Abstract
Pancreaticoduodenectomy is one of the most challenging surgical procedures which requires the highest level of surgical expertise. This procedure has constantly evolved over the years through the meticulous efforts of a number of surgeons before reaching its current state. This review navigates through some of the early limitations and misconceptions and highlights the initial milestones which laid the foundation of this procedure. The current review also provides a few excerpts from the lives and illuminates on some of the seminal contributions of the three great surgeons: William Stewart Halsted, Walther Carl Eduard Kausch and Allen Oldfather Whipple. These surgeons pioneered the nascent stages of this procedure and paved the way for the modern day pancreaticoduodenectomy.
Keywords: history, pancreaticoduodenectomy, early limitations, milestones, Whipple, Halsted, Kausch
Introduction
For a surgical procedure that is extremely complex, the history behind it is no less worthwhile. The history of the pancreaticoduodenectomy extends from the late 19th century with its ominous prohibitive mortality to its current stage where mortality has been reduced to less than 2%.1 This is particularly true for high-volume centres which have significantly lower mortality compared with low-volume centres.2 The maturation of the procedure, that we now call pancreaticoduodenectomy, has been punctuated by the involvement of several giants of surgical heritage. This highly demanding procedure requires the highest level of surgical training and excellent technical skills. The excellent outcomes in the recent past have been very successful in dispelling the nihilism that was attached to this procedure in the 1960s and 1970s. This has led to an increasing number of centres and surgeons that perform pancreaticoduodenectomies on a daily basis. This review chronicles the history of the inception and maturation of the procedure. Although not entirely inclusive, the review attempts to highlight some of the early misconceptions, initial milestones and narrates on the lives and contributions of some of the key surgeons.
Early misconceptions
The dauntless efforts led by earlier surgeons in the 19th and early 20th century lead to a better understanding of several anatomical and physiological dogmas prevailing at the time. One of the early misconceptions was the belief that the duodenum was essential for human survival, which may preclude its complete excision. Desjardins3 (in 1907) and Sauve4 (1908) were the first surgeons to report the feasibility of performing a duodenectomy although it was attempted only on human cadavers. Dragstedt et al.'s5 efforts (1918) further demonstrated that a total duodenectomy was compatible with survival in dogs. Until Dragstedt's study, only partial duodenal excisions were performed. It was about two decades later in 1935, when Whipple et al.6 performed the first reported total duodenectomy as a part of his two-stage procedure.
The question of how to drain the pancreatic juice has been a source of considerable debate since the beginning. It was initially thought that the flow of pancreatic juice was essential for survival and attempts to disrupt continuity may lead to disastrous consequences. However, subsequent work revealed that the flow of pancreatic juice was not essential for survival. At the same time it was mistakenly considered that the pancreatic duct could be permanently ligated without any adverse sequelae. In fact, in 1935 Whipple suggested that attempts to re-establish continuity between the pancreatic duct and intestines would be followed by doom resulting from activation of the pancreatic ferments by the duodenal juice which may subsequently compromise any anastomosis (particularly in humans where the posterior duodenum is devoid of peritoneum). Unfortunately, ligation of the pancreatic duct led to an inordinately high incidence of pancreatic fistulae. As early as 1909, Coffey7 and Kehr8 suggested that the pancreatic stump could be implanted into the distal end of the resected bowel. In 1941, Hunt9 added a pancreaticojejunostomy to reduce the incidence of pancreatic fistulae and this was followed by others as well. In addition, later experiments by Whipple on dogs at Memorial Hospital with one of the surgical residents, John Hawk, showed that pancreatico-jejunal anastomosis is safe. After transecting the pancreas in dogs, the pancreatic duct and jejunal mucosa were anastomosed with two silk sutures only. The anastomoses were examined at 24-h intervals and it was noted that epithelium of the pancreatic duct had overgrown the jejunal mucosa to complete the anastomosis in less than 24 h. This perpetuated the idea of re-implanting rather than ligating the pancreatic duct, to reduce the incidence of pancreatic fistulae.
The initial incidence of marginal ulceration after pancreatic surgery was as high as 36% and was thought to be as a result of multiple factors such as sequencing of anastomoses, inadequate gastric resection, lack of gastric acid inhibition, lack of vagotomy and consequences of duodenal resection.10 Switching of the gastric anastomosis from the most proximal to the most distal, adequate gastrectomy and more recently the pylorus-preserving procedure led to a significant reduction in the incidence of marginal ulceration.
