Abstract
Carcinoembryonic antigen (CEA) levels can be affected by many factors and metabolic syndrome is also a candidate. This study examined the relationship between CEA levels and metabolic syndrome using the data of 32,897 healthy Koreans. Fecal occult blood tests were also performed. Subjects with colorectal carcinoma were excluded. Subjects were classified by their smoking status, metabolic syndrome and its components. Prevalence of metabolic syndrome and its all components showed a significant increase according to the quartile of serum CEA concentration (P < 0.001). Increased numbers of metabolic syndrome components showed a positive association with CEA levels (P-trend < 0.001). The odds ratios for the highest CEA quartile vs the lowest serum CEA quartile significantly increased in the presence of metabolic syndrome and its components. After adjusting for age, gender and smoking status, metabolic syndrome, low high density lipoprotein cholesterol and elevated blood pressure had higher odds ratios (OR) of the highest CEA quartile compared with the lowest serum CEA quartile (OR = 1.125, 95% CI = 1.030 to 1.222, P = 0.009; OR = 1.296, 95% CI = 1.195 to 1.405, P < 0.001; OR = 1.334, 95% CI = 1.229 to 1.448, P < 0.001, respectively). These results indicate that metabolic syndrome is associated with CEA value, which may lead to a misunderstanding of the CEA levels.
Keywords: Metabolic Syndrome, Carcinoembryonic Antigen, Smoking
INTRODUCTION
Carcinoembryonic antigen (CEA) is overexpressed in adenocarcinomas in the colon and other organs including the pancreas, lung, prostate, urinary bladder, ovary, and breast. Therefore, it is used globally as a serological marker of malignant tumors. Serum CEA levels may also be affected by some nonmalignant conditions such as ageing, chronic renal failure, hypothyroidism, cigarette smoking, chronic obstructive pulmonary disease, and obesity (1-5).
Metabolic syndrome is a combination of several metabolic and physiological abnormalities in the same individual, including obesity, insulin resistance, glucose intolerance, hypertension, and dyslipidemia, and is associated with high morbidity and mortality (6, 7). Metabolic syndrome is a common metabolic disorder that results from the increasing prevalence of obesity, and which is an emerging clinical problem of enormous proportions, particularly in light of the rising prevalence of obesity (6, 7). Metabolic syndrome has also been identified as a causal risk factor for cardiovascular disease and several forms of cancer, such as cancers of the breast, pancreas, and colon (8-10). One recent study reported that obesity was associated with decreased CEA levels (11), which may lead to loss of sensitivity and diagnostic accuracy in the CEA test. Thus, interrelationships between CEA levels and individual component of metabolic syndrome may exist partly because of their associations with obesity. However, the relationship between an individual and the combined components of metabolic syndrome and the level of CEA are currently unknown.
The present study was grounded in the hypothesis that components of metabolic syndrome may profoundly affect CEA levels, and should be considered as a factor associated with inappropriately increased or decreased CEA levels. The aim of this study was to examine whether CEA levels were associated with metabolic syndrome in Koreans.
MATERIALS AND METHODS
Study data
From January 2007 to May 2009, the data of 39,906 Koreans aged 10-86 yr, who visited the Health Promotion Center, Ajou University Hospital, Suwon, Gyeonggi-do, Republic of Korea, were reviewed for inclusion in the study. Medical history, demographics, anthropometric, and laboratory data were collected. Data on cigarette smoking were collected by a self-reported questionnaire. Subjects who, at the time of the survey, had quit smoking for ≤ 1 month or > 1 month were considered to be, respectively, current and former smokers. Those without a smoking history were considered to be never smokers. Of the initial 39,906 subjects, 6,619 (16.5%) were excluded because of absent data for any component of metabolic syndrome, serum CEA levels, or smoking history. Subjects with prior diagnosis of colorectal cancer (CRC), gastric cancer, pancreatic cancer, lung cancer, or breast cancer, which are known to affect serum CEA levels, were excluded. Fecal occult blood tests were performed in all subjects. Of these with positive occult blood tests (n = 597), seven subjects with CRC in additional colonoscopic findings were excluded. Thus, a total of 32,897 healthy Korean (13,845 females, 19,052 males) were included in the final analyses.
