Abstract
Retinol binding protein 4 (RBP4) has been postulated to provide a new link between obesity and insulin resistance. We aimed to assess the relationship between serum RBP4 and insulin resistance by investigating serum RBP4 levels in children and adolescents according to degree of obesity and pubertal stage. A total of 103 (30 lean, 39 overweight, 34 obese) were evaluated for serum RBP4, adiponectin, insulin, glucose and lipid profiles. RBP4 levels of obese and overweight groups were higher than those of lean group. RBP4 level was higher in pubertal group than in prepubertal group. RBP4 was positively correlated with age, height, weight, body mass index (BMI), abdominal circumference, systolic blood pressure, fasting insulin, homeostatic model assessment of insulin resistance (HOMA-IR), total cholesterol and triglyceride, and inversely with adiponectin. In the multiple linear regression analysis, RBP4 was found to be independently associated with pubertal stage, BMI and triglyceride but not with HOMA-IR. In conclusion, serum RBP4 level is related with degree of adiposity and pubertal development. The association of RBP4 with insulin resistance is supposed to be secondary to the relation between RBP4 and adipose tissue in children and adolescents.
Keywords: RBP4 protein, Human; Childhood Obesity; Insulin Resistance; Adipose Tissue
INTRODUCTION
Adipose tissue is now considered an active and complex metabolic endocrine organ (1). Adipose tissue secretes various adipokines that play important roles in the modulation of a variety of biological functions. Retinol binding protein 4 (RBP4), a newly discovered adipokine, is the major transport protein for retinol and is strongly expressed in the liver, but also secreted by adipocytes (2). RBP4 has recently been proposed as a new potential link between obesity and insulin resistance (3). Injection of purified RBP4 into mice or transgenic overexpression of RBP4 in mice impairs insulin signaling in muscle tissue and induces the expression of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase in the liver (3). Conversely, lowering RBP4 by genetic knockout improves insulin sensitivity in mice (3).
Several clinical studies in adults confirmed this association (4-7). Interventions that can improve insulin sensitivity, including exercise, may lower serum RBP4 levels in adults (8, 9). In contrast, other studies have found no links between RBP4 levels, obesity and insulin resistance (10, 11). A few studies in children and adolescents, also reported controversial findings concerning the biological functions of RBP4 in obesity (12-14). Park et al. (15) have reported an association between RBP4 and pulmonary function in prepubertal male asthmatics. Recent twin studies suggested that serum RBP4 increased with age and that the association of RBP4 with insulin resistance is secondary and non-casual (16). Hence, it remains unclear, whether RBP4 causes or reflect obesity-related insulin resistance.
In this study, we aimed to assess potential casual relationship between serum RBP4 and obesity-related insulin resistance by investigating serum RBP4 levels according to degree of obesity and pubertal stage and the relationship between serum RBP4 levels and other metabolic or cardiovascular parameters in Korean children and adolescents.
MATERIALS AND METHODS
We recruited children and adolescents who visited Pediatric Endocrinology Clinic at Korea University Ansan Hospital in Korea. Only healthy children and adolescents were included in this study. No participant had cardiovascular disease, diabetes, hypertension or other endocrine disorder according to medical histories and physical examinations that were completed prior to participation in this study.
Anthropometric measurements were taken with the subjects lightly clothed and without shoes. Height was measured to the nearest 0.1 cm using a rigid stadiometer. Weight was measured to the nearest 0.1 kg using a calibrated balance scale. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. All subjects were divided into three groups (lean, overweight, and obese) according to degree of obesity. Obese subjects were defined as having a BMI greater than or equal to the 95th percentile for their age and sex according to 2007 Korean national growth chart. Overweight subjects were defined as having a BMI greater than or equal to the 85th percentile but less than the 95th percentile. Lean subjects were defined as having a BMI less than the 75th percentile. Abdominal circumference was measured at the midpoint between the lower border of the rib cage and the iliac crest. Systolic and diastolic blood pressures (BP) were measured twice on the right arm after a 10 min rest in the supine position.