Early attempts to divert the bile included a cholecystogastrostomy or cholecystoenterostomy because of the ease of the procedure. This was associated with ascending biliary tract infections as a result of the gastric contents and was prone to stenosis.
The second patient in the first series of Whipple6 died from cholangitis as a result of reflux of gastric contents through the cholecystogastrostomy and was found to have stenosis of the anastomosis at autopsy. Whipple11 therefore advocated that choledochoenterostomy using a loop of the jejunum rather than cholecystogastrostomy be used for establishing biliary flow.
Historical milestones
1898: Codivilla4,12 performed the first reported pancreaticoduodenectomy for carcinoma of the pancreas and removed parts of the pancreas, duodenum, distal stomach and distal bile duct. Continuity was restored using Roux-en-Y gastrojejunostomy and cholecystojejunostomy with no anastomosis or closure of the pancreatic stump. Serous drainage of wound on day 5 required evacuation of milky clots and the patient died at 18 days from cachexia resulting from steatorrhea.12
1899: Halsted13 reported the first successful resection for ampullary cancer by excising portions of the duodenum and pancreas in a patient operated upon for gall stones.
1900 and 1904: Mayo-Robson11,14 and Koerte11,15 excised cylindrical segments of the duodenum for ampullary carcinoma but the patients did not survive the procedure.
1907: Desjardins3,11 performed a radical resection of the duodenum and head of pancreas in human cadavers in two stages. He also suggested implantation of a pancreatic stump in the bowel.
1908: Sauve4,11 performed a one-stage procedure in cadavers with various ideas for dealing with the pancreatic stump including suturing it to the abdominal wound to create a fistula.
1912: Walter Kausch11,16 first attempted the resection of the majority of the duodenum en bloc with a significant portion of the pancreas. As a result of the thinking of the time, he did not perform a complete duodenectomy and established continuity with a pancreaticoduodenostomy.
1914: The first reported one-stage partial resection was performed by Hirschel.11,17 He excised parts of the duodenum, ampulla, head of pancreas and the lower part of the common bile duct. Continuity was established by re-implantation of the pancreatic duct into the duodenorrhaphy, posterior gastroenterostomy and bridging of the common bile duct to the duodenum with the aid of a rubber tube. The patients jaundice was relieved and he lived for 1 year. The cause of death or fate of the rubber tube were unknown as an autopsy was never performed. This procedure did not involve complete resection of the duodenum or the pancreas.
1918: Dragstedt et al.5,11 demonstrated in dogs that a total duodenectomy was compatible with survival.
1922: Tenani11,18 carried out a successful two-stage resection for ampullary carcinoma in a 43-year-old male. In the first stage, he performed a posterior gastro enterostomy and choledochoduodenostomy to the lower duodenum. During the second stage, he excised portions of the duodeunum, head of the pancreas wide of the growth and established continuity with a pancreaticoduodenostomy to the lower duodenum. In spite of a stormy post-operative course, the patient lived for 3 years.
1935: Whipple, Parsons and Mullins6,11 published the first seminal report of three patients from Columbia Presbyterian Hospital in New York. The two-stage procedure included a radical resection of the duodenum and head of the pancreas for ampullary cancer. The third patient underwent a total duodenectomy and excision of a large portion of the head of the pancreas. This was the first reported case of complete excision of the duodenum and a large portion of the head of pancreas. The first patient died within 30 h after the operation because of consequences of anastomotic breakdown. The second and third patients lived for 9 and 24 months and succumbed to cholangitis and liver metastasis, respectively.
1937: Nemenyi11,19 performed a partial resection of the duodenum similar to the approach of Walther Kausch, albeit in one stage.
1937: Brunschwig11,20 was the first to perform a radical pancreaticoduodenectomy (i.e. two stage, pylorus-preserving procedure with complete excision of the head of the pancreas to the right of the superior mesenteric vein), for carcinoma of the head of the pancreas.
1940: Vitamin K discovered by Danish physiologist Henrick Dam. Brinkhaus noted that Vitamin K when combined with bile salts reduced the bleeding tendency in jaundiced patients.
