Abstract
Introduction
Trauma-induced hypercatecholaminemia negatively impacts bone marrow (BM) function by suppressing BM hematopoietic progenitor cell (HPC) growth and increasing HPC egress to injured tissue. Beta blockade (BB) given prior to tissue injury alone has been shown to reduce both HPC mobilization and restore HPC colony growth within the BM. In a clinically relevant model, this study examines the effect of BB given following both tissue injury and hemorrhagic shock (HS).
Methods
Male Sprague-Dawley rats underwent lung contusion (LC) with a blast wave percussion. HS was achieved after LC by maintaining the MAP 30-35mmHg for 45 minutes. Propranolol (10 mg/kg) was given once the MAP > 80 mmHg and subsequent doses were given daily (LC/HS/BB). One and seven days post-injury, analysis of BM and lung tissue for the growth of HPCs, hematologic parameters, and histology of lung injury was performed.
Results
LC/HS significantly worsens BM CFU-E growth suppression (15±8 vs. 35±2) and increases CFU-E growth in injured tissue as compared to LC at 1 and 7 days (33±5 vs. 22±9). The use of BB following LC/HS ameliorated BM suppression, the degree of anemia and HPC growth in the injured lung at 1 and 7 days post-injury. Lung injury score shows that there was no worsening of lung healing with BB (LC/HS/BB 3.2±2 vs. LC/HS 3.8±0.8).
Conclusion
In an injury and shock model, administration of propranolol immediately following resuscitation significantly reduced BM suppression and the protective effect is maintained at seven days with daily BB. While BB appears to improve BM function by decreasing HPC mobilization to injured tissue, there was no worsening of lung healing. Therefore, the use of propranolol following trauma and resuscitation may minimize long term BM suppression after injury with no adverse impact on healing.
Introduction
Severe trauma and hemorrhagic shock (HS) initiate a stress response that activates the sympathetic nervous system which leads to the release of catecholamines. A short, acute stress response has many beneficial effects that improve performance, increase blood pressure and heart rate, and enhance the immune response (1). However, when this stress is prolonged there is chronic exposure of catecholamines which triggers many pathologic processes, including cardiovascular dysfunction, metabolic breakdown, an impaired immune response and cancer progression (1). Trauma-induced hypercatecholaminemia has been shown to negatively impact bone marrow (BM) function by suppressing BM hematopoietic progenitor cell (HPC) growth and increasing HPC egress to injured tissue (2). The combined effect of BM progenitor growth suppression and increased mobilization of HPCs from the BM occurs in direct response to the degree and duration of physiologic stress which is mediated by norepinephrine (3-5).Clinically, in severely injured trauma patients this BM dysfunction manifests as a persistent anemia and altered wound healing has been observed (6, 7).
There is strong evidence of bidirectional communication between the sympathetic nervous system, the BM and the immune system (8).The trafficking of HPCs to and from the BM is a highly regulated process and the sympathetic nervous system, particularly norepinephrine, is a crucial regulator of HPC mobilization (5, 9). Concurrently, the BM is the source of both mature and immature cells, including HPCs, mesenchymal stem cells, B- and T-lymphocytes, neutrophils and macrophages. These cells express alpha and beta adrenergic receptors, with a predominance of beta receptors (10, 11). The sympathetic nervous system has been shown to have a direct effect on these cells influencing their cytokine expression, lymphocyte function, and cytotoxic activity (10). The exact mechanism involved in BM HPC mobilization and its role in healing injured tissue has not been fully elucidated.
The regulation of the catecholamine response with BB has been shown to be beneficial in humans after burns, trauma, non-cardiac surgery and traumatic brain injury as measured by improved outcomes (12-15). Previous work, in the context of tissue injury-induced BM dysfunction, has shown that non-selective BB with propranolol reduced suppression of BM HPC growth, reduced HPC mobilization, and reduced HPC growth in injured tissue (16). In addition, the beta-2 and beta-3 adrenergic receptors appear to mediate the effects of reduced BM suppression and HPC mobilization (16). Similar BM protection, with preserved BM cellularity and reduced BM HPC growth suppression, was shown with the use of propranolol given immediately following HS alone (17). Therefore, the aim of this study is to determine the effect of BB given in a more clinically relevant model of combined tissue injury and HS and examine its effects on BM function and injured tissue at one day and seven days post-injury.
Methods
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA), weighing 250-350g were housed under barrier sustained conditions and kept at 25°C with 12 hours light/dark cycles. The rats had free access to water and chow (Teklad 22/5 Rodent Diet W-8640, Harlan Teklad, Madison, WI). All rats were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals. The New Jersey Medical School Animal Care and Use Committee approved all animal protocols.
