Abstract
Background
Previous work demonstrated that maternal haplotypes of the β2-adrenoceptor gene (ADRB2) influence ephedrine requirements during cesarean delivery. The use of ephedrine versus a pure α-adrenergic agonist such as phenylephrine has been associated with lower umbilical artery (UA) pH, thought to be secondary to increased fetal metabolism. There are no data evaluating the effect of fetal/neonatal genotypes on the metabolic response to maternally administered vasopressors. We hypothesized that neonatal ADRB2 genotype would affect the extent of neonatal acidemia. We also examined the effect of maternal ADRB2 and the endothelial nitric oxide synthase gene (NOS3) on ephedrine and phenylephrine requirements for treatment of maternal hypotension.
Methods
The study was performed on 104 Chinese women scheduled for cesarean delivery under spinal anesthesia who were participating in a double-blinded randomized clinical trial evaluating the maternal and neonatal effects of ephedrine versus phenylephrine infusions. Blood samples were drawn from the UA, umbilical vein and maternal radial artery to measure blood gas values, lactate, ephedrine and phenylephrine concentrations, and determine maternal and neonatal genotype at non-synonymous single nucleotide polymorphisms at codons 16 (rs1042713) and 27 (rs1042714) of ADRB2 and codon 298 (rs1799983) of NOS. Clinical variables (UA pH, UA lactate and dose of vasopressors) among genotypes were compared, and regression models were created to assess the effect of genotype on vasopressor dose and fetal acid-base status.
Results
Maternal ADRB2 genotype did not affect the ephedrine dose. Neonatal genotype at codon 16 influenced fetal acid-base status. UA pH was higher in Arg16 homozygous neonates (7.31 ± 0.03 in p.16Arg/Arg vs 7.25 ± 0.11 in p.16 Arg/Gly and p.16 Gly/Gly; p < 0.001, 95% C.I of difference 0.03 ~ 0.09) and UA lactate was lower (2.67 mmol/L ± 0.99 in p.16Arg/Arg vs 4.28 mmol/L ± 2.79 in p.16 Arg/Gly and p.16 Gly/Gly; p < 0.001, 95% C.I of difference −2.40 ~ −0.82). In neonates born to mothers receiving ephedrine, the magnitude of the difference among genotypes was even greater (pH 7.30 ± 0.02 in p.16Arg/Arg vs 7.19 ± 0.10 in p.16 Arg/Gly and p.16 Gly/Gly; p < 0.001, 95% C.I of difference 0.07 ~ 0.14) and UA lactate was lower (3.66 mmol/L ± 1.30 in p.16Arg/Arg vs 5.79 mmol/L ± 2.88 in p.16 Arg/Gly and p.16 Gly/Gly; p = 0.003, 95% C.I of difference −3.48 ~ −0.80). In a multiple linear regression model (R2 = 63.6%; P = 0.03), neonatal ADRB2 genotypes (p.16Arg/Arg and p.27Gln/Glu) and lower neonatal birth weight predicted lower UA lactate concentrations.
Phenylephrine dose was not affected by maternal ADRB2 or NOS3 genotypes, and neonatal NOS3 genotype did not affect UA pH or UA lactate.
Conclusion
In contrast to previous findings in a North American cohort, maternal ADRB2 genotype did not affect ephedrine requirements during elective cesarean delivery in a Chinese cohort. However, our findings suggest that neonatal ADRB2 p.Arg16 homozygosity confers a protective effect against developing ephedrine-induced fetal acidemia.
Introduction
Spinal anesthesia-induced hypotension during cesarean delivery has been the focus of numerous clinical studies searching for the most effective and safe vasopressor to maintain maternal arterial blood pressure and avoid adverse maternal and neonatal outcomes.1,2 It has been established that ephedrine increases the risk for neonatal acidemia due to stimulation of fetal metabolism before delivery3; however, it is unclear whether the degree of neonatal acidemia is proportionate to the dose of ephedrine given to the mother. Recently gathered evidence shows that transplacental transfer of ephedrine exceeds that of phenylephrine and that ephedrine is associated with greater umbilical arterial (UA) and umbilical venous (UV) plasma concentrations of lactate, glucose, epinephrine, and norepinephrine and higher UV PCO2 compared with phenylephrine.4 These findings are consistent with the hypothesis that the underlying mechanism by which ephedrine causes neonatal acidemia is transfer of ephedrine across the placenta and stimulation of metabolic processes in the fetus.