1940: The first recorded one-stage procedure for complete excision of the head of the pancreas and the entire duodenum by Whipple.11
1940: Unaware of Whipple's procedure, Trimble et al.11,21 from The Johns Hopkins hospital performed a similar one-stage radical resection a few weeks after Whipple. Trimble added a distal gastrectomy to avoid blow out of the duodenal stump.
1940: Hunt9 added a pancreaticojejunostomy to avoid leakage of the pancreatic stump.
1946: Whipple11 published his 10-year experience of radical excisions of the head of pancreas and duodenum. In this report he proposed several modifications to his original procedure and advocated a one-stage procedure.
1963: Whipple22 publishes his reminiscences of the procedure that bears his name.
Most of the early surgeons namely Kausch, Hirschel and Tenani resected only portions of the duodenum and pancreas. Whipple was the first surgeon to perform a complete resection of the duodenum and head of the pancreas in his initial two-stage (1935), and subsequent one-stage (1940) procedures. The remainder of the review will narrate on the contributions of William Stewart Halsted, Walther Carl Eduard Kausch and Allen Oldfather Whipple that led to early advancement of this radical surgery of the pancreas and duodenum.
William Stewart Halsted Era (1852–1922)
William Stewart Halsted23 (Fig. 1) was born on 23 September 1852 in New York City. After completing college at Yale (1870–1874) during which period he was better known for his sporting rather than his educational abilities, he entered the College of Physicians and Surgeons in New York in 1874. In contrast to his college days, Halsted excelled in medical school to finish top of his class in 1877.23
Figure 1.

William Steward Halsted by Thomas Corner, Oil on canvas, 1936, Courtesy of The Alan Mason Chesney Medical Archives of The Johns Hopkins Medical Institutions
In 1889, Halsted accepted the invitation of his long-time friend William Welch and moved to Baltimore to join the ranks at The Johns Hopkins Hospital.23 In 1892, he was made the Surgeon-in-Chief and Professor of Surgery. For the next three decades until his death in 1922, Halsted made several monumental contributions to the field of Surgery. These ranged from inventing surgical gloves to the pioneering efforts in the management of hernias, aneurysms, fractures, gall bladder disorders, breast cancer, intestinal surgery and so on. He was also one of the earliest surgeons to emphasise the principles of safe surgery, with particular attention to operative detail and haemostasis.23 His most significant contribution of all was to organise a structured training system for young surgeons which is and has been the accepted model worldwide ever since. Halsted remains one of the brightest stars of the American surgical heritage.
During the 1890s, Halsted developed an interest in the management of diseases of the biliary tree. Halsted is known to have performed one of the first operations for gallstones in the United States in 1882 on his mother.23 Halsted had made great strides in the management of patients with biliary diseases and compiled his series of eight patients that he presented at the Meeting of Surgical Section of the Suffolk Medical Society on 3 May 1899.13 In this series, Halsted also reported the first successful operation for the removal of carcinoma of the ampulla of Vater.13
The patient was a 60-year-old female who presented in February 1898 with signs and symptoms suggestive of obstructive jaundice thought to be as a result of choledocholithiasis.13 She was taken to the operating room on 14 February 1898 where:
‘… a longitudinal opening 2 cm long was made in the common duct. Duct explored with finger. What seems to be a small, very hard stone is felt at site of ampulla. To determine the nature of this body, an incision was made through the wall of the duodenum. No glandular metastasis discoverable. The stone like body proved to be, as was feared, a carcinoma of the papilla’.
He went on to describe the excision by adhering to his new concept of ‘orderly spread of cancer and its en bloc removal –‘to give the growth a wide margin, a large piece of the duodenum was excised, a wedge shaped piece with the apex at the mesenteric border of the intestine. About three-quarters of an inch of the common duct and a shorter piece of the pancreatic duct was excised’. 13 The patient did well and underwent a second operation on 5 May 1898 for proper drainage in the form of a cholecystoduodenostomy and cysticoduodenostomy. The patient was discharged on 9 June 1898 only to present again in early autumn of the same year. At the time of presentation, she was too ill to be operated upon and died within a few weeks. At autopsy, it was discovered that carcinoma had recurred in the head of the pancreas and duodenum closing the common duct and interfering with the perfect action of the cholecystoentererostomy or cystico-enterostomy.13 Although he admitted that the results in this patient were not encouraging, this was a significant milestone that paved the way for other pioneering surgeons.
