Abstract
Norovirus (NV) has caused large outbreaks of gastroenteritis in schools. Studies of NV epidemiology in schools related to NV outbreaks have been frequently reported. However, reports of that in schools without outbreaks are not found. Presently, NV molecular epidemiology surveillance was carried out in asymptomatic food handlers working at nonoutbreak elementary schools in Incheon, Korea, in March, April and December, 2009. NV prevalence was examined by real-time reverse transcription-PCR (RT-PCR) and the positive products were re-evaluated by conventional RT-PCR for sequencing. Fecal samples (n = 776) were collected from 776 food handlers in 60 schools. NV was detected in 26 of them (3.4%). Of these, 17 (65%) were positive for NV GII and 10 (38%) were positive for NV GI. Of the 26 samples, 19 were positive by conventional RT-PCR. Sequencing of these 19 strains revealed GII/4 (n = 5), GI/6 (n = 3), GI/14 (n = 2), GII/8 (n = 2), GI/2 (n = 2), GI/10 (n = 1), GII/1 (n = 1), GII/3 (n = 1), GII/7 (n = 1), and GII/16 (n = 1). In this survey, the food handler population unrelated to NV outbreaks was found to normally contain asymptomatic carriers of NV. The excretion of NV from asymptomatic food handlers should be an infection source of NV outbreaks.
Keywords: Epidemiology, Norovirus, School
INTRODUCTION
Norovirus (NV) is recognized as a worldwide cause of acute non-bacterial gastroenteritis (1). Based on comparisons of the genetic sequences of the viral RNA-dependent RNA polymerase and the capsid protein, NV has been subdivided into five genogroups (GI-GV). Genogroups infecting humans belong to GI, GII, and GIV, whereas genogroups GIII and GV typically infect animals (2). GI and GII comprised of most of the human strains. GI is subdivided into 15 genotypes and GII is subdivided into 18 genotypes (3). NV is resistant to various environmental stresses and is commonly transmitted through ingestion of contaminated food and water, direct contact, or inhalation of aerosols (4). Several studies have reported that not all infections with NV result in clinical symptoms. During a 1994 study of 50 volunteers exposed to NV, 82% became infected; of these infections, 68% resulted in illness, whereas the remaining 32% were asymptomatic (5-7). It is plausible that asymptomatic individuals act as reservoirs, facilitating the transmission of NV. A recent study in Japan reported that 12% of fecal specimens collected from asymptomatic food handlers in a hotel unrelated to NV outbreaks were infected with NV (8). The authors suggested that the population normally contains asymptomatic carriers of NV regardless of the occurrence of NV outbreaks. However, the study had limitation that only a single facility was surveyed so the particular sanitary condition of the facility could affect the results.
Since 2003, there have been several large outbreaks of NV in Korea (9-13). The majority of the outbreaks were related with school lunches. Recently, our epidemiologic study of NV outbreak in Incheon, Korea has reported that the excretion of NV from asymptomatic food handlers may be an infection source of NV outbreak (12). However, the rate of asymptomatic NV excretion and the genetic diversity of NV among food handlers in school food services are largely unknown. The objectives of this study were to determine the prevalence of NV in asymptomatic food handlers working at schools where NV outbreak had not been reported and to identify the NV genotype circulating in asymptomatic school food handlers.
MATERIALS AND METHODS
Subjects and fecal specimens
This study was performed with two investigation periods (first investigation: March 24-April 18, 2009; second investigation: December 4-16, 2009). In the first and second investigation, 23% (51/221) and 24% (54/221) of total elementary schools in Incheon were enrolled, respectively. The other elementary schools in Incheon did not agree to participate in the study. Total subjects for this study were 826 food handlers (first investigation: n = 411, second investigation: n = 415) from 61 elementary schools. Of them, 43 symptomatic food handlers (diarrhea [n = 38], fever [n = 28], vomiting [n = 6]) were excluded. Therefore, the number of subjected food handlers were 783. The final participants in this study were 776 people because 7 food handlers refused to participate in the study.
