Abstract
We investigated the effects of mesenchymal stem cells (MSCs) on wound healing using a three-dimensional (3D) collagen gel scaffold. Three circular full-thickness skin defects were created on the back of Sprague-Dawley rats. One site was covered with a 3D collagen gel containing 2 × 106 MSCs (MSCs+/3D collagen+). Another site was replaced with a 3D collagen gel without MSCs and the third site was left empty. The wound size was significantly reduced in the MSCs+/3D collagen+ sites. MSCs+/3D collagen+ sites exhibited the most neovascularization. FISH showed that Y-chromosome possessing cells were found within the dermis of MSCs+/3D collagen+ sites. Gelatin zymography revealed that the most intense expression of MMP-9 was detected early in the MSCs+/3D collagen+ sites. Our results indicate that MSCs upregulate the early expression of MMP-9 which induces the early mobilization of VEGF. Thus, MSCs appear to accelerate significantly wound healing via early activation of MMP-9 and VEGF.
Keywords: Mesenchymal Stem Cells, Wound Healing
INTRODUCTION
Wound healing requires the complex biomolecular processes of cell migration, proliferation, angiogenesis, and extracellular matrix remodeling (1, 2). This process involves various cells, growth factors, and the extracellular matrix (ECM). During wound healing, cell migration and tissue remodeling necessitate degradation of ECM and release of growth factors (3). The proteolytic degradation of ECM is achieved by extracellular proteases involving the matrix metalloproteinases (MMPs). These MMPs, particularly MMP-9, are thought to play a critical role in degrading ECM to facilitate the necessary initial steps for angiogenesis. The high expression of MMPs stimulates the angiogenic response, subsequently promoting the process of wound healing.
Angiogenesis is subjected to a complex control system with pro-angiogenic and anti-angiogenic factors. One of the most important pro-angiogenic factors is vascular endothelial growth factor (VEGF). During the early period of wound healing, angiogenic capillary sprouts penetrate into the provisional matrix and create microvascular networks (2). Neovascularizaton involves the dynamic interactions of cells, growth factors, and the matrix. VEGF stimulates and increases neovascularization.
Bone marrow-derived mesenchymal stem cells (BM-MSCs) have the capacity to differentiate along three essential mesenchymal lineages: osteoblasts, adipocytes, chondroblasts (4, 5). Also, BM-MSCs can express the phenotypic genes of hepatocytes, cardiomyocytes, astrocytes, neurons, and skin-related tissue (6-10). There have been reports showing wound healing effectively achieved with the application of BM-MSCs (11, 12). However, despite increasing knowledge of MSCs, their contribution to wound healing is not understood, and many questions remain.
We selected the 3D collagen allograft model, which is most similar to the skin dermis structure, to apply rat MSCs in the collagen gel scaffold composed of rat tail type I collagen in an attempt to maintain the functions of stem cells for a prolonged period. The objective of our study was to investigate the effects of MSCs on wound healing using 3D collagen gel scaffold in vivo.
MATERIALS AND METHODS
Experimental animal model
Eighteen adult female Sprague-Dawley rats, weighing 200 g, were used at 6 weeks of age. All animal procedures were approved under the guidelines approved by the institutional animal care and use committee from Soonchunhyang University. This study was carried out from March 2006 to May 2007 at the Soonchunhyang University. Each rat had free access to both sterile water and standard rodent soft chow ad libitum.
Harvest and culture of MSCs
The bone marrow was harvested from specific pathogen-free, 6-week-old male Sprague-Dawley rats (Charles River Technology). In brief, the rats were killed, and femurs and tibias were removed aseptically. A hole was then created in the knee joint end of each bone with a 26-gauge needle, and marrow was flushed with phosphate buffered saline (PBS, Gibco BRL, Grand Island, NY, USA) containing 2% fetal bovine serum (FBS, Gibco BRL). The marrow solution was washed by centrifugation at 400 × g for 5 min. The supernatant was aspirated, and the pellet was resuspended in Dulbecco's modified Eagle's medium-low glucose (DMEM-LG, Gibco BRL) containing 10% FBS and 1% penicillin-streptomycin (Gibco BRL). Cells were seeded into 75 cm2 tissue culture flask and cultured in humidified 5% CO2 incubator at 37℃ for 72 hr. Non-adherent cells were removed. When adherent cells reached subconfluence, they were detached with 0.25% trypsin-EDTA solution, and re-seeded at 2 × 103 cells/cm2. The rat MSCs were used for experiments after the passage 5.
