Abstract
There have been many epidemiological researches of chronic kidney disease (CKD), accompanied by an increase in the incidence of coronary heart disease (CHD). However, as far as we know, little research has been done to examine the extent of the relationship between CKD and CHD as estimated by Framingham risk score (FRS) in Korean men. CKD was defined as either proteinuria or an eGFR of < 60 mL/min per 1.73 m2. The FRS has been used to predict the 10-yr risk of coronary events and usually divided into three levels of risk < 10% (low), 10%-19% (intermediate) and ≥ 20% (high). We defined FRS ≥ 10% as more-than-a-moderate CHD risk group and FRS ≥ 20% as a high CHD risk group, respectively. After adjusting for covariates, multivariable-adjusted logistic regression analyses showed a strong statistical significant relationship between CKD and high risk of CHD (adjusted OR, 1.95 [95% CI, 1.32-2.87]). Dipstick urinalysis and eGFR can be readily measured in most clinical settings. The measurement of kidney function may represent a relatively inexpensive and efficient way to identify individuals at higher risk for CHD.
Keywords: Renal Failure, Chronic Kidney Disease; Coronary Heart Disease; Framingham Risk Score
INTRODUCTION
Chronic kidney disease (CKD) has been known to be associated with end stage renal disease (ESRD), as well as cardiovascular morbidity and mortality (1-3). CKD often progresses to ESRD with its attendant complications; treatment of the earlier stages of CKD is effective in slowing the progression towards ESRD (4, 5). Recently, the number of patients with ESRD is increasing worldwide (6, 7). According to data from the Third National Health and Nutrition Examination Survey, 8.3 million US adults aged 20 yr or older have CKD (8). In 1999, the US Renal Data System documented that the number of long-term ESRD patients would increase up to 651,330 by 2010 (9). In Korea, there also has been a recent dramatic increase in the prevalence of patients with ESRD that require renal replacement therapy, from 303.6 per million population in 1994 to 1,113.6 per million population in 2009 (10). CKD has been found to cause currently a worldwide public health problem (11); and thus, identification and management of the modifiable risk factors for CKD are important for preventing adverse effects and improving patient outcome.
Coronary heart disease (CHD) proves to be the major cause of mortality and morbidity worldwide, estimating CHD risk in clinical settings is of critical importance for primary and secondary prevention strategies. Most of the well-recognized risk factors of CHD have been considered in the development of the Framingham risk score (FRS). The FRS is recognized as an important clinical tool to indicate the intensity of CHD risks and guide the appropriate interventions for risks (12). It is well established that CKD is a single most important risk factor to cardiovascular events.
In fact, Go et al. (1) reported that among the American population patients with mild CKD already showed substantial increases in the frequency of cardiovascular events. There have been many epidemic researches of CKD across the world, accompanied by an increase in the incidence of CHD (13-15). However, as far as we know, little research has been done to examine the extent of the relationship between CKD and CHD as estimated by FRS in Korean men. The aim of this study was to determine the relationship between severity of CKD and FRS in Korean men, which are well known markers for 10-yr risk of CHD incidence.
MATERIALS AND METHODS
Study design
A cross-sectional study was conducted to examine the association between CKD and the risk of CHD in Korean men who were employed at various companies in Korea. All employees participate in an annual health check-up, as is required by Korea's Industrial Safety and Health law. A total of 55,260 men who had visited the Kangbuk Samsung Hospital, Healthcare Center for a medical check-up from January to December in 2008 participated in this study. Study data included laboratory, physical examination and information provided by a questionnaire.
Study population
Among the 55,260 subjects, 2,039 were excluded by various reasons: 1,853 from our study subjects were not calculating FRS; 69 were not assessing serum creatinine; 117 were not possessing information about proteinuria. Ultimately, 53,221 men, aged 21 to 77 yr old, were enrolled in the analysis and were observed the relationship between CKD and the risk of CHD estimated by FRS.