Measurements
Serum CEA was measured using a commercially available immunometric chemiluminescent assay kit (Bayer Medical, Tokyo, Japan) with an interassay coefficient of variation ranging between 2.0% and 3.5%. Blood pressure (BP) was measured using a standard mercury manometer with the participant in a sitting position for 5 min prior to measurement; the average of two measurements was recorded. Circumferential measurements of the waist at the umbilicus were performed with the patients in a standing position. Fasting blood specimens were used for measuring lipids, glucose, and CEA. Metabolic syndrome was defined as three or more of the following criteria, according to the American Heart Association/Updated National Cholesterol Education Program Third Adult Treatment Panel guidelines (NCEP ATP III): waist measurement ≥ 102 cm for males and ≥ 88 cm for females, triglyceride level ≥ 150 mg/dL, high-density lipoprotein cholesterol (HDL cholesterol) < 40 mg/dL for males and < 50 mg/dL for females, BP ≥ 130/85 mmHg or the use of BP medications, and fasting glucose level ≥ 100 mg/dL or undergoing treatment for hyperglycemia (12). Abdominal girth cut-off to determine waist circumference (WC) was defined according to central obesity in Koreans (≥ 90 cm for males and ≥ 85 cm for females) (13). Subjects were grouped by smoking status, the sum of individual metabolic components (0-5), and the presence or absence of metabolic syndrome (yes or no). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2).
Statistics
Analysis of variance (ANOVA) was used to compare baseline characteristics and the prevalence of metabolic syndrome, metabolic syndrome components according to the quartile of serum CEA concentration. We examined the mean serum CEA concentration according to the number of metabolic syndrome components. ANOVA Trend analysis using polynomial contrasts was adapted to perform tests for trend. We also investigated to determine which variables were significant contributors to the serum CEA level in the adjusted state for each variable through ordinal logistic regression analyses using the quartile of serum CEA concentration.
Results of group data are expressed as mean ± standard deviation (SD). All statistical analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). P values < 0.05 were considered statistically significant.
Ethics statement
All participants agreed to the use of their health check-up results. The institutional review board of Ajou University Hospital approved this study (AJIRB-MED-OBS-10-415). Informed consent was waived by the board.
RESULTS
The baseline characteristics of 32,897 subjects according to the quartile of serum CEA concentration are summarized in Table 1. The median (range) of the first to fourth quartiles of CEA levels was 0.35 (0.22-0.48), 0.72 (0.62-0.82), 1.44 (1.01-1.29), and 2.35 (2.0-4.94) ng/mL, respectively. Compared to subjects with the lowest serum CEA quartile, those with higher serum CEA quartile were older and more likely to be male and current smoker. Anthropometric measurements and biochemical measurements were significantly different between the serum CEA quartile (P < 0.001). In case of triglycerides, this difference between the serum CEA quartile remained statistically significant after log-transformation.
Table 1.
Baseline characteristics of study subjects according to the quartile of serum CEA concentration
Data were shown as mean ± SD. *P < 0.001, All values show statistical significance in the comparisons of variables according to the quartile of serum CEA concentration; †Data are presented as a median value. BMI, body mass index; WC, waist circumference; TG, triglyceride; HDLC, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; TC, total cholesterol; Non, non-smoker; Former, former smoker; Current, current smoker.
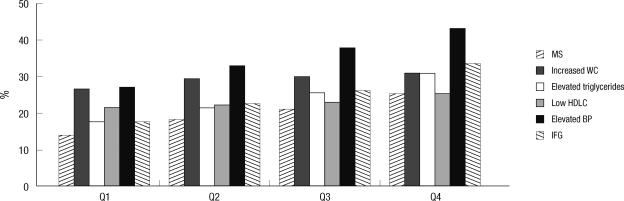
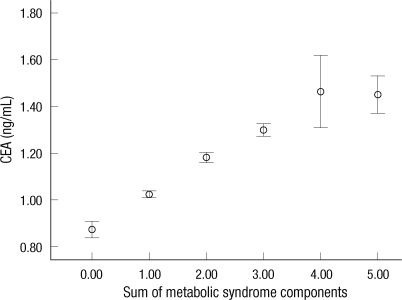
Prevalence of metabolic syndrome and its all components showed a significant increase according to the quartile of serum CEA concentration (P < 0.001; Fig. 1). We then assessed whether CEA levels were affected by the number of metabolic syndrome components. Increasing sums of metabolic syndrome components were significantly associated with linear increasing trends in CEA levels (P-trend < 0.001) (Fig. 2).
