Abstract
Olfaction is one of the most important senses throughout the animal kingdom. It enables animals to discriminate between a wide variety of attractive and repulsive odorants and often plays a decisive role in species specific communication. In recent years the analysis of olfactory systems both invertebrates and invertebrates has attracted much scientific interest. In this context a pivotal question is how the properties and connectivities of individual neurons contribute to a functioning neuronal network that mediates odor-guided behavior. As a novel approach to analyze the role of individual neurons within a circuitry, techniques have been established that make use of light-sensitive proteins. In this review we introduce a non-invasive, optogenetic technique which was used to manipulate the activity of individual neurons in the olfactory system of Drosophila melanogaster larvae. Both channelrhodopsin-2 and the photosensitive adenylyl cyclase PAC α in individual olfactory receptor neurons (ORNs) of the olfactory system of Drosophila larvae allows stimulating individual receptor neurons by light. Depending on which particular ORN is optogenetically activated, repulsion or attraction behavior can be induced, indicating which sensory neurons underlie which type of behavior.
Keywords: optogenetics, Drosophila, channelrhodopsin-2, photo-activated adenylyl cyclase, olfactory behavior
Introduction
Beside the mouse and Caenorhabditis elegans, the fruit fly Drosophila melanogaster has emerged as one of the most important model systems in olfactory research. Studies of the olfactory system of Drosophila have initially focused on the genes involved in olfaction (e.g., Rodrigues, 1980; Monte et al., 1989; Ayyub et al., 1990; Carlson, 1991), on the morphological structures of odor-sensing organs and on the anatomy of neuronal circuits (Stocker, 1994). In a couple of excellent investigations the organization of the odor-sensing parts of the antenna as well as those structures of the brain that process the incoming olfactory information have been described in detail (reviewed by Stocker, 1994; Vosshall and Stocker, 2007; Liang and Luo, 2010). Hence, the neuroanatomy of Drosophila's olfactory system is now fairly well characterized, both in the adult fly and in the larva (Stocker, 1994; Python and Stocker, 2002b; Kreher et al., 2005; Ramaekers et al., 2005; Vosshall and Stocker, 2007). Interestingly, the organization of the olfactory system of fruit flies and other insects shares similarities with the “typical” vertebrate system (Strausfeld and Hildebrand, 1999), thus indicating the existence of general principles of olfactory coding and its underlying neuronal network. Yet, in Drosophila the network that produces an appropriate olfactory-based behavioral response is less complex in terms of cell numbers when compared to vertebrates. The relatively simple anatomical and functional organization and the genetic tractability make Drosophila an excellent model system to study chemosensory perception and to test the functional roles of individual neurons within a neuronal circuit. In this regard the central questions are currently focused on two important aspects: (1) How is a specific chemosensory stimulus translated into an electrical signal, which in turn can be decoded by the olfactory neuronal network? (2) What is the contribution a single neuron makes as part of whole circuits in olfactory behavior coding? Here we will highlight the olfactory system of Drosophila larvae because of its advantageous simplicity in terms of cell numbers and connectivities, the strong and robust olfactory behavior displayed by larvae and the facile accessibility of neuronal function through optogenetic techniques. In the first part of this review the anatomical basis of the olfactory system in larval Drosophila will be described with special emphasis on the organization of the neuronal network. In the following part a non-invasive optogenetic technique will be outlined by which the functional role of individual olfactory receptor neurons (ORNs) can be tested without interfering with the morphology of the neuronal connectivity. To conclude, future possibilities of this new technique will be discussed.
The Neuroanatomy of the Larval Olfactory System
Drosophila larvae are able to detect a variety of diverse odorants which can induce robust odor-guided behaviors (Rodrigues, 1980; Monte et al., 1989). Besides the apparent function of odorous signals for finding food and avoiding toxic substances, investigations suggest that larvae benefit from a primitive way of chemical communication to forage in groups (Wu et al., 2003). The capability of larvae to behaviorally respond to both attractive and repellent odors has been clearly demonstrated (Aceves-Piña and Quinn, 1979; Cobb, 1999; Heimbeck et al., 1999; Boyle and Cobb, 2005; Fishilevich et al., 2005; Kreher et al., 2005; Louis et al., 2008). However, the larval olfactory system is different from that of many other insects in that it operates in a comparatively highly concentrated odor environment. Adult fruit flies lay their eggs directly on rotting fruits, and hatching larvae use these fruits as food sources. Hence, larvae are directly exposed to the fruit odorants and probably do not need very long range odor detection. Rather, larvae have to discriminate and track odorants within a highly concentrated background of smells.
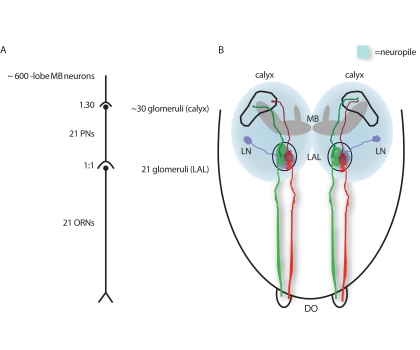
Similar to adult fruit flies the olfactory system of larval stages consists of four main olfactory morphological structures (Figure 1; Vosshall and Stocker, 2007) which show parallels with those of adult flies. At the periphery there are the main olfactory organs, in adults the antennae and the maxillary palps, in larvae the paired dorsal organs (DO). The sensory cells located in these organs express receptors that bind volatile chemical compounds as odorants (Siddiqi, 1987; Carlson, 1996; Heimbeck et al., 1999; de Bruyne et al., 2001; Fishilevich et al., 2005) and transform these stimuli into electrical signals (Kreher et al., 2005). Olfactory information is conveyed to three structures in the adult and the larval brain, first to the antennal lobe, and subsequently to the mushroom body (MB) calyx and the lateral horn. While the MB has been shown to be required for olfactory associative learning not only in adult flies (Heisenberg, 2003) but also in larvae (Honjo and Furukubo-Tokunaga, 2005; Pauls et al., 2010), the role of the lateral horn has been less well investigated as yet.
Figure 1.
Simplified wiring diagrams of the larval olfactory system. (A) Twenty-one ORNs are connected with 21 glomeruli in the LAL, and 21 PNs target ~30 glomeruli in the mushroom body calyx and synapse onto ~600 γ-lobe MB neurons. (B) Schematic illustration of the anatomical situation with individual ORNs (green or red) forming synapses with one PN each (dark green or dark red). PNs project to the calyx of the MB. LNs interconnect glomeruli in the LAL. The MB is shown in gray. DO, dorsal organ; LN, local interneurons; MB, mushroom bodies; PN, projection neurons.