The pubertal stage of each participant was determined by a pediatric endocrinologist and rated according to the Tanner criteria (17). All subjects were divided into three groups (prepuberty, early and late puberty) according to their pubertal stage. Prepubertal subjects were defined as having sexual maturity rating (SMR) 1. Early pubertal subjects were defined as having SMR 2 or 3. Late pubertal subjects were defined as having SMR 4 or 5.
Blood sampling was performed after an eight hour overnight fast. We measured serum insulin, glucose, total cholesterol, triglyceride, LDL-cholesterol and HDL-cholesterol. RBP4 levels were measured with an enzyme immunoassay kit (AdipoGen, Inc., Seoul, Korea). Adiponectin levels were measured using a radioimmune assay kit (Linco Research Inc., St. Charles, MO, USA). Insulin resistance was estimated using the homeostatic model assessment of insulin resistance (HOMA-IR). The equation used was: HOMA-IR = (insulin [mU/L] × glucose [mM/L])/22.5.
Data are expressed as mean ± standard deviation (SD). For comparison of continuous clinical and metabolic variables between two groups, either independent t-test or Mann-Whitney U test, and among three groups, ANOVA's test and for categorical variables, chi-square test were used. Pearson's correlation coefficients were calculated to evaluate the relationship between serum RBP4 levels and other clinical and metabolic variables. Multiple linear regression analysis was performed to investigate whether age, sex, pubertal stage, BMI, systolic BP, triglyceride, and HOMA-IR determine RBP4 independently. SPSS 16.0 software was used for the statistical analyses. P value < 0.05 was considered as statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB number AS0740). Written informed consent was obtained from each subject and his or her parents.
RESULTS
Sixty-one boys and forty-two girls between the ages of 6 and 18 yr were included in this study. There was no significant difference in serum RBP4 between male and female (54.94 ± 20.18 vs 49.67 ± 16.66 mg/L, P = 0.166).
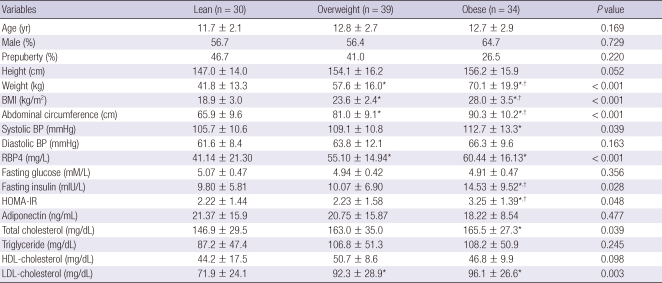
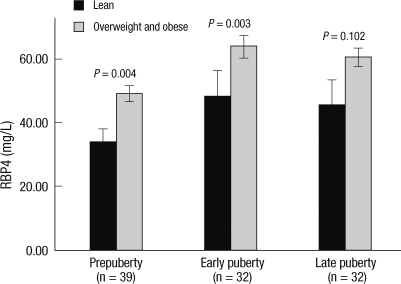
The clinical and metabolic variables of lean (n = 30), overweight (n = 39) and obese (n = 34) groups are shown in Table 1. Obese group had significantly higher weight, BMI, abdominal circumference, fasting insulin, and HOMA-IR than those of overweight and lean groups. Systolic BP, RBP4, total cholesterol, and LDL cholesterol of obese group were significantly higher than those of lean group. Weight, BMI, abdominal circumference, RBP4, and LDL-cholesterol in overweight group were significantly higher compared with lean group. There were no significant differences among lean, overweight, and obese groups for age, sex, puberty, height, diastolic BP, fasting glucose, adiponectin, triglyceride, and HDL-cholesterol. Serum RBP4 levels of obese and overweight groups were higher than those of lean group. Serum RBP4 level was higher in obese group than in overweight group, although it did not reach statistical significance. This tendency was observed regardless of pubertal stage, although it did not reach statistical significance in late puberty group (Fig. 1).