Clinical and laboratory measurements
Study data included a medical history, a physical examination, information provided by a questionnaire, anthropometric measurements and laboratory measurements. The medical history and the history of drug prescription were assessed by the examining physicians. All the participants were asked to respond to a questionnaire on health-related behavior. Questions about alcohol intake included the frequency of alcohol consumption on a weekly basis and the usual amount that was consumed on a daily basis (≥ 20 g/day). We considered persons reporting that they smoked at that time to be current smokers. Blood samples were collected after more than 12 hr of fasting and were drawn from an antecubital vein. The fasting serum glucose, total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and blood urea nitrogen (BUN) were measured enzymatically with an automatic analyzer (Advia 1650 Autoanalyzer, Bayer Diagnostics; Leverkusen, Germany). The fasting serum glucose was measured with the hexokinase method. Total cholesterol and serum triglyceride were measured with enzymatic colorimetric tests, LDL-cholesterol was measured with the homogeneous enzymatic colorimetric test, and HDL-cholesterol was measured with the selective inhibition method (Bayer Diagnostics). Insulin levels were measured with immunoradiometric assays (Biosource, Belgium). Insulin resistance was calculated with the Homeostasis Model Assessment of insulin resistance (HOMA-IR): fasting serum insulin (µU/mL) × fasting blood glucose (mg/dL)/22.5. The serum creatinine was measured with the alkaline picrate (Jaffe) method. The urine protein level was determined at each examination from the results of a single urine dipstick semiquantitative analysis (URiSCAN® Urine strip, YD Diagnostics; Korea). Dipstick urinalysis was performed on fresh, midstream urine samples that were collected in the morning. The results of the urine test were based on a scale that quantified proteinuria as absent, trace, 1+, 2+, 3+, and 4+. The dipstick results of 1+, 2+, 3+, and 4+ corresponded to protein levels of approximately 30, 100, 300, and 1,000 mg/dL, respectively. Kidney function was estimated by the glomerular filtration rate (GFR), which was calculated with the simplified Modification of Diet in Renal Disease Study equation that is defined as estimated glomerular filtration rate (eGFR) = 186.3 × (serum creatinine)-1.154 × age-0.203 (16, 17). Proteinuria was defined as a finding of 1+ or greater. CKD was defined as either proteinuria or an eGFR of < 60 mL/min per 1.73 m2. Trained nurses obtained sitting blood pressure levels with a standard mercury sphygmomanometer. The first and fifth Korotkoff sounds were utilized in order to estimate the systolic blood pressure and the diastolic blood pressure. Height and weight were measured after an overnight fast with the subjects wearing a lightweight hospital gown and no shoes. The BMI was calculated as the weight (kg) divided by the square of the height (m).
Framingham risk score
The FRS was calculated from the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III algorithm, based on six coronary risk factors: gender, age, total cholesterol, HDL-cholesterol, systolic BP and smoking habit (12). Among these factors, age, BP, and cholesterol levels were categorized according to their values and smoking status was classified as either "current smoker" or "non-smoker". Finally, the corresponding point was given to each man and then the total score was used as the individual's CHD risk level. The FRS has been used to predict the 10-yr risk of coronary events (fatal/nonfatal myocardial infarction, coronary-associated mortality or sudden death) (18), and usually divided into three levels of risk < 10% (low), 10%-19% (intermediate) and ≥ 20% (high) (12, 18). In this study, we defined FRS ≥ 10% as more-than-a-moderate CHD risk group and FRS ≥ 20% as a high CHD risk group, respectively.
Statistical analyses
Initially, we compared the characteristics of participants in relation to the proportion of the dipstick urinalysis, eGFR stages and CKD. The one-way ANOVA and chi-square test were used to analyze the statistical differences across the characteristics of the study participants in relation to 10 yr-predicted-risk groups of the FRS. All multiple comparisons were conducted with the tukey method. More-than-a-moderate risk (FRS ≥ 10%) and high risk (FRS ≥ 20%) were considered as dependent variables. The logistic regression analyses were conducted to calculate the odds ratio (OR) and 95% confidence interval (CI). The data were first unadjusted, then adjusted for the multiple covariates. In multivariable model, age, HOMA-IR, BMI, hypertensive medication, diabetic medication and alcohol intake were included for adjustment. The statistical analysis for the data was performed with SPSS version 17.0 (SPSS Inc.). All the reported P values were two-tailed, and those < 0.05 were considered to be statistically significant.
Ethics statement
This study was approved by the institutional review board of the Kangbuk Samsung Hospital, Sungkyunkwan University, School of Medicine in Seoul, Korea (IRB number: KBC10069). All participants gave their written informed consent.