The DO consists of a central dome with 6 peripheral sensillae and 21 ORNs (Gerber and Stocker, 2007). Electrophysiological recordings and experiments combining blocking of individual ORNs with behavioral tests (Heimbeck et al., 1999; Oppliger et al., 2000; Larsson et al., 2004; Fishilevich et al., 2005; Kreher et al., 2005) show that these neurons are the sole larval ORNs. The 21 ORNs are organized in seven triplets and send their axons to the larval antennal lobe (LAL; Tissot et al., 1997; Python and Stocker, 2002b). Although the structure of the larval olfactory system is relatively simple, recent studies demonstrate that the general logic of the expression of olfactory receptors (ORs) in larval ORNs is astoundingly similar to those of mammals and adult flies. Each of the 21 ORNs expresses only one or, in some instances, two specific endogenous ORs out of 25 expressed ORs in the larva (Couto et al., 2005; Fishilevich et al., 2005; Kreher et al., 2005). Interestingly, 13 OR-genes from this set of genes are indeed expressed specifically in larvae (OR1a, OR22c, OR24a, OR30a, OR45a, OR45b, OR59a, OR63a, OR74a, OR83a, OR85c, OR94a, OR94b), indicating specific requirements of the larval olfactory system when compared to the adult state; and 12 ORs are expressed both in the larval and in adult ORNs (OR2a, OR7a, OR10a, OR13a, OR19a, OR33a, OR33b, OR35a, OR42a, OR42b, OR43b, OR67b, and OR88a). Response profiles of many larval ORs to an array of odorants have been characterized by Kreher et al. (2005). Their research results provide a unique overview over the odor-specificity displayed by single receptors in an animal. There are a few cases where ORNs express more than one specific OR, which accounts for the fact that the number of identified OR-genes exceeds the actual number of existing ORNs in the larva (Robertson et al., 2003; Komiyama et al., 2004). However, in all ORNs the gene OR83b is co-expressed along with each specific OR. This is also the case for the majority of ORs in adult flies. OR83b is a cation channel thought to form a multi-dimer with the specific endogenously expressed OR (Sato et al., 2008; Wicher et al., 2008). Binding of an agonist to the putative multi-dimeric OR complex results in an activation of either a purely ionotropic or, additionally, a metabotropic signaling cascade (Sato et al., 2008; Wicher et al., 2008). Loss-of-function of OR83b results in anosmic mutants with abolished olfactory-driven behavior to alcohols, aldehydes ketones, and aromatic molecules (Larsson et al., 2004; Neuhaus et al., 2005; Benton et al., 2006; Masuda-Nakagawa et al., 2010).
The LAL is organized in glomeruli, similar to the anatomical situation of the adult antennal lobe (Figure 1), with the exception that a convergence of many ORNs to few glomeruli in the adult antennal lobe is absent in the larva (Masuda-Nakagawa et al., 2010). This means that each individual ORN expressing a given OR targets a single and specific glomerulus in the LAL (Figure 1; Ramaekers et al., 2005; Vosshall and Stocker, 2007; Masuda-Nakagawa et al., 2010). Conclusively the LAL consists of 21 glomeruli organized in a stereotypic manner with little invariance concerning the position of individual glomeruli (Masuda-Nakagawa et al., 2010). Glomeruli represent the arborization loci of second-order neurons, which are olfactory projection neurons (PNs) that convey the olfactory information to “higher brain centers,” i.e., the MB calyx (Figure 1) and the lateral horn, and, furthermore, inhibitory local interneurons (LNs) that mostly arborize in all glomeruli (Python and Stocker, 2002a). However, the existence of excitatory LNs in larvae cannot be excluded yet. Interestingly, there is an apparent functional arrangement of the larval glomeruli. Aromatic odors are represented in a cluster of more lateral glomeruli, while alcohols map to more medially located glomeruli within the LAL (Masuda-Nakagawa et al., 2010).
Larval PNs arborize their dendritic terminals within individual glomeruli (Ramaekers et al., 2005) and connect single LAL glomeruli with the larval MB, and here in particular with calycal glomeruli (Masuda-Nakagawa et al., 2009, 2010). Usually, one calycal glomerulus is targeted by each PN, but some PNs seem to project to two different calycal glomeruli (Ramaekers et al., 2005). Altogether, there is a rather straightforward connectivity between LAL glomeruli and calyx glomeruli (Figure 1), with one afferent ORN synapsing onto one PN (Figure 1) in the LAL, and most PNs target one calycal glomerulus. Hence, the activity pattern of the LAL should to some degree resemble the pattern in the calyx (Gerber and Stocker, 2007; Masuda-Nakagawa et al., 2009). As a result, a rather strict input–output correlation is generated through which the incoming information from ORNs modified by LNs is channeled to the calycal glomeruli. The calyx itself is an integrated component of the MB and constitutes the main input area of olfactory information to the MB. It consists of about 30 well-demarcated glomeruli (Masuda-Nakagawa et al., 2005, 2009; Ramaekers et al., 2005) and is the site at which incoming PNs form synaptic contacts with intrinsic MB neurons. In contrast to the morphological organization at the level of the LAL, the calyx is a site of divergence as each PN can form synaptic connections with more than one of the many hundreds of intrinsic MB neurons (Technau and Heisenberg, 1982; Masuda-Nakagawa et al., 2009).
Neuronal Populations of the Olfactory Circuitry
How do single neurons within such a network contribute to a specific, odor-evoked behavior? Let's simplify this question and consider only two general types of behavior, attraction toward or repulsion away from an odor source. One could for instance hypothesize two independently operating networks, one generating an appetitive and another producing a repellent olfactory behavior, dependent on the input. In this case the behavior would only be dependent on the stimulated network, and no further information needs to be extracted from the stimulus. Such a model has been proposed for the roundworm C. elegans. By exchanging the odorant receptor ODR-10 from input neurons feeding into a network that usually generates an attractive behavior to the input of a network which produces repellent olfactory behavior, Troemel et al. (1997) could demonstrate that the activity of the ORN already defines the olfactory preference. As an effect of this artificial manipulation, the quality of the ODR-10 ligand changes therefore from an attractant to a repellent.
Alternatively, the decision whether an odorant acts as a repellent or an attractant might be made at different processing levels that evaluate the quality or relevance of the odor. A molecular mechanism at the level of sensory neurons has been demonstrated also in C. elegans by Tsunozaki et al. (2008). Here, the odor preference displayed by the animal is dependent on intracellular signaling cascades that are influenced by previous odor-exposure. Another possibility would be that “higher brain centers” evaluate the quality of the odor on the basis of a massive input from diverse ORNs and induce an appropriate behavior, and the activity of individual ORNs do not cause any specific behavioral response.
