Abstract
The effect of continuous visual flow on the ability to regain and maintain postural orientation was examined. Fourteen young (20–39 years old) and 14 older women (60–79 years old) stood quietly during 3° (30°/s) dorsiflexion tilt of the support surface combined with 30° and 45°/s upward or downward pitch rotations of the visual field. The support surface was held tilted for 30 s and then returned to neutral over a 30-s period while the visual field continued to rotate. Segmental displacement and bilateral tibialis anterior and gastrocnemius muscle EMG responses were recorded. Continuous wavelet transforms were calculated for each muscle EMG response. An instantaneous mean frequency curve (IMNF) of muscle activity, center of mass (COM), center of pressure (COP), and angular excursion at the hip and ankle were used in a functional principal component analysis (fPCA). Functional component weights were calculated and compared with mixed model repeated measures ANOVAs. The fPCA revealed greatest mathematical differences in COM and COP responses between groups or conditions during the period that the platform transitioned from the sustained tilt to a return to neutral position. Muscle EMG responses differed most in the period following support surface tilt indicating that muscle activity increased to support stabilization against the visual flow. Older women exhibited significantly larger COM and COP responses in the direction of visual field motion and less muscle modulation when the platform returned to neutral than younger women. Results on a Rod and Frame test indicated that older women were significantly more visually dependent than the younger women. We concluded that a stiffer body combined with heightened visual sensitivity in older women critically interferes with their ability to counteract posturally destabilizing environments.
Keywords: Posture, Aging, Wavelet transform, Visual sensitivity, Functional principal component analysis
Introduction
Optic flow strongly influences both quiet stance and gait, particularly when somatosensory feedback is fluctuating (Keshner and Kenyon 2000; Warren et al. 2001; Varraine et al. 2002). Although the response to visual information is too slow to be used in the generation of automatic postural reactions (Nashner and Cordo 1981; Vidal et al. 1982; Dijkstra et al. 1994), there is potential for visual field motion to modulate the ensuing compensatory postural behaviors. For example, continuous motion of the optic flow field was shown to modify the kinematics of postural restabilizing behaviors in healthy young adults on a tilted (Wang et al. 2009) or minimized (Streepey et al. 2007) support surface following a support surface disturbance. This suggests that increased visual sensitivity would also have an impact on compensatory postural behaviors and, subsequently, the ongoing postural state.
Visual sensitivity is often increased with aging and sensorimotor impairment, and older women in particular have been shown to be more visually sensitive than younger adults (Wolfson et al. 1994; Bugnariu and Fung 2007; Guerraz and Bronstein 2008; Slaboda et al. 2009). In addition, postural reactions slow with aging (Keshner et al. 1987, 1993; Woollacott 1993; Woollacott and Manchester 1993; Gill et al. 2001), thereby creating even more of a window for the slowly processed visual inputs to modify postural behavior. Postural responses that are more closely matched to the visual environment but not temporally matched to the immediate demands of the postural disturbance (Wright et al. 2005; Dokka et al. 2010) produce a mismatch between the visual motion feedback and somatosensory feedback and rapidly elicit spatial disorientation (Previc 1992; Previc and Donnelly 1993). Sensory conflicts that interfere with the ability to distinguish between visual motion and motion of the body also produce sensations of motion sickness and instability (Dichgans et al. 1975; Lackner and DiZio 1988). Thus, this heightened impact of visual field motion with aging could be a significant factor in the increased occurrence of falls (Isableu et al. 2010).
The repercussions of relying more heavily on visual information to signal a postural disturbance are not as apparent as those from diminished or distorted visual acuity with age (Sturnieks et al. 2008). The purpose of this study was to explore the influence of continuous visual flow, during and following a postural disturbance (i.e., support surface tilt), on the ability to reorient to vertical. We examined a cohort of healthy older women to increase the likelihood of recording from a visually dependent population and compared their postural kinematics to those of healthy young women. An a posteriori method of functional principal component analysis (fPCA) was used to study temporal evolution of the postural response (Slaboda et al. 2011). Rather than divide the trial into time periods defined by initiation and termination of the transient disturbance (Nashner and Cordo 1981), we examined both the imposed disturbance and the emergent behavior as continuous variables to reveal the combined effects of visual field motion and a tilt of the support surface on the postural reorganization.
We also applied a novel method of wavelet transforms to the electromyographic (EMG) activity to study the temporal evolution of lower limb muscle activity. In contrast to Fourier transforms, continuous wavelet transforms extract both power and frequency information from the signal while preserving the original temporal information (Daubechies 1992). Changes in frequency content of the EMG response can then be correlated with other kinematic variables to determine how musculoskeletal responses are adjusted during persistent visual field motion.
We hypothesized that older women would orient themselves to the visual frame of reference and demonstrate postural behaviors that are consistent with both direction and velocity of the visual flow field. We expected that young women would rely on a somatosensory frame of reference and maintain vertical orientation by compensating for the direction and velocity of the support surface tilt. We further hypothesized that young women would be more likely to compensate for the support surface as it returned to a neutral state in order to maintain verticality in space, whereas postural orientation of the older women would be biased toward the visual frame of reference thereby orienting their body toward the direction of optic flow.
Methods
Fourteen healthy young women (20–39 years) and fourteen healthy older women (60–79 years) gave informed consent as approved by the Institutional Review Board at Temple University to participate in this study. Subjects who reported a history of neurological disorders, vision problems, motion sickness, hearing problems, severe to moderate arthritis or any limitations in range of motion at the ankle were excluded from the study.
Rod and frame test
To assess whether this sample of older women was more visually dependent than the younger women, each subject completed the Rod and Frame test (Witkin and Asch 1948a, b; Slaboda et al. 2009; Isableu et al. 2010). The experimental apparatus consisted of a standard, windows-based personal computer, ACDSee 6.0 (ACD Systems) image viewing software, a projector (InFocus, Portland OR), and a rear projection screen. The projection screen measured 2.0 m wide by 1.5 m high and was overlaid with a piece of black, 3/16″ construction board with a 1.12-m-diameter circle cut in its center in order to block out ambient light. The rod and frame display was projected through this circular cutout. The projector was located 3.6 m behind the screen, and the subject stood 2.1 m in front of the screen.