Table 1.
Clinical and metabolic variables of lean, overweight and obese groups
Data are shown as mean ± standard deviation and number. P values were calculated by ANOVA's test. For multiple comparison, Tukey procedure was done. *P < 0.05 compared with lean group; †P < 0.05 obese vs overweight group. BMI, body mass index; BP, blood pressure; RBP4, retinol binding protein 4; HOMA-IR, homeostatic model assessment of insulin resistance.
Fig. 1.
Serum retinol binding protein 4 (RBP4) and obesity in children and adolescents. Serum RBP4 levels of obese and overweight groups were compared with those of lean group according to pubertal stage. Serum RBP4 levels of obese and overweight groups were higher than those of lean group and this tendency was observed regardless of pubertal stage. Data are mean ± standard error of the mean (SEM).
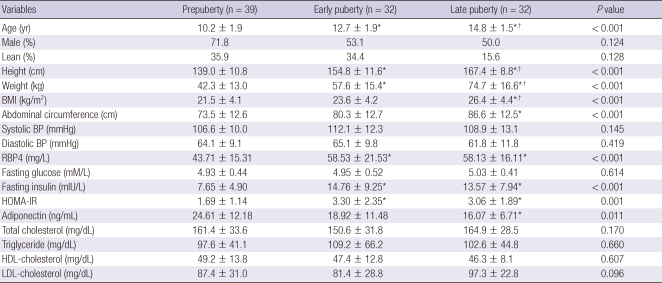
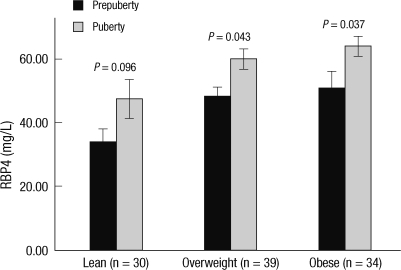
The clinical and metabolic variables of prepuberty (n = 39), early (n = 32) and late puberty (n = 32) groups are shown in Table 2. Late puberty group had significantly higher age, height, weight, and BMI than those of prepuberty and early puberty groups. Abdominal circumference, RBP4, fasting insulin, and HOMA-IR of late puberty group were significantly higher than those of prepuberty group. Age, height, weight, RBP4, fasting insulin, and HOMA-IR in early puberty group were significantly higher compared with prepuberty group. Adiponectin of prepuberty group was significantly higher than that of late pubertal group. There were no significant differences among prepuberty, early and late puberty groups for sex, obesity, systolic, and diastolic BP, fasting glucose, total cholesterol, triglyceride, HDL-cholesterol, and LDL-cholesterol. Serum RBP4 levels were significantly higher in early and late puberty groups than in prepuberty group and there was no significant difference in serum RBP4 level between early and late puberty groups. This tendency was observed regardless of degree of obesity, although it did not reach statistical significance in lean group (Fig. 2).
Table 2.
Clinical and metabolic variables of prepuberty, early puberty and late puberty groups
Data are shown as mean ± standard deviation and number. P values were calculated by ANOVA's test. For multiple comparison, Tukey procedure was done. *P < 0.05 compared with prepuberty group; †P < 0.05 early puberty vs late puberty group. BMI, body mass index; BP, blood pressure; RBP4, retinol binding protein 4; HOMA-IR, homeostatic model assessment of insulin resistance.
Fig. 2.
Serum retinol binding protein 4 (RBP4) and puberty in children and adolescents. Serum RBP4 levels of puberty group were compared with those of prepuberty group according to degree of obesity. Compared with prepuberty group, serum RBP4 levels were higher in puberty group and this tendency was observed regardless of degree of obesity. Data are mean ± standard error of the mean (SEM).