Experimental Design
To study the effects of beta-blockade following injury, animals were assigned to the following experimental groups (N = 4-6 survivors/group): unmanipulated control (UC), lung contusion alone (LC), lung contusion followed by hemorrhagic shock (LC/HS), and lung contusion and hemorrhagic shock followed by BB (LC/HS/BB). Groups were sacrificed via cardiac puncture at 24 hours and 7 days post injury. To induce BB, animals received the first intraperitoneal dose of propranolol at 10 mg/kg (Sigma-Aldrich, St. Louis, MO) after adequate resuscitation from shock (MAP ≥ 80 mmHg) and once every 24 hours thereafter. No additional resuscitation was required in the animals that received BB. Intraperitoneal injection was preferred because of ease of injection and reduced stress associated with sedation and placement of a catheter for long term intravenous access.
Lung Contusion
Rats were weighed and anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg). Unilateral LC was performed by using a blast wave of a percussive nail gun (Craftsman 968514 Stapler, Sears Brands, Chicago, IL) applied to a 12-mm small metal plate placed on the right axilla of the rat. This model has been shown to produce a clinically relevant LC by histology as demonstrated by Badami et al. (2). Animals were allowed to recover from LC and were sacrificed at 24 hours and seven days following injury. The right contused lung, bilateral femurs and peripheral blood were harvested for progenitor cell clonogenic assays and hematologic analysis.
Hemorrhagic Shock
Animals assigned to a combined LC/HS injury were weighed and anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg). First they underwent cannulation of the femoral artery and the internal jugular vein using aseptic techniques. The femoral artery catheter was used for invasive BP and HR monitoring (BP-2 Digital BP Monitor; Columbus Instruments, Columbus, OH). Upon cannulation of the vessels the animals were allowed to equilibrate for 10-15 minutes, the right lung was then contused as described. The animals were then allowed to recover from the LC until their blood pressure and heart rates return to base line (10-15 minutes). HS was then induced via blood withdrawal to a mean arterial pressure of 30-40 mmHg for 45 minutes. The shocked animals were resuscitated by reinfusion of shed blood. Throughout the experiment the animals' temperature was kept at approximately 37°C by using an electric heating pad under the surgical platform. Animals assigned to the LC/HS/BB group received their first dose of intraperitoneal propranolol during resuscitation when the MAP reaches 80 mmHg and subsequent doses were given once daily. At 24 hours and seven days post injury, the right contused lung, bilateral femurs, and peripheral blood were harvested.
Hematopoietic Progenitor Cell Cultures
The BM was harvested from both femurs by removing the epiphysis and flushing each femur with 5 mL of cold MEM-alpha medium (Sigma Chemical, St Louis, MO) to ensure cell separation. The contused lung tissue was mechanically shredded using two 18-gauge needles in 1 ml of cold MEM-alpha medium. Both lung and BM samples were centrifuged at 1500 rpm (400g) for 15 minutes, the supernatant was discarded, and the pellet was resuspended in 1ml of Dulbecco's MEM containing 10% fecal calf serum. Lung and BM mononuclear cells were plated in duplicate (2 × 106) in Iscoves media containing 30% fetal calf serum, 2% bovine serum albumin, 1% methylcellulose, rat growth factor, penicillin/streptomycin (GIBCO, Grand Island, NY), 2 × 10-4 mol/L 2-mercaptoethanol, and glutamine (Cellgro, Mediatech, Herndon, VA). BFU-E and CFU-E cultures were supplemented with 1.3 U/mL rhEpo and 6 U/mL rhIL-3 (Genetics Institute, Cambridge, MA) and CFU-GEMM cultures were supplemented with 3 U/mL rhGM-CSF (Genetics Institute, Cambridge, MA).Cultures were incubated at 37°C in 5% CO2. CFU-E colonies were counted at day 7, CFU-GEMM at day 10, and BFU-E at day 15 by an observer blinded to the origin of the samples.
Lung Injury Scores
Lung injury scores (LIS) were obtained in all animals at seven days post injury. Upon euthanizing the animals, the contused lungs were immediately harvested and fixed in 10% buffered formalin. After fixation, the tissue samples were dehydrated and embedded in paraffin blocks. 4μm thickness sections were cut and stained by hematoxylin and eosin (H&E). The slides were read under standard light microscope and the degree of injury scored by a modified quantitative LIS that accounted for inflammatory cells, interstitial edema, pulmonary edema and alveolar integrity (Table 1) (18). The LIS ranges between zero to eleven. Slides were evaluated under the standard light microscope and 30 random fields in each sample were graded in a blinded fashion.