Differentiation of MSCs
For osteogenic differentiation of MSCs, MSCs were maintained with DMEM medium containing 0.1 µM dexamethasone (Sigma, St. Louis, MO, USA), 50 µM ascorbate (Sigma), 10 mM β-glycerol phosphate (Sigma), and 10% FBS for 3 weeks, with the increase of calcium examined using Von Kossa staining.
For adipogenic differentiation of MSCs, MSCs were cultured in DMEM medium (Adipogenic induction, MDI + I) containing 0.1 µM dexamethasone (Sigma), 10 µg/mL insulin (Sigma), 100 mM indomethacin (Sigma), 0.5 mM methyl-isobuthylxanthine (Sigma), and 10% FBS for 72 hr. The medium was changed to DMEM medium (Adipogenic maintenance, AM), containing insulin (10 µg/mL) and 10% FBS for 24 hr. The cells were then re-treated with MDI + I for a second treatment. The cultures were then maintained in AM for 1 week before fixation. Adipogenic differentiation was demonstrated by the accumulation of lipid vesicles with Oil-Red O staining.
For chondrogenic differentiation of MSCs, MSCs were treated with trypsin-EDTA. Then, 2 × 105 cells were added to 500 µL of DMEM medium containing 10-7 M dexamethasone (Sigma), 1 µg/mL TGF-β (R&D Systems, Minneapolis, MN, USA), and 50 µM ascorbic acid 2-phosphate (Sigma), and centrifuged at 1,000 rpm for 5 min. The cells were grown as a pelleted micromass for 3 weeks. The cell pellet was stained with toluidine blue.
Flow cytometric analysis of MSCs
MSCs were trypsinized, washed with PBS, and incubated with antibodies against fluorescein isothiocyanate (FITC)-conjugated CD44H (Pgp-1, BD Biosciences Pharmingen), CD90 (Thy-1, BD), CD11b (Integrin, BD), phycoerythrin (PE)-conjugated CD106 (VCAM-1, BD), and CD45 (BD). Analysis was performed with a flow cytometer (Beckman Coulter, Inc., CA, USA).
Preparation of the three-dimensional collagen gel scaffold
Collagen gel scaffolds were prepared in a sterile tube, by mixing an appropriate volume of rat tail type I collagen (BD Biosciences) to yield a final concentration of 1 mg/mL with medium cocktail (DMEM, NaHCO3 [44 mM/L], L-glutamine [4 mM/L], and folic acid [9 mM/L], neutralized with 1N NaOH to pH 7.2). Collagen solution (1 mL per well of a 6-well plate) was placed in an incubator at 37℃ for 30 min to allow polymerization of the collagen.
Assembly of stem cell/collagen tissue construct implants
Control scaffolds were prepared as above. Early passage primary rat MSCs (2 × 106/well) were placed on top of each collagen scaffold and allowed to attach. After attachment, collagen scaffolds were covered with 1 mL of media (DMEM, 10% FBS) and incubated for 72 hr at 37℃, and 5% CO2. The circular MSCs/3D collagen gel scaffolds, at a density of 2 × 106 cells for implantation, were 20-mm diameter.
Surgical procedures
In an aseptic surgical room, the rats were anesthetized with intraperitoneal ketamine (60 mg/kg) and xylazine (8 mg/kg). After induction of general anesthesia, the dorsal regions of the rats were shaved. The dorsal skin was disinfected with 10% povidoneiodine and 70% alcohol. Three circular full-thickness defects 20-mm in diameter, including the panniculus carnosus muscle, were created on the back of each rat. One site was covered with 3D collagen gel containing 2 × 106 male rat MSCs (MSCs+/3D collagen+ site). Another site replaced with 3D collagen gel without MSCs (MSCs-/3D collagen+) and the third site was left empty (MSCs-/3D collagen-). All wounds were covered with a sterile transparent silicone membrane (Tegaderm, 3M Health Care, St. Paul, MN, USA) to prevent desiccation. Each animal was then housed in its own cage to avoid damage of the wound. Digital pictures were taken to visualize the wound. Wound healing was calculated based on the remaining wound area.