It is ironic that Halsted actually died from complications relating to choledocholithiasis – the same disease that he so valiantly treated in so many patients. In 1919, Halsted underwent a cholecystectomy performed by one of his former students, Richard Follis.24 In 1922, he developed choledocholithiasis, for which he was operated on again by his students, Heuer and Mont Reid.25 They practiced the same principles that Halsted had advocated. Despite these efforts, on 7 September 1922, he died from a gastrointestinal haemorrhage and pneumonia in the post-operative period.
Walther Carl Eduard Kausch Era (1867–1928)
Walther Kausch (Fig. 2a) was born in 1867 in Koenisgsberg.26 His medical career began in 1890 in the field of psychiatry and continued until 1892 when under the influence of Bernhard Naunyan, he switched disciplines to internal medicine. In 1896, upon the insistence of Naunyan, he unenthusiastically went to Breslau to work under Johannes von Mikulicz-Radecki.26 Although he was hired in the capacity of an Internist, within 2 years he was made Professor of Surgery and published the seminal paper on benign diseases of the stomach, ‘The Functional Outcomes of Operations on the stomach for benign diseases’. During his career at Strassburg, Breslau and Berlin, he published more than 150 journal articles including his seminal papers on the first pancreaticoduodenectomy.
Figure 2.
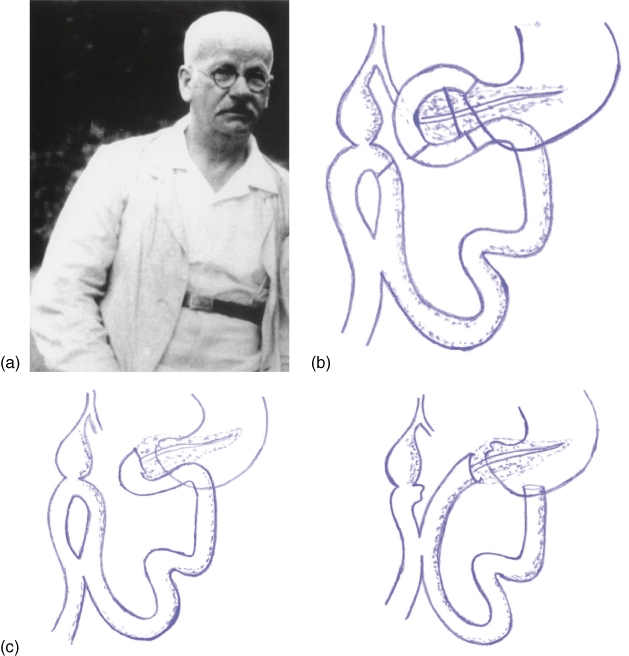
(a) Walther Carl Eduard Kausch.26 Published with permission. Originally published in: Specht G, Stinshoff K. (2001) Walther Kausch (1867–1928) und seine Bedeutung für die Pankreaschirurgie. Zentralbl Chir 126:479–481, Georg Thieme Verlag KG. (b) Cholecystoenterostomy16 (Figure based on the original drawing of Kausch16). (c) Variations in restoring continuity: pancreatico-duodenostomy and pancreatico-jejunostomy respectively (Figure based on the original drawing of Kausch16)
At the time of his first operation in 1909,16 there were only three prior reports (Halsted, Koerte and Mayo) describing the management of carcinoma of the papilla vateri and none were complete in the extent of resection. Kausch had remarked in exasperation that only a few understood the operation well enough and went on to describe his procedure:16
'49 year old male, Ernst G Kurativ, a cash messenger by profession presented with icterus for 6 weeks and weight loss of 17 kg,
First operation: June 15th 1909 (Fig. 2b)
At exploration, I felt a bean-sized lump in the papilla on transduodenal palpation, which obviously was not a gallstone. There was no evidence of metastasic disease. A cholecystoenterostomy was performed. Icterus improves and patient gains weight to the tune of ½ kilogram.
Second operation: August 21, 1909 (Fig. 2c)
Adhesions from prior operation add to the difficulty. After Kocherization, I verified resectability. Having confirmed that it was resectable, I went on to, fashion a gastroenterostomy, close the pylorus, resect the duodenum and part of the pancreatic head the size of a walnut, ligate the choledochus, suture the cut end of the duodenum to the pancreas'.