Subjects were standing freely in the upright position in the dark. They were instructed to look straight ahead at the projection screen that displayed a luminous frame tilted 22.5° clockwise or counterclockwise from horizontal. A luminous rod was digitally rotated from an initial position of 20° or 45° from vertical or horizontal. Subjects verbally identified when they perceived the rod as reaching pure vertical or horizontal. Mean angular differences from pure vertical and horizontal were compared across the two populations with Wilcoxon nonparametric test since the variances are not homogeneous and significance accepted at P < 0.05.
Postural task
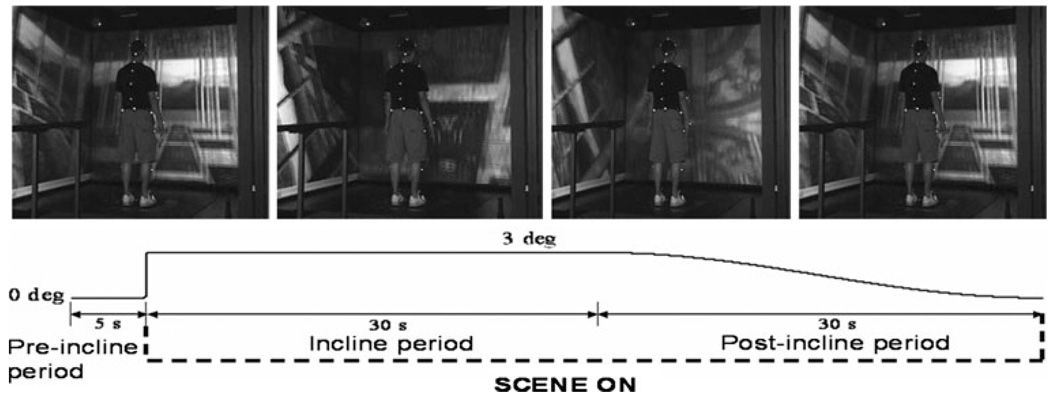
Subjects were instructed to look straight ahead and maintain an upright posture while standing quietly with feet side-byside and arms at their sides. After 5 s of quiet stance, a transient 3° tilt of the support surface produced dorsiflexion of the feet at a constant velocity of 30°/s. This was followed by 30 s of quiet stance on the tilted surface and a 30-s return of the support surface to a neutral position at a constant velocity of 0.1°/s (Fig. 1). A small backward tilt was chosen in order to produce postural destabilization without the need to take a step (Keshner et al. 1987; Buchanan and Horak 1999). The visual field was either dark or rotated in continuous upward or downward pitch at the same velocity as the platform (30°/s) or faster (45°/s) resulting in a total of five trials with the tilt perturbation. Onset of visual field rotation and support surface tilt was synchronized, and visual field motion was maintained throughout the trial. Five catch trials with a stationary support surface were included for a total of 10 trials randomized across direction and velocity of support surface and visual scene.
Fig. 1.
Top Snapshots of a young adult standing in the virtual environment as the visual scene continuously rotates in the pitch direction. Bottom Time frame of the experimental trial indicating the motion of the platform and the visual scene over the course of the trial
Data collection
Three transparent 1.2 × 1.6 mscreens were placed 90 cm in front and to the right and left of a 3-degree of freedom platform (Neurocom International Inc., Clackamas OR) with integrated dual triaxial force plates (AMTI, Watertown, MA) from which center of pressure (COP) was calculated. Two Panasonic PT-D5600U DLP-based projectors located behind each screen projected a full-color workstation field (1,024 × 768 stereo) at 60 Hz onto each screen. Polarized filters placed in front of the projector provided left eye and right eye views of the image on each screen, and passive stereo glasses delivered the correct view to each eye. Three dual processor computers created the imagery projected in the virtual environment and were synchronized via the CAVELib application (MechDyne, Virginia Beach, VA).
Muscle electromyographic (EMG) responses were recorded with pairs of 2.5-mm-diameter Ag–AgCl surface electrodes (Noraxon, USA) placed bilaterally on the tibialis anterior (TA) and medial gastrocnemius (GAS) muscles. Placement of EMG electrodes was determined using anatomical landmarks and verified with isometric contractions of the muscle. EMG data were amplified, bandpass filtered at 10–500 Hz, and sampled at 1,080 Hz.
Three-dimensional kinematic data from the head, trunk, lower, and upper limbs were collected using a Motion Analysis (Santa Rosa, CA) 6-camera infrared Hawk system sampling at 120 Hz. Center of mass (COM) of the body was calculated (Winter 1990) from anterior–posterior displacement of infrared reflective markers placed on the body. Segmental angles of the ankle were calculated as the angle between the foot and the shank. Hip ankle was calculated as the angle between thigh and the trunk.
Wavelet analysis of the EMG
A continuous wavelet transform (CWT) was applied to the EMG signal from each muscle using the Morlet wavelet as the mother function, defined as:
| (1) |
where η represents a nondimensional time parameter, and ω0 is the nondimensional frequency (Lauer et al. 2005, 2007a, b, 2008). The Morlet had an increasing linear scale of 1 and 128 to encompass all frequencies between 0.1 and 540 Hz. The output of the CWT analysis is a scalogram, which is a three-dimensional representation of time (s) on the x-axis, frequency (scale) on the y-axis, and power (amplitude) on the z-axis. Reduction of the 3-dimensional scalogram to a representative EMG activation equation was performed by calculating instantaneous mean frequency (IMNF) for each time interval as:
| (2) |
where P(t, f) represents the range of powers at a given frequency at each time interval, and f represents frequency range of the EMG signal (Karlsson et al. 2003; Lauer et al. 2005, 2007a, b, 2008). The IMNF produced a representative curve of muscle activity over time that could be compared with kinematic changes at the hip and ankle.
Functional principal component analysis
An fPCA was applied to COM, COP, segmental angles, and IMNF data to identify trial periods in which the two populations were differentially affected by visual conditions (Slaboda et al. 2011). A “standard” multivariate principal component analysis is a mathematical least squares statistical procedure where the first principal component accounts for as much of the variability in the data set as possible; each succeeding component accounts for the maximum amount of variance left. The fPCA also converts large numbers of correlated variables into a smaller number of principal components. However, the fPCA ‘variable’ is a functional relationship of data points and not a single data point. Thus, the fPCA yields a measure of variability across an entire curve captured as a small subset of functional principal components (fPC). COM, COP, IMNF curves, and segmental angle data were transformed into functions by fitting a 6th order B-spline basis function using a least squared maximization procedure.