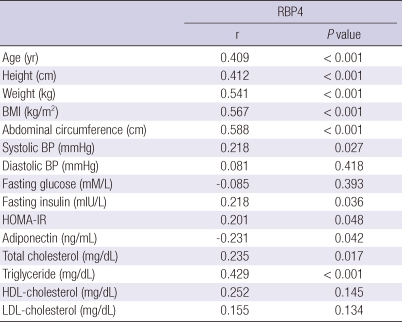
The correlations between serum RBP4 and other clinical and metabolic variables are shown in Table 3. Serum RBP4 was positively correlated with age, height, weight, BMI, abdominal circumference, systolic BP, fasting insulin, HOMA-IR, total cholesterol, and triglyceride. Serum RBP4 was negatively correlated with adiponectin.
Table 3.
Correlations between RBP4 levels and other clinical and metabolic variables
Coefficients (r) and P values are calculated using Pearson's correlation analysis. BMI, body mass index; BP, blood pressure; RBP4, retinol binding protein 4; HOMA-IR, homeostatic model assessment of insulin resistance.
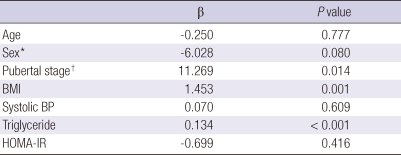
In the multiple linear regression analysis, RBP4 was found to be independently associated to pubertal stage, BMI and triglyceride, whereas RBP4 demonstrated no significant correlation to age, sex, systolic BP, and HOMA-IR as shown in Table 4.
Table 4.
Results of multiple regression analysis to assess independent relationships between RBP4 and clinical and metabolic variables (R2 = 0.477, P < 0.0001)
Unstandardized coefficients (β) and P values are presented. *Male = 0, female = 1; †Prepubertal = 0, pubertal = 1. BMI, body mass index; BP, blood pressure; RBP4, retinol binding protein 4; HOMA-IR, homeostatic model assessment of insulin resistance.
DISCUSSION
To the best of our knowledge, this is the first study on serum RBP4 levels according to degree of obesity and pubertal stage in Korean children and adolescents. In this study, we found that serum RBP4 level increased with progression of degree of obesity and also increased in children with pubertal development. We also found that serum RBP4 level was correlated with indices of adiposity (BMI, abdominal circumference), insulin resistance (fasting insulin, HOMA-IR), cardiovascular risk factors (systolic BP, triglyceride) and inversely with adiponectin in accordance with previous studies (8, 13, 18). However, most variables correlated with serum RBP4 in univariate analysis except for pubertal stage, BMI, and triglyceride were abolished after multiple linear regression analysis.
RBP4 has been postulated to be a determinant of insulin resistance associated with obesity but, controversies exist as to whether RBP4 is an adipocyte-derived mediator of insulin resistance or whether these associations are rather secondary to underlying obesity. Some previous studies reported a positive correlation between RBP4 levels and HOMA-IR in adults (8, 11). Lee et al. (18) found a positive correlation between RBP4 levels and HOMA-IR in non-obese adolescents, but not in obese adolescents. In recent studies, RBP4 was also correlated with parameters of cardiovascular dysfunction (19). Furthermore, longitudinal significant relationships between RBP4 levels, insulin levels and HOMA-IR before and after weight loss were previously detected in a pediatric cohort (13). However other studies have failed to find such a correlation (10, 20). Kanaka-Gantenbein et al. (14) also found no significant correlation between RBP4 levels and HOMA-IR and BMI SDS.
We found that RBP4 was independently associated with BMI but not with HOMA-IR in multiple regression analysis. Our results indicate that serum RBP4 level is significantly associated with adipose tissue mass and the relationship with insulin resistance was secondary to an underlying association with obesity. In a recent study, RBP4 mRNA and protein expression during adipogenesis was analyzed. With progression of differentiation from human preadipocytes into adipocytes, RBP4 production increased by several magnitudes (21). These in vitro results suggest that RBP4 expression increases with increasing adipose tissue mass. Another previous study found a higher mRNA expression in obese compared with lean subjects (22). Our findings are consistent with results of these studies.