One fecal sample (N = 776) were collected from each food handler in elementary school food services in which no case of acute gastroenteritis had been reported in Incheon Metropolitan City, Korea. Sixty schools were enrolled in the study. The food handlers were classified into three groups (dietician, food distributor, and food service employee). In this study, the dieticians and food distributors were not excluded because they were partly involved in the food handling. These subjects provided informed consent. The collection periods of fecal sample were March 24-April 8, 2009 (394 samples) and December 4-16, 2009 (382 samples). The average temperature and humidity in Incheon during the investigation periods was obtained from Incheon Meteorological Administration. Asymptomatic infection was defined as NV detection in stools with no clinical symptom of acute gastroenteritis for at least 14 days prior to and at least 2 days after the day of stool collection. The clinical symptom of acute gastroenteritis was defined as a diarrhea and/or vomiting and/or fever. Fever was defined when the body temperature was over 37.8℃. The body temperature of food handler was checked when the investigator collected the stool specimen. Diarrhea was defined when watery stool developed more than three times a day. We noticed the asymptomatic food handlers to report their symptoms if they develop any new symptoms in 2 days after the day of stool collection. However, there was no report of newly developed symptom. Self-administered questionnaires were distributed to food handlers and information was collected on gender, age, type of food handler, the location of school, symptoms, time of onset and duration of illness, using groundwater in the school, and the collection date of specimen.
RNA extraction and real-time quantitative reverse transcription-PCR (RT-PCR)
Viral RNA was extracted from 150 µL of supernatant with a QIAamp viral RNA mini kit (Qiagen, Valencia, CA, USA). Real-time quantitative RT-PCR was performed as described previously (14). Two sets of specific primers, COG1F and COG1R, and COG2F and COG2R were used to detect NV GI and GII, respectively. Real-time RT-PCR was performed with an ABI Prism 7500 sequence detector (Applied Biosystems, Foster City, CA, USA) under the following conditions: RT incubation at 45℃ for 40 min, initial denaturation at 95℃ for 10 min, 45 cycles of amplification with denaturation at 95℃ for 15 sec, and annealing and extension at 56℃ for 1 min. Amplification data were collected and analyzed with the Sequence Detector software version 2.3 (Applied Biosystems). The cut off value for positive NV specimens of real-time RT-PCR was described as these: Ct value ≤ 35, delta Rn ≥ 5,000.
Conventional RT-PCR and sequencing analysis
Specimens that tested positive for NV by real-time quantitative RT-PCR were tested with conventional RT-PCR for NV. Conventional RT-PCR was performed to amplify the sequences of the RNA dependent RNA polymerase and the capsid gene by using one-step RT-PCR premix (Bioneer, Daejeon, Korea). The cycling conditions were: one cycle of RT at 48℃ for 40 min and, for PCR, denaturation for 15 min at 95℃; 35 amplification cycles with denaturation for 30 sec at 95℃, annealing for 30 sec at 55℃, extension for 45 sec at 72℃; and a final cycle of incubation at 72℃ for 5 min. The amplicons obtained from RT-PCR were purified using a QIA PCR product purification kit (Qiagen). Sequencing was carried out with the primers using an ABI PRISM Big DYE Terminator cycle sequencing kit v3.0 and an automated sequencer ABI PRISM (Applied Biosystems). The partial nucleotide sequence of capsid genes were aligned with the Clustal W program and compared to reference strains obtained from the NCBI Genbank database: GI/2 (Southampton, L07418), GI/6 (Sindlesham, AJ277615), GI/10 (Boxer, AF538679), GI/14 (SaitamaT25, AB097911), GII/1 (Hawaii, U07611), GII/3 (Toronto, U02030), GII/4 (Grimsby, AJ004864), GII/7 (Leeds, AJ277608), GII/8 (Wortley, AJ277618), and GII/16 (SaitamaT53, AB112260).
Statistical analysis
Statistical analyses were performed using the chi-square test or Fisher's exact test to compare categorical variables and analysis of variance (ANOVA) to compare continuous variables. P value < 0.05 was considered significant. All analyses were carried out using the software packge SPSS for Windows ver. 12.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study protocol was approved by the institutional review board of Gachon University of Medicine and Science (GIRBA2239, 2010.2.25) and conformed to the ethical guidelines of the Helsinki Declaration (revised 1989). Each study participant signed an informed consent.