RESULTS
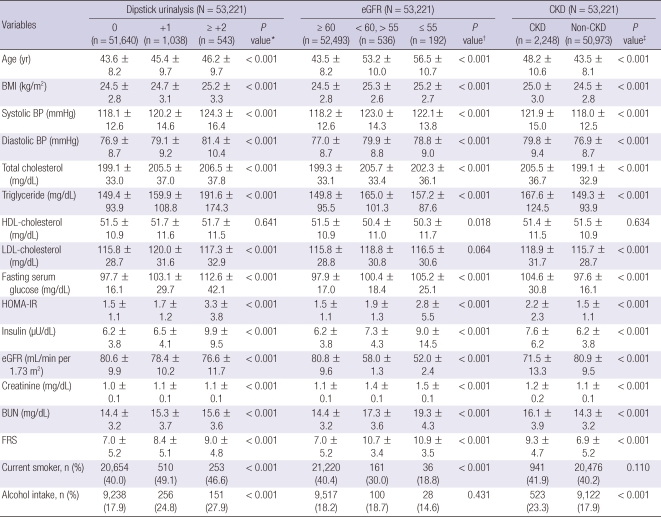
Overall, the mean age and eGFR were 43.7 ± 8.3 yr and 80.5 ± 9.9 mL/min per 1.73 m2, respectively (data mean ± S.D.). The overall prevalence of CKD was 4.2% (data not shown). Anthropometric, clinical, and laboratory data of those in relation to the proportion of the dipstick urinalysis, eGFR stages and CKD were shown in Table 1.
Table 1.
Comparison of baseline characteristics of proteinuria, eGFR and CKD (N = 53,221)
All values are the mean ± SD or the number of subjects (percent of the total). The dipstick urinalysis of trace was regarded as absent. *P value by ANOVA-test for continuous variables and Chi square test for categorical variables among dipstick urinalysis categories; †P value by ANOVA-test for continuous variables and Chi square test for categorical variables among eGFR categories; ‡P value by t-test for continuous variables and Chi square test for categorical variables between CKD and non-CKD. BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, Homeostasis Model Assessment of insulin resistance; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; FRS, Framingham risk score.
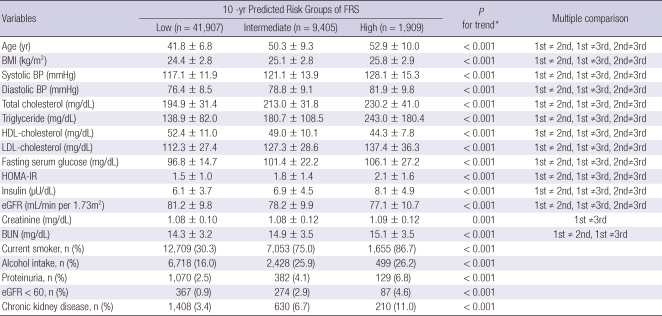
The risk factors found to be significantly associated with CKD were as follows: age, BMI, systolic and diastolic BP, total cholesterol, triglyceride, LDL-cholesterol, fasting serum glucose, HOMA-IR, insulin level, BUN, FRS and alcohol intake. The clinical characteristics of the study participants in relation to 10 yr-predicted-risk groups of the FRS were shown in Table 2. All clinical variables showed statistical significance. In the categorical analyses, the risk for CKD significantly increased with 10 yr-predicted-risk groups of the FRS. Table 3 presented the OR and 95% CI of the risk of CHD.
Table 2.
Characteristics of the study subjects stratified for 10-yr predicted risk groups (N = 53,221)
All values are the mean ± SD or the number of subjects (percent of the total). *P value by ANOVA-test for continuous variables and Chi square test for categorical variables. BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, Homeostasis Model Assessment of insulin resistance; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen.
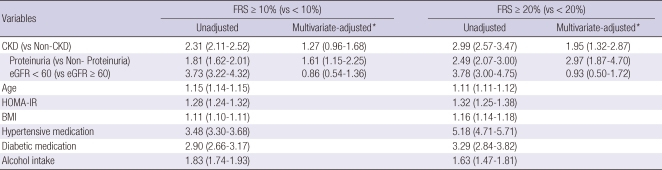
Table 3.
Odds ratio and 95% confidence intervals assessing the independent influence of CKD on more-than-a-moderate risk (FRS ≥ 10%) and high risk (FRS ≥ 20%) of CHD
*Multivariate-adjusted for age, HOMA-IR, BMI, hypertensive medication, diabetic medication and alcohol intake. CKD, chronic kidney disease; FRS, Framingham risk score; CHD, coronary heart disease; HOMA-IR, Homeostasis Model Assessment of insulin resistance; BMI, body mass index.
