Abstract
A large reservoir of bacterial lipopolysaccharide (LPS) is available in the colon and this could promote colon cancer metastasis by enhancing tumor cell adhesion, intravasation, and extravasation. Furthermore, adhesion molecules like ICAM-1, VCAM-1, and E-selectin play important roles in the adhesion of tumor cells to endothelium. This study was designed to determine whether morphine can attenuate the expressions of adhesion molecules up-regulated by the supernatant of LPS-stimulated HCT 116 colon cancer cells (LPS-Sup). In this study, we divided to three groups by cell-growth medium of human umbilical vascular endothelial cells (HUVECs): the control group was incubated in growth factor-free endothelial medium, the Sup group was incubated in the supernatant of HCT 116 cells (Sup), and the LPS-Sup group was incubated in LPS-Sup. To observe effect of morphine to the adhesion molecules expressions in the LPS-Sup group, we co-treated morphine with LPS or added it to LPS-Sup. Adhesion molecule expressions on HUVECs in all three groups were measured during incubation period. Consquentially, ICAM-1, VCAM-1, and E-selectin expressions on HUVECs were significantly lower when morphine was co-treated with LPS than not co-treated. Thus, we suggest that morphine affects the expressions of adhesion molecules primarily by attenuating LPS stimuli on tumor cells.
Keywords: Lipopolysaccharides, Morphine, Adhesion Molecule, Supernatant, HUVEC
INTRODUCTION
The metastasis of tumor cells is a multistep process. The important steps in the metastatic cascade of tumor cell are intravasation, dissemination, and extravasation (1). During intravasation tumor cells traverse the basement membrane and the endothelium of blood vessel walls into the blood stream. During extravasation tumor cells penetrate the vascular endothelium and the underlying basement membrane from their luminal sides. Importantly, both of these steps require adhesion between tumor cells and endothelium. This adhesion is made possible by the expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM-1), and E-selectin on endothelium. The first step in the cell adhesion cascade, so-called rolling, is regulated by selectins like E-selectin. Adhesion and migration predominantly involve integrins and members of the immunoglobulin supergene family such as ICAM-1 and VCAM-1. Tumor cells are often surrounded by inflammatory cells which secrete various cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) and bacterial lipopolysaccharide (LPS). These cytokines are known to be involved in tumor promotion, and cell adhesion (2). A large reservoir of LPS is available in the colon, because bacteria like Escherichia coli typically produce copious amounts of LPS, and thus inflammation around colon cancer cells can cause much LPS release. This LPS stimulates to secrete various cytokines and pro-inflammatory mediators (3, 4). The effects of cytokines and pro-inflammatory mediators on adhesion molecules expressions are not clear, but in a previous study, it was demonstrated that the supernatant of LPS-stimulated HT-29 colon cancer cells up-regulates adhesion molecules on endothelial cells.
Morphine has been used for analgesia in cancer for many years. Furthermore, Sasamura et al. (5) found that morphine can inhibit the growth and metastasis of tumor cells in a mouse model, and Harimaya et al. (6) showed that morphine can also inhibit the adhesion, invasion and metastasis of metastatic colon carcinoma cells. In addition, other studies (7-9) have demonstrated that morphine attenuates the expressions of adhesion molecules on endothelial cells. However, no previous study has examined the inhibitory effect of morphine on the vascular invasion of tumor cells. In the present study, we investigated whether morphine can attenuate the expressions of endothelial adhesion molecules induced on HUVECs by the supernatant of LPS-stimulated HCT 116 colon cancer cells.
MATERIALS AND METHODS
Cell lines and reagents
The human umbilical vascular endothelial cell (HUVEC) line (JG-C2517A, single donor) and its medium (EGM®-2; endothelial growth medium with BulletKit® [CC-3162]) were purchased from Lonza (Walkersville, MD, USA). The HCT 116 colon carcinoma cell (CCL-247) line was from ATCC (Manassas, VA, USA) and was cultured in RPMI-1640 (R1145). PE Mouse Anti-Human CD54, CD62E, CD 106 antidodies are from Becton Dickinson (BD) (cat. 555749; Franklin Lakes, NJ, USA), and all the other reagents were from Sigma Chemicals (St. Louis, MO, USA).