Fig. 1.
Prevalence of metabolic syndrome and its components according to the quartile of serum CEA concentration. Prevalence of metabolic syndrome and its all components show a significant increase according to the quartile of serum CEA concentration (P < 0.001). WC, waist circumference; HDLC, high-density lipoprotein cholesterol; BP, blood pressure; IFG, impaired fasting glucose. Q1, 1st quartile; Q2, 2nd quartile; Q3, 3rd quartile; Q4, 4th quartile.
Fig. 2.
The relationship between carcinoembryonic antigen (CEA) level and the sum of metabolic syndrome components. Vertical bar indicates 95% confidence interval. White circle indicates mean. Lower and upper bar indicates 95% confidence interval (P-trend < 0.001).
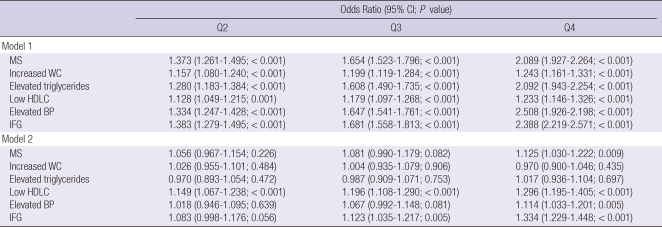
Table 2 reports the ordinal logistic regression models used to evaluate the association between metabolic syndrome, each components and serum CEA levels. The odds ratios (OR) for the highest CEA quartile for the lowest serum CEA quartile were significant in the presence of metabolic syndrome and its components. After adjusting for age, gender and smoking status, metabolic syndrome, low HDLC and elevated BP had higher odds of the highest CEA quartile compared with the lowest serum CEA quartile (OR = 1.125, 95% confidence interval [CI] = 1.030 to 1.222, P = 0.009; OR = 1.296, 95% CI = 1.195 to 1.405, P < 0.001; OR = 1.334, 95% CI = 1.229 to 1.448, P < 0.001, respectively). Former and current smokers also displayed higher odds of the highest CEA quartile compared with the lowest serum CEA quartile; the association was statistically significant (P < 0.05; data not shown).
Table 2.
Logistic regression analysis of the metabolic syndrome and its components as independent variables and CEA quartiles as a dependent variable
Reference group; no metabolic syndrome and normal metabolic components. Model 1, before adjustment; Model 2, after adjustment for age, sex, and smoking status. MS, presence of metabolic syndrome; BMI, body mass index; WC, waist circumference; HDLC, high-density lipoprotein cholesterol; BP, blood pressure; IFG, impaired fasting glucose; Q2, 2nd quartile; Q3, 3rd quartile; Q4, 4th quartile. CI, confidence interval.
DISCUSSION
The present data demonstrate that the sum of metabolic syndrome components is positively associated with serum CEA levels, and metabolic syndrome and its components have higher odds ratios of the highest CEA quartile compared with the lowest serum CEA quartile. In particular, serum CEA levels were found to be inversely significant to HDLC. This association remained statistically significant even after adjusting for age, BMI, and smoking status.
Over the past several years, the prevalence of metabolic syndrome has sharply increased worldwide (7, 14, 15), and is associated with the international epidemic of diabetes and obesity (6, 7, 14). Metabolic syndrome is associated with the increased risks of cardiovascular disease and various cancers, including colorectal carcinoma (CRC) (8-10, 16). As well, individual components of metabolic syndrome have been linked to an increased risk of CRC (9, 17). Recent studies have provided information concerning the association between CRC incidence and the number of metabolic syndrome components, especially BMI, lipid levels, plasma glucose, and glycosylated hemoglobin (HbA1c) (18, 19). One study demonstrated that there was a dose-response association between CRC incidence and the number of metabolic syndrome components present at baseline (P for trend = 0.006) after multivariate adjustment (18). Thus, it is possible that metabolic syndrome itself could confer an increased risk of CRC.