The rats were sacrificed with an overdose of sodium pentobarbital at either 3, 7, 14, or 21 days after wounding. Full-thickness wound tissues, including the adjacent skin, were then harvested, fixed immediately in 10% formalin for 12 hr, embedded in paraffin and sliced into 6-micrometer-thick sections for subsequent immunohistochemistry. For positive controls, normal healthy rat tissues were harvested.
Measurement of wound size
The wound sizes in a standard prone position were photographed using a 334 million d.p.i. digital camera (Canon) and an image analyzer (Image Pro Plus, ver. 5.1; Media Cybernetics) on a personal Microsoft computer. The macroscopic sizes of the remaining wounds were calculated three times for each animal at 0, 3, 7, 14, and 21 days after initial wounding.
Immunohistochemical staining
Paraffin sections were deparaffinized with xylene, rehydrated with 10 mM citrate buffer (pH 6.0) solution, and then treated in a microwave oven for 15 min to restore antigens. To suppress the reaction of endogenous peroxidase within the tissues, the samples were treated with 3% peroxide for 5 min and then with a blocking solution for 30 min. Slides were then placed in a humid chamber and incubated for 60 min with the anti-VEGF antibody (sc-152; Santa Cruz Biotec., Santa Cruz, CA, USA). Tissue staining was visualized with a 3,3'-diaminobenzidine (ACK500; ScyTek, Logan, UT, USA) substrate chromogen solution.
Gelatin zymography
Protein samples (20 µg of protein) were mixed with 2× sample buffer (62.5 mM Tris-HCl pH 6.8, 10% glycerol, 2% SDS, 0.0025 bromphenol blue) and added to the gel without boiling. MMP-2 and MMP-9 activities were analyzed using 10% zymogram gelatin gel (EC6175Box, Invitrogen). Following electrophoresis, gels were washed twice, first with a 1 × renaturing buffer (1 M Tris-HCl [pH 7.4], Triton X-100) for 30 min, after the gel was washed with distilled water for 30 min. Next, gels were reacted with a 1 × developing buffer (50 mM Tris base, 40 mM HCl, 200 mM NaCl, 5 mM CaCl2, 0.02% [w/v] Brij35) for 30 min, by incubating overnight at 37℃. Gels were treated with 0.5% Coomassie blue R-250 in destaining solution (40% ethanol, 8% acetic acid) for 3 hr and destained in the same solution without Coomassie blue.
X and Y chromosome fluorescence in situ hybridization
X and Y chromosome fluorescence in situ hybridization (FISH) was performed in frozen tissues in order to confirm the existence of stem cells. Probes for rat DNA (ID Labs Inc.) were applied. Briefly, six-micrometer thick frozen tissue sections were rehydrated for 5 min each in 100% EtOH, 90% EtOH, and 70% EtOH, and washed in 4 × standard saline citrate (SSC)/0.5% Tween 20 for 30 min in rotating shaker. Pre-fixated slides were incubated in a solution of protease (32-801260, VYSIS™) for 20 min at 37℃, and washed in PBS, PBS/MgCl2, and formaldehyde/PBS/MgCl2. Slides were dehydrated in 70%, 90%, and 100% EtOH and dried in air. Ten µL probe was dropped onto the slide, and sealed with a cover slip and rubber cement. Pre-treated sections and probes were denatured at 80℃ for 5 min and incubated in an incubator for 4 hr at 37℃. Post-hybridization washes were performed for 2 min in a 0.4 × SSC solution and 30 sec in 2 × SSC/0.05% Tween 20. Tissue sections were counter-stained with 7 µL of 4',6-diamidino-2-phenylindole (DAPI). Results were analyzed under a fluorescent microscope and VYSIS™ direct-labeled FISH probe (VYSIS™, USA).