A Varimax rotation was used so that the fPC curves expressed a more focused, descriptive characterization of variations in the curve, thus maximizing differences between the groups. With Varimax rotation, the values remain orthogonal but may no longer be uncorrelated. Therefore, the percent variance explained by each fPC may not decrease monotonically with rising principal components (Ramsay and Silverman 2002, 2005).
Statistics
Once principal component curves were generated, each of the original data sets was given a “weight” by integrating over the area of the time period described by each fPC. These weights were a measure of how closely that individual data set agreed with, or deviated from, the calculated fPC. Separate mixed models repeated measures ANOVAs with one between group measures (older women and young women) and five within-group measures (visual conditions) were performed on the COP, COM, segmental angular displacement, and IMNF weights for the TA and GAS muscles. Responses in the dark were compared to each visual scene rotation with paired t test post hoc analyses that contained a Bonferrroni correction and reported as significant at P < 0.05.
Results
Rod and frame results
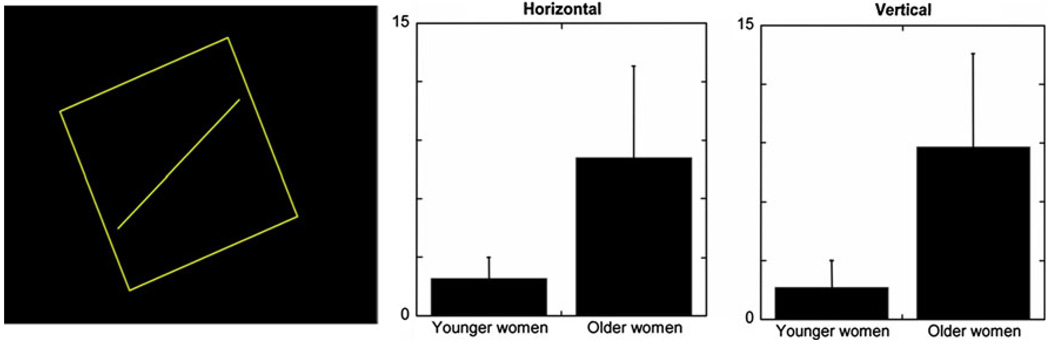
Our population of older women did select significantly greater angular deviations of the rod than younger women in both the vertical (older women μ = 8.8 ± 4.7 and young women μ = 2.4 ± 2; P < 0.002) and horizontal (older women μ = 8.1 ± 4.8 and young women μ = 2.1 ± 2 P < 0.002) orientations (Fig. 2). Thus, the older women tested as more visually dependent; however, no significant correlation between COM responses and the angular deviations of the rod was found.
Fig. 2.
Left Projected rod and frame image with the rod at a 22.5° angle relative to vertical. Means and standard deviation of the absolute angular deviations of the rod from gravitational horizontal (center) and vertical (right) of young adults and older women in the Rod and Frame test
COM and COP responses
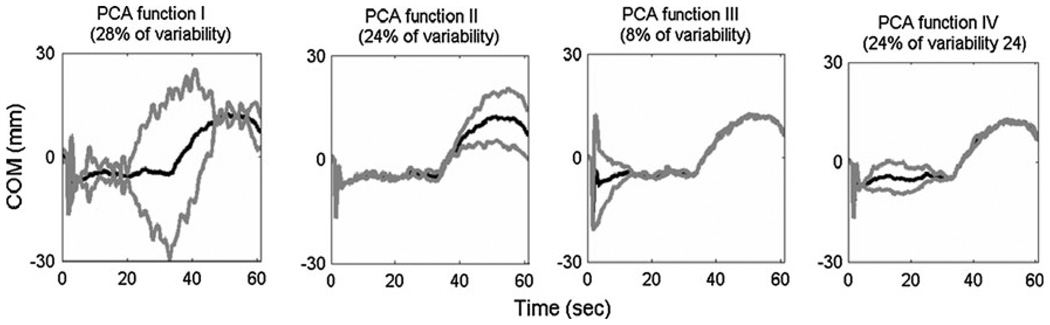
The fPC analysis identified the first four principal components with the greatest differences in the COM and COP responses as occurring: (1) during the 20- to 50-s time period that contained both the sustained tilt and the platform return to neutral, (2) over the period that the platform return to neutral (40–60 s into the trial), (3) during and several seconds following the support surface disturbance (0–10 s into the trial), and (4) during the period of sustained platform tilt (4–30 s into the trial; Fig. 3 and Table 1).
Fig. 3.
Four principal components in chronological order describing the COM and COP data of all subjects. Each component identifies a different time period that was highly variable in the data indicating differences either between the groups and trial conditions. Mean trajectory of all subjects and conditions (black line) plus and minus 2 standard deviations from the mean (gray lines) are shown
Table 1.