He went on to describe the different methods of restoring intestinal continuity. This procedure lasted under 4 h with minimal documented blood loss. The patient was seen again on 12 September 1909 and was noted to have gained weight to the tune of 2½ kilograms.
This operation was another significant milestone and some still regard that the apt name for the so-called Whipple procedure should be the Kausch–Whipple procedure. For it was not until another 25 years that Whipple described his procedure. In spite of this, posterity of reputation is not attached to the name of Kausch as is to Halsted and Whipple. Kausch suffered many major personal setbacks after this achievement.26 These personal tragedies had a detrimental effect on the promising future of Kausch of which the loss of his son Wolfi was the greatest. This gradual decline culminated in his death on 24 March 1928. He presented with abdominal pain and self-diagnosed it as cholecystitis, although at laparotomy it was noted to be as a result of a perforated appendicitis. He was recovering well from the operation and he even took a walk in the park with his son Johannes on 23 March 1928. Unfortunately the same night he succumbed to a massive pulmonary embolism26.
Allen Oldfather Whipple Era (1881–1963)
Allen Oldfather Whipple27 (Fig. 3a) was born on 2 September 1881 to Christian missionaries while on mission in Azerbaijan, Iran. Whipple28,29 went to medical school at the Columbia's College of Physicians and Surgeons in New York and received his MD in 1908, following which he was appointed as faculty in 1911. Within 10 years he rose to the rank of Professor and Surgeon-in-Chief of the Columbia-Presbyterian Medical Center in New York.27 As the Valentine Mott Chair in Surgery, he led the department for 25 years before retiring in 1946. Whipple continued to make significant contributions until his death in 1963.27
Figure 3.
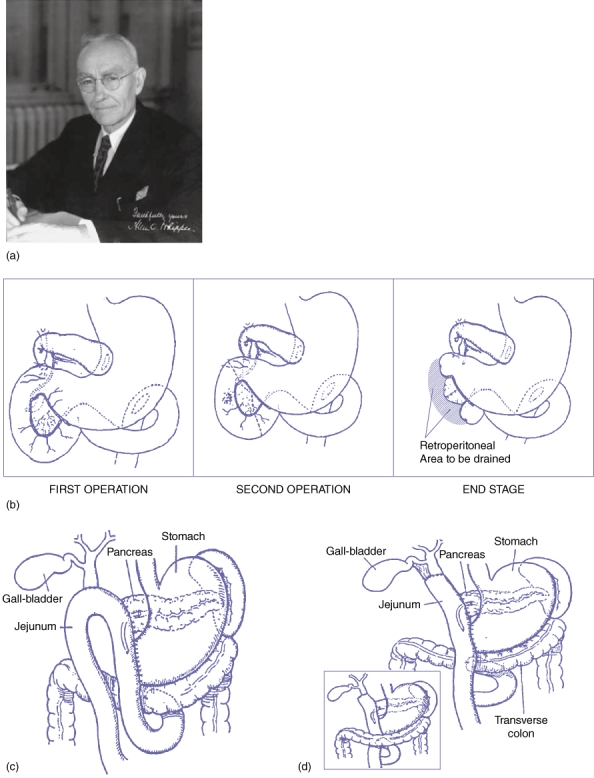
(a) Allen Oldfather Whipple (Reprinted with permission from Dr Richard J. Bing). (b) First two-stage operation as described by Whipple in his original report in 1936.6 (c) Antecolic anastomosis with the loop of the jejunum in a one-stage procedure11 (Reprinted with permission from Elsevier. Originally published in Whipple AO. Observations on radical surgery for lesions of the pancreas. Surg Gynecol Obstet 1946; 82: 623–631). (d) Antecolic or retrocolic anastomosis with the vertical limb of a resected jejunum in a one-stage procedure11 (Reprinted with permission from Elsevier. Originally published in Whipple AO. Observations on radical surgery for lesions of the pancreas. Surg Gynecol Obstet 1946; 82: 623–631)
Although Whipple made significant contributions to the diagnosis and management of insulinomas, he will be remembered most for the procedure that bears his name.30,31 Whipple wrote in 1946 that the removal of islet cell tumours had a determining influence in developing radical surgery for malignant tumours of the pancreas. His first report on a two-stage pancreaticoduodenectomy was a compilation of three patients that was presented in 1935 at the American Surgical Association meeting in Boston.6 The pre-operative findings, operative details and post-operative course of these three patients have been summarised in Table 1 and Fig. 3b.