To address the question how the olfactory neuronal network in the Drosophila larva is organized the functional properties of neurons within the network must be taken into consideration. One way to examine such functional properties is to determine the neurotransmitters, excitatory or inhibitory, or to monitor the odor-evoked activity of neurons. As regards neurotransmitters, several studies have shown that acetylcholine (ACh) and γ-aminobutyric acid (GABA) as well as biogenic amines act as neurotransmitters and neuromodulators in the olfactory system of adult and larval insects, including Drosophila (Bicker, 1999; Homberg and Müller, 1999; Blenau and Baumann, 2001). ORNs as well as most PNs are cholinergic and therefore excitatory, while LNs are mostly GABAergic and inhibitory. However, there are also cholinergic LNs in the adult olfactory system (Olsen et al., 2007; Root et al., 2007; Shang et al., 2007; Yaksi and Wilson, 2010), but this type of LNs has not been described in the LAL so far. LNs mediate cross-communication between glomeruli at the level of the LAL. Based on electrophysiological and calcium imaging studies on the adult antennal lobe, the function of such GABAergic LNs can be interpreted as mediating a transformation of the odor representation in the antennal lobe, ultimately enhancing discriminability between odors (Sachse and Galizia, 2002; Lei et al., 2004; Wilson et al., 2004; Wilson and Laurent, 2005; Bhandawat et al., 2007; Olsen and Wilson, 2008). Moreover, using genetically encoded calcium sensitive reporters Silbering et al. (2008) have found that subpopulations of LNs in adult flies are either specialized to certain ORN inputs or parts of a more “global” inhibitory network, which implies a role of particular LNs in the intraglomerular computation of specific glomeruli response patterns and in overall gain control. Whereas these physiological experiments provide crucial insights into the computational mechanisms within the neuronal circuitry, the contribution of distinct neurons to the ultimate output, the behavior, has not yet been clearly determined.
An alternative strategy to investigate the roles of individual neurons in the olfactory network is to manipulate single neurons in the olfactory network and to directly test for changes in olfactory behavior. Three different genetic approaches have been established: functional knockout, rescue of function and exogenous activation. Using the first approach, several ORNs in the larva (OR1a, OR49a, or Or42a, respectively) have been individually knocked out through the expression of diphtheria toxin (Fishilevich et al., 2005) which resulted in a change in chemotaxis. Whereas the ablation of OR1a and OR49a caused a reduced chemotaxis to only one odorant each out of 20 tested, the ablation of OR42a resulted in a chemotaxis defect toward more odorants. These effects are consistent with the finding that some ORNs have a more narrow, others a more broad ligand tuning (Imai et al., 2010); and it also underlines that there is some degree of redundancy with respect to the ORNs required for detecting an odor source. In a second, reverse experiment one or two ORNs were functionally restored in otherwise anosmic larvae (Fishilevich et al., 2005). The chemosensory behavior rescued by individual OR expression was dependent on the type of OR. Whereas restoring OR1a or OR49a did not cause any rescue of chemotaxis toward the tested odorants, the expression of OR42a rescued chemotaxis to a larger degree, confirming that individual Ors with broader or narrower ligand tuning contribute differentially to odor detection. Interestingly, a rescue of OR1a and in addition OR42a caused a rescue of behavior in response to more odorants than the sum of a rescue with the two individual ORs, which speaks for a cooperative effect between the two types of ORNs (Fishilevich et al., 2005). Neuronal crosstalk at the level of the LAL accomplished by LNs (see above) could perhaps contribute to this chemotaxis behavior.
Since the analysis of the contribution of individual ORNs to the net outcome is obviously difficult due to the differential ligand tuning of the diverse ORs and potential crosstalk between ORNs a tool would be favorable that allows one to circumvent odorant binding and to activate individual ORNs directly. For such an approach, optogenetic techniques to manipulate neuronal activity within neuronal circuits can be used in a non-invasive manner. This quickly developing field combines optical methods with the cell-specific expression of protein-based probes with the aim to manipulate neural circuits (Fiala et al., 2010). Different strategies with respect to the photosensitive proteins applied have been established both in vertebrates and invertebrates (Fiala et al., 2010). They can be categorized into three groups: (1) light-dependent uncaging of neurotransmitters or other receptor-binding ligands along with appropriate receptor expression. The caged compounds used for this approach include, e.g., ATP, glutamate, GABA, or glycine as well as components of specific signaling cascades, e.g., Ca2+ or IP3 (Dalva and Katz, 1994; Wieboldt et al., 1994; Kantevari et al., 2006; Ellis-Davies, 2007, 2008; Shembekar et al., 2007); (2) small molecule photoswitches that rely on a coupling of photoisomerizable molecules to ion channels or receptors, rendering them light-sensitive (Bartels et al., 1971; Lester et al., 1980; Banghart et al., 2004, 2009; Fortin et al., 2008); and (3) natural photosensitive proteins such as channelrhodopsin-2 (ChR2; Nagel et al., 2003; Boyden et al., 2005; Li et al., 2005; Schroll et al., 2006; Gorostiza and Isacoff, 2008; Bellmann et al., 2010), halorhodopsin (NpHR; Han and Boyden, 2007; Zhang et al., 2007a), photosensitive adenylyl cyclases (PAC α; Schroder-Lang et al., 2007), or even an almost entire rhodopsin-dependent signaling cascade (Zemelman et al., 2002). In Drosophila several optogenetic techniques have been established by now and include ATP-dependent channels in combination with the light-dependent uncaging of ATP (Clyne and Miesenbock, 2008) as well as natural photosensitive proteins (Schroll et al., 2006; Schroder-Lang et al., 2007; Suh et al., 2007; Zhang et al., 2007b; Bellmann et al., 2010).
The Smell of Blue Light: Non-Invasive Optogenetic Activation of Olfactory Neurons
In general larvae exhibit a robust appetitive olfactory behavior toward a variety of odorants, but only few odors are repellent (Cobb and Dannet, 1994; Heimbeck et al., 1999; Hallem et al., 2004; Kreher et al., 2005; Louis et al., 2008; Asahina et al., 2009; Table 1).
Table 1.
Overview of LAL glomerlui, their functional properties, and the resulting behavior.
