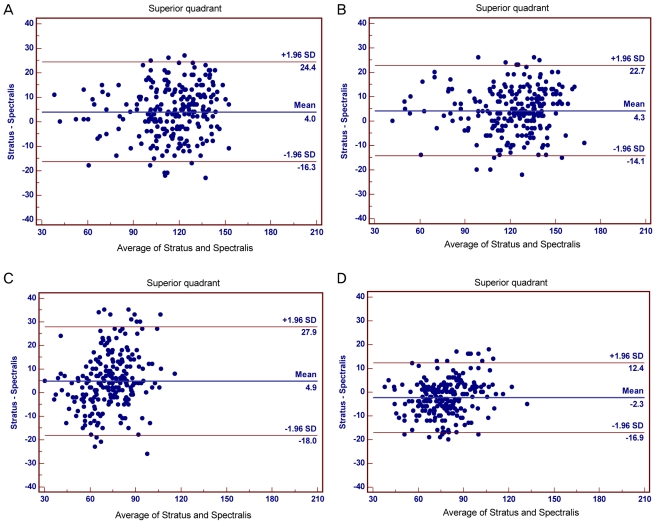
A Bland-Altman plot of the mean paired difference was performed to assess agreement between the two imaging modalities. Fig. 2 demonstrates the Bland-Altman plot for the individual quadrants. The 95% agreement limit for each comparison was -16.3 µm to 24.4 µm for the superior quadrant, -14.1 µm to 22.7 µm for the inferior quadrant, -18.0 µm to 27.9 µm for the nasal quadrant, and -16.9 µm to 12.4 µm for the temporal quadrant. There was greater variation in the superior and nasal plots, as evidenced by a wide spread; agreement of the temporal quadrant was greater than the other quadrants.
Fig. 2.
Bland-Altman plots of quadrant retinal nerve fiber layer (RNFL) thickness values between Stratus optical coherence tomography (OCT) and Spectralis OCT for the: (A) superior, (B) inferior, (C) nasal, and (D) temporal quadrants. The difference (Stratus OCT RNFL thickness - Spectralis OCT RNFL thickness) was plotted against the average of both measurements (Stratus OCT RNFL thickness + Spectralis OCT RNFL thickness/2) for all participants in each quadrant. SD = standard deviation.
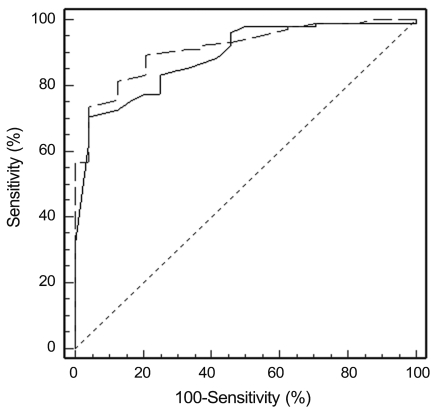
Table 5 shows the AUC values of the average RNFL thickness and quadrant RNFL thickness in normal control and glaucoma patients (NTG and POAG groups). At a specificity of 95%, the sensitivities were 73.8% for Stratus and 70.6% for Spectralis. The AUC of average RNFL thickness in Stratus was 0.913 (95% confidence intervals, 84.9% to 95.6%, p < 0.001) and that of Spectralis was 0.888 (95% confidence intervals, 80.9% to 93.7%, p < 0.001). The average RNFL thickness parameter demonstrated the best diagnostic performance for the OCT compared to any other quadrant (Savg, 0.858; Iavg, 0.859; Navg, 0.702; Tavg, 0.858 in Stratus / Savg, 0.800; Iavg, 0.854; Navg, 0.794; Tavg, 0.708 in Spectralis). In average RNFL thickness, the AUC of stratus OCT was greater than that of spectralis OCT. However, there was no statistically significant difference (p = 0.232) (Fig. 3).
Table 5.
Area under the receiver operating characteristic curve (95% confidence Intervals) for Stratus OCT and Spectralis OCT
OCT = optical coherence tomography; AvgT = thickness measurements of average; Savg = superior quadrant; Iavg = inferior quadrant; Navg = nasal quadrant; Tavg = temporal quadrant.
Fig. 3.
Comparison of the receiver operating characteristic curves of average retinal nerve fiber layer thickness in Stratus optical coherence tomography (OCT) (dash-dotted line) and Spectralis OCT (solid line).
Discussion
Since its introduction in 1991, OCT has quickly become an integral part of glaucoma diagnosis [2]. As a quick, high-resolution imaging device, OCT uses laser light to acquire an in vivo image of the retina [11]. Currently, the most widely used TD-OCT machine, the Stratus OCT, has a theoretical resolution of <10 µm [12]. It acquires images by evaluating the interference pattern created by the echo time delays of backscattered light from the patient's retina and those from a moving reference mirror. In the most recent development in SD-OCT, the Spectralis gathers depth information from the spectral data by Fourier transformation, eliminating the need for a moving reference mirror and allowing for more efficient data acquisition [13,14].