Cell cultures
HUVECs were cultured in EGM containing 10% fetal bovine calf serum, endothelial growth supplement (CC-4113); hEGF 0.5 mL, Hydrocortisone 0.5 mL, a 5% penicillin/streptomycin (CC-4381), and 25 µg/mL heparin (CC-4936), and maintained at 37℃ in 5% CO2 humidified atmosphere. All experiments utilized cells grown between two and three passages. The HCT 116 cell line was cultured in RPMI-1640 medium supplemented with L-glutamine (300 mg/L), 25 mM HEPES and 25 mM NaHCO3, 10% heat-inactivated fetal bovine serum, and 100 U/mL penicillin/100 µg/mL streptomycin, and maintained at 37℃ in 5% CO2 humidified atmosphere. All cells were subcultured following enzymatic digestion using trypsin/EDTA solution.
Cell viabilities
Cell viabilities were measured by cell counting. First, cells were removed from cultures and placed in test tubes. The samples were diluted four times and stained with trypan blue, cover-slipped in a hemocytometer, and observed under an optical microscope. Finally, cells with an intact membrane were counted.
Experimental protocols
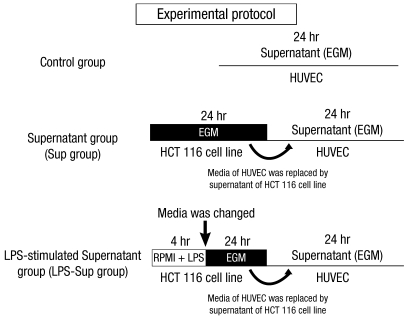
HUVECs were grown in EGM until they coated 70%-80% of dish surfaces. The EGM was then replaced with growth factor-free EGM. In the control group, ICAM-1, VCAM-1 and E-selectin were measured at 0, 4, 8, 12, and 24 hr of incubation period in growth factor-free EGM (Fig. 1).
Fig. 1.
Experimental protocol. The control group; HUVECs were incubated in growth factor-free endothelial medium. The Sup group; HUVECs were incubated in the supernatant of HCT 116 cells. The LPS-Sup; HUVECs were incubated in the supernatant of LPS-stimulated HCT 116 cells.
HCT 116 cancer cells were grown in RPMI 1640 medium until they coated 80%-90% of dish surfaces. The RPMI 1640 medium was then replaced with growth factor-free EGM, and cells were incubated for 24 hr and supernatant was then harvested. In the Sup group, HUVECs were incubated for 24 hr in the supernatant of HCT 116 cancer cells. On the other hand, in the LPS-Sup group, HCT 116 cancer cells were stimulated with 1 µg/mL of LPS for 4 hr. Cells were then throughly washed and growth hormone-free EGM was added to ensure that observed effects on HUVECs were due to substances produced by the tumor cells and not by residual stimulants. HUVECs were then incubated for 24 hr in LPS-Sup. ICAM-1, VCAM-1 and E-selectin were measured at 0, 4, 8, 12, and 24 hr of incubation period (Fig. 1).
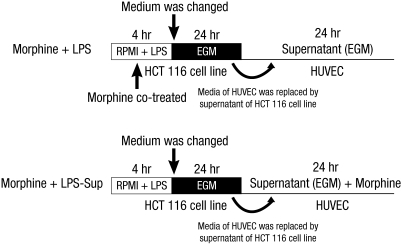
In addition, HUVECs were also treated as follows: 1) 0.01, 0.1, or 1 µM of morphine was co-treated with LPS during LPS-stimulation on the HCT 116 cells (Morphine + LPS), or 2) 0.01, 0.1, or 1 µM of morphine was added to LPS-Sup during incubation period (Morphine + LPS-Sup) (Fig. 2).
Fig. 2.