We have previously demonstrated that genetic variability (sequence variability) of ADRB2 influences the dose of ephedrine administered to treat hypotension during elective cesarean delivery under spinal anesthesia.5 Women carrying two common haplotypes that were present in 20% of a North American cohort were found to require substantially lower doses of ephedrine. We hypothesized that while maternal genetic variability will influence ephedrine requirement, neonatal ADRB2 genotype will directly influence the degree of neonatal acidemia in response to ephedrine given to the mother before delivery. We present here the results of the genetic analysis of mothers and neonates participating in the randomized controlled trial of placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery.4
Methods
Ethics committee approval was obtained from the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee, Shatin, Hong Kong, China for the previously reported aspects of this work4 and the genetic analysis. Ethics committee approval was obtained from the University Hospitals of Geneva, Geneva, Switzerland for genetic analysis of de-identified samples. Written informed consent was obtained from all women for participation in the randomized clinical trial and genetic analysis. One hundred four women with term singleton pregnancies who were scheduled for elective cesarean delivery under spinal anesthesia were enrolled. As previously reported4, clinical management included spinal anesthesia with hyperbaric 0.5% bupivacaine (10 mg) and fentany15 μg. Women were then placed in the left-tilt supine position, and arterial blood pressure was recorded every 1 min beginning 1 min after spinal injection. Women were randomly allocated to infusions of ephedrine (8 mg/ml) or phenylephrine (100 μg/ml) according to sequentially numbered sealed opaque envelopes that each contained a computer-generated randomization code (Statview for Windows 5.0.1;SAS Institute Inc, Cary, NC). To facilitate double-blinding, the drugs were prepared in identical syringes by one of the investigators who was not involved with subsequent patient management or data collection. The vasopressors were administered by infusion using a syringe pump (Graseby 3500 Anaesthesia Pump; Graseby Medical Ltd., Watford, Herts, United Kingdom) connected via fine-bore tubing to the IV cannula by a 3-way stopcock, aiming to maintain blood pressure near the baseline value.4 Maternal radial artery (MA), UA and UV blood samples were drawn at the time of delivery, for assays including blood gas analysis and plasma concentrations of lactate, ephedrine and phenylephrine.4 As initially planned at the time of study design, an aliquot of MA blood and umbilical cord blood was obtained and sent to Geneva, Switzerland for maternal and neonatal DNA isolation and analysis.
DNA Collection and Purification, and Genotyping
Maternal arterial (3 ml, EDTA tubes) and umbilical cord (1 ml, EDTA tubes) blood samples underwent DNA purification and genotyping of ADRB2 and endothelial nitric oxide synthase (NOS3) at the University Hospitals of Geneva, Switzerland. DNA was purified by a Puregene extraction Kit (Gentra, Minneapolis, MN) and tested for quantity, purity, and quality by optical densitometry (ratio, 260/280 nm) and gel electrophoresis. For the identification of the polymorphisms of the ADRB2 gene, 60 ng DNA was amplified by polymerase chain reaction (PCR) (96-well microtiter plate block thermocycler; Biometra, Göttingen, Germany) using specific primers. Primers were chosen in single-copy DNA regions surrounding polymorphisms p.16Arg/Gly and p.27Gln/Glu located in the single exon of ADRB2 using Oligo6-primer designing software (Molecular Biology Insight, Cascade, CO) with specificity checking by sequence comparison as previously described.5 Each assay was tested for specificity and reliability by sequencing before its use for the entire cohort. Polymorphism genotypes were determined by Sanger sequencing reaction and electrophoresis on a fluorescent DNA fragment analyzer apparatus. For p.298Glu/Asp of NOS3 gene, the PCR–pyrosequencing analysis was used, as we have previously reported.6
Statistical analysis
The study was powered to detect differences in UA pH.4 Based on an anticipated difference of 0.03 in UA pH and assuming a standard deviation of 0.04, a sample size of 38 patients per group would be required to have 90% power with a two-sided value of 0.05 to detect a difference in neonatal pH between fetuses exposed to ephedrine versus phenylephrine. In anticipation that obtaining sufficient MA and umbilical cord blood could be difficult in some cases, the sample size was increased to 52 women per group. 4 Based on previous genetic studies examining the frequency of the p.16Arg/Gly and p.27Gln/Glu in various cohorts, we hypothesized that 20% of mothers/neonates would carry one of two haplotype that were previously shown to influence the response to ephedrine. 5 Therefore we anticipated at least 10 mothers/babies of 52 receiving ephedrine would be either heterozygous p.27Gln/Glu or homozygous p.27Glu/Glu.
The vasopressor dose (ephedrine or phenylephrine), time interval from induction to delivery, UA pH, and UA lactate were compared among genotypes using one-way ANOVA analysis. The paired t-test was used for comparing UA pH among genotypes (homozygous p.16Arg/Arg versus homozygotes p.16Gly/Gly - and heterozygous p.16Arg/Gly). Equal variances were assumed if p ≧ 0.05 with Levene’s test; equal variances were not assumed if p < 0.05 with Levene’s test.