Period of trial described and % variability explained by each of the four functional principal components (fPC) for each dependent variable across all subjects
| fPC1 | fPC2 | fPC3 | fPC4 | |
|---|---|---|---|---|
| COM | Sustained tilt and return to neutral (28%) | Return to neutral (24%) | Tilt + 10 s (8%) | Sustained tilt (24%) |
| COP | Sustained tilt and return to neutral (25%) | Return to neutral (21%) | Tilt + 10 s (6%) | Sustained tilt (22%) |
| TA | Sustained tilt and return to neutral (28%) | Return to neutral (39%) | Sustained tilt (20%) | Tilt + 10 s (3%) |
| GAS | Sustained tilt (24%) | Return to neutral (24%) | Tilt + 10 s (9%) | Sustained tilt and return to neutral (30%) |
| Ankle angle | Sustained tilt and return to neutral (30%) | Return to neutral (31%) | Tilt + 10 s (5%) | Sustained tilt (23%) |
| Hip angle | Return to neutral (36%) | Sustained tilt (20%) | Tilt (5%) | Sustained tilt and return to neutral (32%) |
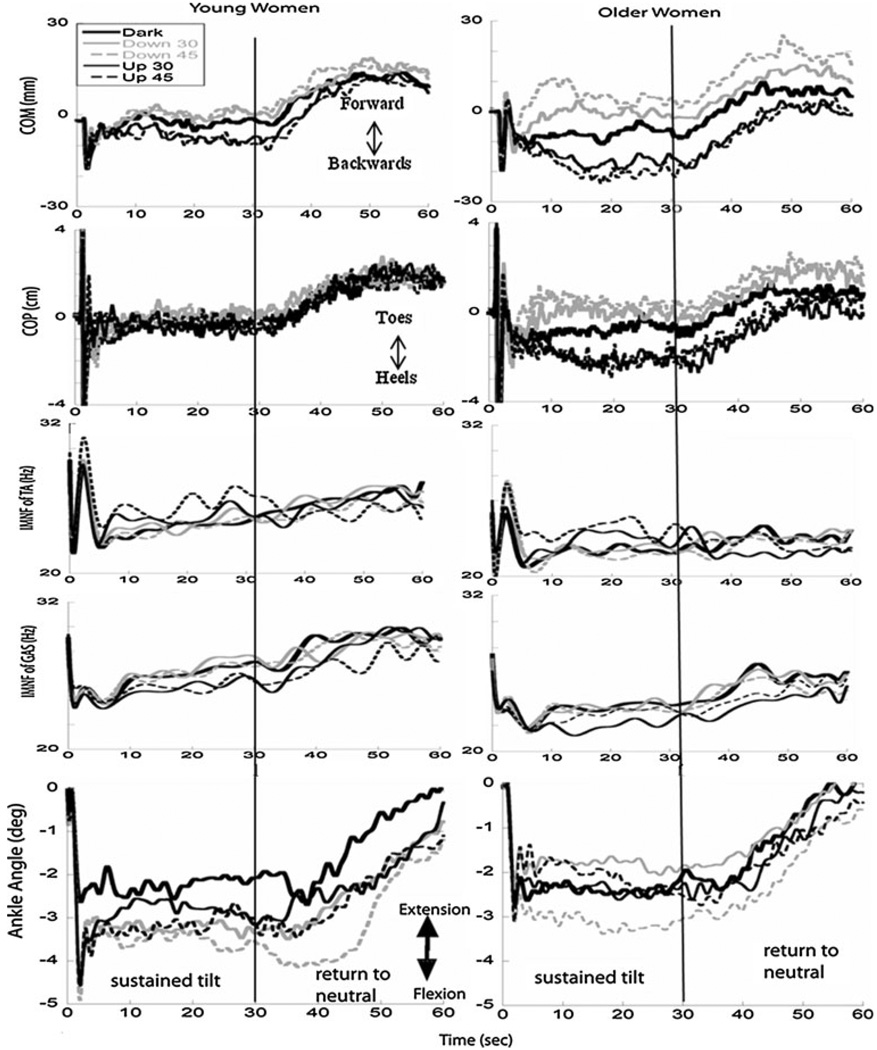
With pitch up rotations of the visual scene at both velocities, both younger and older women displaced their COM further backwards than when in the dark (P < 0.05) starting 4 s after the tilt disturbance and continuing over 20 s of the platform return to neutral (first fPC and fourth fPC, Fig. 3). In the COP excursions, older women significantly shifted their weight toward their heels starting 4 s after the tilt and continuing over 20 s of the platform return to neutral when the scene rotated in pitch up direction at 45°/s compared to in the dark (first and fourth fPCs, Fig. 4). Similar results were found with pitch up rotation at 30°/s relative to responses in the dark with older women shifting their COP to their heels starting 20 s after the tilt and continuing over the initial 20 s that the platform returned to neutral (first fPC). No significant differences in the COP responses due to visual condition were found in young women.
Fig. 4.
Average responses of COM, COP, IMNF of TA, and GAS, and the mean ankle angle displacement over the entire trial in each visual condition (see legend) for young women (left) and older women (right). The vertical black line indicates the time at which the platform began its return to neutral
With pitch down rotations of the visual scene at 45°/s, COM was positioned more forwards than when in the dark starting 4 s after the tilt and continuing until the platform returned to neutral in both young and older women (P < 0.05). No significant differences in responses were found during pitch down rotation at 30°/s when compared to in the dark for either group.
Significant group differences were also found in COM displacement starting 4 s after the tilt and continuing over the entire period that platform returned to neutral (COM: first fPC: F(1,26) = 6.9, P < 0.014; second fPC: F(1,26) = 8.2, P < 0.008 and fourth fPC: F(1,26) = 5, P < 0.034). These results indicated that although both groups were responsive to the direction and velocity of the visual scene, the older women exhibited larger displacements of COM in the direction of visual scene rotation across all visual conditions compared with younger women (Fig. 4).
Frequency responses of the GAS and TA muscles
The four functional principal components that described the IMNF response of the GAS and TA muscles over the trial were not the same as those identified for the COM and COP data (Table 1). Whereas fPCs of COM and COP reflected the concurrent actions of these variables, time shifts in the fPCs reflected reciprocal actions of the two muscles. For both muscles, the smallest percent of variability was described by the period of the tilt disturbance.
The greatest percent variability of the GAS muscle (57%) emerged in the second and fourth fPCs over 20–60 s of the trial period as the tilted platform began and completed its return to a neutral position. The first fPC described 24% of the variability in the GAS for the period of sustained tilt. For the TA muscle, the greatest percent variability was described by the 1st and 3rd fPCs (48%) immediately following the tilt and for the whole period of sustained tilt (4–40 s of the trial). The 2nd fPC described 39% of the variability across the whole period that the platform returned to a neutral position (30–60 s of the trial). Thus, the principal action of each of these muscles was to stabilize the orientation of the body against the position or motion of the support surface.
Although the IMNF responses of the TA muscles were larger in the younger women compared with older women, there were no significant effects of age or visual condition. In the GAS, IMNF responses were significantly larger in younger women than older women over the last 20 s that the platform returned to neutral (F(1,26) = 4.6, P = 0.041; Fig. 4).