After adjusting for age, HOMA-IR, BMI, hypertensive medication, diabetic medication and alcohol intake, multivariable-adjusted logistic regression analyses showed a strong statistical significant relationship between CKD and high risk of CHD (adjusted OR, 1.95 [95% CI, 1.32-2.87]). In unadjusted analyses, the OR of eGFR < 60 was bigger than that of proteinuria in both more-than-a-moderate risk and high risk group of CHD. After adjusting for confounding variables, the OR of proteinuria only remained statistically significant.
DISCUSSION
The aim of this study was to determine whether individuals with CKD have an elevated 10-yr risk of CHD estimated by FRS. By multivariate logistic regression analysis, after adjusting for age, HOMA-IR, BMI, hypertensive medication, diabetic medication, and alcohol intake, CKD was found to be independently related to CHD. The results of our study identified that CKD was independently associated with an elevated CHD risk in Korean men, regardless of known coronary risk factors such as age, HOMA-IR, BMI, hypertension, diabetes mellitus and alcohol intake.
FRS provides estimates of total CHD events (defined as angina pectoris, myocardial infarction and coronary-associated mortality). Thus, a higher FRS reflects a greater 10-yr risk of developing CHD. FRS is transformed using the Framingham equation to 10-yr CHD risk estimates, that is, low risk < 10%, 10% ≤ moderate risk < 20% and high risk ≥ 20%. In this study, we defined FRS ≥ 10% as more-than-a-moderate CHD risk group and FRS ≥ 20% as a high CHD risk group. The FRS was used as a dependent variable, and it is the first study that using FRS as dependent variable instead of CHD events or mortality.
Although the exact mechanisms by which CKD relates to CHD remain unclear, it is possible that renal dysfunction may have led to CHD. Subtle decrements in kidney function may activate the renin-angiotensin-aldosterone system and the sympathetic nervous system. Upregulation of the renin-angiotensin-aldosterone system may lead to CHD by promoting Inflammatory process including atherosclerosis, nephropathy and cardiomyopathy (19, 20).
This study had some limitations, despite being conducted on a large representative sample of the general population. First, FRS should be applied to individuals who are over 30 yr old (21), but in this study, study participants were ≥ 20 yr old and FRS may less accurately predict the risk of CHD in younger individuals. The number of study participants aged between 20 and 29 was only 378, which occupied 0.71%. Thus, that could not cause bias. Second, this study was just confined to relatively racially homogeneous male of individuals of Korean ancestry who were recruited at a single urban hospital, which introduced the possibility of bias. Additionally, the participants were self-selected, so this study might show participant selection bias. Some studies have shown that ethnicity affects the presence and severity of CHD independently of atherosclerotic risk factors (22, 23). In fact, the original Framingham equations overestimated the risk of 5-yr CHD events in Chinese, Japanese American and Hispanic men and Native American women, and thus, specific population risk equations are required to take into account different prevalence of risk factors and underlying rates of developing CHD (24, 25). It may also overestimate in Koreans with significant different genetic and environmental backgrounds. However, in this study, FRS was used as a risk stratification tool, not for calculating absolute risks of CHD among racially different groups. Third, we used an eGFR instead of a directly measured GFR to define CKD. A recent review article reported that current eGFR had a greater inaccuracy in populations without known CKD than in those with the disease (26). Nonetheless, current eGFR facilitates the detection, evaluation, and management of CKD, and many organizations recommend the use of equations that estimate GFR in epidemiologic studies and in clinical practice for the evaluation of renal function (26). Therefore, our findings were applicable to clinical and public health practice settings. Regardless of these limitations, strength of the present study is that the number of relatively healthy male participants is large enough to show the evidence for correlations between CKD and CHD.
In conclusion, as far as we know, this is the first study to explore the relation between CKD and CHD, as determined using FRS in a large population. Those with CKD were found to have an elevated 10-yr risk of CHD. Furthermore, CKD was found to be independently related to CHD regardless of classical atherosclerotic risk factors. These findings highlight the importance of regular surveillance and monitoring of renal function for prevention of CHD (27). Mild to moderate kidney dysfunction is highly prevalent. Dipstick urinalysis and eGFR can be readily measured in most clinical settings. If kidney dysfunction precedes the onset of CHD, the measurement of kidney function may represent a relatively inexpensive and efficient way to identify individuals at higher risk for CHD.