Table 1.
Pre-operative findings, operative details and post-operative course of three patients with a two-stage pancreaticoduodenectomy presented by Whipple at the American Surgical Association meeting in Boston in 1935 (summarised from Whipple AO, Parsons WB, Mullins CR. Treatment of Carcinoma of the Ampulla of Vater. Ann Surg 1935; 102: 763–779)
| WS, 60-year-old female | EW, 53-year-old male | CC, 49-year-old male | |
|---|---|---|---|
| Pre op | 1) Jaundice-10 weeks | 1) Jaundice-8 weeks | 1) Jaundice-8 weeks |
| 2) Epigastric fullness-7 years | 2) Gonorrhoea | 2) Gonorrhoea | |
| 3) Temp: 101.4 F | 3) Temp: 100.4 F | 3) Temp: 98.8 F | |
| 1st operation | 03/16/1934 | 07/18/1934 | 01/25/1935 |
| Choledochoduodenostomy | 1) Cholecystogastrostomy | 1) Gastroenterostomy | |
| Cholecystostomy | 2) Right upper rectus incision | 2) Cholecystogastrostomy | |
| Suture: Silk | 3) Ligation of Common bile duct (CBD) | ||
| Right upper quadrant incision | |||
| Suture: Silk | |||
| Post op | Fistula closed-3rd week | Sutures out-7th day | Jaundice cleared |
| Jaundice cleared | Retentions out-12th day | ||
| Ambulating-15th day | |||
| Discharged-26th day | |||
| 2nd operation | 05/07/1934 | 08/21/1934 | 02/07/1935 |
| 1) Resection of duodenal wall, ampulla and part of the pancreas. | 1) Partial duodenectomy | 1) Near total duodenectomy | |
| 2) Pancreas sutured to duodenal defect: Catgut | 2) Partial pancreatectomy | 2) Large excision- head of pancreas | |
| 3) CBD not visualised | 3) DuodenoduodenostomyTransverse incision | 3) Duodenum inverted | |
| Suture: Silk | 4) Pancreatic stump closed | ||
| Transverse incision | |||
| Suture: Silk | |||
| Post op | Patient died in 30 h | Persistent emesis >2 l/day | Pancreatic fistula |
| Ambulated-12th day | |||
| Discharged-18th day | |||
| 3rd operation | 08/29/1935 | 03/25/1935 | |
| 1) Anterior gastro-enterostomy | Exploratory laparotomy | ||
| Left upper rectus incision | Drainage of abscess | ||
| Suture: Silk | |||
| Post op | No complications | No complications | |
| Long term | 03/25/1935 | Survived 25 months | |
| 1) Fever (104 F), chills, pain, jaundice. | |||
| 2) Blood Cultures- B lactic aerogenes | |||
| 3) Death- 4/18/1935 | |||
| Pathology | Adenocarcinoma-Ampulla of Vater | Adenocarcinoma-Ampulla of Vater | Adenocarcinoma-Ampulla of Vater |
| Autopsy | Leakage, breakdown of anastomosis, peritonitis. | 1) Stenosis of cholecystogastrostomy | Liver metastasis |
| No metastasis | 2) Multiple liver abscesses | ||
| 3) Abscesses in pancreas, lungs and kidneys | |||
| 4) No gross recurrence, Microscopic recurrence at distal CBD | |||
This report of Whipple which was a significant advancement in the field of Surgery proved that, the pancreas can be operated upon in a relatively safe manner.
Whipple's first one-stage procedure was an un-planned procedure in a patient explored for a pyloric channel ulcer.30,31 During surgery, discovering that she actually had a mass in the head of the pancreas, Whipple performed a resection of the lesion, distal gastrectomy and choledochoduodenostomy. Although in this patient he did not perform a pancreatic anastomosis, in subsequent patients he did. The discovery of Vitamin K and development of blood banks helped overcome the bleeding tendencies in jaundiced patients. These advancements along with difficulties encountered in the second stage of the operation (due to adhesions) led Whipple to advocate a one-stage procedure. In another of his seminal reports on the topic in 1946,11 he went on to summarise the significant steps of the one-stage procedure, that included: (i) complete resection of the duodenum and the head of pancreas; (ii) restoration of pancreatico-enteric continuity by anastomosing the pancreatic duct to a jejunal loop; and (iii) performing a choledochoenterostomy rather than using the gallbladder as a conduit to avoid ascending biliary tract infections and stenosis (Fig. 3c–d).