Hip and ankle angular displacement
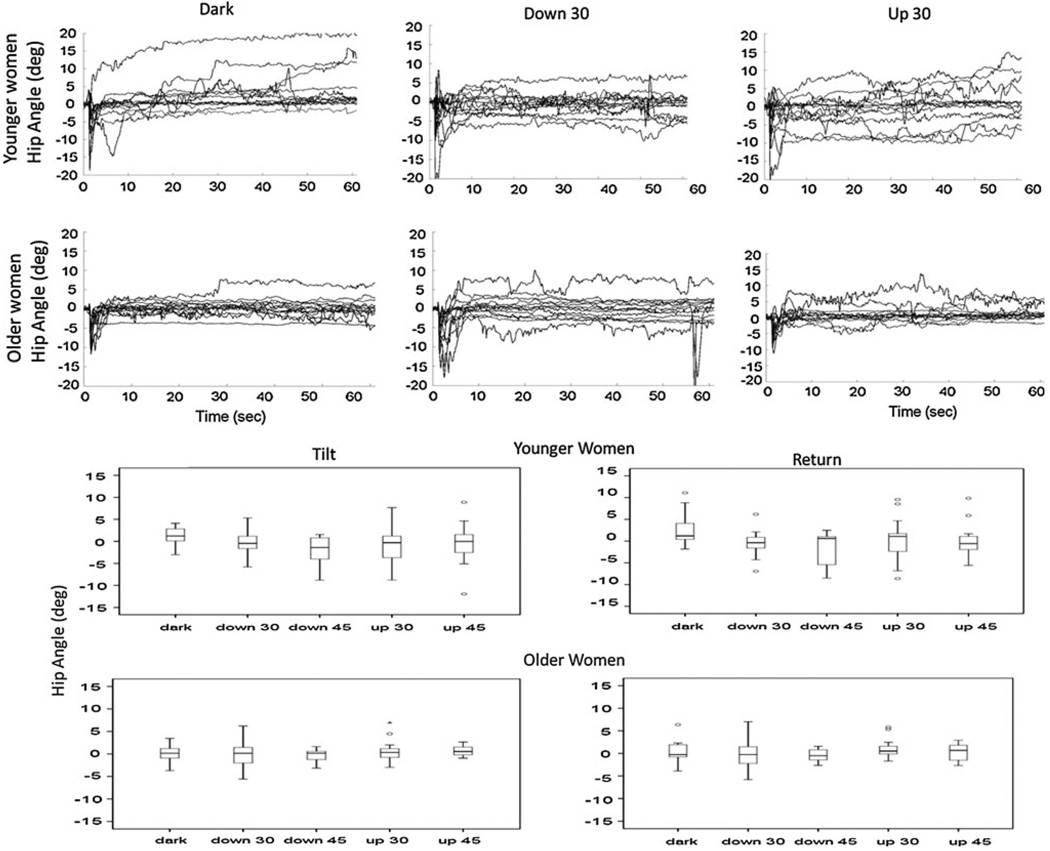
The four fPCs of ankle angle mirrored the fPCs that described the COP. Younger women demonstrated greater ankle flexion when the visual scene was rotating compared to in the dark, but only ankle angle excursions during the initial response to the tilt (third fPC, P < 0.05) when the scene was pitching upwards at 45°/s were significant due to variability across the subjects (Fig. 4). Large amounts of variability across subjects were also observed in the profiles of the hip angle (top, Fig. 5) even after these angles were corrected for variations in the initial position that were found to occur prior to the tilt disturbances (Schweigart and Mergner 2008). Five younger and three older women exhibited less than 2° of change in their hip angle following the initial response to the tilt disturbance in all visual conditions; all others shifted their hip angle as much as 6–10° from their initial position.
Fig. 5.
Top Hip angles across the whole period of the trial for all younger (top row) and older (bottom row) women in the dark (left), during pitch down 30°/s (middle) and pitch up 30°/s (right) of the visual scene. Bottom Box plots of the average hip angles over the period of sustained tilt (left) and over the return to neutral (right) of the platform for the younger (upper) and older women (lower). The box indicates the inter-quartile range (lower hinge = 25% and the upper hinge = 75%), the black line indicates the median, and the whiskers are the upper and lower data point. Points outside the whiskers are outliers as defined by 1.5–3 box lengths from the upper and lower edge on the box
The fPCs that described the hip angle also differed from all of the other measures (Table 1). The 1st and 4th fPCs of the hip described 68% of the variability as occurring across the period that the platform transitioned from a tilted to a neutral position (20–60 s of the trial). The 2nd and 3rd fPCs identified 25% of the variability at the hip following the tilt disturbance and during the sustained period of support surface tilt. Average hip angles across each of these fPC time periods for each population (bottom, Fig. 5) reflected greater variability in the hip position of the younger women compared with the older women. Older women kept a median hip angle close to zero degrees for each visual condition and had smaller variation across all visual conditions. Younger women, however, exhibited larger and more variable hip flexion angles with visual field motion than when in the dark. No significant differences between populations or visual conditions were found in the hip angles, and there were no correlations with the Rod and Frame results.
Discussion
Variability in postural response behavior is a natural consequence of acting in busy environments with constantly changing demands. We have explored this variability, and the impact of visual field motion on postural recovery and maintenance, by employing a time series approach that incorporated wavelet analysis and functional principal component analyses. We have chosen these methods in order to assess how the current postural state may influence ensuing behaviors. Our results clearly demonstrated that both the direction and velocity of visual flow modified postural restabilization following the support surface tilt and continued to affect the postural state while the support surface was returning to a neutral position. The effects of visual field motion were more pronounced in the older than in the younger women tested here.
More than 50% of the variability between the two populations was described by the functional principal components as occurring across the period of sustained tilt and as the platform began its return to a neutral position. In fact, we would expect vision to play a significant role in postural orientation during these two response periods. The tilt period because the body was being statically tilted off-vertical and either visual or vestibular information would be necessary to identify a vertical orientation in space (Kluzik et al. 2005; Kluzik et al. 2007). The return to neutral period because the platform was moving at a velocity that was sub-threshold for recognition of vestibular signals (Fitzpatrick and McCloskey 1994), therefore visual or tactile/proprioceptive receptors would be essential to identify orientation in space. With constant rotation of the visual field, we might predict that the subjects would weight the more reliable somatosensory information more heavily in each of these trial periods (Peterka 2002; Creath et al. 2008). This was not the case for the older women who exhibited larger and quicker responses to visual flow following compensation to the support surface tilt than did the younger women.