Serum CEA was elevated in subjects with CRC as well as gastric carcinoma, pancreatic carcinoma, lung carcinoma and breast carcinoma. CEA levels may also be raised in some non-neoplastic diseases like chronic inflammatory conditions. A recent study documented the association between serum CEA and carotid atherosclerosis, independent of atherogenic risk factors and markers of inflammation (20). Another study reported that increased CEA concentrations are an independent determinant for acute coronary syndrome (OR = 3.1, 95% CI = 1.2-7.9, P < 0.05) after correction for other significant risk factors (21). Taken together, the data indicate the plausibility that serum CEA may be associated with metabolic syndrome and increased insulin resistance. Presently, the serum CEA level was also associated with components of the metabolic syndrome and the syndrome itself.
What is the possible underlying mechanism to explain the observed link between metabolic syndrome, individual components, and serum CEA? First, insulin has been shown to affect growth of normal and neoplastic epithelial cells, and to have mitogenic actions in vitro and in experimental models, either directly or indirectly through insulin-like growth factor-1 (IGF-1) (18). One study reported that an antisense IGF-I gene that blocks the corresponding IGF sequences may be the reason for the decrease of CEA (22). Thus, insulin resistance may be associated with the increase of CEA. Second, visceral adiposity is positively correlated with clinical manifestations associated with insulin resistance, including type 2 diabetes and dyslipidemia. Visceral fat releases greater quantities of adipokines, which are related to either metabolic syndrome or cancer (23), and they may serve as a link between metabolic syndrome, individual components, and serum CEA. Third, while the relationship between high BP, low HDL cholesterol, and serum CEA is still unknown, increased risk of CRC and chronic inflammation has been linked with high BP (9, 17, 18, 24) and inversely associated with HDL cholesterol concentration and established cardiovascular disease (25). CEA is a tumor-associated antigen of gastrointestinal cancer that is associated with chronic inflammatory conditions. This may explain the finding of an association between high BP, low HDL cholesterol, and serum CEA.
The present study also has revealed a positive association of former/current smoking status with serum CEA levels, compared with never smoking. This finding is supported by previous observations that serum CEA levels were significantly higher in smokers and that cigarette smoking is associated with increased serum CEA levels in a dose- and duration-dependent manner, with the association being more prominent in current smokers than in former smokers (20).
There are some limitations with this study. First, because the study was cross-sectional, we cannot determine whether there was a causal or resultant relationship between the individual and the combined components of metabolic syndrome and the elevation of serum CEA. Second, it is not clear whether the analysis of this restricted patient group introduced a selection bias, and these findings may or may not reflect the situation in the overall population. Third, serum CEA is known to be elevated in other nonmalignant conditions, such as hypothyroidism and end-stage lung diseases. We did not include these disorders as confounding variables, because their prevalence is considered to be very low among the study population.
In conclusion, the present study reports a positive association between former or current smoking status, metabolic syndrome, some components of metabolic syndrome and elevation of serum CEA, and demonstrates that serum CEA levels are inversely significant by HDL cholesterol levels. Thus, metabolic syndrome might be a factor that is associated with CEA levels, which may lead to a misunderstanding of the CEA levels.
Footnotes
This research is supported by the Ubiquitous Computing and Network (UCN) Project, the Ministry of Knowledge and Economy (MKE) Knowledge and Economy Frontier R&D Program in Korea, by the Korea Breast Cancer Foundation.
AUTHOR SUMMARY
Carcinoembryonic Antigen Level Can be Overestimated in Metabolic Syndrome
Kyu-Nam Kim, Nam-Seok Joo, Sang-Yeon Je, Kwang-Min Kim, Bom-Taeck Kim, Sat-Byul Park, Doo-Yeoun Cho, Rae-Woong Park and Duck-Joo Lee
Carcinoembryonic antigen (CEA) levels can be affected by various factors including metabolic syndrome. This study examined the relationship between CEA levels and metabolic syndrome using the data of 32,897 healthy Koreans. Prevalence of metabolic syndrome and its all components showed a significant increase according to the quartile of serum CEA concentration. Metabolic syndrome, low high density lipoprotein cholesterol and elevated blood pressure were found to be significantly associated with the highest CEA quartile compared with the lowest serum CEA quartile. These results indicate that metabolic syndrome is associated with CEA levels.