Statistical analyses
Data were evaluated by analysis of variance (Stacel, add-in software to Microsoft® Excel 2000). Statistical analyses were performed using the one-way ANOVA followed by Scheffé's multiple-comparison test, and P value of less than 0.05 was considered statistically significant.
RESULTS
Characterization of cultured rat MSCs
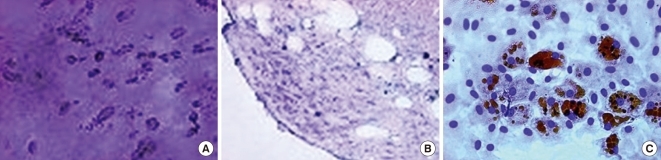
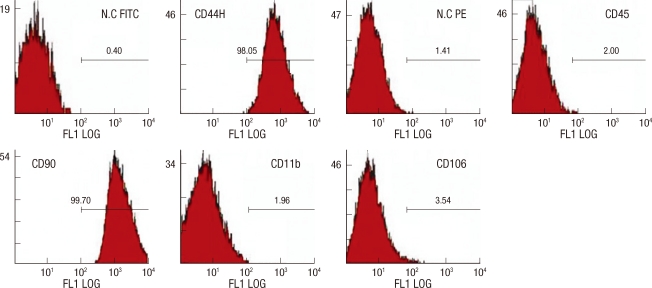
When cultured in osteogenic, chondrogenic, and adipogenic medium, they differentiated into osteocytes, chondrocytes, and adipocytes (Fig. 1). Flow cytometer analysis confirmed expression of stem cell-related surface markers. Cultured adherent cells expressed CD44H and CD90, but not hematopoietic markers such as CD11b, CD45, and CD106 (Fig. 2). Thus, we confirmed that the adherent cells were mesenchymal stem cells.
Fig. 1.
Characterization of MSCs in vitro. Differentiation of MSCs. (A) Cultured in appropriate differentiate media, MSCs differentiated osteocytes. (B) Chondrocytes in pellet culture which were positive for toluidine blue. (C) Adipocytes were demonstrated by the accumulation of lipid vesicles with oil red O staining (original magnification, × 40).
Fig. 2.
Flow cytometer analysis of rat mesenchymal stem cells. MSCs were analyzed by flow cytometer after staining with FIFC- or PE-conjugated antibodies against indicated cell surface proteins. MSCs did not produce CD11b, CD45, CD106 antigens, but produced CD44H, CD90 antigens.
Wound size measurements
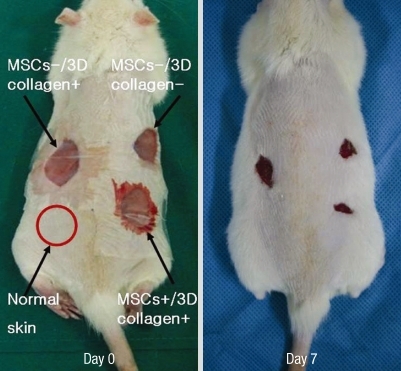
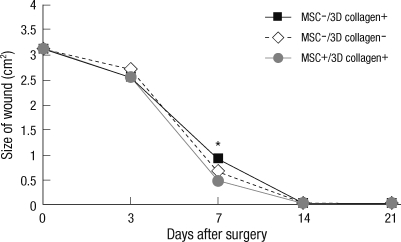
Each experimental site was relatively of the same size at day 3 due to excision of the panniculus carnosus muscle. The most effective wound healing was observed at day 7 (Fig. 3). At day 7, the wound size of MSCs+/3D collagen+ sites was significantly decreased (0.48 ± 0.01 cm2: P < 0.05), which were significantly smaller than the MSCs-/3D collagen- sites (0.92 ± 0.01 cm2) or the MSCs-/3D collagen- sites (0.66 ± 0.02 cm2). Also, there was no significant difference between MSCs-/3D collagen+ sites and MSCs-/3D collagen- sites (Fig. 4). MSCs+/3D collagen+ sites demonstrated accelerated wound healing. Since there was a possibility that the rate of wound healing could vary with the defect position, we repeated the same procedure by changing the positions of the defect sites, which yielded similar results.