Table 1.
Histologic grading for lung injury scores (LIS). HPF=high power field.
| Degree of injury | Points | |
|---|---|---|
| Inflammatory cells/HPF | <5 | 0 |
| 6-10 | 1 | |
| 11-15 | 2 | |
| 16-20 | 3 | |
| >20 | 4 | |
| Interstitial edema | None | 0 |
| Minimal | 1 | |
| Moderate | 2 | |
| Severe | 3 | |
| Pulmonary edema | <5% | 0 |
| 5-25% | 1 | |
| >25% | 2 | |
| Alveolar Integrity | Normal | 0 |
| Abnormal moderate | 1 | |
| Abnormal severe | 2 |
Peripheral blood analysis
Peripheral white and red blood cell counts, hemoglobin and mean corpuscular volume (MCV) were measured in all animals seven days following injury. Peripheral blood was obtained by via cardiac puncture using a 10ml heparinized syringe. 300μl aliquots were then sampled and analyzed within 10 minutes of collection using a veterinary Hematrue hematology bench top analyzer (Heska Corporation, Loveland, CO). Samples were run in duplicate.
Statistical Analysis
All data are represented as mean±standard deviation (SD). The data were subjected to repeated measures one-way analysis of variance (ANOVA) followed by Tukey-Kramer's multiple comparison post test using GraphPad Prism statistical package (version 4.0; Graph Pad, San Diego, CA). A p value of < 0.05 was considered significant.
Results
The Impact of BB on BM Hematopoietic Colony Formation
Lung injury alone (LC) results in approximately a 50% suppression of BM CFU-E growth at 24 hours when compared to UC (Figure 1A). LC alone has significantly similar growth reduction for earlier HPCs, CFU-GEMM and BFU-E, one day following injury (GEMM 26±10* vs. 61±2 and BFU-E: 30±8* vs. 63±6; * P <0.05 vs. UC). The addition of HS to LC resulted in further suppression of BM CFU-E growth to 78% that of UC at one day (Figure 1A). HPC growth suppression is seen in LC/HS with earlier progenitors as well (GEMM: 22±8* vs. 61±2 and BFU-E: 26±11* vs. 63±6; * P <0.05 vs. UC). The use of BB immediately after resuscitation (LC/HS/BB) significantly increased BM CFU-E growth at 1 day and restored it to near control levels (Figure 1A). The addition of BB to LC/HS similarly restored CFU-GEMM and BFU-E colony growth when compared to LC/HS one day post-injury (GEMM: 50±7** vs. 22±8 and BFU-E: 67±9** vs. 26±11; ** P <0.05 vs. LC/HS).
Figures 1A and 1B.
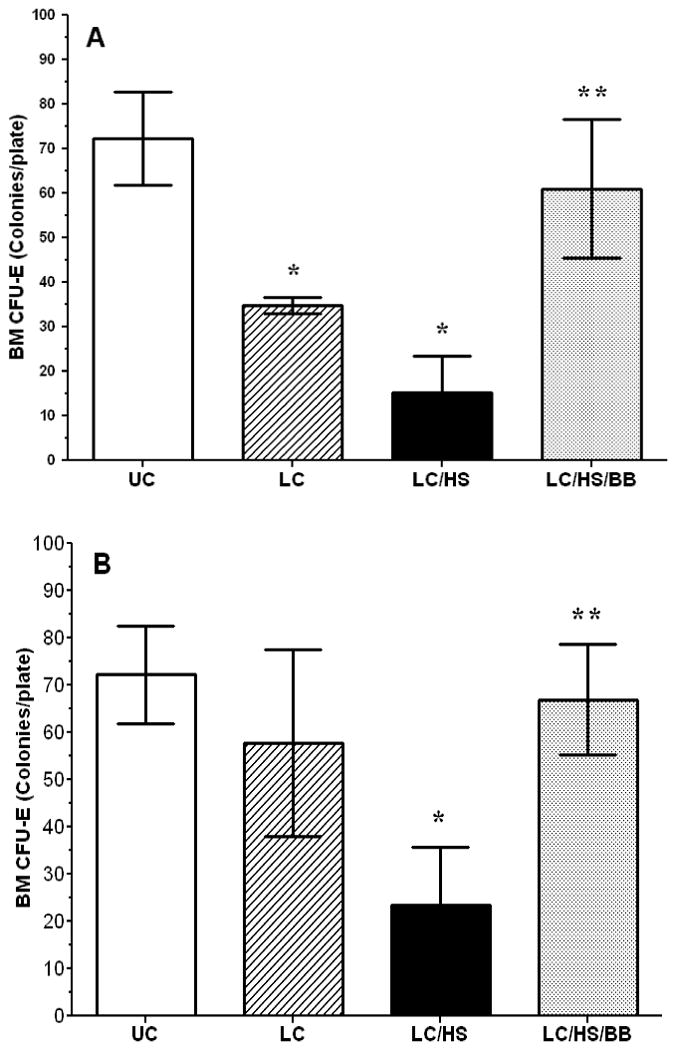
BM CFU-E colony growth one day (A) and seven days (B) following injury. (N = 4-6/group) UC=unmanipulated control; LC=lung contusion; LC/HS=lung contusion followed by hemorrhagic shock; LC/HS/BB=LC/HS followed by beta blockade after resuscitation * P <0.05 vs. UC; **P <0.05 vs. LC/HS