ACKNOWLEDGMENTS
We thank the fine efforts of the members of the health screening center at the Kangbuk Samsung Hospital, Seoul, Korea.
AUTHOR SUMMARY
Relationship between Chronic Kidney Disease and Risk of Coronary Heart Disease in Korean Men
Jae-Hong Ryoo, Soo-Geun Kim, Byung-Seong Suh, Dong-Il Kim and Sung Keun Park
Chronic kidney disease (CKD) has been known to be associated with end stage renal disease (ESRD). It is well established that CKD is a single most important risk factor to coronary heart disease (CHD). Estimating CHD risk in clinical settings is of critical importance for prevention. Most of the well-recognized risk factors of CHD have been considered in the development of the Framingham risk score (FRS). The aim of this study is to determine the relationship between severity of CKD and FRS in Korean men. Multivariable-adjusted logistic regression analyses showed a strong statistical significant relationship between CKD and high risk of CHD (adjusted OR, 1.95 [95% CI, 1.32-2.87]). Dipstick urinalysis and eGFR can be readily measured in clinical settings. These findings highlight the importance of regular surveillance and monitoring of renal function for prevention of CHD.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Kiberd B. The chronic kidney disease epidemic: stepping back and looking forward. J Am Soc Nephrol. 2006;17:2967–2973. doi: 10.1681/ASN.2006020123. [DOI] [PubMed] [Google Scholar]
- 3.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis. 2000;36:415–418. doi: 10.1053/ajkd.2000.8996. [DOI] [PubMed] [Google Scholar]
- 5.Iseki K, Ikemiya Y, Fukiyama K. Risk factors of end-stage renal disease and serum creatinine in a community based mass screening. Kidney Int. 1997;51:850–854. doi: 10.1038/ki.1997.119. [DOI] [PubMed] [Google Scholar]
- 6.US Renal Data System. The National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: United States Renal Data System; 2003. USRDS 2003 Annual Data Report. [Google Scholar]
- 7.Stengel B, Billon S, Van Dijk PC, Jager KJ, Dekker FW, Simpson K, Briggs JD. Trends in the incidence of renal replacement therapy for end-stage renal disease in Europe, 1990-1999. Nephrol Dial Transplant. 2003;18:1824–1833. doi: 10.1093/ndt/gfg233. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 9.Xue JL, Ma JZ, Louis TA, Collins AJ. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol. 2001;12:2753–2758. doi: 10.1681/ASN.V12122753. [DOI] [PubMed] [Google Scholar]
- 10.Korean Society of Nephrology, Registry Committee. Renal replacement therapy in Korea: Insan Memorial Dialysis Registry. 2009. [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Prevalence of chronic kidney disease and associated risk factors-United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007;56:161–165. [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 13.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 14.Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 15.Wannamethee SG, Shaper AG, Perry IJ. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke. 1997;28:557–563. doi: 10.1161/01.str.28.3.557. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 19.Whaley-Connell A, Pavey BS, Chaudhary K, Saab G, Sowers JR. Renin-angiotensin-aldosterone system intervention in the cardiometabolic syndrome and cardio-renal protection. Ther Adv Cardiovasc Dis. 2007;1:27–35. doi: 10.1177/1753944707082697. [DOI] [PubMed] [Google Scholar]
- 20.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Circulation prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 22.Budoff MJ, Nasir K, Mao S, Tseng PH, Chau A, Liu ST, Flores F, Blumenthal RS. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis. 2006;187:343–350. doi: 10.1016/j.atherosclerosis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Hong Y, D'Agostino RB, Sr, Wu Z, Wang W, Sun J, Wilson PW, Kannel WB, Zhao D. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA. 2004;291:2591–2599. doi: 10.1001/jama.291.21.2591. [DOI] [PubMed] [Google Scholar]
- 25.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 26.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 27.Leung WY, So WY, Tong PC, Chan NN, Chan JC. Effects of structured care by a pharmacist-diabetes specialist team in patients with type 2 diabetic nephropathy. Am J Med. 2005;118:1414. doi: 10.1016/j.amjmed.2005.07.050. [DOI] [PubMed] [Google Scholar]