References
- 1.Fukuda I, Yamakado M, Kiyose H. Influence of smoking on serum carcinoembryonic antigen levels in subjects who underwent multiphasic health testing and services. J Med Syst. 1998;22:89–93. doi: 10.1023/a:1022643102208. [DOI] [PubMed] [Google Scholar]
- 2.Amino N, Kuro R, Yabu Y, Takai SI, Kawashima M, Morimoto S, Ichihara K, Miyai K, Kumahara Y. Elevated levels of circulating carcinoembryonic antigen in hypothyroidism. J Clin Endocrinol Metab. 1981;52:457–462. doi: 10.1210/jcem-52-3-457. [DOI] [PubMed] [Google Scholar]
- 3.Bulut I, Arbak P, Coskun A, Balbay O, Annakkaya AN, Yavuz O, Gülcan E. Comparison of serum CA 19.9, CA 125 and CEA levels with severity of chronic obstructive pulmonary disease. Med Princ Pract. 2009;18:289–293. doi: 10.1159/000215726. [DOI] [PubMed] [Google Scholar]
- 4.Witherspoon LR, Shuler SE, Alyea K, Husserl FE. Carcinoembryonic antigen: assay following heat compared with perchloric acid extraction in patients with colon cancer, non-neoplastic gastrointestinal diseases, or chronic renal failure. J Nucl Med. 1983;24:916–921. [PubMed] [Google Scholar]
- 5.Herbeth B, Bagrel A. A study of factors influencing plasma CEA levels in an unselected population. Oncodev Biol Med. 1980;1:191–198. [PubMed] [Google Scholar]
- 6.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 7.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 8.Furberg AS, Veierød MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96:1152–1160. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 9.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–391. [PubMed] [Google Scholar]
- 10.Michaud DS, Liu S, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Dietary sugar, glycemic load, and pancreatic cancer risk in a prospective study. J Natl Cancer Inst. 2002;94:1293–1300. doi: 10.1093/jnci/94.17.1293. [DOI] [PubMed] [Google Scholar]
- 11.Park JS, Choi GS, Jang YS, Jun SH, Kang H. Influence of obesity on the serum carcinoembryonic antigen value in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2461–2468. doi: 10.1158/1055-9965.EPI-10-0569. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Kim MH, Kim MK, Choi BY, Shin YJ. Prevalence of the metabolic syndrome and its association with cardiovascular diseases in Korea. J Korean Med Sci. 2004;19:195–201. doi: 10.3346/jkms.2004.19.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu SY, Kweon SS, Park HC, Shin JH, Rhee JA. Obesity and the metabolic syndrome in Korean adolescents. J Korean Med Sci. 2007;22:513–517. doi: 10.3346/jkms.2007.22.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, Adam HO. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 17.Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006;107:28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 19.Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F Risk Factors and Life Expectancy Research Group. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev. 2001;10:937–941. [PubMed] [Google Scholar]
- 20.Ishizaka N, Ishizaka Y, Toda E, Koike K, Yamakado M, Nagai R. Are serum carcinoembryonic antigen levels associated with carotid atherosclerosis in Japanese men? Arterioscler Thromb Vasc Biol. 2008;28:160–165. doi: 10.1161/ATVBAHA.107.155465. [DOI] [PubMed] [Google Scholar]
- 21.Vassalle C, Pratali L, Ndreu R, Battaglia D, Andreassi MG. Carcinoembryonic antigen concentrations in patients with acute coronary syndrome. Clin Chem Lab Med. 2010;48:1339–1343. doi: 10.1515/CCLM.2010.243. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Li SN, Wang XN. CEA and AFP expression in human hepatoma cells transfected with antisense IGF-I gene. World J Gastroenterol. 1998;4:30–32. doi: 10.3748/wjg.v4.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006;12:1623–1635. doi: 10.2174/138161206776843313. [DOI] [PubMed] [Google Scholar]
- 25.Cea-Calvo L, Lozano JV, Fernández-Pérez C, Llisterri JL, Martí-Canales JC, Aznar J, Gil-Guillén V, Redón J Investigators of PREV-ICTUS study. Prevalence of low HDL cholesterol, and relationship between serum HDL and cardiovascular disease in elderly Spanish population: the PREV-ICTUS study. Int J Clin Pract. 2009;63:71–81. doi: 10.1111/j.1742-1241.2008.01902.x. [DOI] [PubMed] [Google Scholar]