Seven days following injury in LC alone, BM CFU-E growth is only 15% suppressed as compared to UC (Figure 1B). Similarly, seven days post-LC, BM CFU-GEMM and BFU-E growth is no longer suppressed as compared to UC (57±9 vs. 61±2 and 62±14 vs. 63±6). In contrast, there was minimal improvement in BM HPC growth in the LC/HS group at seven days with a persistent 50–70% reduction in colony growth when compared to UC (Figure 1B) (CFU-GEMM: 38±9* vs. 61±2 and BFU-E: 30±9* vs. 63±6; *P <0.05 vs. UC). The restoration of BM CFU-E growth to control levels persists seven days after injury with the continued use of BB daily (Figure 1B).
Analysis of Hematologic Parameters
The findings in the BM parallel that of peripheral blood hematologic parameters. Seven days following LC, there were no significant changes in RBC or Hb as compared to control animals (Table 2). Whereas, LC/HS results in a significant reduction of RBC and Hb levels when compared to UC (RBC: 5.3±1* vs. 7.2±0.3 and Hb: 10.2±1.7* vs. 14.4±0.9; *P <0.05 vs. UC). Table 2 shows that at seven days following injury the use of BB significantly restored both RBC levels and Hb. In the LC/HS/BB group the Hb was greater than 13 g/dL which is only slightly below their pre-injury level. All experimental groups had no significant change in WBC and MCV as compared to controls.
Table 2.
Peripheral blood hematologic parameters seven days after injury. (N = 4-6/group) UC=unmanipulated control; LC=lung contusion; LC/HS=lung contusion followed by hemorrhagic shock; LC/HS/BB=LC/HS followed by beta blockade after resuscitation WBC=white blood cell; RBC=red blood cell; Hb=hemoglobin; MCV=mean corpuscular volume *P <0.05 vs. UC; **P <0.05 vs. LC/HS
| UC | LC | LC/HS | LC/HS/BB | |
|---|---|---|---|---|
| WBC (103/μl) | 7.3±2 | 6±2 | 5.2±1 | 7.8±2 |
| RBC (106/ μl) | 7.2±0.3 | 6.9±0.5 | 5.3±0.9* | 6.7±0.5** |
| Hb (g/dL) | 14.4±0.9 | 13.6±0.5 | 10.2±1.7* | 13.4±0.8** |
| MCV (fl) | 59±2 | 57±2 | 56±1 | 58±2 |
The Impact of BB on HPC Growth in Injured Tissue
There is a seven-fifteen fold increase in HPC growth in injured tissue one day following LC when compared to UC (CFU-GEMM: 12±4* vs. 2±1, BFU-E: 20±6* vs. 2±1, CFU-E: 22±9* vs. 2±0.7; *P <0.05 vs. UC). LC/HS had a significantly higher increase in CFU-E growth in the injured lung at one day as compared to UC (Figure 2A). LC/HS also increased earlier erythroid progenitor growth in injured tissue (GEMM: 25±7 and BFU-E: 28±4).
Figure 2A and 2B.