Experimental protocol. Morphine was co-treated in two ways: 1) Morphine + LPS; co-treating morphine with LPS during stimulation of HCT 116 cells or 2) Morphine + LPS-Sup; adding morphine to LPS-Sup prior to incubation of HUVECs. EGM is an endothelial cell growth medium without endothelial growth hormone.
Flow cytometry
The surface expressions of adhesion molecules were measured by flow cytometry (Facsflow from BD) by using an ICAM-1 specific fluorescein isothiocyanate-labeled antibody from BD (Franklin Lakes, NJ, USA). Briefly, cells were washed with phosphate buffered saline (PBS) and then trypsinized. After detaching cells and transferring them into tubes, the digestion was stopped by the adding medium 199 that contained 10% fetal calf serum. After centrifugation at 218 g, (4℃, 5 min), tubes were washed with PBS. The cells were incubated with PE Mouse Anti-Human CD54, CD62E, and CD 106 for 30 min at 4℃, rewashed with PBS and resuspended in 500 µL PBS for flow cytometric analysis. To adjust instrument settings, control cells were prepared by using the procedure described above, but omitting antibody. At least three different sets of experiments with cells from different isolations were performed in triplicate.
Statistical analysis
We used SPSS for Windows version 10.0 (SPSS, Chicago, IL, USA) for the statistical analysis. All results, which are expressed as means ± SDs, are representative of three different experiments. Flow cytometry results were evaluated by one-way ANOVA. Significance was accepted for P values of < 0.05.
RESULTS
Cell viabilities
The cell survival rates of HUVECs in growth factor-free EGM, in the supernatant of HCT 116 cancer cells, and in the supernatant of LPS-stimulated HCT 116 cancer cells were 96%. No significant time-dependent differences were observed between these treatments.
The expressions of ICAM-1, VCAM-1, and E-selectin on HUVECs after incubation in growth hormone-free EGM, Sup, or LPS-Sup
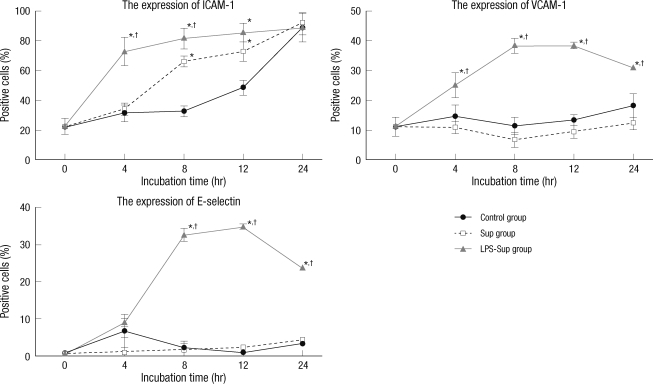
In the Sup group, the expressions of VCAM-1 and E-selectin were not significantly increased as compared to the control group, but the expression of ICAM-1 was significantly higher after incubation for 8 and 12 hr. In the LPS-Sup group, the expressions of ICAM-1, VCAM-1 and E-selectin were significantly greater than in the control group througout 24-hr incubation period (Fig. 3).
Fig. 3.
Up-regulations of the expressions of the adhesion molecules on the HUVEC cells by LPS-Sup. Graphs show the expressions of ICAM-1, VCAM-1, and E-selectin on HUVECs during a 24-hr of incubation period. ICAM-1, VCAM-1, and E-selectin expressions in the LPS-Sup and Sup groups were compared versus those in the control group. Percentages of positive cells were recorded at 0, 4, 8, 12, and 24 hr of incubation period. Values are expressed as means ± SD. *P < 0.05 vs the control group; †P < 0.05 vs the Sup group.