Fisher’s exact test was used for comparisons of neonatal acidemia (defined as UA pH < 7.20) among genotypes. Regression models were constructed to adjust for possible confounders affecting neonatal outcomes (UA pH and UA lactate). Dummy variables were created for ADRB2 genotype, setting p.16Arg/Gly as reference for codon 16 and p.27Gln/Gln as reference for codon 27. Dependent variables were dose of vasopressor, UA pH and UA lactate. Independent variables included maternal genotypes, neonatal genotypes, neonatal birth weight, maternal weight, maternal height, body mass index, baseline diastolic blood pressure, baseline systolic blood pressure, and baseline heart rate. In addition, dose of ephedrine was used as an independent variable when UA pH and UA lactate were used as dependent variables. Data were analyzed using SPSS 18.0.0 (SPSS Chicago IL).
Results
One hundred four mothers/baby pairs participated in this study. Technical problems occurred in 10 cases during ADRB2 genotyping and in 8 mothers and 3 neonates during NOS3 sequencing. Haplotypes for ADRB2 at codon 16 and 27 are presented in Table 1 for both mothers and neonates. Due to known linkage disequilibrium between codon 16 and 27 (Glu27 almost never occurs in the presence of Arg16), only six genotype combinations (instead of the theoretical 9) were found in this cohort. Only one neonate was found to be homozygote for Glu27 (p.16Gly/Gly/p.27Glu/Glu) and he was born to a mother with p.16Arg/Gly/p.27Gln/Glu. Due to the overall rare occurrence of Glu at codon 27 in both mothers and neonates, all clinical data were analyzed according to codon 16 only and not per haplotype of codons 16 and 27 of ADRB2. Genetic distribution in mothers and neonates at codon 298 of NOS3 gene is presented in Table 2. Genotype distribution for both mothers and neonates for both ADRB2 and NOS3 appeared to be in Hardy-Weinberg equilibrium.
Table 1.
Overall distribution of ADRB2 haplotypes (codons 16 and 27)
| Genotypic combination | Mothers (N = 94) | Neonates (N = 94) |
|---|---|---|
| p.16Arg/Arg-p.27Gln/Gln | 34 (36.2%) | 32 (34.0%) |
| p.16Arg/Arg-p.27Gln/Glu | 0 | 0 |
| p.16Arg/Arg-p.27GluGlu | 0 | 0 |
| p.16ArgGly-p.27Gln/Gln | 33 (35.1%) | 40 (42.6%) |
| p.16Arg/Gly-p.27Gln/Glu | 10 (10.6%) | 6 (6.4%) |
| p.16ArgGly-p.27Glu/Glu | 0 | 0 |
| p.16Gly/Gly-p.27Gln/Gln | 10 (10.6%) | 10 (10.6%) |
| p.16Gly/Gly-p.27Gln/Glu | 7 (7.4%) | 5 (5.3%) |
| p.16Gly/Gly-p.27Glu/Glu | 0 | 1 (1.1%) |
Values presented are N = number of subjects, and percentage
Table 2.
Overall distribution of NOS3 genotype (p.298Glu/Asp)
| Genotype | Mothers (N = 96) | Neonates (N = 101) |
|---|---|---|
| p.298Glu/Glu | 69 (71.9%) | 75 (74.3%) |
| p.298Glu/Asp | 24 (25.0%) | 22 (21.8%) |
| p.298Asp/Asp | 3 (3.1%) | 4 (4.0%) |
Values presented are N = number of subjects, and percentage.
NOS3 = endothelial nitric oxide synthase.
Overall, induction-to-delivery-time was similar between maternal genotypic groups for codon 16 of ADRB2 (28.2 min ± 7.9 in p.16Arg/Arg, 30.4 min ± 10.9 in p.16Arg/Gly, 30.2 min ± 8.7 in p.16Gly/Gly). Maternal genotype at codon 16 of ADRB2 did not influence UA pH (7.27 ± 0.09 in p.16Arg/Arg, 7.29 ± 0.07 in p.16Arg/Gly, 7.26 ± 0.10 in p.16Gly/Gly, p = 0.42).
Based on neonatal genotype at codon 16 of ADRB2, neonates homozygous for Arg16 had higher UA pH values (7.31 ± 0.11 vs 7.25 ± 0.11, p < 0.001, difference of 0.06 ± 0.15, 95% C.I of difference 0.03 ~ 0.09) and lower UA lactate concentrations with an order of magnitude of 30% (2.67 mmol/L ± 0.99 vs 4.28 ± 2.79, with a difference of −1.61 ± 0.40, 95% C.I −2.40 ~ −0.82) compared to neonates carrying the two other genotypes (Table 3). There was no difference in UA lactate between p.16Arg/Arg neonates and p.16Gly/Gly neonates (no dose-gene effect, p = 0.74).
Table 3.