RESULTS
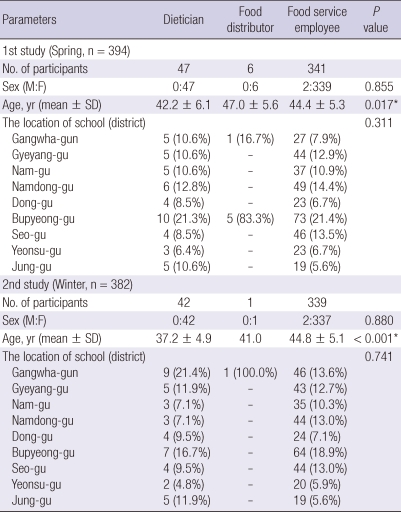
The mean age of food handlers was 44.1-yr-of-age (range 24-60-yr-of-age). Of the 776 individuals, 772 (99.5%) were females. Among the 776 food handlers, 680 (87.6%) were food service employees, 89 (11.5%) were dieticians, and seven (0.9%) were food distributors. The demographic characteristics of the food handlers according to the role are showed in Table 1. There was significant difference of age among the three food handler groups.
Table 1.
The demographic characteristics of final participants according to the type of food handlers
*Statistical significances were tested by one way analysis of variances among groups.
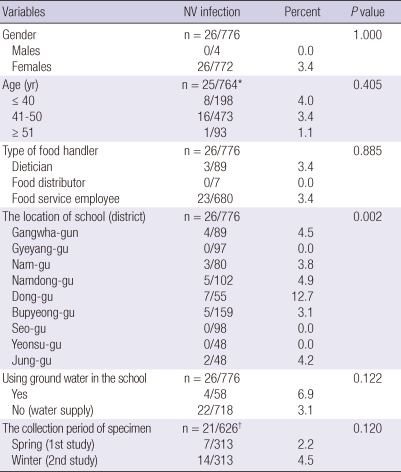
Among the 776 specimens screened by real-time RT-PCR, NV was detected in 26 samples (3.4%). We performed the univariate analysis of risk factors for asymptomatic NV infection according to gender, age, type of food handler, the location of school, using groundwater in the school, and the collection period of specimen. The location of school (district) was a significant risk factor (P = 0.002). The other variables were not risk factors for asymptomatic NV infection. There was no significant differences of NV detection rate among dietician, food distributor and food service employee (3.4%, 0.0%, 3.4%, P = 0.885) (Table 2).
Table 2.
Univariate analysis of risk factors for asymptomatic NV infection in food handlers working at the elementary school in some regions of Incheon, Korea
*Age was not recorded for 12 subjects; †The subjects, who were examined for NV twice in spring and winter, were analyzed.
In the first investigation period (March and April, 2009), the average temperature and humidity were 6.9℃ and 60.1%, respectively. During the period, 11 of 394 samples (2.8%) were positive for NV. In the second investigation period (December, 2009), the average temperature and humidity were 2.1℃ and 59.6%, respectively. During the period, 15 of 382 samples (3.9%) were positive for NV.
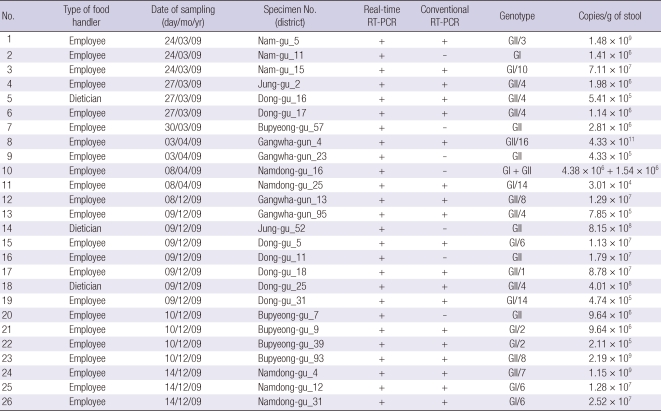
Ten of 776 (1.2%) stool specimens were positive for the NV GI genogroup, and 17 of 776 (2.2%) stool specimens were positive for NV GII. One sample was positive for both GI and GII. The 26 NV positive specimens were tested with conventional RT-PCR for NV and the positive PCR products were confirmed by the sequence analysis to contain NV partial genomic sequences. Seven of the 26 (27%) specimens that tested positive for NV tested negative by conventional RT-PCR. The remaining 19 specimens that had tested NV positive by the conventional RT-PCR were sequenced. Sequencing of these 19 strains revealed GII/4 (n = 5), GI/6 (n = 3), GI/14 (n = 2), GII/8 (n = 2), GI/2 (n = 2), GI/10 (n = 1), GII/1 (n = 1), GII/3 (n = 1), GII/7 (n = 1), and GII/16 (n = 1) (Table 3).
Table 3.