The expression of ICAM-1 on HUVECs after 0.01, 0.1, or 1 µM of morphine was added to LPS-Sup or co-treated with LPS
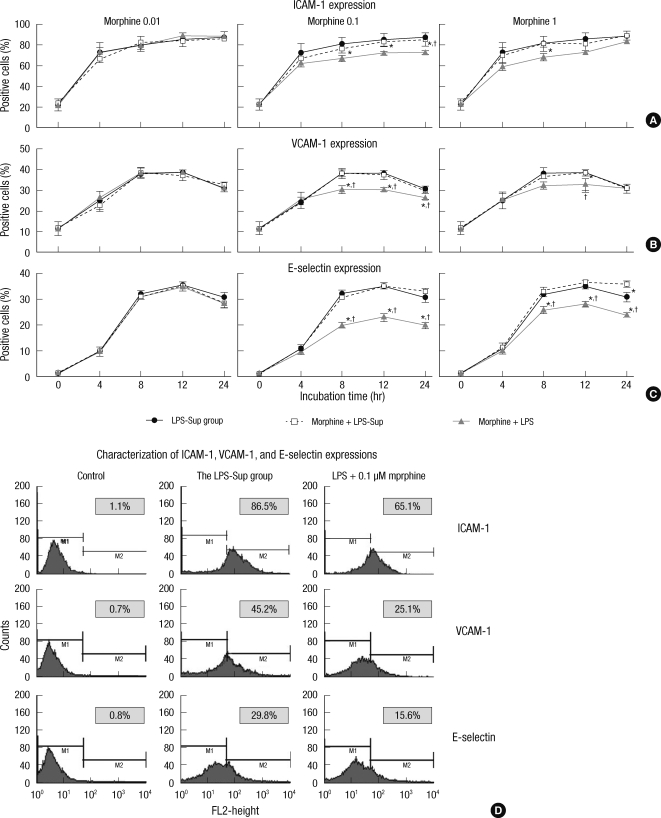
ICAM-1 expression on HUVECs was significantly attenuated at 8, 12, and 24 hr of incubation period by co-treating 0.1 µM of morphine with LPS and at 8 hr of incubation period by co-treating 1 µM of morphine with LPS. Otherwise, we could not observe down-regulated of ICAM-1 expression on HUVECs by adding morphine to LPS-Sup or co-treating 0.01 µM of morphine with LPS (Fig. 4A).
Fig. 4.
The effect of morphine on the expressions of adhesion molecules on HUVECs. (A) Graphs show the expressions of ICAM-1 on HUVECs incubated in LPS-Sup, Morphine + LPS-Sup and supernatant of Morphine + LPS. (B) Graphs show the expressions of VCAM-1 on HUVECs incubated in LPS-Sup, Morphine + LPS-Sup and supernatant of Morphine + LPS. (C) Graphs show the expressions of E-selectin on HUVECs incubated in LPS-Sup, Morphine + LPS-Sup and supernatant of Morphine + LPS. Values are Aexpressed as means ± SD. (D) Histograms show the characterization of ICAM-1, VCAM-1, and E-selectin expressions on HUVECs at 8-hr of incubation period. Control means unstained value. LPS + 0.1 µM of morphine means the value of when 0.1 µM morphine was co-treated with LPS in the LPS-Sup group. The percentage of positive cells from each group was recorded at 0, 4, 8, 12, and 24 hr of incubation period. In gray box, values are expressed as percentages of gated positive cells. *P < 0.05 versus the LPS-Sup group; †P < 0.05 versus Morphine + LPS-Sup.
The expression of VCAM-1 on HUVECs after 0.01, 0.1, or 1 µM of morphine was added to LPS-Sup or co-treated with LPS
VCAM-1 expression on HUVECs was significantly attenuated at 8 and 12 hr of incubation period by co-treating 0.1 or 1 µM of morphine with LPS, but this was also observed at 24 hr of incubation period by co-treating 0.1 µM of morphine. Otherwise, we could not observe down-regulated VCAM-1 expression on HUVECs by adding morphine to LPS-Sup or co-treating 0.01 µM of morphine with LPS (Fig. 4B).