Visual field motion continued to dominate the response orientation in the older women as the platform returned to neutral. Younger women, however, were able to match their responses to the motion of the platform rather than to the visual field. From this, we infer that the older women relied on an allocentric frame of reference to adjust their position in space, whereas younger women relied on an egocentric frame of reference (Barnett-Cowan and Harris 2008). Vestibular information in older adults has been reported to be less reliable than that of young adults (Rosenhall and Rubin 1975), and therefore, they would be more susceptible to the visual field motion, even when it did not match their self-motion (Tran et al. 1998; Haibach et al. 2009).
As previously reported, the older women tested as significantly more visually dependent than the younger women (Witkins 1954; Lambrey and Berthoz 2003); thus, we might assume that the visual frame of reference became more dominant with age. But previous results from our laboratory have demonstrated a significant effect of visual field velocity on the peak angular velocities of the head and trunk in healthy young adults within 250 ms of a platform tilt (Keshner et al. 2007; Keshner and Dhaher 2008). Within 2 s after a support surface tilt, the velocity of visual scene motion was observed to modulate both direction and amplitude of the linear displacement of whole body center of mass and angular displacement of head, hip, and ankle (Wang et al. 2009; Dokka et al. 2010) in healthy young adults. In our current results, both older and younger women responded more strongly when the direction of visual field motion was not appropriate for their postural behavior (i.e., when the body pitches backwards the world should move downward). Therefore, other changes that occur with age must be factored into the selection or dependence on a particular frame of reference.
Physiological and mechanical limitations that have previously been identified with aging, including insufficiency in muscle responses (Allum et al. 2002; Lin and Woollacott 2002; Melzer et al. 2009) and stiffer body mechanics (Wu 1998), may underlie the more rapid and larger postural response to visual flow in older women. We did observe that when vision was available, younger women incrementally increased ankle muscle activity and hip angles as the platform returned to neutral, most likely in order to counteract the visual field disturbance and calibrate their body position to the support surface frame of reference (Keshner et al. 2004). This increased muscle activity did not appear in the older women, and, in fact, ankle muscle responses in the older women suggest that they engaged in synchronous muscle activation (i.e., cocontraction) at the ankle as a compensatory strategy to improve the accuracy of ankle stabilization (Seidler-Dobrin et al. 1998). Increased activation of the muscles around a joint may increase neuromuscular noise through increasing force variability (Meulenbroek et al. 2005; Faisal et al. 2008), but stiffening at a joint also increases stabilization (Gribble et al. 2003; Faisal et al. 2008; Selen et al. 2009). Thus, the choice of limiting motion at the ankle and hip may be an attempt by older women to decrease sensorymotor noise and enhance behavioral precision for the somatosensory disturbances (Wong et al. 2009).
We did not find a significant correlation between the Rod and Frame results and the postural kinematics. Weak correlations between a Rod and Frame test and postural behaviors in the medial–lateral plane have been previously reported in young adults, especially as the postural task became more difficult such as when standing in a sharpened Romberg position or in unipedal stance (Isableu et al. 2010). In fact, stronger correlations only emerged when a visual orientating task was combined with the postural task. This implies that the Rod and Frame task is not a good predictor of postural orientation although there are other variables that will need to be further investigated such as the plane of visual motion (Lord and Webster 1990; Previc 1992; Previc and Donnelly 1993; Keshner and Kenyon 2000) and the impact of meaningful content in the visual field that might supply cues to vertical (Dijkstra et al. 1994; Isableu et al. 2010).
Our results indicate that the organization of the postural responses to a transient support surface disturbance has a lesser impact on subsequent postural orientation in space than the ongoing environmental array. Disorienting effects of continuous visual field rotations across the continuum of the trial can critically impact successive postural stabilizing actions of older women due to the musculoskeletal and sensory reception changes that occur with age. We conclude that when faced with multiple postural demands, such as a surface that changes in gradient and frictional characteristics in a busy visual environment, compensation for one disturbance will impinge on how our responses are organized to meet successive disturbances, and this influence becomes more overpowering as we age.
Acknowledgments
This work was supported by NIH-NIDCD grant DC05235 and NIH-NIA grant AG26470. We gratefully acknowledge the permission to use the CAVElib and TrackD software to generate and control the virtual scene from Mechdyne, Virginia Beach, VA. The authors also thank Dr. Justin Shi for his assistance with programming of the visual field, Dr. Eugene Komaroff for statistical advice and Tarrè Ferrell and Lois Lanaria for their help with data collection and reduction.
Contributor Information
Jill C. Slaboda, Email: jslaboda@temple.edu, Department of Physical Therapy, College of Health Professions and Social Work, Temple University, Philadelphia, PA 19140, USA; 1800 North Broad Street, 40 Pearson Hall, Philadelphia, PA 19121, USA.
Richard T. Lauer, Department of Physical Therapy, College of Health Professions and Social Work, Temple University, Philadelphia, PA 19140, USA Department of Electrical and Computer Engineering, College of Engineering, Temple University, Philadelphia, PA 19121, USA.
Emily A. Keshner, Department of Physical Therapy, College of Health Professions and Social Work, Temple University, Philadelphia, PA 19140, USA Department of Electrical and Computer Engineering, College of Engineering, Temple University, Philadelphia, PA 19121, USA.