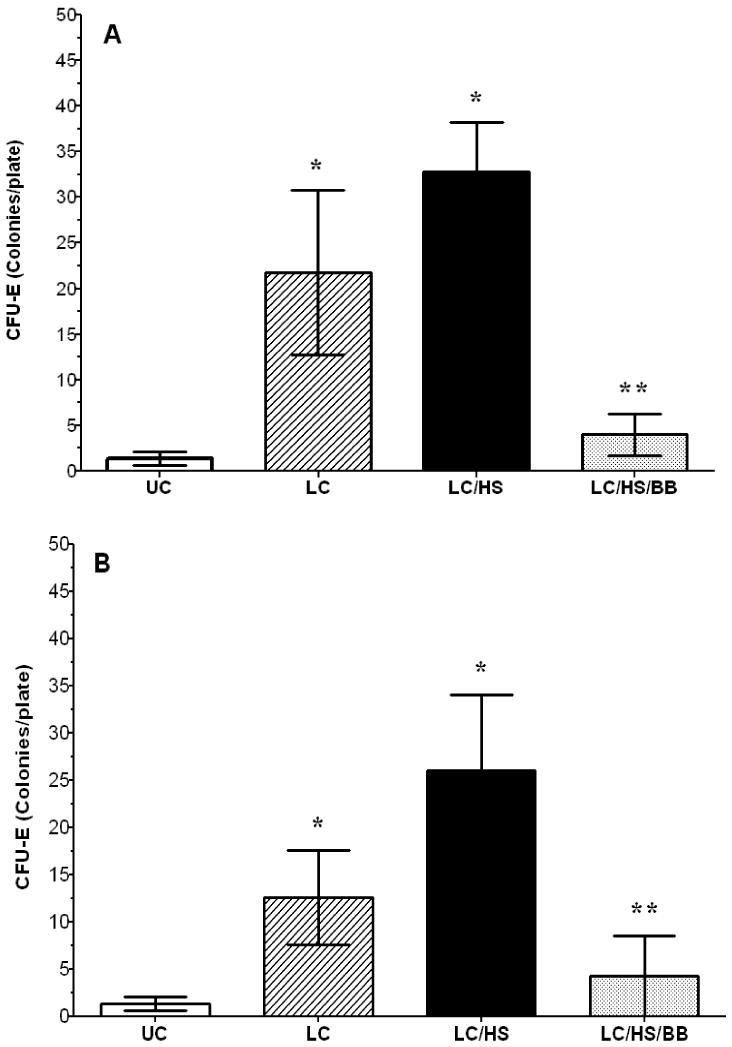
Lung CFU-E colony growth one day (A) and seven days (B) following injury. (N = 4-6/group) UC=unmanipulated control; LC=lung contusion; LC/HS=lung contusion followed by hemorrhagic shock; LC/HS/BB=LC/HS followed by beta blockade after resuscitation * P <0.05 vs. UC; **P <0.05 vs. LC/HS
Seven days after LC alone, HPC growth in injured tissue remains elevated seven-nine fold when compared to UC (CFU-GEMM: 11±6*, BFU-E: 19±11*, and CFU-E: 13±5*; *P <0.05 vs. UC). In the LC/HS group, Figure 2B shows that CFU-E growth is persistently elevated in the injured lung seven days post injury. Both CFU-GEMM and BFU-E are also persistently elevated seven days after LC/HS (GEMM: 49±10 and BFU-E: 24±9).
Rats treated with BB following LC/HS show a lack of CFU-E growth and sequestration of HPCs in injured tissue both one and seven days following injury (Figure 2A-B). Similarly, BB following LC/HS reduces CFU-GEMM and BFU-E colony growth one and seven days following injury (CFU-GEMM: 6±3 and 5±6, BFU-E: 4±2 and 4±2)
Histologic Assessment of Lung Injury
Due to reduced HPC growth in injured tissue with BB, it was important to determine whether BB worsened healing of injured lung tissue. Seven days following LC there is near complete healing of injured lung tissue with a measured LIS of 0.8±0.4. Figure 3 shows LC histologically seven days following injury as and is compared to UC. The addition of shock to LC (LC/HS) prevents lung healing at seven days with evidence of pulmonary edema, alveolar disruption, and neutrophil infiltration and the LIS remains elevated at 3.8±0.8 (Figure 3). The use of BB did not worsen lung healing at seven days and there was little histologic difference between LC/HS and LC/HS/BB (Figure 3). There was no statistically significant difference in the LIS of LC/HS/BB as compared to LC/HS (3.2±2 vs. 3.8±0.8).
Figure 3.
