Abstract
In diverse eukaryotic species from yeast to human, TOR (Target Of Rapamycin) protein kinase operates in signaling pathways that link extracellular stimuli to the control of cell growth and metabolism. TOR kinase functions in two distinct protein complexes, TOR complex 1 (TORC1) and 2 (TORC2). While TORC1 is known to be under the control of the Ras-like small GTPase Rheb, our knowledge about TORC2 regulation is very limited. We thus set out to identify TORC2 activators through genetic approaches in the fission yeast Schizosaccharomyces pombe. Here we briefly review our study that has identified a Rab-family GTPase, Ryh1 and its GEF (guanine nucleotide exchange factor) as positive regulators of TORC2 signaling in S. pombe. Considering the evolutionary conservation of the TOR pathways, it is conceivable that Rabfamily GTPases also play a role in the regulation of human TORC2 in cellular proliferation and insulin signaling.
Key words: TOR, Akt, Gad8, Rab GTPase, Rab6, Ryh1, fission yeast, Schizosaccharomyces pombe, phosphorylation
TOR kinase was originally identified in a yeast genetic screen for mutants that are resistant to the immunosuppresant rapamycin.1 Subsequent identification of TOR kinase genes in diverse eukaryotes revealed the evolutionary conservation of the TOR kinase from yeast to human. In many species, two types of protein complexes containing TOR, TOR complex 1 (TORC1) and 2 (TORC2), have been reported.2 The different subunit compositions of TORC1 and TORC2 are likely to define their distinct cellular functions as well as regulations.
TORC2 was recently identified as a key component of the PI3K (phosphatidylinositol-3-kinase)-Akt signaling pathway that controls cellular proliferation and insulin response in animals.3,4 Receptor tyrosine kinases stimulated by insulin or growth factors activate PI3K, which generates the lipid second messenger PIP3 (phosphatidylinositol-3-phosphate) in the plasma membrane. Increased PIP3 recruits the Akt protein kinase (also known as protein kinase B, PKB) to the plasma membrane, resulting in activation of Akt.5 Full activation of Akt in response to PIP3 requires phosphorylation of two residues in Akt; a threonine residue within the T-loop is phosphorylated by PDK1 (Phosphoinositide-Dependent Kinase 1), while the TOR kinase in TORC2 phosphorylates the hydrophobic motif at the C-terminus of Akt. These regulatory phosphorylation sites are characteristic of the AGC (protein kinase A/protein kinase G/protein kinase C) family that Akt belongs to.
Despite the pivotal role of Akt in cancerous cell proliferation and insulin signaling,5 the regulation of its activator, TORC2, is poorly understood. Our recent studies focus on TORC2 in a model system provided by the fission yeast Schizosaccharomyces pombe,6,7 because of the following two reasons. First, S. pombe TORC2 is not essential for cellular viability under normal growth conditions,6 allowing simple genetic analyses of TORC2. In contrast, mutational inactivation of TORC2 in the budding yeast Saccharomyces cerevisiae, another popular model organism, results in cellular lethality. Second, and more importantly, the TORC2 pathway is well conserved between S. pombe and higher eukaryotes including human. Fission yeast TORC2 is composed of the TOR kinase Tor1 and its regulatory subunits, Sin1, Ste20, Wat1 and Bit61,8,9 which are homologous to mSin1, Rictor, mLst8 and Protor/PRR5 in mammals, respectively.10 We demonstrated that loss of functional TORC2 in fission yeast causes a defect in phosphorylating the hydrophobic motif of Gad8, an AGC-family protein kinase.6 Activation of Gad8 kinase is also dependent on the phosphorylation of its T-loop by Ksg1, a fission yeast ortholog of mammalian PDK1.11 Thus, the TORC2-Gad8 pathway appears to be the S. pombe counterpart of the TORC2-Akt pathway in animals. Fission yeast mutants lacking functional TORC2 or Gad8 are sensitive to various stress, such as osmostress and high temperature.6 Those mutants are also sterile as a result of their defective response to nitrogen starvation.11–13
Aiming to discover factors required for TORC2 activation, we searched for fission yeast mutants that show stress-sensitive phenotypes as well as a defect in Gad8 phosphorylation at the hydrophobic motif.7 sat1, sat4 and sat7 (starvation-induced arrest) mutants previously reported by Takashi Toda and colleagues14 appeared to be excellent candidates, because of their phenotypic resemblance to the TORC2 mutants, including sterility and stress sensitivity. As expected, phosphorylation of the hydrophobic motif of Gad8 is significantly compromised in those sat mutants, indicating that the sat1+, sat4+ and sat7+ genes are important for TORC2 signaling. We also performed an independent screen for mutants defective in the TORC2 function, using a haploid mutant library that includes approximately 80% of the viable null mutants in fission yeast.15 This screen re-isolated sat1 and sat7 as the only mutants that exhibited both stress-sensitivity and compromised Gad8 phosphorylation, while the sat4 mutant was not in the library.