References
- Allum JH, Carpenter MG, Honegger F, Adkin AL, Bloem BR. Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man. J Physiol. 2002;542:643–663. doi: 10.1113/jphysiol.2001.015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett-Cowan M, Harris LR. Perceived self-orientation in allocentric and egocentric space: effects of visual and physical tilt on saccadic and tactile measures. Brain Res. 2008;1242:231–243. doi: 10.1016/j.brainres.2008.07.075. [DOI] [PubMed] [Google Scholar]
- Buchanan JJ, Horak FB. Emergence of postural patterns as a function of vision and translation frequency. J Neurophysiol. 1999;81:2325–2339. doi: 10.1152/jn.1999.81.5.2325. [DOI] [PubMed] [Google Scholar]
- Bugnariu N, Fung J. Aging and selective sensorimotor strategies in the regulation of upright balance. J Neuroeng Rehabil. 2007;4:19. doi: 10.1186/1743-0003-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak FB, Jeka JJ. The role of vestibular and somatosensory systems in intersegmental control of upright stance. J Vestib Res. 2008;18:39–49. [PMC free article] [PubMed] [Google Scholar]
- Daubechies I. Society for industrial and applied mathematics. Philadelphia: 1992. Ten lectures on wavelets. [Google Scholar]
- Dichgans J, Wist E, Diener HC, Brandt T. The Aubert-Fleischl phenomenon: a temporal frequency effect on perceived velocity in afferent motion perception. Exp Brain Res. 1975;23:529–533. doi: 10.1007/BF00234920. [DOI] [PubMed] [Google Scholar]
- Dijkstra TM, Schoner G, Gielen CC. Temporal stability of the action-perception cycle for postural control in a moving visual environment. Exp Brain Res Experimentelle Hirnforschung. 1994;97:477–486. doi: 10.1007/BF00241542. [DOI] [PubMed] [Google Scholar]
- Dokka K, Kenyon RV, Keshner EA, Kording KP. Self versus environment motion in postural control. PLoS Comput Biol. 2010;6:e1000680. doi: 10.1371/journal.pcbi.1000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Allum JH, Carpenter MG, Held-Ziolkowska M, Adkin AL, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of age. J Gerontol A Biol Sci Med Sci. 2001;56:M438–M447. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- Gribble PL, Mullin LI, Cothros N, Mattar A. Role of cocontraction in arm movement accuracy. J Neurophysiol. 2003;89:2396–2405. doi: 10.1152/jn.01020.2002. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Bronstein AM. Mechanisms underlying visually induced body sway. Neurosci Lett. 2008;443:12–16. doi: 10.1016/j.neulet.2008.07.053. [DOI] [PubMed] [Google Scholar]
- Haibach P, Slobounov S, Newell K. Egomotion and vection in young and elderly adults. Gerontology. 2009;55:637–643. doi: 10.1159/000235816. [DOI] [PubMed] [Google Scholar]
- Isableu B, Ohlmann T, Cremieux J, Vuillerme N, Amblard B, Gresty MA. Individual differences in the ability to identify, select and use appropriate frames of reference for perceptuo-motor control. Neuroscience. 2010;169:1199–1215. doi: 10.1016/j.neuroscience.2010.05.072. [DOI] [PubMed] [Google Scholar]
- Karlsson JS, Ostlund N, Larsson B, Gerdle B. An estimation of the influence of force decrease on the mean power spectral frequency shift of the EMG during repetitive maximum dynamic knee extensions. J Electromyogr Kinesiol. 2003;13:461–468. doi: 10.1016/s1050-6411(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Dhaher Y. Characterizing head motion in three planes during combined visual and base of support disturbances in healthy and visually sensitive subjects. Gait Posture. 2008;28:127–134. doi: 10.1016/j.gaitpost.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshner EA, Kenyon RV. The influence of an immersive virtual environment on the segmental organization of postural stabilizing responses. J Vestib Res. 2000;10:207–219. [PubMed] [Google Scholar]
- Keshner EA, Allum JH, Pfaltz CR. Postural coactivation and adaptation in the sway stabilizing responses of normals and patients with bilateral vestibular deficit. Exp Brain Res. 1987;69:77–92. doi: 10.1007/BF00247031. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Allum JH, Honegger F. Predictors of less stable postural responses to support surface rotations in healthy human elderly. J Vestib Res. 1993;3:419–429. [PubMed] [Google Scholar]
- Keshner EA, Kenyon RV, Langston J. Postural responses exhibit multisensory dependencies with discordant visual and support surface motion. J Vestib Res. 2004;14:307–319. [PubMed] [Google Scholar]
- Keshner EA, Streepey J, Dhaher Y, Hain T. Pairing virtual reality with dynamic posturography serves to differentiate between patients experiencing visual vertigo. J Neuroeng Rehabil. 2007;4:24. doi: 10.1186/1743-0003-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluzik J, Horak FB, Peterka RJ. Differences in preferred reference frames for postural orientation shown by after-effects of stance on an inclined surface. Exp Brain Res. 2005;162:474–489. doi: 10.1007/s00221-004-2124-6. [DOI] [PubMed] [Google Scholar]
- Kluzik J, Peterka RJ, Horak FB. Adaptation of postural orientation to changes in surface inclination. Exp Brain Res. 2007;178:1–17. doi: 10.1007/s00221-006-0715-0. [DOI] [PubMed] [Google Scholar]
- Lackner JR, DiZio P. Gravitational effects on nystagmus and on perception of orientation. Ann NY Acad Sci. 1988;545:93–104. doi: 10.1111/j.1749-6632.1988.tb19557.x. [DOI] [PubMed] [Google Scholar]
- Lambrey S, Berthoz A. Combination of conflicting visual and non-visual information for estimating actively performed body turns in virtual reality. Int J Psychophysiol. 2003;50:101–115. doi: 10.1016/s0167-8760(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Stackhouse C, Shewokis PA, Smith BT, Orlin M, McCarthy JJ. Assessment of wavelet analysis of gait in children with typical development and cerebral palsy. J Biomech. 2005;38:1351–1357. doi: 10.1016/j.jbiomech.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Smith BT, Shewokis PA, McCarthy JJ, Tucker CA. Time-frequency changes in electromyographic signals after hamstring lengthening surgery in children with cerebral palsy. J Biomech. 2007a;40:2738–2743. doi: 10.1016/j.jbiomech.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Stackhouse CA, Shewokis PA, Smith BT, Tucker CA, McCarthy J. A time-frequency based electromyographic analysis technique for use in cerebral palsy. Gait Posture. 2007b;26:420–427. doi: 10.1016/j.gaitpost.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Johnston TE, Smith BT, Lee SC. Lower extremity muscle activity during cycling in adolescents with and without cerebral palsy. Clin Biomech. 2008;23:442–449. doi: 10.1016/j.clinbiomech.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SI, Woollacott MH. Postural muscle responses following changing balance threats in young, stable older, and unstable older adults. J Mot Behav. 2002;34:37–44. doi: 10.1080/00222890209601929. [DOI] [PubMed] [Google Scholar]