Fetal acid-base status according to neonatal ADRB2 codon 16 genotype
| p.16Arg/Arg | p.16Arg/Gly | p.16Gly/Gly | |
|---|---|---|---|
| All Neonates | N = 32 | N = 46 | N = 16 |
| UA pH | 7.31 ± 0.03§ | 7.24 ± 0.12 | 7.27 ± 0.07 |
| UA lactate (mmol/L) | 2.67 ± 0.99§§ | 4.55 ± 3.05 | 3.51 ± 1.71 |
Values are mean ± standard deviation.
One-way ANOVA, post hoc test: Bonferroni, and paired t-test.
p.16Arg/Arg different from p.16Arg/Gly, p = 0.001 (95% C.I of difference 0.03 ~ 0.10); p.16Arg/Arg different from p.16Arg/Gly and p.16Gly/Gly combined (7.25± 0.11; p < 0.001, 95% C.I of difference 0.03 ~ 0.09).
p.16Arg/Arg different from p.16Arg/Gly, p < 0.001 (95% C.I of difference −2.85 ~ −0.91); p.16Arg/Arg different from p.16Arg/Gly and p.16Gly/Gly combined (4.28± 2.79; p < 0.001, 95% C.I of difference −2.40 ~ −0.82).
UA = umbilical artery.
In the ephedrine group (N = 45), maternal genotype of ADRB2 at codon 16 did not affect UA pH (7.23 ± 0.11 in p.16Arg/Arg, 7.23 ± 0.08 in p.16Arg/Gly, 7.18 ± 0.09 in p.16Gly/Gly, p = 0.39). Maternal genotype of ADRB2 at codon 16 did not affect the dose of administered ephedrine (Table 4) and neither maternal nor neonatal genotype affected the concentration of ephedrine in the UA, UV, or MA, or the UA/UV or UV/MA ratios of ephedrine concentration. Ten neonates were Arg16 homozygotes; pH values at birth were available for 9 neonates. Of these 9 Arg16 homozygous neonates, none had a pH lower than 7.28. In the 35 neonates carrying the Gly16 allele, 17 (49%) where found to have a pH≤ 7.20. In comparing Arg16 homozyous neonates and neonates with the two other genotypes, neonatal acidosis defined as pH<7.20 was significantly less frequent with Arg16 homozygosity (p = 0.008, Fisher’s exact test). In the linear regression model, the dose of ephedrine administered to the mother was associated with fetal acidemia (lower UA pH) in neonates carrying one or two Gly16 alleles (p = 0.002, r = −0.50); however, this association was not present in neonates who were Arg16 homozygous (p = 0.12, r = −0.56) (Figure 1).
Table 4.
Ephedrine group
| p.16Arg/Arg | p.16Arg/Gly | p.16Gly/Gly | |
|---|---|---|---|
| Mothers | N = 18 | N = 19 | N = 8 |
| Dose of ephedrine (mg) | 61 ± 26 | 72 ± 20 | 60 ± 29 |
| MA ephedrine concentration (ng/ml) | 368.3 ± 186.4 | 449.2 ± 144.9 | 428.0 ± 243.2 |
| UV ephedrine concentration (ng/ml) | 449.9 ± 250.9 | 488.2 ± 156.2 | 442.3 ± 122.2 |
| UA/UV ratio | 0.83 ± 0.17 | 0.83 ± 0.12 | 0.77 ± 0.11 |
| UV/MA ratio | 1.22 ± 0.27 | 1.09 ± 0.16 | 1.26 ± 0.24 |
| Neonates | N = 10 | N = 28 | N = 7 |
| UA ephedrine concentration (ng/ml) | 374.0 ± 204.4 | 402.8 ± 211.0 | 314.5 ± 101.0 |
| UV ephedrine concentration (ng/ml) | 406.7 ± 191.2 | 481.3 ± 223.9 | 384.7 ± 163.6 |
| UA/UV ratio | 0.90 ± 0.15 | 0.86 ± 0.20 | 0.83 ± 0.13 |
| UV/MA ratio | 1.06 ± 0.21 | 1.19 ± 0.26 | 1.13 ± 0.14 |
| N = 9 | N = 28 | N = 7 | |
| UA pH | 7.30 ± 0.02§ | 7.18 ± 0.11 | 7.22 ± 0.07 |
| UA lactate (mmol/L) | 3.66 ± 1.30§§ | 6.10 ± 3.04 | 4.60 ± 1.86 |
Values are mean ± standard deviation.
One-way ANOVA, post hoc test: Bonferroni, and paired t-test.
p.16Arg/Arg different from p.16Arg/Gly p < 0.001 (95% C.I of difference 0.07 ~ 0.16); p.16Arg/Arg different from p.16Arg/Gly and p.16Gly/Gly combined (7.19± 0.10; p < 0.001, 95% C.I of difference 0.07 ~ 0.14).
p.16Arg/Arg different from p.16Arg/Gly, p = 0.002 (95% C.I of difference −3.92 ~ −0.96); p.16Arg/Arg different from p.16Arg/Gly and p.16Gly/Gly combined (5.79 ± 2.88; p = 0.003, 95% C.I of difference −3.48 ~ −0.80)
UA = umbilical artery; MA = maternal artery; UV = umbilical vein.