Gene cloning determined that the sat7+ gene encodes Ryh1, a Rab GTPase homologous to the budding yeast Ypt6 and human Rab6 GTPases.16 Sat1 and Sat4 turned out to be S. pombe orthologs of the budding yeast Rgp1 and Ric1 proteins, respectively, both of which form a complex that functions as GEF to activate Ypt6.17 Consistently, we successfully collected genetic and biochemical data to support the notion that Sat1 and Sat4 form a GEF complex for Ryh1 GTPase in S. pombe.7
Rab-family Ryh1 GTPase is implicated in the vesicle transport between the endosome and Golgi compartments,18 and its identification as a regulator of TORC2 signaling was somewhat unanticipated. We initially suspected that the ryh1/sat7 mutation might alter the intracellular membrane traffic, disturbing the cellular localization of TORC2 or its substrate Gad8. However, the characteristic cortical localization of S. pombe TORC2 is insensitive to ryh1 mutations, and so is the cytoplasmic distribution of Gad8.7 Furthermore, TORC2 signaling is hyper-active in a strain that expresses only the GTP-locked mutant of Ryh1 and therefore, Ryh1's cycling between the GTP- and GDP-bound states is not required to activate TORC2.7 This is in a stark contrast with how Rab GTPases function in the intracellular membrane traffic, where switching between the GDP- and GTP-bound forms is essential.19 Hence the action of Ryh1 GTPase on the TORC2 pathway appears to be independent of the intracellular membrane traffic.
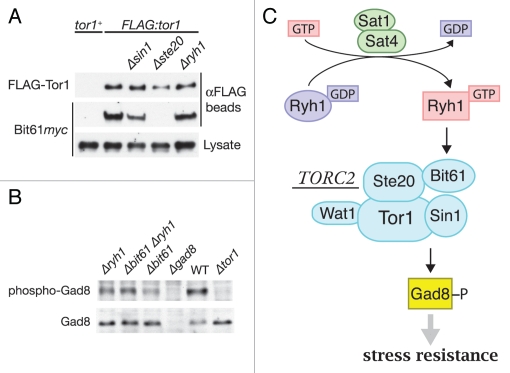
Direct regulation of TORC2 by Ryh1 was also suggested by the weak but significant physical interaction between Ryh1 and TORC2.7 The effector domain of Ryh1 is important for the interaction, because I44E, a point mutation to the effector domain, notably impairs the association of Ryh1 with TORC2. Moreover, the Ryh1 I44E mutant fails to promote TORC2 signaling, implying that GTP-dependent interaction of Ryh1 with TORC2 via the effector domain drives TORC2-Gad8 signaling. It should be noted, however, that the I44E mutation does not completely abrogate the association of Ryh1 with TORC2; the regulatory interaction of Ryh1 with the multi-subunit complex TORC2 may be more convoluted than the typical one-to-one interaction between a small GTPase and its effector. Indeed, our data suggest that the Bit61 subunit of TORC2 is involved in the Ryh1-mediated TORC2 regulation. Bit61,8 is structurally related to mammalian Protor/PRR5, which binds to the Rictor subunit of TORC2.20–22 Also in S. pombe, association of Bit61 to TORC2 is dependent on the Rictor ortholog, Ste20 (Fig. 1A), and the Ste20-Bit61 interaction is detectable in yeast two-hybrid assays (data not shown). Like the ryh1 mutant, the bit61 null mutant shows reduced phosphorylation of Gad8 (Fig. 1B), indicating compromised TORC2-Gad8 signaling in the absence of Bit61. Importantly, Gad8 phosphorylation in the Δbit61 Δryh1 double mutant was not lower than that in the individual single mutants (Fig. 1B); such an epistasis data often indicates that the two genes function in the same pathway. We found that Bit61 is not required for the physical interaction between Ryh1 and TORC2, and the exact molecular mechanisms by which Ryh1 and Bit61 stimulate TORC2-Gad8 signaling remains to be elucidated. Loss of Ryh1 does not affect the intrinsic kinase activity of Tor1, the catalytic subunit of TORC2. Interestingly, however, overexpression of GTP-locked Ryh1 appears to promote the physical interaction between TORC2 and Gad8; together with Bit61, GTP-bound Ryh1 may stimulate TORC2 signaling by enhancing the interaction between TORC2 and its substrate.7
Figure 1.