Detection of NV using conventional RT-PCR and real-time RT-PCR in asymptomatic food handlers in schools
Viral load of the 26 stool specimens were quantified; NV GII had a slightly higher median viral load than NV GI (1.29 × 107 vs 7.01 × 106 copies/g of stool) (Table 3). In this study, 313 sebject were examined for NV in both the first and second investigation. Among the 313 individuals, no sebject was positive for NV in both investigations.
DISCUSSION
In this study, 3.4% and 2.4% of the stool samples collected from asymptomatic food handlers who worked at elementary schools where NV outbreak had not been reported contained NV, as indicated by real-time RT-PCR and conventional RT-PCR, respectively. This is markedly less than the conventional RT-PCR detection of NV in 20 of 159 (12%) samples in a study conducted in Japan (8). The difference may be attributed to climatic factor. The present study collected fecal samples from March-April and December, while sample collection in the Japanese study was in the colder and drier months of January-March. Cold and dry weather conditions are associated with increased NV infections, with the temperature and humidity being possible contributors to the transmission of enteric infections (15). Our study was performed twice in spring and winter. The prevalence of NV in asymptomatic food handlers was 2.8% in spring and 3.9% in winter. We compared the prevalence of asymptomatic NV infection for the subjects who were duplicatedly assayed for NV in spring and winter. Although the difference between them (2.2% vs 4.5%, P = 0.120) was statistically insignificant, there was a tendency that the prevalence of asymptomatic NV infection in winter was higher than that of spring. The difference between them could be due to temperature and humidity. In this study, the gap of average humidity between both investigation periods was only 0.5% (60.1% vs 59.6%). However, the gap of average temperature between both investigation periods was 4.8℃ (6.9℃ vs 2.1℃). In a previous study, inverse linear associations with daily temperature (rate ratio [RR] = 0.85 for every 1℃ increase) and relative humidity (RR = 0.98 for every 1% increase) were found, with temperature having a greater overall effect (15). Another possible contributing factor to the differences between the present and previous (8) studies could be the sanitation management status of the facilities and the sanitation knowledge of food handlers. Sanitation education and knowledge tend to be higher in school food service workers than commercial food service workers (16).
In the univariate analysis of risk factors for asymptomatic NV infection in food handlers, the location of school (especially, Dong-gu, Incheon) was the only significant risk factor. However, we could not perform the additional epidemiologic study for the schools where we found the NV positive food handlers to prevent possible disadvantages to the schools. Therefore we could not confirm whether there are any differences of environment like sanitation status among the districts or the schools. The food handler population who work at the schools using ground water had higher prevalence of asymptomatic NV infection than those working at the schools using water supply (6.9% vs 3.1%, P = 0.122). However, the difference between them was not significant (Table 2).
The present study indicates a significant number of asymptomatic, NV-infected food handlers may be present in Korea. In India, NV was detected from asymptomatic children at a rate of 4.6% (17). In contrast, a large study involving 2,205 asymptomatic individuals in the general population in England detected NV in 358 participants (16%) (18). In addition, almost 30% of asymptomatic children living in a Mexican community were infected with NV (19). These results indicate that the prevalence of asymptomatic NV infections may differ by population in terms of ethnicity, age, and occupation. We found that there is no significant difference of asymptomatic NV infection according to the type of food handlers (Table 2). However, we could not presently confirm whether the prevalence of asymptomatic NV infections in food handlers is higher than that in the general population. Further study comparing the prevalence of asymptomatic NV infections by different population is needed to evaluate the risk factor of asymptomatic NV infection.
Several genotypes belonging to GI or GII were detected in this study. GII was the dominant genogroup. Among the genotypes, GII/4 was predominant (5/19, 26.3%). This is similar to the previous Japanese study that reported all sequence-positive samples collected from asymptomatic food handlers in a hotel belonging to NV GII (8). Accordingly, it is possible to assume that GII is the dominant genogroup in an asymptomatic food handler population. This might be related to the fact that genogroup II strains (especially GII/4) are more transmissible than the others (20, 21). It is possible that the clinical manifestations (e.g., increased vomiting) of GII/4 strain infections or physical characteristics (e.g., environmental persistence) of this strain might facilitate spread (22). As well, the higher cDNA viral load accounts for the higher transmissibility of genogroup II strains (20).