Fig. 3.
Effects of MSCs. The wound size was significantly smaller in the MSCs+/3D collagen+ site at day 7.
Fig. 4.
Wound size measurement. MSCs+/3D collagen+ sites were significantly decreased (0.48 ± 0.01 cm2: *P < 0.05), which were significantly smaller than the MSCs-/3D collagen+ sites (0.92 ± 0.01 cm2) or MSCs-/3D collagen- sites (0.66 ± 0.02 cm2).
Immunohistochemistry
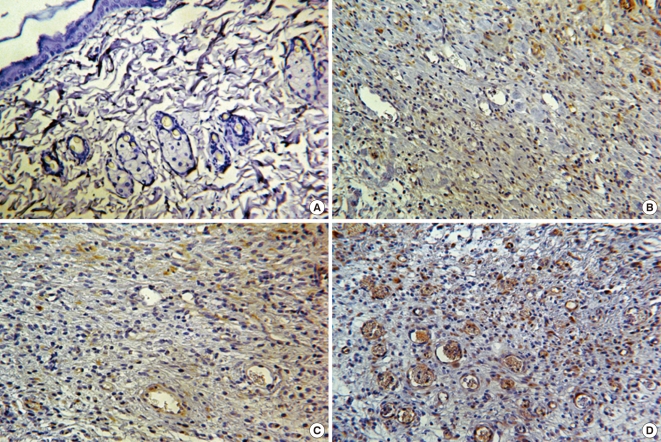
The tissue samples from each experimental group at day 7, when stained with VEGF antibody, showed that MSCs+/3D collagen+ sites exhibited the most distinct neovascularization compared to other sites (Fig. 5).
Fig. 5.
Immunohistochemistry. VEGF was not detected in the normal rat skin tissue. The MSCs+/3D collagen+ site was showed the most expression of VEGF than MSCs-/3D collagen+ site and MSCs-/3D collagen- site (A, Normal rat skin; B, MSCs-/3D collagen+ site; C, MSCs-/3D collagen- site; D, MSCs+/3D collagen+ site; A to D, × 400 magnification).
X and Y chromosome fluorescence in situ hybridization
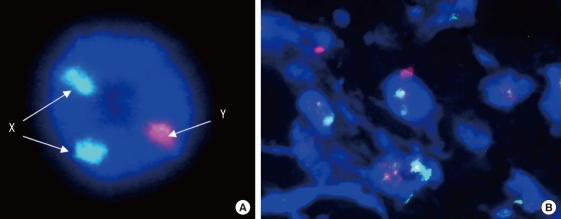
Y-chromosomes were probed with spectrum red (Cy3), whereas X-chromosomes with spectrum green (FITC). FISH analysis showed that many Y-chromosome positive cells were identified within the dermis of MSCs+/3D collagen+ sites at day 7 (Fig. 6).
Fig. 6.
Fluorescence in situ hybridization. (A) Positive control. Probe (Whole chromosome Painting Probe): Rat X/Y (FITC/Cy3) Probe. (B) Y-chromosome positive cells were identified within the dermis of MSCs+/3D collagen+ site at day 7. Y-chromosomes were probed with spectrum red (Cy3), X-chromosomes with spectrum green (FITC).
Gelatin zymography
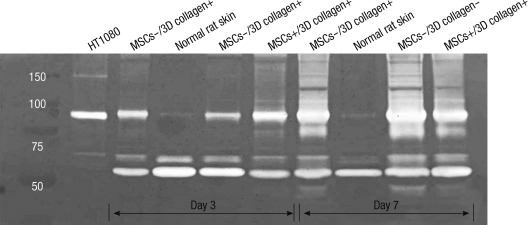
We used the gelatin zymography to assess the expression status of MMP-2 (72-kDa/gelatinase A) and MMP-9 (92-kDa/gelatinase B). MMP-2 was expressed in all experimental sites, including normal rat skin tissue, at day 3 and day 7. MMP-9 was not detected in normal tissue, but expressed in MSCs-/3D collagen+ sites, MSCs-/3D collagen- sites, and MSCs+/3D collagen+ sites. Interestingly, MMP-9 was expressed most intensively early in MSCs+/3D collagen+ sites at day 3. However, by day 7, MMP-9 was weakly expressed in MSCs+/3D collagen+ sites compared to MSCs-/3D collagen+ and MSCs-/3D collagen- sites (Fig. 7).