The results of the current study demonstrate that Spectralis RNFL measurements correlate well with those from the Stratus OCT. H owever, the absolute measures of peripapillary RNFL thickness differed between the Stratus and Spectralis modalities. These results are similar to previous data, suggesting that there is a systemic difference in measurement values between another SD-OCT, the Cirrus OCT (Carl Zeiss Meditec) and the Stratus OCT [15,16]. Knight et al. [15] determined RNFL thickness differences existed between the Stratus OCT and Cirrus OCT, and thus their data cannot be directly compared. The Stratus OCT delivers thicker RNFL measurements than the Cirrus OCT, although the correlation between these instruments was good.
Han and Jaffe [17] studied 290 patients and obtained foveal thickness measurements from TD-OCT (Stratus) and SD-OCT (Spectralis and Cirrus) instruments. They determined that foveal thickness measurements obtained using SD-OCT is consistently greater than those obtained using TD-OCT [17]. This difference occurred because the segmentation algorithms for the two instrument sets differ in their respective outer retinal boundaries (Stratus, the outer segment line overlies the photoreceptor inner segment / outer segment junction; Spectralis, the outer segmentation line overlies the retinal pigment epithelium / Bruch membrane). However, in peripapillary RNFL thickness measurements, contrary to the foveal thickness measurements, the TD-OCT RNFL thickness is greater than that measured by SD-OCT. The reason for this finding is unclear. We speculate that there are several possible reasons for this finding.
First, Stratus OCT measures RNFL thickness within a 3.4 mm diameter at the center of the optic disc, while Spectralis OCT measures within a diameter 3.5 mm. At greater distance from optic disc, the RNFL thickness is thinner hence, different ways of measuring RNFL thickness could yield the discrepancy.
Second, as signal strength has been demonstrated to affect RNFL thickness measurements using TD-OCT [18,19], differences in signal strength between the two OCT systems are capable of creating considerable discrepancy. In the current study, we did not adjust for signal strength as there were no reliable data regarding the correlation between signal strength and RNFL thickness changes using the Spectralis method. However, only scans with adequate signal strength and image quality were included (Stratus OCT, signal strength ≥ 6; Spectralis OCT, image quality score ≥ 16).
Third, another possibility for this discrepancy could be related to differences in detection algorithms and data analysis methods for measurements between foveal and peripapillary thicknesses. Also, in the current study, the temporal quadrant measurements demonstrated the strongest agreement between the two OCT systems; the temporal quadrant was the only sector where the RNFL thickness, as measured by Spectralis, was greater than that measured by Stratus. These findings suggest that the temporal quadrant measurements reflect the actual RNFL thickness most accurately.
It is likely that the Spectralis method provides higher resolution than the Stratus method, and may also provide more accurate measurements. However, the hypothesis that SD-OCT is more sensitive to detect decreased RNFL thickness is debatable. Despite the technological improvements of SD-OCT, in actual clinical settings, TD-OCT is still more widely used to differentiate between healthy eyes and eyes with glaucoma, due to more versatile software. Beyond technological improvements with SD-OCT, this modality should allow for more diagnostic parameters for glaucoma.
The current study has several limitations. PACG patients were not included because there were few PACG participants (nine patients) in this study. Second, as the OCT images were obtained on the same day and in a single session, this may underestimate the actual variation in measurements; nevertheless, quality assessment was carefully performed. Third, usually RNFL thickness measured with OCT was significantly correlated with age and axial length [20]. However, in this study we did not consider potential influential factors for RNFL measurements, including optic disc area and ethnic variation. Future studies should address which instrument offers greater accuracy in estimating the true RNFL thickness. Also, the reproducibility, sensitivity, and specificity between two OCT instruments needs to be further studied. More large-scale analysis trials including PACG patients and considering variant potential influential factors will be necessary in the future.
The results of the current study demonstrate that SD-OCT and TD-OCT thickness measurements are highly correlated. The correlation was particularly strong in the POAG group when compared with the normal, glaucoma suspects, and NTG groups. Although good correlation was found for all parameters, TD-OCT RNFL measurements were thicker than SD-OCT measurements; this was especially true in the POAG group. Thus, absolute measures of peripapillary RNFL thickness using these two instruments should not be considered interchangeable. The temporal quadrant was the only sector where the RNFL thickness measured by Spectralis was thicker than that measured by Stratus; this quadrant also demonstrated a greater agreement than other quadrants. It is possible that the temporal quadrant measurement is the most sensitive parameter for reflecting the true peripapillary RNFL thickness. Future studies are required to obtain more information and confirm these results.