- Lord SR, Webster IW. Visual field dependence in elderly fallers and non-fallers. Int J Aging Hum Dev. 1990;31:267–277. doi: 10.2190/38MH-2EF1-E36Q-75T2. [DOI] [PubMed] [Google Scholar]
- Melzer I, Benjuya N, Kaplanski J, Alexander N. Association between ankle muscle strength and limit of stability in older adults. Age Ageing. 2009;38:119–123. doi: 10.1093/ageing/afn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbroek RG, Van Galen GP, Hulstijn M, Hulstijn W, Bloemsaat G. Muscular co-contraction covaries with task load to control the flow of motion in fine motor tasks. Biol Psychol. 2005;68:331–352. doi: 10.1016/j.biopsycho.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Cordo PJ. Relation of automatic postural responses and reaction-time voluntary movements of human leg muscles. Exp Brain Res. 1981;43:395–405. doi: 10.1007/BF00238382. [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Previc FH. The effects of dynamic visual stimulation on perception and motor control. J Vestib Res. 1992;2:285–295. [PubMed] [Google Scholar]
- Previc FH, Donnelly M. The effects of visual depth and eccentricity on manual bias, induced motion, and vection. Perception. 1993;22:929–945. doi: 10.1068/p220929. [DOI] [PubMed] [Google Scholar]
- Ramsay J, Silverman B. Applied functional data analysis. New York: Springer; 2002. [Google Scholar]
- Ramsay J, Silverman B. Functional data analysis. New York: Springer; 2005. [Google Scholar]
- Rosenhall U, Rubin W. Degenerative changes in the human vestibular sensory epithelia. Acta Otolaryngol. 1975;79:67–80. doi: 10.3109/00016487509124657. [DOI] [PubMed] [Google Scholar]
- Schweigart G, Mergner T. Human stance control beyond steady state response and inverted pendulum simplification. Exp Brain Res. 2008;185:635–653. doi: 10.1007/s00221-007-1189-4. [DOI] [PubMed] [Google Scholar]
- Seidler-Dobrin RD, He J, Stelmach GE. Coactivation to reduce variability in the elderly. Mot Control. 1998;2:314–330. doi: 10.1123/mcj.2.4.314. [DOI] [PubMed] [Google Scholar]
- Selen LP, Franklin DW, Wolpert DM. Impedance control reduces instability that arises from motor noise. J Neurosci. 2009;29:12606–12616. doi: 10.1523/JNEUROSCI.2826-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaboda JC, Barton JE, Maitin IB, Keshner EA. Visual field dependence influences balance in patients with stroke. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:1147–1150. doi: 10.1109/IEMBS.2009.5333916. [DOI] [PubMed] [Google Scholar]
- Slaboda JC, Lauer R, Keshner EA. Time series analysis of postural responses to combined visual pitch and support surface tilt. Neurosci Lett. 2011;491:138–142. doi: 10.1016/j.neulet.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streepey JW, Kenyon RV, Keshner EA. Visual motion combined with base of support width reveals variable field dependency in healthy young adults. Exp Brain Res. 2007;176:182–187. doi: 10.1007/s00221-006-0677-2. [DOI] [PubMed] [Google Scholar]
- Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467–478. doi: 10.1016/j.neucli.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Tran DB, Silverman SE, Zimmerman K, Feldon SE. Age-related deterioration of motion perception and detection. Graefes Arch Clin Exp Ophthalmol. 1998;236:269–273. doi: 10.1007/s004170050076. [DOI] [PubMed] [Google Scholar]
- Varraine E, Bonnard M, Pailhous J. Interaction between different sensory cues in the control of human gait. Exp Brain Res. 2002;142:374–384. doi: 10.1007/s00221-001-0934-3. [DOI] [PubMed] [Google Scholar]
- Vidal PP, Berthoz A, Millanvoye M. Difference between eye closure and visual stabilization in the control of posture in man. Aviat Space Environ Med. 1982;53:166–170. [PubMed] [Google Scholar]
- Wang Y, Kenyon RV, Keshner EA. Identifying the control of physically and perceptually evoked sway responses with coincident visual scene velocities and tilt of the base of support. Exp Brain Res. 2009;201:663–672. doi: 10.1007/s00221-009-2082-0. [DOI] [PubMed] [Google Scholar]
- Warren WH, Jr, Kay BA, Zosh WD, Duchon AP, Sahuc S. Optic flow is used to control human walking. Nat Neurosci. 2001;4:213–216. doi: 10.1038/84054. [DOI] [PubMed] [Google Scholar]
- Winter D. Biomechanics and motor control of human movement. New York: Wiley-Interscience; 1990. [Google Scholar]
- Witkin HA, Asch SE. Studies in space orientation; further experiments on perception of the upright with displaced visual fields. J Exp Psychol. 1948a;38:762–782. doi: 10.1037/h0053671. [DOI] [PubMed] [Google Scholar]
- Witkin HA, Asch SE. Studies in space orientation; perception of the upright in the absence of a visual field. J Exp Psychol. 1948b;38:603–614. doi: 10.1037/h0055372. [DOI] [PubMed] [Google Scholar]
- Witkins HA. Personality through perception: an experimental and clinical study. Westport: Greenwood Press; 1954. [Google Scholar]
- Wolfson L, Whipple R, Derby CA, Amerman P, Nashner L. Gender differences in the balance of healthy elderly as demonstrated by dynamic posturography. J Gerontol. 1994;49:M160–M167. doi: 10.1093/geronj/49.4.m160. [DOI] [PubMed] [Google Scholar]
- Wong J, Wilson ET, Malfait N, Gribble PL. The influence of visual perturbations on the neural control of limb stiffness. J Neurophysiol. 2009;101:246–257. doi: 10.1152/jn.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott MH. Age-related changes in posture and movement. J Gerontol. 1993;48:56–60. doi: 10.1093/geronj/48.special_issue.56. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Manchester DL. Anticipatory postural adjustments in older adults: are changes in response characteristics due to changes in strategy? J Gerontol. 1993;48:M64–M70. doi: 10.1093/geronj/48.2.m64. [DOI] [PubMed] [Google Scholar]
- Wright WG, DiZio P, Lackner JR. Vertical linear self-motion perception during visual and inertial motion: more than weighted summation of sensory inputs. J Vestib Res. 2005;15:185–195. [PubMed] [Google Scholar]
- Wu G. Age-related differences in body segmental movement during perturbed stance in humans. Clin Biomech. 1998;13:300–307. doi: 10.1016/s0268-0033(98)00068-0. [DOI] [PubMed] [Google Scholar]