The expression of E-selectin on HUVECs after 0.01, 0.1, or 1 µM of morphine was added to LPS-Sup or co-treated with LPS
E-selectin expression on HUVECs was significantly attenuated at 8, 12, 24 hr of incubation period by co-treating 0.1 or 1 µM of morphine with LPS. Otherwise, we could not observe down-regulated E-selectin expression on HUVECs by adding morphine to LPS-Sup or co-treating 0.01 µM of morphine with LPS (Fig. 4C).
DISCUSSION
In cancer patients, morphine is mainly used to relieve pain in order to improve the quality of life. However, the analgesic dosages administered vary substantially (10), and thus, we choose a morphine dosage, based on a previous clinical study (11). In the previous study, the effective dosage of morphine required for analgesia was achieved at the blood concentrations between 0.1 and 0.4 µM. Therefore, we used 0.01, 0.1, or 1 µM of morphine in this study.
When tumor cells are surrounded by inflammatory cells, they secrete various soluble factors such as IL-1β, TNF-α, and LPS. Simiantonaki et al. (12) reported that LPS-stimulated tumor cells supernatant increased adhesion molecules expressions, such as, of ICAM-1, VCAM-1, and E-selectin on HUVECs as compared with the nonstimulated, or IL-1β, or TNF-α stimulated tumor cells supernatant. In particular, LPS-stimulated supernatant of colon cancer cells such as HT-29 and HRT-18 more highly stimulated the expressions of ICAM-1, VCAM-1, and E-selectin when LPS was used as a stimulant. In the present study we observed that LPS-Sup induced higher expressions of ICAM-1, VCAM-1, and E-selectin than medium of the control group (Fig. 3). Vidal-Vanaclocha et al. (13) demonstrated that systemic inflammation induced by LPS enhances the metastatic potential of B16 melanoma cells in vivo and in vitro, and Andrews et al. (14) found that the metastatic colon carcinoma cell line LS174T showed increased adhesion to HUVECs after exposure to LPS and also found that these adhesions involve a NF-κB-dependent pathway. Furthermore, it is known that the transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) controls the gene expressions of E-selectin, ICAM-1, and VCAM-1 (15).
In colon cancer cells, LPS causes the synthesis and secretion of a variety of cytokines and other pro-inflammatory mediators (16, 17), though the effect of LPS stimulation to up-regulation of adhesion molecules on tumor cells is still unclear. Furthermore, IL-8, IL-6, and vascular endothelial growth factor are promoted by LPS in ovarian cancer cells (3) and IL-8 synthesis is increased by LPS stimulation in non-small cell lung cancer cells (4). Moreover, it is known that IL-1β and IL-6 are highly induced by tumors that metastasize to the liver, such as breast cancer and colon carcinoma (18). Thornton et al. (19) reported that the endothelial expressions of ICAM-1 and VCAM-1 are induced by IL-1β via an NF-κB-dependent pathway and Weber et al. (9) described that TNF-α significantly induces messenger RNA (mRNA) and protein expression of adhesion molecules like ICAM-1, VCAM-1 and E-selectin on HUVECs.
The anti-tumor and anti-metastatic effects of morphine are considered to be mainly due to indirect mechanisms involving pain relief (20) and to the elimination of pain-associated stress (21). However, Hariyama et al. (6) found that the metastasis of colon 26-L5 cells was reduced by morphine, although no evidence of hyperalgesia at cancer-inoculated sites was observed in their mouse model. These findings suggest that morphine directly down-regulates the invasive and metastatic potential of tumor cells. Wang et al. (8) demonstrated that serum levels of ICAM-1 and L-selectin were significantly elevated in patients with a myocardial infarction and that these elevated serum levels of ICAM-1 and L-selectin were attenuated by morphine. In an vitro study, Weber et al. (9) recently found that morphine can attenuate the expression of ICAM-1 on HUVECs by reducing NF-κB activity and we also demonstrated that morphine can down-regulate the expression of ICAM-1 and neutrophil adhesion on HUVECs during ischemic-reperfusion period (22). Moreover, Ohno et al. (23) reported that DHMEQ (NF-κB inhibitor) inhibits adhesion between HUVECs and HT-29 colon cancer cells by reducing the expressions of ICAM-1, VCAM-1 and E-selectin on HUVECs. In our study, morphine was not found to down-regulate the expression of ICAM-1, VCAM-1, and E-selectin on HUVECs directly, and yet the expressions of ICAM-1, VCAM-1, and E-selectin were significantly reduced on HUVECs when morphine was co-treated during LPS-stimulation (Fig. 4). It seems that morphine affects the expressions of adhesion molecules primarily by attenuating LPS stimuli on tumor cells rather than by directly down-regulating the expressions of adhesion molecules on HUVECs.