Acknowledgements
This work was supported by the Konkuk University Medical Center Research Grant 2009.
Footnotes
This study was presented as a post at the World Glaucoma Congress meeting, July 8-11, 2009, Boston, USA.
No potential conflict of interest relevant to this article was reported.
References
- 1.Bowd C, Zangwill LM, Berry CC, et al. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci. 2001;42:1993–2003. [PubMed] [Google Scholar]
- 2.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budenz DL, Chang RT, Huang X, et al. Reproducibility of retinal nerve fiber thickness measurements using the stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–2443. doi: 10.1167/iovs.04-1174. [DOI] [PubMed] [Google Scholar]
- 4.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest Ophthalmol Vis Sci. 2004;45:1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuman JS, Pedut-Kloizman T, Pakter H, et al. Optical coherence tomography and histologic measurements of nerve fiber layer thickness in normal and glaucomatous monkey eyes. Invest Ophthalmol Vis Sci. 2007;48:3645–3654. doi: 10.1167/iovs.06-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal EZ, Parikh RS, Pe'er J, et al. Retinal nerve fibre layer imaging compared with histological measurements in a human eye. Eye (Lond) 2009;23:171–175. doi: 10.1038/sj.eye.6702942. [DOI] [PubMed] [Google Scholar]
- 7.Bowd C, Weinreb RN, Williams JM, Zangwill LM. The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch Ophthalmol. 2000;118:22–26. doi: 10.1001/archopht.118.1.22. [DOI] [PubMed] [Google Scholar]
- 8.Williams ZY, Schuman JS, Gamell L, et al. Optical coherence tomography measurement of nerve fiber layer thickness and the likelihood of a visual field defect. Am J Ophthalmol. 2002;134:538–546. doi: 10.1016/s0002-9394(02)01683-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen TC, Cense B, Pierce MC, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005;123:1715–1720. doi: 10.1001/archopht.123.12.1715. [DOI] [PubMed] [Google Scholar]
- 10.de Boer JF, Cense B, Park BH, et al. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett. 2003;28:2067–2069. doi: 10.1364/ol.28.002067. [DOI] [PubMed] [Google Scholar]
- 11.Ray R, Stinnett SS, Jaffe GJ. Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am J Ophthalmol. 2005;139:18–29. doi: 10.1016/j.ajo.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe GJ, Caprioli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol. 2004;137:156–169. doi: 10.1016/s0002-9394(03)00792-x. [DOI] [PubMed] [Google Scholar]
- 13.Cense B, Chen TC, Nassif N, et al. Ultra-high speed and ultra-high resolution spectral-domain optical coherence tomography and optical Doppler tomography in ophthalmology. Bull Soc Belge Ophtalmol. 2006;(302):123–132. [PubMed] [Google Scholar]
- 14.Nassif N, Cense B, Park BH, et al. In vivo human retinal imaging by ultrahigh-speed spectral domain optical coherence tomography. Opt Lett. 2004;29:480–482. doi: 10.1364/ol.29.000480. [DOI] [PubMed] [Google Scholar]
- 15.Knight OJ, Chang RT, Feuer WJ, Budenz DL. Comparison of retinal nerve fiber layer measurements using time domain and spectral domain optical coherent tomography. Ophthalmology. 2009;116:1271–1277. doi: 10.1016/j.ophtha.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung KR, Kim DY, Park SB, Kook MS. Comparison of retinal nerve fiber layer thickness measured by Cirrus HD and Stratus optical coherence tomography. Ophthalmology. 2009;116:1264–1270. 1270.e1. doi: 10.1016/j.ophtha.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Han IC, Jaffe GJ. Comparison of spectral- and time-domain optical coherence tomography for retinal thickness measurements in healthy and diseased eyes. Am J Ophthalmol. 2009;147:847–858. 858.e1. doi: 10.1016/j.ajo.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Vazeen M, Varma R, et al. Factors associated with variability in retinal nerve fiber layer thickness measurements obtained by optical coherence tomography. Ophthalmology. 2007;114:1505–1512. doi: 10.1016/j.ophtha.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 19.Cheung CY, Leung CK, Lin D, et al. Relationship between retinal nerve fiber layer measurement and signal strength in optical coherence tomography. Ophthalmology. 2008;115:1347–1351. 1351.e1–1351.e2. doi: 10.1016/j.ophtha.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Bendschneider D, Tornow RP, Horn FK, et al. Retinal nerve fiber layer thickness in normals measured by spectral domain OCT. J Glaucoma. 2010;19:475–482. doi: 10.1097/IJG.0b013e3181c4b0c7. [DOI] [PubMed] [Google Scholar]