| ORN type | Main sensitivity to odorant class | LAL glomerulus | Calyx glomerulus | Exclusive larval expression | Induced olfactory behavior |
|---|---|---|---|---|---|
| OR1a | 1a | A4 + L8 | + | Attraction2 | |
| OR13a | Alcohol | 13a | L1 | − | Attraction1 |
| OR22c | Aromatic | 22c | L4 | + | |
| OR24a | Aromatic | 24a | L2 | + | |
| OR30a | Aromatic | 30a | L6 or M4 | + | Attraction1 |
| OR33a | 33a | I3 | − | ||
| OR33b within the | Ester | 33b | D1 | − | Repulsion1 |
| OR47a same ORN | Ester | 47a | D1 | − | |
| OR35a | Alcohol, aldehyde, ketone | 35a | V1 | − | |
| OR42a | Alcohol, aldehyde, ketone, ester | 42a | V9 | − | Attraction2 |
| OR42b | Ketone, ester | 42b | L9 | − | |
| OR45a | Ketone, ester | 45a | A3 + L11 | + | Repulsion1 |
| OR45b | Aromatic | 45b | D4 | − | Attraction1 |
| OR49a | 49a | D3 | − | Attraction2 | |
| OR59a | Aromatic | 59a | A5 | + | Attraction1 |
| OR63a | 63a | V5 | + | Attraction1 | |
| OR67b | Alcohol, aldehyde, ketone, aromatic | 67b | A2 | − | Attraction1 |
| OR74a | Alcohol | 74a | L5 | + | Attraction1 |
| OR82a | 82a | A1 | − | ||
| OR85c | Alcohol, ketone, ester | 85c | L10 | + | Attraction1 |
| OR94b | 94b | L3 | + | Attraction1 |
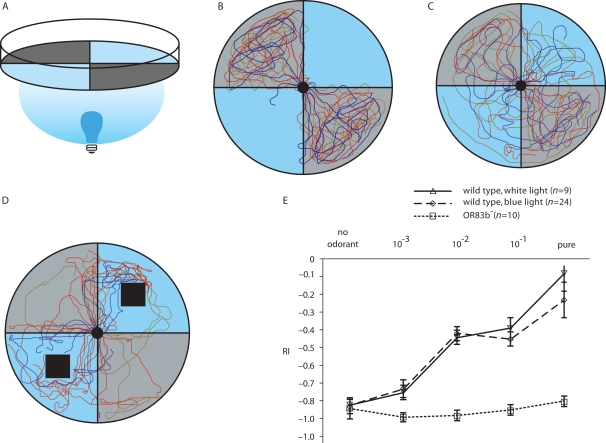
Behavioral assays to test for odor-evoked locomotion are very easy to perform. Moreover, the relatively simple neuronal organization of the olfactory system in the Drosophila larva in combination with optogenetic techniques makes it possible to address the question at which processing level within the larval olfactory circuit an aversive or appetitive olfactory behavior can be induced. Such a specific behavior might be generated by processes within the “central brain structures,” e.g., the LAL or the MB. Alternatively, ORNs might be hardwired to specific behavioral outputs as has been shown for two types of ORNs of the adult olfactory system (Semmelhack and Wang, 2009) or for a CO2-sensitive ORN (Suh et al., 2007). Two properties make the larva a suitable model organism for optogenetic stimulation: (1) Larvae display a robust negative phototactic behavior (Figures 2A,B; Movie S1 in Supplementary Material; Sawin-McCormack et al., 1995) that can be discriminated from an optogenetic stimulation of olfactory neurons. And (2) the larval transparency is ideal for a light-induced stimulation. Of course, the entire genetic toolbox available for adult flies can also be used in larvae. In particular, the Gal4/UAS system in Drosophila (Brand and Perrimon, 1993; Duffy, 2002) facilitates targeted and cell type-specific gene expression, in this case the expression of either the natural photosensitive proteins ChR2 and PAC α without changing the functional circuitry. The activation of ChR2 causes a cation-mediated depolarization, while the photo-stimulation of PAC α induces an increase in intracellular cAMP-level. In our recent report the driver line OR83b-Gal4 was used to either express ChR2 or PAC α in all larval ORNs (Bellmann et al., 2010). Photoactivation at a wavelength of 480 nm leads to a depolarization of ORNs which ultimately causes the “illusion” of an odor stimulus. When subjected to a choice situation in which two quadrants were illuminated and two opposing quadrants were dark (Figure 2A) larvae crawled into the illuminated areas of a Petri dish (Figure 2C; Movie S2 in Supplementary Material) to search for the odor source. Apparently, the stimulus sensed through the optogenetically activated olfactory pathway interferes with the aversive, visual signal input. Similarly, wild type larvae are also attracted by odorants applied in an illuminated environment (Bellmann et al., 2010). Application of blue light did not result in a completely attractive stimulation of the transformed larvae since several larvae remained in the dark quadrants or returned into the dark areas after having been attracted by the blue light (Figure 2D). Obviously the optogenetically mediated stimulus is not as attractive as the odorant itself, and in addition the aversive visual input remains present. However, by applying a repulsive odorant in the dark quadrants wild type larvae could be “driven” into the illuminated areas, indicating that this aversive olfactory input is more effective than visual cues (Figure 2E). In contrast the anosmic mutant OR83b− was incapable to detect the aversive odor and performed a normal phototactic behavior.
Figure 2.
Odor-induced or optogenetically induced behavior of wild type and transgenic larvae. (A) Experimental setup. A Petri dish is divided into four quadrants. Blue quadrants represent illuminated areas while gray sections are light-impermeable. (B) Negative phototaxis behavior of wild type larvae shown as individual crawling traces of 35 larvae. (C) The same experimental test as in (B), but this time transgenic larvae are used that express ChR2 under the control of OR83b-Gal4 in all ORNs. Larvae show less pronounced negative phototaxis behavior. (D) Wild larvae exposed to blue light and simultaneously to the attractive odorant benzaldehyde in illuminated areas, indicated by the black squares, show some attraction toward the odor source. (E) In a similar experimental setup a response index (RI) is calculated indicating the degree of attraction toward or repulsion from an odor source. A RI of −1 indicates complete repulsion. In this experimental setup the repulsive odorant octyl acetate is applied in the dark quadrants at various concentrations. With increasing odor concentration wild type larvae escape from the dark into illuminated areas (blue or white light) as is indicated by the increasing RI. In contrast, anosmic mutants (Or83b−) show a normal negative phototaxis behavior and strongly avoid blue or white light (modified from Bellmann et al., 2010).
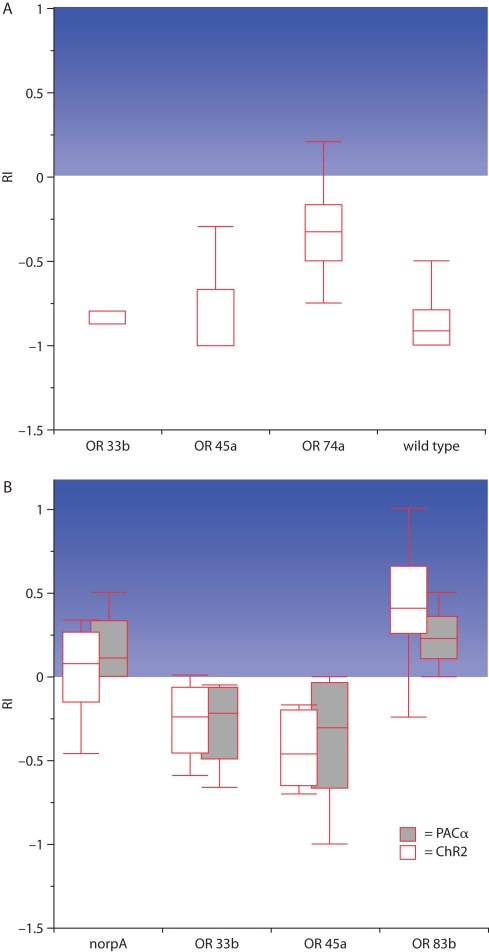
Using specific Gal4-driver lines it was now possible to narrow down the photo-stimulation to individual ORNs (Figure 3A; Table 1). With the exception of two driver lines, OR33b-Gal4 and OR45a-Gal4, larvae were allured by the blue light which indicates that the stimulation of a single ORN is sufficient to generate a specific attractive olfactory behavior (Table 1). In accordance with that finding the endogenous ORs of the appropriate stimulated ORNs detect ligands that act as attractants for larvae (Table 1; Bellmann et al., 2010). However, when the driver lines OR33b-Gal4 and OR45a-Gal4 were used to express ChR2 or PAC α, larvae showed a behavior that was not distinguishable from the negative phototactic behavior of wild type larvae (Bellmann et al., 2010). Thus, to avoid any visual inputs, photoactivation of single ORNs was performed in a genetic background of a mutation (norpA) that makes the animals blind. When ChR2 or PAC α was expressed in OR33b or OR45a ORNs blind norpA larvae avoided the light. In contrast, the blind control line did not show any preference to either dark or illuminated quadrants in the Petri dish (Figure 3B; Bellmann et al., 2010). In accordance with that finding, ligands of the receptor OR33b or OR45a (octyl acetate or hexyl acetate) are repellent odors for larvae. In conclusion, specific ORNs already define the olfactory preference or avoidance. Whereas the activation of most OSNs causes attraction behavior toward the source of stimulation, only two ORs have an opposite effect (Bellmann et al., 2010). The fact that the activation of individual ORNs causes a directed locomotor output demonstrates a determined input–output relationship between individual ORNs and ultimate behavior, and appetitive and aversive functions can be assigned to individual ORNs.