Although the effect of morphine on tumor metastasis is unclear, based on our observations, we believe that morphine can reduce LPS-stimulated metastasis in colon cancer by attenuating the expressions of adhesion molecules on endothelial cells. In the future, we plan to evaluate the mechanism responsible for the effect of morphine on LPS-stimulated metastasis in vitro and to investigate the anti-metastatic effect of morphine in vivo.
Footnotes
This study was supported by a Korea University grant (2010, K1032501).
AUTHOR SUMMARY
Morphine Attenuates Endothelial Cell Adhesion Molecules Induced by the Supernatant of LPS-Stimulated Colon Cancer Cells
Too Jae Min, Sang-Hee Park, Yi-Hwa Ji, Yoon-Sook Lee, Tae Woo Kim, Jae Hwan Kim, Woon-Young Kim and Young-Cheol Park
A large source of bacterial LPS is available in colon and the LPS might promote colon cancer metastasis by enhancing tumor cell adhesion to endothelium. We tested whether morphine treatment could attenuate the expressions of adhesion molecules in endothelial cells (HUVEC) that was stimulated by the supernatant of LPS-stimulated HCT 116 colon cancer cells. The expressions of adhesion molecules on HUVECs were significantly lower when morphine was co-treated with LPS than without morphine co-treatment. Thus, we suggest that morphine could reduce the LPS-stimulated metastasis in colon cancer by attenuating the expressions of adhesion molecules on endothelial cells.
References
- 1.Lafrenie RM, Buchanan MR, Orr FW. Adhesion molecules and their role in cancer metastasis. Cell Biophys. 1993;23:3–89. doi: 10.1007/BF02796507. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007;14:377–386. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]
- 3.Szajnik M, Szczepanski MJ, Czystowska M, Elishaev E, Mandapathil M, Nowak-Markwitz E, Spaczynski M, Whiteside TL. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–4363. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandel U, Heygster D, Sibelius U, Fink L, Sigel S, Seeger W, Grimminger F, Hattar K. Amplification of lipopolysaccharide-induced cytokine synthesis in non-small cell lung cancer/neutrophil cocultures. Mol Cancer Res. 2009;7:1729–1735. doi: 10.1158/1541-7786.MCR-09-0048. [DOI] [PubMed] [Google Scholar]
- 5.Sasamura T, Nakamura S, Iida Y, Fujii H, Murata J, Saiki I, Nojima H, Kuraishi Y. Morphine analgesia suppresses tumor growth and metastasis in a mouse model of cancer pain produced by orthotopic tumor inoculation. Eur J Pharmacol. 2002;441:185–191. doi: 10.1016/s0014-2999(02)01450-4. [DOI] [PubMed] [Google Scholar]
- 6.Harimaya Y, Koizumi K, Andoh T, Nojima H, Kuraishi Y, Saiki I. Potential ability of morphine to inhibit the adhesion, invasion and metastasis of metastatic colon 26-L5 carcinoma cells. Cancer Lett. 2002;187:121–127. doi: 10.1016/s0304-3835(02)00360-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang TL, Chang H, Hung CR, Tseng YZ. Morphine preconditioning attenuates neutrophil activation in rat models of myocardial infarction. Cardiovasc Res. 1998;40:557–563. doi: 10.1016/s0008-6363(98)00192-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang TL, Chang H, Hung CR, Tseng YZ. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am J Cardiol. 1997;80:1532–1535. doi: 10.1016/s0002-9149(97)00788-1. [DOI] [PubMed] [Google Scholar]