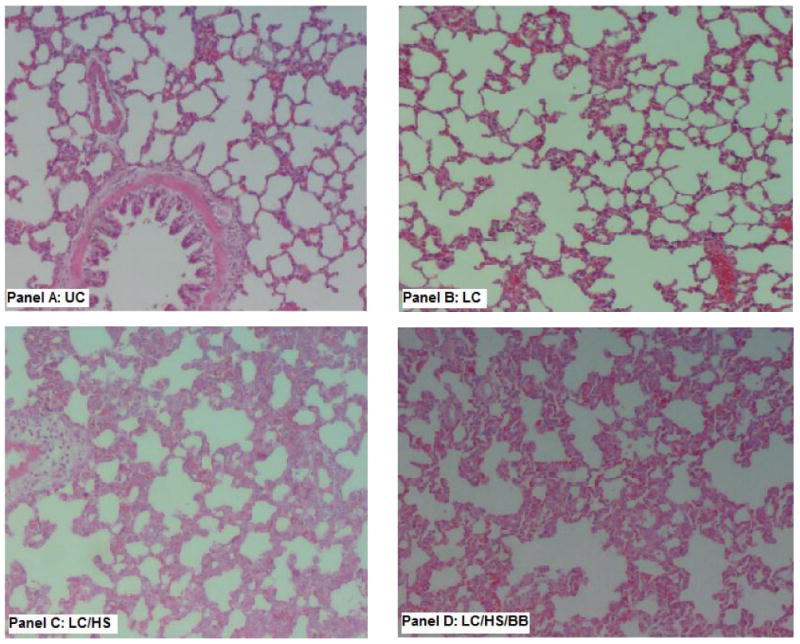
Histology of the contused lung tissue seven days post injury as compared to uninjured lung (Panel A: UC). Panel B (LC) shows near complete healing of the contused lung. The addition of HS to LC delayed healing at seven days (Panel C) and the use of BB did not worsen LIS (Panel D). (N = 4-6/group) LIS=lung injury score UC=unmanipulated control; LC=lung contusion; LC/HS=lung contusion followed by hemorrhagic shock; LC/HS/BB=LC/HS followed by beta blockade after resuscitation
Discussion
Severe traumatic injury and shock lead to the development of an inflammatory response that is associated with a sustained release of catecholamines (19). An excessive elevation of urinary epinephrine and norepinephrine has been found for up to seven-ten days following severe traumatic injury in humans (3). In the BM, both the degree and duration of catecholamine elevation have been shown to impact BM HPC growth (3, 4, 9). The dose dependent effects of norepinephrine on BM HPC growth have previously been shown both in vitro and in vivo (4, 20). Schraml et al. (21) also found that the degree of BM suppression directly correlates with the severity of adrenergic stress. They utilized adrenergic agonists in increasing doses to affect HPC cell division and found that it decreases HPC colony growth in a dose dependent manner (21). In the current study using different injury models as surrogates of higher norepinephrine elevation, LC alone resulted in a 50% suppression of BM HPC growth one day post-injury and by day seven the suppression was reduced to only 15%. This is consistent with previous studies showing that LC leads to 40% suppression of BM HPC growth three hours after injury (2, 16, 22). In a more severe injury model, the addition of HS to lung injury significantly worsened BM suppression and led to increased HPC growth in injured tissue. There was a persistent 50-70% reduction in BM HPC growth seven days following injury as well as continued growth of HPCs in injured tissue. No previous studies have shown the effect of a combined tissue injury with shock on BM HPC growth. However, HS alone has been shown to result in 50-60% BM HPC growth reduction three and twenty-four hours after shock (17, 23). Overall, these observations support the concept of a norepinephrine mediated impact on BM function that is both dose and duration dependent.
Previous work in a lung injury model revealed that selective beta-2 and beta-3 blockers and nonselective BB propranolol given pre-injury decreased the catecholamine surge which protected BM HPC growth and prevented HPC mobilization three hours after injury (16). Similarly, in a HS alone model, the use of propranolol prior to HS or immediately following resuscitation demonstrated BM protection (17). In our study using a combined lung injury and hemorrhagic shock model, the use of propranolol immediately following resuscitation with subsequent daily dosing resulted in increased BM HPC growth both at one and seven days. This protection of BM was associated with a statistically significant increase in RBC number and higher hemoglobin in peripheral blood seven days following injury. Our findings in the peripheral blood are supported by the work of Zyuz'kov et al. (24) who also demonstrated an increase in both RBC number and hemoglobin with the use of propranolol in a murine model of hypoxia and acute blood loss. In a model of rodent footshock stress, the total number RBCs were found to be decreased three days after stress and in the group treated with propranolol subcutaneously the total number of RBCs increased significantly (25). In the short study period of three days no difference in MCV, mean cell hemoglobin or mean cell hemoglobin concentration were noted (25).