Figure 3.
Behavioral responses to blue light in the four-quadrant assay. The response index (RI) indicates the degree of attraction toward the blue illuminated areas of the Petri dish, illustrated by the blue background. (A) Wild type as well as larvae in which ChR2 is expressed in ORNs that endogenously express the receptors OR33b or OR45a strongly escape from the light. In contrast, when ChR2 is expressed in those ORNs that endogenously express the receptor OR74a larvae are significantly more attracted by blue light (n = 20 each). (B) Blind larvae that express ChR2 or PAC α in all ORNs (OR83b) are attracted by blue light, while the expression of the same proteins in single ORNs with the endogenous receptors OR33b or OR45a results in an avoidance of the light. The blind mutant control (norpA) shows no preference (n = 10 each; modified from Bellmann et al., 2010).The median is indicated as line in the box. Boxes locate the 25 and 75 percentiles and the whiskers indicate the minimum and maximum values.
However, the experiments outlined above represent only a first step toward the dissection of the olfactory system of Drosophila larvae. As mentioned above, ORs can bind more or less natural ligands, and some odorants can activate several ORNs. In conclusion, an integration of the multiple inputs causing a net output is apparently required. From the anatomical organization one can conclude that the stimulation of a single ORN initially leads to the activation of a single glomerulus in the LAL, and perhaps the activation pattern at the level of the LAL is to some degree mirrored in the calyx (see above). Based on the relative simple organization of the olfactory system in larvae, it can be assumed that the escape reaction evoked by repulsive odorants is the result of the olfactory stimulation of a specialized neuronal wiring through defined ORNs rather than through the quality of the repellent odorant that is extracted through a “higher brain” network. In this case the neuronal network in larvae would resemble the neuronal network found in C. elegans. The above-mentioned results and experimental findings in which adaptive processes could not be found for repulsive odorants in the larvae also speak in favor of such a specification (Colomb et al., 2007). However, further investigation will have to elucidate to which degree the combinatorial activity of several ORNs specifies a distinct behavior and what contributions further processing steps at the level of the LAL, i.e., cross-communication via LNs, make for the behavioral output. In fact, even larvae are able to change their odor-guided behavior through associative or non-associative learning (Honjo and Furukubo-Tokunaga, 2005; Gerber and Stocker, 2007; Larkin et al., 2010; Pauls et al., 2010), showing that even hardwired input–output connections are subject to modulation.
Outlook: Optogenectics in Olfactory Research
Optogenetic stimulation has now been established in a variety of model organisms (Li et al., 2005; Herlitze and Landmesser, 2007; Fiala et al., 2010). The advantage of these quickly developing techniques relies on the spatial and temporal precision as well as on a defined dosage-dependent application of the stimulus, properties that are usually difficult to achieve using odorants as stimuli. In addition, its non-invasiveness might make optogenetics a powerful tool for Drosophila larvae and might help to explore the functional properties of individual PNs or LNs in the larval olfactory system. However, the activation of neurons through ChR2 and PAC α has to be compatible with the endogenous signal transduction. With respect to ChR2, there is no doubt that neuronal depolarization through cation influx represents a “natural” signal for most neurons. Concerning PAC α the situation is not as clear, because the functional role(s) of cAMP in olfactory sensory neurons still remains controversial (Nakagawa and Vosshall, 2009). To sum up: optogenetic tools provide a promising approach to dissect an olfactory circuitry and to correlate neuronal activity with a resulting behavior. The Drosophila larva represents an ideal model organism for this task.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Movies 1 and 2 for this article can be found online at http://www.frontiersin.org/neuroscience/10.3389/fnins.2011.00072/abstract/
Acknowledgments
This review is dedicated to V. Rodrigues (1953–2010; Department of Biology at NCBS Bangalore and Department of Biological Sciences at TIFR, Mumbai). This work was supported by grants of the Deutsche Forschungsgemeinschaft (SFB 554/A2 to André Fiala and STO 283/11 to Klemens Störtkuhl).
Key Concepts
- Olfactory receptor neurons
These are specialized neurons that reside in either the antennae of adult or the dorsal organs of larval fruit flies. One olfactory receptor neuron expresses one specific olfactory receptor protein that specifies to which odorants the receptor neuron responds.
- Mushroom body
This neuronal structure of insect brains consists of hundreds of densely packed intrinsic neurons and is anatomically subdivided into calyx, columnar stalk, and lobes. The mushroom body has been show to be a structure crucially involved in associative olfactory learning.
- Projection neurons
These are neurons that relay signals form the ORNs in the antennal lobe to more central brain regions, namely the calyx of the mushroom body and the lateral horn.
- Inhibitory local interneurons
These specialized neurons are located in the larval antennal lobes. Typically, these neurons interconnect different glomeruli of the larval antennal lobes, release the transmitter GABA and inhibit neuronal activity in the glomerular subunits of the antennal lobe, thereby modifying the olfactory information detected by receptor neurons.
- Gal4/UAS system
This genetic tool is based on mobile DNA elements that make a specific germline transformation of Drosophila with transgenes possible. Two fly strains are used: one strain determines which transgene of interest is expressed under the control of an “upstream activator sequence” (UAS), e.g., an optogenetic protein as ChR2 or PAC α. The second fly strain determines where the transgene is expressed. In our case promoter regions of the DNA coding for olfactory receptors restrict the expression of the yeast transcription factor to particular olfactory receptor neurons of interest. By crossing the two fly strains a cell type-specific expression of transgenes can be achieved, in our case the expression of optogenetic, light-sensitive proteins in particular olfactory receptor neurons.