The Bit61 subunit is important for TORC2 regulation by Ryh1. (A) Ste20-dependent association of Bit61 with TORC2. tor1+, FLAG:tor1, FLAG:tor1 Δsin1, FLAG:tor1 Δste20 and FLAG:tor1 Δryh1 strains carrying the bit61:myc allele were subjected to anti-FLAG immunoprecipitation, and the isolated FLAG-Tor1 and Bit61myc were detected by immunoblotting. (B) Reduced Gad8 phosphorylation at the hydrophobic motif in Δbit61 cells. Crude lysate from wild-type, Δtor1, Δgad8, Δryh1, Δbit61 and Δbit61 Δryh1 strains was analyzed by anti-phospho-Gad8 and anti-Gad8 immunoblotting.7 (C) GTP-bound Ryh1 stimulates phosphorylation and activation of Gad8 kinase by TORC2. Activity of Gad8 kinase is essential for cellular resistance to environmental stress, such as high osmolarity and high temperature.
Rab proteins constitute the largest subfamily of the Ras-like small GTPase, and their discrete distributions establish compartmental specificity within the eukaryotic endomembrane system.23 It is likely that the localization of Ryh1 GTPase18 as well as those of its GEF and GAP (GTPase-activating protein) confer spatial regulation on TORC2 activity. Intriguingly, we observed that Rab6 GTPase, a mammalian ortholog of Ryh1, can stimulate TORC2 signaling when expressed in fission yeast.7 To date, no TORC2 activator has been reported in animals, despite the significant interest in how human TORC2 is regulated in the upstream of Akt kinase that controls cellular proliferation and insulin response. It will be of great interest to investigate if Rab6 and other Rab-family members regulate the TORC2-Akt pathway in cancers and insulin signaling.
Acknowledgements
We thank T. Toda for kindly providing S. pombe sat1, sat4 and sat7 mutant strains. K.S. acknowledges support by the National Institute of Health grant GM059788.
Extra View to: Tatebe H, Morigasaki S, Murayama S, Zeng CT, Shiozaki K. Rab-family GTPase regulates TOR complex 2 signaling in fission yeast. Curr Biol. 2010;20:1975–1982. doi: 10.1016/j.cub.2010.10.026.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/14936
References
- 1.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 2.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 4.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 5.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda K, Morigasaki S, Tatebe H, Tamanoi F, Shiozaki K. Fission yeast TOR complex 2 activates the AGC-family Gad8 kinase essential for stress resistance and cell cycle control. Cell Cycle. 2008;7:358–364. doi: 10.4161/cc.7.3.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatebe H, Morigasaki S, Murayama S, Zeng CT, Shiozaki K. Rab-family GTPase regulates TOR complex 2 signaling in fission yeast. Curr Biol. 2010;20:1975–1982. doi: 10.1016/j.cub.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi T, Hatanaka M, Nagao K, Nakaseko Y, Kanoh J, Kokubu A, et al. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells. 2007;12:1357–1370. doi: 10.1111/j.1365-2443.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo T, Otsubo Y, Urano J, Tamanoi F, Yamamoto M. Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol Cell Biol. 2007;27:3154–3164. doi: 10.1128/MCB.01039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci. 2009;34:620–627. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo T, Kubo Y, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 2003;22:3073–3083. doi: 10.1093/emboj/cdg298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai M, Nakashima A, Ueno M, Ushimaru T, Aiba K, Doi H, et al. Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity and high temperature. Curr Genet. 2001;39:166–174. doi: 10.1007/s002940100198. [DOI] [PubMed] [Google Scholar]
- 13.Weisman R, Choder M. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J Biol Chem. 2001;276:7027–7032. doi: 10.1074/jbc.M010446200. [DOI] [PubMed] [Google Scholar]
- 14.Kominami K, Seth-Smith H, Toda T. Apc10 and Ste9/Srw1, two regulators of the APC-cyclosome, as well as the CDK inhibitor Rum1 are required for G1 cell cycle arrest in fission yeast. EMBO J. 1998;17:5388–5399. doi: 10.1093/emboj/17.18.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol. 2010;28:617–623. doi: 10.1038/nbt.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengst L, Lehmeier T, Gallwitz D. The ryh1 gene in the fission yeast Schizosaccharomyces pombe encoding a GTP-binding protein related to ras, rho and ypt: structure, expression and identification of its human homologue. EMBO J. 1990;9:1949–1955. doi: 10.1002/j.1460-2075.1990.tb08322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siniossoglou S, Peak-Chew SY, Pelham HR. Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p. EMBO J. 2000;19:4885–4894. doi: 10.1093/emboj/19.18.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Sugiura R, Ma Y, Kita A, Deng L, Takegawa K, et al. Genetic and functional interaction between Ryh1 and Ypt3: two Rab GTPases that function in S. pombe secretory pathway. Genes Cells. 2006;11:207–221. doi: 10.1111/j.1365-2443.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- 19.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce LR, Huang X, Boudeau J, Pawłowski R, Wullschleger S, Deak M, et al. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jenö P, et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS ONE. 2007;2:1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo SY, Kim DH, Jun CB, Kim YM, Haar EV, Lee SI, et al. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem. 2007;282:25604–25612. doi: 10.1074/jbc.M704343200. [DOI] [PubMed] [Google Scholar]
- 23.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]