Figure 1. Regression between uterine artery (UA) pH and ephedrine dose (mg) according to neonatal ADRB2 genotype at codon 16.
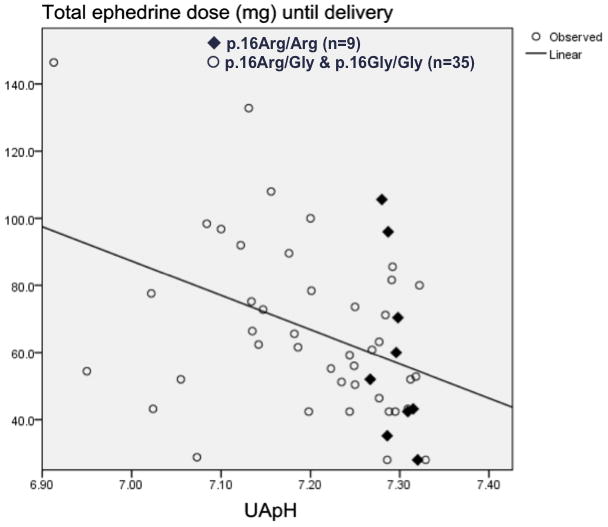
The X-axis represents UA pH, Y-axis represents total ephedrine dose (mg) given to the mothers from the time of the spinal injection until delivery of the baby.
The line represents the “fit line” for p.16Arg/Gly and p.16Gly/Gly.
Using Pearson’s correlation rank, UA pH and ephedrine dose were correlated in p.16Arg/Gly and p.16Gly/Gly neonates (p = 0.002, r = −0.50), but not in p.16Arg/Arg neonates (p = 0.12, r = −0.56).
◆ p.16Arg/Arg (N = 9) (r2 = 31.2%, p = 0.12)
○ p.16Arg/Gly and p.16Gly/Gly (N = 35) (r2 = 24.9%, p = 0.002)
No Arg16 homozygous neonate had UA pH < 7.28.
In a multiple linear regression model in the ephedrine group (R2 = 63.6%; P = 0.03), only neonatal genotypes at codon 16 (p.16Arg/Arg, partial correlation coefficient = −0.54, P = 0.008) and 27 (heterozygous p.27Gln/Gln, partial correlation coefficient = −0.50, P = 0.02) and lower neonatal birth weight (partial correlation coefficient = 0.52, P = 0.01) had significant effects on UA lactate (lower UA lactate concentrations). Other factors (maternal genotype at codon 16 and 27 of ADRB2, dose of ephedrine, weight, height, body mass index, baseline systolic or diastolic blood pressure, and heart rate) were not significant predictors of UA pH (R2 = 53.5%; P = 0.15). (Supplemental Digital Content 1, illustrating the regression models with ADRB2 and NOS3). In the phenylephrine group (N = 49), the dose of phenylephrine administered to maintain baseline maternal blood pressure was not affected by maternal genotype at codon 16 of ADRB2 (Table 5). Maternal ADRB2 genotype at codon 16 did not predict UA pH (7.32 ± 0.04 in p.16Arg/Arg, 7.33 ± 0.03 in p.16Arg/Gly, 7.32 ± 0.05 in p.16Gly/Gly, p = 0.56). Neonatal ADRB2 genotype at codon 16 did not affect UA pH or UA lactate concentrations.
Table 5.
Phenylephrine Group
| p.16Arg/Arg | p.16Arg/Gly | p.16Gly/Gly | |
|---|---|---|---|
| Mothers | N = 16 | N = 24 | N = 9 |
| Dose of phenylephrine (μg) | 1342 ± 436 | 1403 ± 494 | 1260 ± 691 |
| Neonates | N = 22 | N = 18 | N = 9 |
| UA pH | 7.32 ± 0.03 | 7.34 ± 0.03 | 7.31 ± 0.05 |
| UA lactate (mmol/L) | 2.27 ± 0.43 | 2.12 ± 0.40 | 2.67 ± 1.02 |
Values are mean ± standard deviation.
One-way ANOVA, post hoc test: Bonferroni, and paired t-test. There were no differences among groups.
UA = umbilical artery.
Overall, UA pH was neither predicted by maternal NOS3 genotype (7.30 ± 0.02 in p.298Glu/Glu, 7.27 ± 0.10 in p.298Glu/Asp, 7.27 ± 0.10 in p.298Asp/Asp, p = 0.78), nor by neonatal NOS3 genotype (7.27 ± 0.09 in p.298Glu/Glu, 7.27 ± 0.11in p.298Glu/Asp, 7.26 ± 0.05 in p.298Asp/Asp, p = 0.98) (Table 6).