Presently, real-time RT-PCR results were used to analyze the viral loads by genotype and genogroup. In a recent study of asymptomatic NV infections with outbreaks, NV GII had a slightly higher mean viral load than NV GI (i.e., 3.81 × 108 vs 2.79 × 107 copies/g of stool) (23). Another study found median viral loads of 3.0 × 108 and 8.4 × 105 copies/g of stool specimen for NV GII and GI, respectively, in the fecal specimens of patients with NV outbreaks, and speculated that the higher viral loads of GII strains were due to their higher transmissibility (20). In our study, NV GII had a slightly higher median viral load than NV GI. This result coincides with the findings of previous studies.
Our study is the first molecular epidemiology investigation in Korea showing asymptomatic NV infections in school food service handlers. Our study has several limitations. First, the findings in this report are not enough to be representative of Incheon. Only 27.1% (60/221) of elementary schools in Incheon were enrolled in this study. The other schools did not agree to participate in the study due to several reasons (prejudice or misunderstanding about the purpose of this study, concern about loss of honor in case that there is NV positive specimen, the difficulty of specimen transportation in case of schools in island). Second, stool specimens were collected from individuals without clinical symptoms for at least 14 days prior to the day of stool collection. It is not certain whether NV was shed from a recent symptomatic gastroenteritis or primary asymptomatic infections. In addition, some food handlers might not report their symptoms out of fear of any disadvantage.
Despite these limitations, this report demonstrates that the excretion of NV from asymptomatic food handlers may be an infection source of NV outbreaks, especially in school food service facilities where NV outbreak has not been reported. In addition, among the NV strains circulating in asymptomatic food handlers, GII genotypes (especially, GII/4 genotype), which have higher transmissibility than other genotypes, were dominant. The excretion of NV from asymptomatic food handlers should be an infection source of NV outbreaks.
ACKNOWLEDGMENTS
We thank all the research workers from the local public health office and all the food handlers who participated in the study for help in data collection.
AUTHOR SUMMARY
Norovirus Infections in Asymptomatic Food Handlers in Elementary Schools without Norovirus Outbreaks in Some Regions of Incheon, Korea
Jun-Hwan Yu, Na-Yeon Kim, Eun-Jung Lee and In-Sang Jeon
Although Norovirus (NV) outbreak in schools have been frequently reported, epidemiological study of NV in schools without NV outbreaks is not found. We performed NV molecular epidemiologic study in asymptomatic food handlers (n = 776) working at elementary schools (n = 60) in Incheon, Korea, in March, April and December of 2009. NV was detected in 26 of the 776 samples (3.4%) by real-time RT-PCR. Of these, 17 (65%) were positive for NV GII and 10 (38%) were positive for NV GI. Of the 26 samples, 19 were positive by conventional RT-PCR. Sequencing of these 19 strains revealed GII/4 (n = 5) was dominant. In conclusion, a significant population of food handlers in elementary schools are asymptomatic carriers of NV.
References
- 1.Rockx B, De Wit M, Vennema H, Vinje J, De Bruin E, Van Duynhoven Y, Koopmans M. Natural history of human calicivirus infection: a prospective cohort study. Clin Infect Dis. 2002;35:246–253. doi: 10.1086/341408. [DOI] [PubMed] [Google Scholar]
- 2.La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. Detection of genogroup IV noroviruses in environmental and clinical samples and partial sequencing through rapid amplification of cDNA ends. Arch Virol. 2008;153:2077–2083. doi: 10.1007/s00705-008-0241-4. [DOI] [PubMed] [Google Scholar]
- 3.Okada M, Ogawa T, Kaiho I, Shinozaki K. Genetic analysis of noroviruses in Chiba Prefecture, Japan, between 1999 and 2004. J Clin Microbiol. 2005;43:4391–4401. doi: 10.1128/JCM.43.9.4391-4401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widdowson MA, Sulka A, Bulens SN, Beard RS, Chaves SS, Hammond R, Salehi ED, Swanson E, Totaro J, Woron R, Mead PS, Bresee JS, Monroe SS, Glass RI. Norovirus and foodborne disease, United States, 1991-2000. Emerg Infect Dis. 2005;11:95–102. doi: 10.3201/eid1101.040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Wit MA, Koopmans MP, Kortbeek LM, Wannet WJ, Vinje J, van Leusden F, Bartelds AI, van Duynhoven YT. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am J Epidemiol. 2001;154:666–674. doi: 10.1093/aje/154.7.666. [DOI] [PubMed] [Google Scholar]