Biography
 Klemens F. Störtkuhl, worked in the laboratories of Professor E. Buchner and Professor M. Heisenberg in Würzburg, Germany and of Professor R. Stocker at the Université de Fribourg, where he received his Ph.D. After a postdoc research period spent at the laboratory of Professor J. Carlson at Yale University he founded his own research group at the Ruhr-University in Bochum. Since 2004 he has been Professor at the Ruhr-University. His main scientific research interest focuses on the functional analysis of olfactory system of Drosophila melanogaster at the biochemical and electrophysiological level as well as the study of olfactory behavior.
Klemens F. Störtkuhl, worked in the laboratories of Professor E. Buchner and Professor M. Heisenberg in Würzburg, Germany and of Professor R. Stocker at the Université de Fribourg, where he received his Ph.D. After a postdoc research period spent at the laboratory of Professor J. Carlson at Yale University he founded his own research group at the Ruhr-University in Bochum. Since 2004 he has been Professor at the Ruhr-University. His main scientific research interest focuses on the functional analysis of olfactory system of Drosophila melanogaster at the biochemical and electrophysiological level as well as the study of olfactory behavior.
 André Fiala, is Professor at the Georg-August-University of Göttingen. Research in his lab focuses on neuronal mechanisms underlying olfactory coding and olfactory learning using Drosophila melanogaster as a model organism. He has worked in the laboratory of Professor R. Menzel, at the Free University of Berlin, where he also received his PhD, at the Memorial Sloan Kettering Institute in Professor Gero Miesenböck's laboratory in New York and in Professor E. Buchner's laboratory at the University of Würzburg.
André Fiala, is Professor at the Georg-August-University of Göttingen. Research in his lab focuses on neuronal mechanisms underlying olfactory coding and olfactory learning using Drosophila melanogaster as a model organism. He has worked in the laboratory of Professor R. Menzel, at the Free University of Berlin, where he also received his PhD, at the Memorial Sloan Kettering Institute in Professor Gero Miesenböck's laboratory in New York and in Professor E. Buchner's laboratory at the University of Würzburg.
References
- Aceves-Piña E., Quinn W. (1979). Learning in normal and mutant Drosophila larvae. Science 206, 93–96 10.1126/science.206.4414.93 [DOI] [PubMed] [Google Scholar]
- Asahina K., Louis M., Piccinotti S., Vosshall L. B. (2009). A circuit supporting concentration-invariant odor perception in Drosophila. J. Biol. 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyub C., Paranjape J., Rodrigues V., Siddiqi O. (1990). Genetics of olfactory behavior in Drosophila melanogaster. J. Neurogenet. 6, 243–262 10.3109/01677069009107114 [DOI] [PubMed] [Google Scholar]
- Banghart M., Borges K., Isacoff E., Trauner D., Kramer R. H. (2004). Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart M. R., Mourot A., Fortin D. L., Yao J. Z., Kramer R. H., Trauner D. (2009). Photochromic blockers of voltage-gated potassium channels. Angew. Chem. Int. Ed. Engl. 48, 9097–9101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E., Wassermann N. H., Erlanger B. F. (1971). Photochromic activators of the acetylcholine receptor. Proc. Natl. Acad. Sci. U.S.A. 68, 1820–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann D., Richardt A., Freyberger R., Nuwal N., Schwarzel M., Fiala A., Stortkuhl K. F. (2010). Optogenetically induced olfactory stimulation in Drosophila larvae reveals the neuronal basis of odor-aversion behavior. Front. Behav. Neurosci. 4:27. 10.3389/fnbeh.2010.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R., Sachse S., Michnick S. W., Vosshall L. B. (2006). Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, e20. 10.1371/journal.pbio.0040020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V., Olsen S. R., Gouwens N. W., Schlief M. L., Wilson R. I. (2007). Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 10, 1474–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker G. (1999). Histochemistry of classical neurotransmitters in the antennal lobes and mushroom bodies of the honeybee. Microsc. Res. Tech. 45, 174–183 [DOI] [PubMed] [Google Scholar]
- Blenau W., Baumann A. (2001). Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch. Insect Biochem. Physiol. 48, 13–38 [DOI] [PubMed] [Google Scholar]
- Boyden E. S., Zhang F., Bamberg E., Nagel G., Deisseroth K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 [DOI] [PubMed] [Google Scholar]
- Boyle J., Cobb M. (2005). Olfactory coding in Drosophila larvae investigated by cross-adaptation. J. Exp. Biol. 208, 3483–3491 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- Carlson J. (1991). Olfaction in Drosophila : genetic and molecular analysis. Trends Neurosci. 14, 520–524 10.1016/0166-2236(91)90004-E [DOI] [PubMed] [Google Scholar]
- Carlson J. R. (1996). Olfaction in Drosophila : from odor to behavior. Trends Genet. 12, 175–180 10.1016/0168-9525(96)10015-9 [DOI] [PubMed] [Google Scholar]
- Clyne J. D., Miesenbock G. (2008). Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 133, 354–363 10.1016/j.cell.2008.01.050 [DOI] [PubMed] [Google Scholar]
- Cobb M. (1999). What and how do maggots smell? Biol. Rev. Camb. Philos. Soc. 74, 425–459 [Google Scholar]
- Cobb M., Dannet F. (1994). Multiple genetic control of acetate-induced olfactory responses in Drosophila melanogaster larvae. Heredity 73(Pt 4), 444–455 10.1038/hdy.1994.192 [DOI] [PubMed] [Google Scholar]
- Colomb J., Grillenzoni N., Stocker R. F., Ramaekers A. (2007). Complex behavioral changes associated to oder exposure in Drosophila larvae. Anim. Behav. 73, 587–594 10.1016/j.anbehav.2006.04.016 [DOI] [Google Scholar]
- Couto A., Alenius M., Dickson B. J. (2005). Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547 [DOI] [PubMed] [Google Scholar]
- Dalva M. B., Katz L. C. (1994). Rearrangements of synaptic connections in visual cortex revealed by laser photostimulation. Science 265, 255–258 10.1126/science.7912852 [DOI] [PubMed] [Google Scholar]
- de Bruyne M., Foster K., Carlson J. R. (2001). Odor coding in the Drosophila antenna. Neuron 30, 537–552 10.1016/S0896-6273(01)00289-6 [DOI] [PubMed] [Google Scholar]
- Duffy J. B. (2002). GAL4 system in Drosophila : a fly geneticist's Swiss army knife. Genesis 34, 1–15 10.1002/gene.10150 [DOI] [PubMed] [Google Scholar]
- Ellis-Davies G. C. (2007). Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods 4, 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies G. C. (2008). Neurobiology with caged calcium. Chem. Rev. 108, 1603–1613 [DOI] [PubMed] [Google Scholar]
- Fiala A., Suska A., Schlüter O. M. (2010). Optogenetic approaches in neuroscience. Curr. Biol. 20, 897–903 [DOI] [PubMed] [Google Scholar]