Table 6.
Outcomes according to NOS3 genotype
| Ephedrine Group | ||
|---|---|---|
| p.298Glu/Glu | p.298Glu/Asp and p.298Asp/Asp | |
| Mothers | N = 34 | N = 13 |
| Dose of ephedrine (mg) | 64.1 ± 24.8 | 66.8 ± 32.4 |
| Neonates | N = 37 | N = 12 |
| UA pH | 7.21 ± 0.10 | 7.22 ± 0.12 |
| UA lactate (mmol/l) | 5.40 ± 2.71 | 4.77 ± 2.64 |
| Phenylephrine Group | ||
| Mothers | N = 35 | N = 14 |
| Dose of phenylephrine (μg) | 1356.6 ± 496.4 | 1252.9 ± 566.2 |
| Neonates | N = 38 | N = 12 |
| UA pH | 7.33 ± 0.04 | 7.32 ± 0.04 |
| UA lactate (mmol/l) | 2.25 ± 0.61 | 2.37 ± 0.50 |
Values are mean ± standard deviation.
Paired t-test. There were no differences among groups.
UA = umbilical artery
Discussion
This Chinese cohort of healthy women scheduled for cesarean delivery had a distribution of ADRB2 genotypes and allele (genotype) combination at codon 16 and 27 that was significantly different from that described in other obstetrical cohorts.5,7,8 In particular, in our previous work assessing hypotension and vasopressor requirement in a North American cohort of women undergoing spinal anesthesia for cesarean delivery, 5 20% of women carried at least one Glu27 allele (heterozygous p.27Gln/Glu or homozygous p.27Glu/Glu). In this current cohort of Chinese women, only 7% of women were found to be heterozygous at codon 27 and no mother was Glu27 homozygous. A comparison between the American and Chinese cohorts reveals an overall haplotype distribution with a significant difference (p < 0.001). This relatively low occurrence of Glu27 homozygosity among Chinese cohorts has been reported.9,10
Another significant finding, also in contrast with our findings in the North American cohort, is that genotype of ADRB2 did not influence the dose of ephedrine administered to maintain maternal blood pressure during spinal anesthesia for cesarean delivery. Our previous work had described a presumed pharmacogenetic effect of ADRB2, with Glu27 carriers requiring lower doses of ephedrine to treat spinal hypotension.5 There are several possible explanations for these discrepant findings. The difference in genotype distribution according to ethnic background could explain why we could not find a pharmacogenetic effect in this Chinese group, because the two combinations (p.16Gly/Gly-p.27Gln/Glu and p.16Gly/Gly-p27Glu/Glu) that were found to reduce the ephedrine requirement in the North American cohort were “under-represented” in the current study. Alternatively, the dose response to adrenoceptor-agonists could be attenuated in Asians.11
Second, ephedrine was not given in a similar manner in both studies (bolus in the North American cohort versus continuous infusion in the current study) and the criteria applied for treatment of hypotension, and therefore targeted systolic blood pressure, were different (systolic blood pressure decrease more than 20% or to less than 90 mmHg in the North American study versus near baseline values in the current study). As a consequence, the total ephedrine dose was significantly higher in the current study compared to the doses used in our previous work, and this strategy might overwhelm differences among genotype groups. It is also of course possible that our previous finding was a Type 1 error (false positive) and there is no effect of ARDB2 genotype on ephedrine requirements.
The most clinically relevant and intriguing finding was that UA pH was overall higher and UA lactate was lower in neonates that were Arg16 homozygous as compared to neonates with the two other genotypes of ADRB2. Furthermore, among babies born to mothers receiving ephedrine, ephedrine dose was associated with neonatal acidemia (decreased UA pH) only in neonates carrying a Gly16 allele, but not in neonates who were Arg16 homozygous. Since there was no significant difference in ephedrine concentration as determined by maternal and umbilical cord assays (MA, UA, UV or ratio of UA/UV and UV/MA) among genetic groups, any difference in metabolic markers are unlikely to have resulted from differential transplacental transfer of drug or a pharmacokinetic effect. Arg16 homozygous neonates seem to be protected from the risk of developing acidemia when exposed to ephedrine, irrespective of the dose given to the mother. Previous studies have demonstrated differential metabolic responses based on ADRB2 genotype,12–14 so it is reasonable to postulate that such genetic variants in the fetus could lead to altered responses to a given dose of a cardiac and vascular stimulant such as ephedrine.