- 6.Hutson AM, Atmar RL, Graham DY, Estes MK. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis. 2002;185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 7.Graham DY, Jiang X, Tanaka T, Opekun AR, Madore HP, Estes MK. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 8.Okabayashi T, Yokota S, Ohkoshi Y, Ohuchi H, Yoshida Y, Kikuchi M, Yano K, Fujii N. Occurrence of norovirus infections unrelated to norovirus outbreaks in an asymptomatic food handler population. J Clin Microbiol. 2008;46:1985–1988. doi: 10.1128/JCM.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HJ, Lee SW, Bang JH, Lim DJ, En SH, Park MS, Sin HJ Service DoEI, editors. Norwalk-virus food poisoning epidemiological investigation, Seoul, Korea, 2003. Seoul: Korea Centers for Disease Control and Prevention; 2003-2004. pp. 103–113. [Google Scholar]
- 10.Gong YW, Oh BY, Kim H, Lee M, Kim YH, Go JM, Lee JM, Jeong HS, Cheon DS. Molecular epidemiologic investigation of Norovirus infections in Incheon City, Korea, from 2005 to 2007. J Bacteriol Virol. 2008;38:249–257. [Google Scholar]
- 11.Korea Centers for Disease Control and Prevention; Service DoEI, editors. Epidemiological investigation report of mass food poisoning in the Metropolitan area, Korea, 2006. Seoul: Korea Centers for Disease Control and Prevention; 2006-2007. pp. 165–181. [Google Scholar]
- 12.Yu JH, Kim NY, Koh YJ, Lee HJ. Epidemiology of foodborne Norovirus outbreak in Incheon, Korea. J Korean Med Sci. 2010;25:1128–1133. doi: 10.3346/jkms.2010.25.8.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh SJ, Cho HG, Kim BH, Choi BY. An outbreak of gastroenteritis caused by norovirus-contaminated groundwater at a waterpark in Korea. J Korean Med Sci. 2011;26:28–32. doi: 10.3346/jkms.2011.26.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003;41:1548–1557. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopman B, Armstrong B, Atchison C, Gray JJ. Host, weather and virological factors drive norovirus epidemiology: time-series analysis of laboratory surveillance data in England and Wales. PLoS One. 2009;4:e6671. doi: 10.1371/journal.pone.0006671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SH, Jung HA, Bae HJ, Joo N. A study on differences of sanitation education and sanitation knowledge between dietitians in school foodservice and managers in commercial foodservice. Korean J Community Nutr. 2009;14:306–315. [Google Scholar]
- 17.Monica B, Ramani S, Banerjee I, Primrose B, Iturriza-Gomara M, Gallimore CI, Brown DW, Fathima M, Moses PD, Gray JJ, Kang G. Human caliciviruses in symptomatic and asymptomatic infections in children in Vellore, South India. J Med Virol. 2007;79:544–551. doi: 10.1002/jmv.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993-1996) Eur J Clin Microbiol Infect Dis. 2007;26:311–323. doi: 10.1007/s10096-007-0290-8. [DOI] [PubMed] [Google Scholar]
- 19.García C, DuPont HL, Long KZ, Santos JI, Ko G. Asymptomatic norovirus infection in Mexican children. J Clin Microbiol. 2006;44:2997–3000. doi: 10.1128/JCM.00065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan MC, Sung JJ, Lam RK, Chan PK, Lee NL, Lai RW, Leung WK. Fecal viral load and norovirus-associated gastroenteritis. Emerg Infect Dis. 2006;12:1278–1280. doi: 10.3201/eid1208.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siebenga JJ, Vennema H, Duizer E, Koopmans MP. Gastroenteritis caused by norovirus GGII.4, The Netherlands, 1994-2005. Emerg Infect Dis. 2007;13:144–146. doi: 10.3201/eid1301.060800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widdowson MA, Cramer EH, Hadley L, Bresee JS, Beard RS, Bulens SN, Charles M, Chege W, Isakbaeva E, Wright JG, Mintz E, Forney D, Massey J, Glass RI, Monroe SS. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus--United States, 2002. J Infect Dis. 2004;190:27–36. doi: 10.1086/420888. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa K, Oka T, Takeda N, Hansman GS. Norovirus infections in symptomatic and asymptomatic food handlers in Japan. J Clin Microbiol. 2007;45:3996–4005. doi: 10.1128/JCM.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]