Shah et al. (22) has shown that the BM HPCs mobilize to peripheral blood, sequester in injured tissue and are likely involved in tissue repair. In injured tissue, both LC and LC/HS were shown to increase HPC growth 15-25 fold respectively. At seven days post-injury the LC alone model demonstrated healing of the lung injury seen both histologically and confirmed quantitatively with an LIS of 0.8±0.4. Whereas the addition of HS to LC delayed lung healing as demonstrated by a higher LIS of 3.8±0.8 seven days after injury. Therefore the mobilization of HPCs after injury appears to represent a range of physiologic response that is based on the degree and timing of stress that leads to either normal wound healing, delayed wound healing or fibrosis. Spiegel et al. (9) similarly demonstrated the influence of adrenergic stimuli on HPC motility, proliferation and repopulation. Katayama et al. (5) found that HPC mobilization following G-CSF injection was significantly reduced following chemical sympathectomy with 6-hydroxydopamine thus demonstrating a direct link between norepinephrine and HPC egress from the BM under normal conditions. Katayama's work is similar to our findings in normal rats showing that both the absence of norepinephrine and high levels of norepinephrine have an impact on BM function (4, 5).
In our previous work, we found that propranolol and selective beta-2 and beta-3 blockers prevented the mobilization and sequestration of HPCs in injured tissue following LC (16). Therefore, there is a concern that the lack of HPC mobilization from the BM would lead to impaired tissue healing. Rather, we found that while propranolol given following LC/HS did decrease HPC growth in injured tissue, this did not adversely impact wound healing both qualitatively by histology and quantitatively by an LIS score of 3.2±2. In models of cutaneous wound healing, the role of BB has been controversial (26-28). However in stress models of wound healing, both rodent and human, have shown that BB actually improved epithelialization and wound healing (29, 30).
The anemia seen after trauma is multifactorial, due to acute and ongoing blood loss, decreased RBC survival, nutritional deficiencies, inhibition of proliferation and differentiation of erythroid progenitor cells and loss of HPCs to peripheral blood and sites of injury (6). Various circulating mediators, including erythropoietin, catecholamines, and cytokines, impact the proliferation and differentiation of erythroid progenitor cells (31-32). Both TNF-alpha and IFN-gamma have been shown to inhibit growth and differentiation of erythroid precursors by inhibiting erythropoietin mediated signaling pathways (33). The BM is a complex milieu of multiple cells types, both hematopoeitic and stromal, and regulatory mediators which are all impacted by the duration and severity of traumatic injury making its study and therapeutic options challenging. While a restrictive transfusion practice is more common in the intensive care unit (ICU), many severely injured patients still receive transfusion during a prolonged ICU length of stay. Therefore, strategies to minimize blood loss and increase RBC production and hemoglobin remain important. BB may potentially be the first therapeutic agent to prevent the suppression of BM erythroid progenitor cells and limit the egress of HPCs which can lead to increased RBC numbers and higher hemoglobin levels.
In summary, following severe injury and shock there are surges in catecholamines that disrupt the BM milieu leading to increased HPC mobilization to injured tissue, suppression of BM HPC growth and a prolonged anemia. The use of propranolol acts to abrogate the detrimental effects of a hypercatecholamine state and by reducing HPC mobilization to injured tissue more HPCs are retained in the BM allowing for both restored BM HPC growth and ultimately higher RBC and Hb levels with no adverse effect on tissue healing. Consequently, the use of propranolol after severe trauma may reduce the prolonged anemia seen, thus potentially avoiding the long term need for blood transfusions.
Acknowledgments
This research was supported by the National Institutes of Health grant K08 NIH GM078304 and the Clowes American College of Surgeons/American Association for the Surgery of Trauma Award.