One potential mechanistic explanation for the apparent protective effect of Arg16 homozygosity against neonatal acidemia could be increased desensitization of the β-adrenergic receptor in response to ephedrine. Arg16 homozygous individuals have been shown to undergo rapid desensitization in response to β-agonists;15 therefore, it is possible that the continuous infusion of ephedrine could have resulted in a greater degree of desensitization, i.e., tachyphylaxis, in p.16Arg/Arg neonates. It should be noted that there is considerable controversy in the literature regarding the assumption of increased desensitization of Arg16 homozygotes in vivo,12,15 and the time course of desensitization in vivo is unclear. Therefore, conclusions about mechanism must be viewed as preliminary.
Genetic distribution of NOS3 p.298Glu/Asp was similar to previous reports in a Chinese population.16 A pharmacogenetic effect of NOS3 with an enhanced response to phenylephrine in subjects carrying the Asp298 allele has been shown in a study in Caucasian patients undergoing cardiopulmonary bypass.17 We did not find a difference in phenylephrine dose according to NOS3 genotype in this study, although our study was underpowered for this clinical outcome due to the low prevalence of the rare variant in this ethnic group. Other considerations include the very different study population and conditions (healthy pregnant women undergoing cesarean delivery rather than a cardiac population undergoing cardiopulmonary bypass), ethnicity (Chinese rather than Caucasian), and different mode of phenylephrine administration (infusion in our study rather than increasing bolus dosing) and the targeted blood pressure. Neonatal acidemia and other markers of fetal metabolism were also not associated with any specific genotype of NOS3 in either the phenylephrine or ephedrine groups. Based on our findings, NOS3 genotype may not play an important role in determining the response to phenylephrine given as an infusion to maintain baseline systolic blood pressure in pregnant Chinese women under spinal anesthesia.
Obvious limitations of this study relate to the overall small sample of patients. In addition, the possible effect of p.27Gln/Glu on maternal ephedrine response could not be examined because contrary to our expectations, no mother was found to be homozygous for Glu at codon 27 (only 5/9 possible combined genotypes were found in this cohort instead of the expected 6/9). Furthermore, due to the study design, only half of the neonates were exposed to ephedrine, and a smaller proportion of neonates exposed to ephedrine were found to be Arg16 homozygotes (10/45) as compared to the proportion of Arg16 homozygous neonates in the phenylephrine group (22/49). Thus, although we found that Arg16 homozygous neonates had higher pH values, and none had a pH below 7.28 we must acknowledge that this neonatal cohort (Arg16 neonates receiving ephedrine) only consisted of 9 babies. The proportion of Arg16 homozygous women was similar in the ephedrine (18/45) and phenylephrine groups (16/49), which was expected since women were randomly assigned to one treatment group or the other, so this uneven distribution of neonatal genotype is almost certainly a chance occurrence.
In conclusion, maternal genotypes of ADRB2 and NOS3 did not impact on the total dose of ephedrine or phenylephrine infusions administered to maintain maternal systolic blood pressure close to baseline during spinal anesthesia for cesarean delivery in a healthy cohort of Chinese women. Neonatal genotype and birth weight and not maternal genotypes or ephedrine dose were found to predict acid-base status and neonatal acidemia. Neonatal homozygosity for Arg16 of ADRB2, which was found to occur in more than 30% of babies in this Chinese cohort, seemed to protect from the risk of developing neonatal acidemia when mothers were treated with ephedrine. Whether this finding is specific to this Chinese cohort and can be replicated in this or other ethnic groups remains to be determined.
Supplementary Material
Acknowledgments
Funding: This project was funded by Swiss National Foundation Grant (SNF #3200B0-114129) and National Institutes of Health R01HD48805 (RMS).
Footnotes
The authors declare no conflicts of interest.
Name: Ruth Landau, MD
Contribution: Design of genetic portion of the study, data analysis, manuscript preparation
Name: Shih-Kai Liu, MD
Contribution: Data analysis, manuscript preparation
Name: Jean-Louis Blouin, PhD
Contribution: Conduct of genetic analysis, data analysis, manuscript preparation
Name: Richard M. Smiley, MD, PhD
Contribution: Data analysis, manuscript preparation
Name: Warwick D. Ngan Kee, MBChB, MD
Contribution: Study design, conduct of study, data analysis, manuscript preparation
Contributor Information
Ruth Landau, Department of Anesthesia and Pain Medicine, University of Washington, Seattle, WA.
Shih-Kai Liu, Department of Anesthesia and Pain Medicine, University of Washington, Seattle, WA.
Jean-Louis Blouin, Genetic Medicine, University Hospitals of Geneva, Geneva, Switzerland.
Richard M. Smiley, Department of Anesthesiology, Columbia University College of Physicians and Surgeons, NY.
Warwick D. Ngan Kee, Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Hong Kong, China.