For studies in children and adolescents, pubertal development should be considered because it is associated with changes in body composition, endocrine and metabolic alterations. RBP4 was reported to correlated with increasing gonadotropin levels in healthy adult women (23). In addition, Bottner et al. (24) showed that serum adiponectin levels decreased with pubertal development in boys and androgens were closely correlated with adiponectin. We found that RBP4 levels were higher in pubertal group than in prepubertal group and pubertal stage was an independent predictor for RBP4 level in multiple linear regression analysis. In contrast, no differences in circulating RBP4 levels across different pubertal stages were observed in other study (25). The increase in fat mass and sex hormones during pubertal development may account for the difference in RBP4 level between prepubetal and pubertal groups. Our findings suggest that RBP4 is may be involved in insulin resistance of obese children during puberty.
As reported in previous studies (8, 18, 26, 27), serum RBP4 levels were positively associated with triglyceride levels. This suggests that RBP4 is likely to dysregulate fatty acid metabolism. Dysregulation of fatty acid metabolism is closely related with insulin resistance (28). However, it is not clear whether RBP4 affect insulin action through a dysregulated fatty acid metabolism.
In previous studies, it has been suggested that sex-specific differences in RBP4 levels may exist, although the mechanism for this is not clear (5, 18). There were no significant sex-specific differences in RBP4 levels in this study, although the male subjects had slightly higher RBP4 levels compared to the female subjects.
The subjects of this study were limited to children and adolescents because they represent a unique study population, as they present early stages of the pathogenic development and they are relatively free of co-morbidities and treatment commonly seen in adults. Because this was a cross sectional study, interpretation of the results is limited. Therefore it is difficult to prove causality based on the findings. Further longitudinal studies are needed to confirm our results.
In conclusion, serum RBP4 level is related with degree of adiposity and pubertal development in children and adolescent. There is a causal relation between serum RBP4 and adipose tissue mass and serum RBP4 is a marker of adipose tissue mass. The association of RBP4 with insulin resistance is secondary to the underlying relation with adiposity.
Footnotes
This work was supported by a Korea University Grant
AUTHOR SUMMARY
Association of Serum Retinol Binding Protein 4 with Adiposity and Pubertal Development in Korean Children and Adolescents
Young Jun Rhie, Byung-Min Choi, So Hee Eun, Chang Sung Son, Sang Hee Park and Kee-Hyoung Lee
Retinol binding protein 4 (RBP4) has been postulated to provide a new link between obesity and insulin resistance. We aimed to assess potential casual relationship between serum RBP4 and obesity- related insulin resistance by investigating serum RBP4 levels according to degree of obesity and pubertal stage. RBP4 is a marker of adipose tissue mass and the association of RBP4 with obesity- related insulin resistance appears to be secondary to the underlying relation with adipose tissue.