- Fishilevich E., Domingos A. I., Asahina K., Naef F., Vosshall L. B., Louis M. (2005). Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr. Biol. 15, 2086–2096 [DOI] [PubMed] [Google Scholar]
- Fortin D. L., Banghart M. R., Dunn T. W., Borges K., Wagenaar D. A., Gaudry Q., Karakossian M. H., Otis T. S., Kristan W. B., Trauner D., Kramer R. H. (2008). Photochemical control of endogenous ion channels and cellular excitability. Nat. Methods 5, 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B., Stocker R. F. (2007). The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem. Senses 32, 65–89 10.1093/chemse/bjl030 [DOI] [PubMed] [Google Scholar]
- Gorostiza P., Isacoff E. Y. (2008). Optical switches for remote and noninvasive control of cell signaling. Science 322, 395–399 10.1126/science.1166022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Ho M. G., Carlson J. R. (2004). The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965–979 10.1016/j.cell.2004.05.012 [DOI] [PubMed] [Google Scholar]
- Han X., Boyden E. S. (2007). Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2, e299. 10.1371/journal.pone.0000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbeck G., Bugnon V., Gendre N., Haberlin C., Stocker R. F. (1999). Smell and taste perception in Drosophila melanogaster larva: toxin expression studies in chemosensory neurons. J. Neurosci. 19, 6599–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. (2003). Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275 [DOI] [PubMed] [Google Scholar]
- Herlitze S., Landmesser L. T. (2007). New optical tools for controlling neuronal activity. Curr. Opin. Neurobiol. 17, 87–94 [DOI] [PubMed] [Google Scholar]
- Homberg U., Müller U. (1999). “Neuroactive substances in the antennal lobe,” in Insect Olfaction, ed. Hansson B. S. (New York: Springer; ), 81–206 [Google Scholar]
- Honjo K., Furukubo-Tokunaga K. (2005). Induction of cAMP response element-binding protein-dependent medium-term memory by appetitive gustatory reinforcement in Drosophila larvae. J. Neurosci. 25, 7905–7913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Sakano H., Vosshall L. B. (2010). Topographic mapping – the olfactory system. Cold Spring Harb. Perspect. Biol. 2, a001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantevari S., Hoang C. J., Ogrodnik J., Egger M., Niggli E., Ellis-Davies G. C. (2006). Synthesis and two-photon photolysis of 6-(ortho-nitroveratryl)-caged IP3 in living cells. Chembiochem 7, 174–180 10.1002/cbic.200500345 [DOI] [PubMed] [Google Scholar]
- Komiyama T., Carlson J. R., Luo L. (2004). Olfactory receptor neuron axon targeting: intrinsic transcriptional control and hierarchical interactions. Nat. Neurosci. 7, 819–825 [DOI] [PubMed] [Google Scholar]
- Kreher S. A., Kwon J. Y., Carlson J. R. (2005). The molecular basis of odor coding in the Drosophila larva. Neuron 46, 445–456 10.1016/j.neuron.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Larkin A., Karak S., Priya R., Das A., Ayyub C., Ito K., Rodrigues V., Ramaswami M. (2010). Central synaptic mechanisms underlie short-term olfactory habituation in Drosophila larvae. Learn. Mem. 17, 645–653 [DOI] [PubMed] [Google Scholar]
- Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H., Vosshall L. B. (2004). OR83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 10.1016/j.neuron.2004.08.019 [DOI] [PubMed] [Google Scholar]
- Lei H., Christensen T. A., Hildebrand J. G. (2004). Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe. J. Neurosci. 24, 11108–11119 10.1523/JNEUROSCI.3677-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Krouse M. E., Nass M. M., Wassermann N. H., Erlanger B. F. (1980). A covalently bound photoisomerizable agonist: comparison with reversibly bound agonists at electrophorus electroplaques. J. Gen. Physiol. 75, 207–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Gutierrez D. V., Hanson M. G., Han J., Mark M. D., Chiel H., Hegemann P., Landmesser L. T., Herlitze S. (2005). Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. PNAS 102, 17816–17821 10.1073/pnas.0509030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Luo L. (2010). The olfactory circuit of the fruit fly Drosophila melanogaster. Sci. China Life Sci. 53, 472–484 10.1007/s11427-010-0099-z [DOI] [PubMed] [Google Scholar]
- Louis M., Huber T., Benton R., Sakmar T. P., Vosshall L. B. (2008). Bilateral olfactory sensory input enhances chemotaxis behavior. Nat. Neurosci. 11, 187–199 [DOI] [PubMed] [Google Scholar]
- Masuda-Nakagawa L. M., Awasaki T., Ito K., O'Kane C. J. (2010). Targeting expression to projection neurons that innervate specific mushroom body calyx and antennal lobe glomeruli in larval Drosophila. Gene Expr. Patterns 10, 328–337 10.1016/j.gep.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Masuda-Nakagawa L. M., Gendre N., O'Kane C. J., Stocker R. F. (2009). Localized olfactory representation in mushroom bodies of Drosophila larvae. Proc. Natl. Acad. Sci. U.S.A. 106, 10314–10319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Nakagawa L. M., Tanaka N. K., O'Kane C. J. (2005). Stereotypic and random patterns of connectivity in the larval mushroom body calyx of Drosophila. Proc. Natl. Acad. Sci. U.S.A. 102, 19027–19032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte P., Woodard C., Ayer R., Lilly M., Sun H., Carlson J. (1989). Characterization of the larval olfactory response in Drosophila and its genetic basis. Behav. Genet. 19, 267–283 [DOI] [PubMed] [Google Scholar]
- Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E. (2003). Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U.S.A. 100, 13940–13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Vosshall L. B. (2009). Controversy and consensus: noncanonical signaling mechanisms in the insect olfactory system. Curr. Opin. Neurobiol. 19, 284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus E. M., Gisselmann G., Zhang W., Dooley R., Stortkuhl K., Hatt H. (2005). Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 8, 15–17 [DOI] [PubMed] [Google Scholar]
- Olsen S. R., Bhandawat V., Wilson R. I. (2007). Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron 54, 89–103 10.1016/j.neuron.2007.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S. R., Wilson R. I. (2008). Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452, 956–960 10.1038/nature06864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppliger F. Y., M Guerin P., Vlimant M. (2000). Neurophysiological and behavioural evidence for an olfactory function for the dorsal organ and a gustatory one for the terminal organ in Drosophila melanogaster larvae. J. Insect Physiol. 46, 135–144 10.1016/S0022-1910(99)00109-2 [DOI] [PubMed] [Google Scholar]