References
- 1.Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2002;97:1582–90. doi: 10.1097/00000542-200212000-00034. [DOI] [PubMed] [Google Scholar]
- 2.Ngan Kee WD. Prevention of maternal hypotension after regional anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2010;23:304–9. doi: 10.1097/ACO.0b013e328337ffc6. [DOI] [PubMed] [Google Scholar]
- 3.Ngan Kee WD, Lee A. Multivariate analysis of factors associated with umbilical arterial pH and standard base excess after Caesarean section under spinal anaesthesia. Anaesthesia. 2003;58:125–30. doi: 10.1046/j.1365-2044.2003.02888.x. [DOI] [PubMed] [Google Scholar]
- 4.Ngan Kee WD, Khaw KS, Tan PE, Ng FF, Karmakar MK. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–12. doi: 10.1097/ALN.0b013e3181b160a3. [DOI] [PubMed] [Google Scholar]
- 5.Smiley RM, Blouin JL, Negron M, Landau R. β2-adrenoceptor genotype affects vasopressor requirements during spinal anesthesia for cesarean delivery. Anesthesiology. 2006;104:644–50. doi: 10.1097/00000542-200604000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Landau R, Xie HG, Dishy V, Wood AJ, Stein CM, Smiley RM. No association of the Asp298 variant of the endothelial nitric oxide synthase gene with preeclampsia. Am J Hypertens. 2004;17:391–4. doi: 10.1016/j.amjhyper.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Landau R, Morales MA, Antonarakis SE, Blouin JL, Smiley RM. Arg16 homozygosity of the β2-adrenergic receptor improves the outcome after beta2-agonist tocolysis for preterm labor. Clin Pharmacol Ther. 2005;78:656–63. doi: 10.1016/j.clpt.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Landau R, Xie HG, Dishy V, Stein CM, Wood AJ, Emala CW, Smiley RM. β2-Adrenergic receptor genotype and preterm delivery. Am J Obstet Gynecol. 2002;187:1294–8. doi: 10.1067/mob.2002.128524. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell TJ, Ameyaw MM, Pritchard S, Thornton N, Folayan G, Githang’a J, Indalo A, Tariq M, Mobarek A, Evans DA, Ofori-Adjei D, Templeton AR, McLeod HL. β2adrenergic receptor genotypes and haplotypes in different ethnic groups. Int J Mol Med. 2005;16:573–80. [PubMed] [Google Scholar]
- 10.Xie HG, Stein CM, Kim RB, Xiao ZS, He N, Zhou HH, Gainer JV, Brown NJ, Haines JL, Wood AJ. Frequency of functionally important β2adrenoceptor polymorphisms varies markedly among African-American, Caucasian and Chinese individuals. Pharmacogenetics. 1999;9:511–6. [PubMed] [Google Scholar]
- 11.Kapoor C, Singarajah C, Zafar H, Adubofour KO, Takahashi B, Vajo Z, Dachman WD. Impaired β2-adrenergic agonist-induced venodilation in Indians of Asian origin. Clin Pharmacol Ther. 1996;59:569–76. doi: 10.1016/S0009-9236(96)90185-X. [DOI] [PubMed] [Google Scholar]
- 12.Hesse C, Eisenach JH. Genetic variation in the beta(2)-adrenergic receptor: impact on intermediate cardiovascular phenotypes. Curr Pharmacogenomics Person Med. 2008;6:160–70. doi: 10.2174/1875692110806030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder EM, Beck KC, Dietz NM, Eisenach JH, Joyner MJ, Turner ST, Johnson BD. Arg16Gly polymorphism of the β2-adrenergic receptor is associated with differences in cardiovascular function at rest and during exercise in humans. J Physiol. 2006;571:121–30. doi: 10.1113/jphysiol.2005.098558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenach JH, Barnes SA, Pike TL, Sokolnicki LA, Masuki S, Dietz NM, Rehfeldt KH, Turner ST, Joyner MJ. Arg16/Gly β2-adrenergic receptor polymorphism alters the cardiac output response to isometric exercise. J Appl Physiol. 2005;99:1776–81. doi: 10.1152/japplphysiol.00469.2005. [DOI] [PubMed] [Google Scholar]
- 15.Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM, Wood AJ. The effect of common polymorphisms of the β2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–5. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 16.Tso AW, Tan KC, Wat NM, Janus ED, Lam TH, KSLL Endothelial nitric oxide synthase G894T (Glu298Asp) polymorphism was predictive of glycemic status in a 5-year prospective study of Chinese subjects with impaired glucose tolerance. Metabolism. 2006;55:1155–8. doi: 10.1016/j.metabol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Philip I, Plantefeve G, Vuillaumier-Barrot S, Vicaut E, LeMarie C, Henrion D, Poirier O, Levy BI, Desmonts JM, Durand G, Benessiano J. G894T polymorphism in the endothelial nitric oxide synthase gene is associated with an enhanced vascular responsiveness to phenylephrine. Circulation. 1999;99:3096–8. doi: 10.1161/01.cir.99.24.3096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