Fig. 7.
Gelatin zymography. The level of MMP-9 (92-kDa/gelatinase B) was detect early in MSCs+/3D collagen+ site at day 3. MMP-2 (72-kDa/gelatinase A) was expressed in all experimental sites (HT1080, positive control: Human fibrosarcoma cell line).
DISCUSSION
Wound healing is a complex process requiring the proper expression of signaling molecules and receptors, cellular adhesion molecules, and ECM proteins. These molecules are processed by MMPs and regulate many processes of wound healing (13). MMPs contribute too many aspects of tissue regeneration and angiogenesis by promoting the release of ECM-bound cytokines or angiogenesis-modulating ECM fragments (14). Recent studies have reported that implanted MSCs accelerate the restoration of damaged tissue (15-17). With regard to tissue regeneration, this potency of MSCs may play a more principal role than trans-differentiation (18). In the present study, MSCs+/3D collagen+ sites significantly reduced the wound size and exhibited the most intense expression of VEGF. VEGF is a potent angiogenic factor that stimulates angiogenesis in vivo (19-21). Angiogenesis is a complex and dynamic process which is affected by many factors including growth factors, cytokines, and ECM (22). The expression of VEGF is thought to be potentiated in response to the activation of bioactive molecules, and VEGF is considered to mediate angiogenic activity during the early period of wound healing.
There are interesting differences between MMP-2 and MMP-9 by gelatin zymography. MMP-2 was expressed in all experimental sites including normal rat skin tissue. This finding indicates that MMP-2 plays a less important role in early tissue metabolism. MMP-9 was expressed in MSCs+/3D collagen+ sites, MSCs-/3D collagen+ sites, and MSCs-/3D collagen+ sites, but not in normal rat skin tissue. Interestingly, during the early healing period (day 3), MMP-9 was most intensively expressed in MSCs+/3D collagen+ sites. In the later period (day 7), however, MMP-9 was more weakly expressed in MSCs+/3D collagen+ sites, compare to MSCs-/3D collagen+ sites and MSCs-/3D collagen- sites. This suggests that MMP-9 is involved in the early period of wound healing which coincides with enhanced wound healing process via angiogenesis. This is in good agreement with the findings that many processes are involved in the early period of wound healing require the action of proteases to facilitate cell movement, tissue degradation, granulation tissue formation and angiogenesis (1, 2). Hence, MMP-9, which is expressed early in MSCs+/3D collagen+ sites, appears to play critical roles in initiating angiogenesis.
There are reports that MMP-9, but not MMP-2, stimulates the production and secretion of VEGF in retinal pigment epithelial cells (23), and that inhibition of MMP-9 attenuates VEGF-induced intracerebral hemorrhage (24). Upon secretion, VEGF becomes bound to the ECM (25), and the interaction of VEGF with ECM proteins is mediated via ECM-binding domain. VEGF is encoded by a single gene and the coding portion contains eight exons (26). Exon 6 and 7 have been demonstrated to encode the ECM-binding domain of the protein. This domain can bind matrix proteins and is considered to account for the sequestration of VEGF within the ECM (27, 28). Thus, VEGF becomes liberated by ECM degradation through MMPs. Their effects are due to the mobilization of VEGF release through the degradation of ECM proteins by MMPs. In this study, VEGF and MMP were expressed in MSCs+/3D collagen+ sites. These factors may have an effect in a single manner or rather interact with each other. Early expressed MMP-9 promotes the release of extracellular matrix-bound cytokines, VEGF, which regulates angiogenesis. In the present study, MSCs upregulate the early expression of MMP-9, not MMP-2. This early upregulation of MMP-9 seems to be the crucial steps in angiogenesis.