- Pauls D., Selcho M., Gendre N., Stocker R. F., Thum A. S. (2010). Drosophila larvae establish appetitive olfactory memories via mushroom body neurons of embryonic origin. J. Neurosci. 30, 10655–10666 10.1523/JNEUROSCI.1281-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Python F., Stocker R. F. (2002a). Immunoreactivity against choline acetyltransferase, gamma-aminobutyric acid, histamine, octopamine, and serotonin in the larval chemosensory system of Dosophila melanogaster. J. Comp. Neurol. 453, 157–167 10.1002/cne.10383 [DOI] [PubMed] [Google Scholar]
- Python F., Stocker R. F. (2002b). Adult-like complexity of the larval antennal lobe of D. melanogaster despite markedly low numbers of odorant receptor neurons. J. Comp. Neurol. 445, 374–387 10.1002/cne.10188 [DOI] [PubMed] [Google Scholar]
- Ramaekers A., Magnenat E., Marin E. C., Gendre N., Jefferis G. S., Luo L., Stocker R. F. (2005). Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr. Biol. 15, 982–992 [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Warr C. G., Carlson J. R. (2003). Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 100(Suppl. 2), 14537–14542 10.1073/pnas.2335847100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues V. (1980). Olfactory behavior of Drosophila melanogaster. Basic Life Sci. 16, 361–371 [DOI] [PubMed] [Google Scholar]
- Root C. M., Semmelhack J. L., Wong A. M., Flores J., Wang J. W. (2007). Propagation of olfactory information in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 104, 11826–11831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse S., Galizia C. G. (2002). Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J. Neurophysiol. 87, 1106–1117 [DOI] [PubMed] [Google Scholar]
- Sato K., Pellegrino M., Nakagawa T., Vosshall L. B., Touhara K. (2008). Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 10.1038/nature06850 [DOI] [PubMed] [Google Scholar]
- Sawin-McCormack E. P., Sokolowski M. B., Campos A. R. (1995). Characterization and genetic analysis of Drosophila melanogaster photobehavior during larval development. J. Neurogenet. 10, 119–135 10.3109/01677069509083459 [DOI] [PubMed] [Google Scholar]
- Schroder-Lang S., Schwarzel M., Seifert R., Strunker T., Kateriya S., Looser J., Watanabe M., Kaupp U. B., Hegemann P., Nagel G. (2007). Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 4, 39–42 [DOI] [PubMed] [Google Scholar]
- Schroll C., Riemensperger T., Bucher D., Ehmer J., Voller T., Erbguth K., Gerber B., Hendel T., Nagel G., Buchner E., Fiala A. (2006). Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 [DOI] [PubMed] [Google Scholar]
- Semmelhack J. L., Wang J. W. (2009). Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature 459, 218–223 10.1038/nature07983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Claridge-Chang A., Sjulson L., Pypaert M., Miesenbock G. (2007). Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell 128, 601–612 10.1016/j.cell.2006.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembekar V. R., Chen Y., Carpenter B. K., Hess G. P. (2007). Coumarin-caged glycine that can be photolyzed within 3 microseconds by visible light. Biochemistry 46, 5479–5484 10.1021/bi700280e [DOI] [PubMed] [Google Scholar]
- Siddiqi O. (1987). Neurogenetics of olfaction in Drosophila melanogaster. Trends Genet. 3, 137–142 10.1016/0168-9525(87)90204-6 [DOI] [Google Scholar]
- Silbering A. F., Okada R., Ito K., Galizia C. G. (2008). Olfactory information processing in the Drosophila antennal lobe: anything goes? J. Neurosci. 28, 13075–13087 10.1523/JNEUROSCI.2973-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R. F. (1994). The organization of the chemosensory system in Drosophila melanogaster : a review. Cell Tissue Res. 275, 3–26 10.1007/BF00305372 [DOI] [PubMed] [Google Scholar]
- Strausfeld N. J., Hildebrand J. G. (1999). Olfactory systems: common design, uncommon origins? Curr. Opin. Neurobiol. 9, 634–639 [DOI] [PubMed] [Google Scholar]
- Suh G. S., Ben-Tabou de Leon S., Tanimoto H., Fiala A., Benzer S., Anderson D. J. (2007). Light activation of an innate olfactory avoidance response in Drosophila. Curr. Biol. 17, 905–908 [DOI] [PubMed] [Google Scholar]
- Technau G., Heisenberg M. (1982). Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature 295, 405–407 10.1038/295405a0 [DOI] [PubMed] [Google Scholar]
- Tissot M., Gendre N., Hawken A., Stortkuhl K. F., Stocker R. F. (1997). Larval chemosensory projections and invasion of adult afferents in the antennal lobe of Drosophila. J. Neurobiol. 32, 281–297 [DOI] [PubMed] [Google Scholar]
- Troemel E. R., Kimmel B. E., Bargmann C. I. (1997). Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–169 10.1016/S0092-8674(00)80399-2 [DOI] [PubMed] [Google Scholar]
- Tsunozaki M., Chalasani S. H., Bargmann C. L. (2008). A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preferences in C. elegnas. Neuron 59, 959–971 10.1016/j.neuron.2008.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall L. B., Stocker R. F. (2007). Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 [DOI] [PubMed] [Google Scholar]
- Wicher D., Schafer R., Bauernfeind R., Stensmyr M. C., Heller R., Heinemann S. H., Hansson B. S. (2008). Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007–1011 10.1038/nature06861 [DOI] [PubMed] [Google Scholar]
- Wieboldt R., Gee K. R., Niu L., Ramesh D., Carpenter B. K., Hess G. P. (1994). Photolabile precursors of glutamate: synthesis, photochemical properties, and activation of glutamate receptors on a microsecond time scale. Proc. Natl. Acad. Sci. U.S.A. 91, 8752–8756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. I., Laurent G. (2005). Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 25, 9069–9079 10.1523/JNEUROSCI.2070-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. I., Turner G. C., Laurent G. (2004). Transformation of olfactory representations in the Drosophila antennal lobe. Science 303, 366–370 10.1126/science.1090782 [DOI] [PubMed] [Google Scholar]
- Wu Q., Wen T., Lee G., Park J. H., Cai H. N., Shen P. (2003). Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron 39, 147–161 10.1016/S0896-6273(03)00396-9 [DOI] [PubMed] [Google Scholar]
- Yaksi E., Wilson R. I. (2010). Electrical coupling between olfactory glomeruli. Neuron 67, 1034–1047 10.1016/j.neuron.2010.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelman B. V., Lee G. A., Ng M., Miesenbock G. (2002). Selective photostimulation of genetically chARGed neurons. Neuron 33, 15–22 10.1016/S0896-6273(01)00574-8 [DOI] [PubMed] [Google Scholar]
- Zhang F., Wang L. P., Brauner M., Liewald J. F., Kay K., Watzke N., Wood P. G., Bamberg E., Nagel G., Gottschalk A., Deisseroth K. (2007a). Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 10.1038/nature05744 [DOI] [PubMed] [Google Scholar]
- Zhang W., Ge W., Wang Z. (2007b). A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur. J. Neurosci. 26, 2405–2416 10.1111/j.1460-9568.2007.05862.x [DOI] [PubMed] [Google Scholar]