References
- 1.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 2.Blaner WS. Retinol-binding protein: the serum transport protein for vitamin A. Endocr Rev. 1989;10:308–316. doi: 10.1210/edrv-10-3-308. [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 4.Basualdo CG, Wein EE, Basu TK. Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr. 1997;16:39–45. doi: 10.1080/07315724.1997.10718647. [DOI] [PubMed] [Google Scholar]
- 5.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida A, Matsutani Y, Fukuchi Y, Saito K, Naito M. Analysis of the factors contributing to serum retinol binding protein and transthyretin levels in Japanese adults. J Atheroscler Thromb. 2006;13:209–215. doi: 10.5551/jat.13.209. [DOI] [PubMed] [Google Scholar]
- 7.Munkhtulga L, Nakayama K, Utsumi N, Yanagisawa Y, Gotoh T, Omi T, Kumada M, Erdenebulgan B, Zolzaya K, Lkhagvasuren T, Iwamoto S. Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Hum Genet. 2007;120:879–888. doi: 10.1007/s00439-006-0264-4. [DOI] [PubMed] [Google Scholar]
- 8.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 9.Haider DG, Schindler K, Prager G, Bohdjalian A, Luger A, Wolzt M, Ludvik B. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2007;92:1168–1171. doi: 10.1210/jc.2006-1839. [DOI] [PubMed] [Google Scholar]
- 10.Janke J, Engeli S, Boschmann M, Adams F, Böhnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 11.Gavi S, Stuart LM, Kelly P, Melendez MM, Mynarcik DC, Gelato MC, McNurlan MA. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:1886–1890. doi: 10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 12.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab. 2007;92:1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 13.Reinehr T, Stoffel-Wagner B, Roth CL. Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93:2287–2293. doi: 10.1210/jc.2007-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaka-Gantenbein C, Margeli A, Pervanidou P, Sakka S, Mastorakos G, Chrousos GP, Papassotiriou I. Retinol-binding protein 4 and lipocalin-2 in childhood and adolescent obesity: when children are not just "small adults". Clin Chem. 2008;54:1176–1182. doi: 10.1373/clinchem.2007.099002. [DOI] [PubMed] [Google Scholar]
- 15.Park YH, Kim KW, Lee KE, Kim ES, Sohn MH, Kim KE. Clinical implications of serum retinol-binding protein 4 in asthmatic children. J Korean Med Sci. 2009;24:1010–1014. doi: 10.3346/jkms.2009.24.6.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribel-Madsen R, Friedrichsen M, Vaag A, Poulsen P. Retinol-binding protein 4 in twins: regulatory mechanisms and impact of circulating and tissue expression levels on insulin secretion and action. Diabetes. 2009;58:54–60. doi: 10.2337/db08-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanner J. Growth at adolescence. 2nd ed. Oxford, England: Blackwell Scientific Publications; 1962. [Google Scholar]
- 18.Lee DC, Lee JW, Im JA. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism. 2007;56:327–331. doi: 10.1016/j.metabol.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Ingelsson E, Lind L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care. 2009;32:733–735. doi: 10.2337/dc08-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin MJ, Kang SM, Jang Y, Lee JH, Oh J, Chung JH, Chung N. Serum retinol binding protein 4 levels are associated with serum adiponectin levels in non-diabetic, non-obese subjects with hypercholesterolemia. Clin Chim Acta. 2007;378:227–229. doi: 10.1016/j.cca.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Friebe D, Neef M, Erbs S, Dittrich K, Kratzsch J, Kovacs P, Blüher M, Kiess W, Körner A. Retinol binding protein 4 (RBP4) is primarily associated with adipose tissue mass in children. Int J Pediatr Obes. 2010 doi: 10.3109/17477166.2010.491228. doi: 10.3109/17477166.2010.491228. [DOI] [PubMed] [Google Scholar]
- 22.Klöting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schön MR, Stumvoll M, Blüher M, Kahn BB. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Makimura H, Wei J, Dolan-Looby SE, Ricchiuti V, Grinspoon S. Retinol-binding protein levels are increased in association with gonadotropin levels in healthy women. Metabolism. 2009;58:479–487. doi: 10.1016/j.metabol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Böttner A, Kratzsch J, Müller G, Kapellen TM, Blüher S, Keller E, Blüher M, Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 25.Santoro N, Perrone L, Cirillo G, Brienza C, Grandone A, Cresta N, Miraglia del. Variations of retinol binding protein 4 levels are not associated with changes in insulin resistance during puberty. J Endocrinol Invest. 2009;32:411–414. doi: 10.1007/BF03346477. [DOI] [PubMed] [Google Scholar]
- 26.Erikstrup C, Mortensen OH, Pedersen BK. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006;355:1393–1394. [PubMed] [Google Scholar]
- 27.Takashima N, Tomoike H, Iwai N. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006;355:1392. doi: 10.1056/NEJMc061863. [DOI] [PubMed] [Google Scholar]
- 28.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]