In the present study, we used a three-dimensional collagen gel scaffold. In vivo, cells within the tissue are surrounded by complex three-dimensional microenvironments. Three-dimensional scaffolds possibly give an appropriate cell density and mimic ECM microenvironments in vivo. The main structural element of the ECM is collagen type-I which is why 3D collagen scaffolds have been frequently used. Up to our knowledge, there is no prior study dealing with 3D collagen and MSCs in wound healing. The 3D collagen gel scaffold is constitutionally suited, therefore, stably introduces MSCs to the experimental sites.
There have been reports stating that adult stem cells demonstrate a low level of engraftment (29, 30). At day 7, within the dermis of MSCs+/3D collagen+ sites, we detected Y-chromosome possessing cells in FISH and VEGFs were expressed in large amounts by immunohistochemistry. These Y-chromosome possessing MSCs and increased VEGFs, are expressed early in MSCs+/3D collagen+ sites, are most likely to promote dermal remodeling, since they were observed in the dermis of MSCs+/3D collagen+ sites. MSCs+/3D collagen+ site enhances pro-angiogenic properties by early induction of MMP-9 and VEGF.
In conclusion, early expression of MMP-9 and VEGF, as part of an underlying mechanism of accelerated wound healing, play crucial roles in increasing angiogenesis and dermal remodeling.
AUTHOR SUMMARY
Mesenchymal Stem Cells Improve Wound Healing In Vivo via Early Activation of Matrix Metalloproteinase-9 and Vascular Endothelial Growth Factor
Chul Han Kim, Jang Hyun Lee, Jong Ho Won and Moon Kyun Cho
We investigated the effects of mesenchymal stem cells (MSCs) on wound healing using a three- dimensional (3D) collagen gel scaffold. Full-thickness skin defects in Sprague-Dawley rats were effectively recovered with neovascularization by MSCs+3D collagen. The transplanted MSCs were found within the dermis of wound sites. Gelatin zymography revealed intense expression of MMP-9 in the MSCs+3D collagen-treated sites. We suggest that the early expression of MMP-9 and VEGF as part of an underlying mechanism of accelerated wound healing by MSCs+3D collagen.
References
- 1.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Vassalli JD, Saurat JH. Cuts and scrapes? Plasmin heals! Nat Med. 1996;2:284–285. doi: 10.1038/nm0396-284. [DOI] [PubMed] [Google Scholar]
- 4.Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 7.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 8.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo T, Johnson SA, Yoder MC, Romand R, Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc Natl Acad Sci USA. 2005;102:4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu M, Yang L, Liu S, Li H, Hui N, Wang F, Liu H. Differentiation potential of human embryonic mesenchymal stem cells for skin-related tissue. Br J Dermatol. 2006;155:282–291. doi: 10.1111/j.1365-2133.2006.07357.x. [DOI] [PubMed] [Google Scholar]
- 11.Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005;55:414–419. doi: 10.1097/01.sap.0000178809.01289.10. [DOI] [PubMed] [Google Scholar]
- 12.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 13.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10:136–141. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 16.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 17.Redmond DE, Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR, Jr, Sidman RL, Snyder EY. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prockop DJ. "Stemness" does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 19.Flamme I, von Reutern M, Drexler HC, Syed-Ali S, Risau W. Overexpression of vascular endothelial growth factor in the avian embryo induces hypervascularization and increased vascular permeability without alterations of embryonic pattern formation. Dev Biol. 1995;171:399–414. doi: 10.1006/dbio.1995.1291. [DOI] [PubMed] [Google Scholar]
- 20.Hippenstiel S, Krüll M, Ikemann A, Risau W, Clauss M, Suttorp N. VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol. 1998;274:L678–L684. doi: 10.1152/ajplung.1998.274.5.L678. [DOI] [PubMed] [Google Scholar]
- 21.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- 22.Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther. 1991;52:407–422. doi: 10.1016/0163-7258(91)90034-j. [DOI] [PubMed] [Google Scholar]
- 23.Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48:4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 24.Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- 25.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincenti V, Cassano C, Rocchi M, Persico G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation. 1996;93:1493–1495. doi: 10.1161/01.cir.93.8.1493. [DOI] [PubMed] [Google Scholar]
- 27.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 28.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 29.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 30.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]