- 9.Weber NC, Kandler J, Schlack W, Grueber Y, Fradorf J, Preckel B. Intermitted pharmacologic pretreatment by xenon, isoflurane, nitrous oxide, and the opioid morphine prevents tumor necrosis factor alpha-induced adhesion molecule expression in human umbilical vein endothelial cells. Anesthesiology. 2008;108:199–207. doi: 10.1097/01.anes.0000299441.32091.ed. [DOI] [PubMed] [Google Scholar]
- 10.Penson RT, Joel SP, Gloyne A, Clark S, Slevin ML. Morphine analgesia in cancer pain: role of the glucuronides. J Opioid Manag. 2005;1:83–90. doi: 10.5055/jom.2005.0021. [DOI] [PubMed] [Google Scholar]
- 11.Dershwitz M, Walsh JL, Morishige RJ, Connors PM, Rubsamen RM, Shafer SL, Rosow CE. Pharmacokinetics and pharmacodynamics of inhaled versus intravenous morphine in healthy volunteers. Anesthesiology. 2000;93:619–628. doi: 10.1097/00000542-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Simiantonaki N, Jayasinghe C, Kirkpatrick CJ. Effect of pro-inflammatory stimuli on tumor cell-mediated induction of endothelial cell adhesion molecules in vitro. Exp Mol Pathol. 2002;73:46–53. doi: 10.1006/exmp.2002.2440. [DOI] [PubMed] [Google Scholar]
- 13.Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello CA. Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst. 1996;88:198–205. doi: 10.1093/jnci/88.3-4.198. [DOI] [PubMed] [Google Scholar]
- 14.Andrews EJ, Wang JH, Winter DC, Laug WE, Redmond HP. Tumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of beta-1 integrin expression. J Surg Res. 2001;97:14–19. doi: 10.1006/jsre.2001.6090. [DOI] [PubMed] [Google Scholar]
- 15.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 16.Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 2003;221:42–49. doi: 10.1016/s0008-8749(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Liu Q, Sun Q, Zhang C, Chen T, Cao X. TLR4 signaling in cancer cells promotes chemoattraction of immature dendritic cells via autocrine CCL20. Biochem Biophys Res Commun. 2008;366:852–856. doi: 10.1016/j.bbrc.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Fujii N, Nitta Y, Sakihara H, Nakayama K, Rikiishi H, Kumagai K. Murine tumor cells metastasizing selectively in the liver: ability to produce hepatocyte-activating cytokines interleukin-1 and/or -6. Jpn J Cancer Res. 1991;82:1299–1308. doi: 10.1111/j.1349-7006.1991.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton P, McColl BW, Cooper L, Rothwell NJ, Allan SM. Interleukin-1 drives cerebrovascular inflammation via MAP kinase-independent pathways. Curr Neurovasc Res. 2010;7:330–340. doi: 10.2174/156720210793180800. [DOI] [PubMed] [Google Scholar]
- 20.Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54:21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 21.Kanno J, Wakikawa A, Utsuyama M, Hirokawa K. Effect of restraint stress on immune system and experimental B16 melanoma metastasis in aged mice. Mech Ageing Dev. 1997;93:107–117. doi: 10.1016/s0047-6374(96)01827-1. [DOI] [PubMed] [Google Scholar]
- 22.Min TJ, Kim JI, Kim JH, Noh KH, Kim TW, Kim WY, Lee YS, Park YC. Morphine postconditioning attenuates ICAM-1 expression on endothelial cells. J Korean Med Sci. 2011;26:290–296. doi: 10.3346/jkms.2011.26.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohno O, Shima Y, Ikeda Y, Sakurai K, Watanabe K, Kawai Y, Umezawa K. Inhibition of cellular adhesion in human umbilical vein endothelial cells by NF-kappaB inhibitor DHMEQ under flow. Oncol Res. 2005;15:189–197. doi: 10.3727/096504005776382314. [DOI] [PubMed] [Google Scholar]