Footnotes
This was presented as an oral presentation at the Sixty-Ninth Annual Meeting of the American Association for the Surgery of Trauma to be held in Boston, MA on September 22-25, 2010
References
- 1.Godbout JP, Glaser R. Stress-induced immune dysregulation:implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–427. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 2.Badami CD, Livingston DH, Sifri ZC, et al. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63:596–602. doi: 10.1097/TA.0b013e318142d231. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca RB, Mohr AM, Wang L, et al. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Inf. 2004;5:385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 4.Penn A, Mohr AM, Shah SG, et al. Dose response relationship between norepinephrine and erythropoiesis: Evidence for a critical threshold. J Surg Res. 2010 Apr 18; doi: 10.1016/j.jss.2010.03.051. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katayama Y, Battista M, Kao W, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 6.Livingston DH, Anjaria D, Wu J, et al. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care. 2006;12:325–332. doi: 10.1097/01.ccx.0000235210.85073.fc. [DOI] [PubMed] [Google Scholar]
- 8.Dwight MN, Virginia MS. Autonomic innervations and regulation of the immune system. Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nature Imm. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 10.Muthu K, Iyer S, He LK, et al. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohm AP, Sanders VM. Norepinephrine and beta-2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharm Reviews. 2001;53:487–525. [PubMed] [Google Scholar]
- 12.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Eng J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 13.Arbabi S, Ahrns KS, Wahl WL, et al. Beta-blockade use is associated with improved outcomes in adult burn patients. J Trauma. 2004;56:265–271. doi: 10.1097/01.TA.0000109859.91202.C8. [DOI] [PubMed] [Google Scholar]
- 14.Auerback AD, Goldman L. Beta-blockers and reduction in cardiac events in non-cardiac surgery: clinical applications. JAMA. 2002;287:1445–1447. doi: 10.1001/jama.287.11.1445. [DOI] [PubMed] [Google Scholar]
- 15.Inaba K, Teixeira P, David JS, et al. Beta-Blockers in isolated blunt head Injury. J Am Coll Surg. 2008;206:432–438. doi: 10.1016/j.jamcollsurg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Beiermeister KA, Keck BM, Sifri ZC, et al. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 adrenergic receptors following injury. J Trauma. 2010;69:338–343. doi: 10.1097/TA.0b013e3181e5d35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elhassan IO, Hannoush EJ, Alzate W, et al. Beta blockade prevents hematopoietic progenitor cell suppression following hemorrhagic shock. Surg Infections. doi: 10.1089/sur.2010.043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claridge JA, Enelow RI, Young JS. Hemorrhage and resuscitation induce delayed inflammation and pulmonary dysfunction in rats. J Surg Res. 2000;92:206–213. doi: 10.1006/jsre.2000.5899. [DOI] [PubMed] [Google Scholar]
- 19.Molina PE. Neurobiology of the stress response: contribution of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock. 2005;24:3–10. doi: 10.1097/01.shk.0000167112.18871.5c. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca RB, Mohr AM, Wang L, et al. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–890. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 21.Schraml E, Fuchs R, Kotzbeck P, Grillari J, Schauenstein K. Acute adrenergic stress inhibits proliferation of murine hematopoietic progenitor cells via p38/MAPK signaling. Stem cells and develop. 2009;18:215–227. doi: 10.1089/scd.2008.0072. [DOI] [PubMed] [Google Scholar]
- 22.Shah S, Ulm J, Sifri ZC, et al. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma. 2009;67:315–322. doi: 10.1097/TA.0b013e3181a5c9c7. [DOI] [PubMed] [Google Scholar]
- 23.Livingston DH, Gentile PS, Malangoni MA. Bone marrow failure after hemorrhagic shock. Circ Shock. 1990;30:255–263. [PubMed] [Google Scholar]
- 24.Zyuz'kuv GN, Abramova EV, Dygai AM, Gol'dberg ED. Role of adrenergic mechanisms of erythropoiesis regulation during severe hypoxia. Bull Exper Biol Med. 2005;140:13–17. doi: 10.1007/s10517-005-0399-7. [DOI] [PubMed] [Google Scholar]
- 25.Lucian H. Effects of beta-adrenergic receptor blockade on stress-induced changes in hematological parameters of rats. Turk J Hematol. 2006;23:90–93. [PubMed] [Google Scholar]
- 26.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Gosain A, Jones SB, Shankar R, et al. Norepinephrine modulates the inflammatory and proliferative phases of wound healing. J Trauma. 2006;60:736–744. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 28.Leicht M, Greipel N, Zimmer H. Comitogenic effect of catecholamines on rat fibroblasts in culture. Cardiovasc Res. 2000;48:274–284. doi: 10.1016/s0008-6363(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 29.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing:potential new indication for beta blockers. PLoS Med. 2009 Jan 13;6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammadi AA, Bakhshaeekia A, Alibeigi P, et al. Efficacy of propranolol in wound healing for hospitalized burn patients. J Burn Care Res. 2009;30:1013–1017. doi: 10.1097/BCR.0b013e3181b48600. [DOI] [PubMed] [Google Scholar]
- 31.Lapidot T, Petit I. Current understanding of stem cell mobilization: the role of chemokines, proteolytic enzymes, adhesion molecules, cytokines and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 32.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem/progenitor cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 33.Morceau E, Dicato M, Diederich M. Pro-inflammatory cytokine-mediated anemia: Regarding molecular mechanisms of erythropoiesis. Mediators Inflamm. 2009:405016. doi: 10.1155/2009/405016. Epub 2010 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]