Whipple performed a total of 37 pancreaticoduodenectomies in his career of which 30 were for periampullary carcinoma and 7 for chronic pancreatitis. His contributions not only advanced the management of pancreatic malignancies but also paved the way for significant developments in the field of Surgery as a whole.
It used to be said in the past that God had placed the pancreas in the back as he did not want anyone to mess with it. These three giants of surgical heritage combined with the efforts of several other surgical pioneers not only had the courage to mess with it but also to leave a legacy that has put the operation within the reach of several aspiring surgeons.
Conflicts of interest
None declared.
References
- 1.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meguid RA, Ahuja N, Chang DC. What constitutes a ‘high-volume’ hospital for pancreatic resection? J Am Coll Surg. 2008;206:622 e1–622 e9. doi: 10.1016/j.jamcollsurg.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Desjardins A. Technique de la Pancréatectomie. Rev chir. 1907;35:945–973. [Google Scholar]
- 4.Sauve L. Des pancréatectomies et spécialement de la pancréatectomie céphalique. Rev chir. 1908;37:113–152. and 335–85. [Google Scholar]
- 5.Dragstedt LR, Dragstedt C, McClintock JT, Chase CS. Extirpation of the duodenum. Am J Physiolo. 1918;46:584–590. [Google Scholar]
- 6.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935;102:763–779. doi: 10.1097/00000658-193510000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffey RCXV., II Pancreato-enterostomy and pancreatectomy: a preliminary report. Ann Surg. 1909;50:1238–1264. doi: 10.1097/00000658-190912000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehr H. Die gut- und bösartigen Neubildungen der Gallenblase und der Gallengänge unter besonderer Berücksichtigung eigener Erfahrungen (Das Karzinom der Papilla Vateri) Ergebn d Chir u Orth. 1914;8:471–624. [Google Scholar]
- 9.Hunt VC. Surgical Management of Carcinoma of the Ampulla of Vater and of the Periampullary Portion of the Duodenum. Ann Surg. 1941;114:570–602. doi: 10.1097/00000658-194110000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owens FM. The problem of peptic ulcer following pancreatectomy. Ann Surg. 1948;128:15–20. doi: 10.1097/00000658-194807000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whipple AO. Observations on radical surgery for lesions of the pancreas. Surg Gynecol Obstet. 1946;82:623–631. [PubMed] [Google Scholar]
- 12.Schnelldorfer T, Adams DB, Warshaw AL, Lillemoe KD, Sarr MG. Forgotten pioneers of pancreatic surgery: beyond the favorite few. Ann Surg. 2008;247:191–202. doi: 10.1097/SLA.0b013e3181559a97. [DOI] [PubMed] [Google Scholar]
- 13.Halsted WS. Contributions to the surgery of the bile passages, especially of the common bile-duct. Boston Med Surg J. 1899;141:645–654. [Google Scholar]
- 14.Mayo-Robson AW. La chirurgie du pancréas. XIIIe Congrés International de Médecine. 1900;10:149–150. [Google Scholar]
- 15.Koerte W. Arch f Klin Chir. 1900;89:1–54. [Google Scholar]
- 16.Kausch W. Das Carcinom der Papilla duodeni und seine radikale Entfernung. Beitrage zur Klinische Chirurgie. 1912;78:439–486. [Google Scholar]
- 17.Hirschel G. Die Resektion des Duodenums mit der Papille wegen Karzinoms. Munchen Med Wochenschr. 1914;61:1728–1729. [Google Scholar]
- 18.Tenani O. Contributo alla chirurgia della papilla del Vater. Policlinico. 1922;29:291–300. [Google Scholar]
- 19.Nemenyi G. Zur operationstechnik des Papillen karzinoms. Zentralbl f Chir. 1937;64:1337–1339. [Google Scholar]
- 20.Brunschwig A. Resection of head of pancreas and duodenum for carcinoma-pancreatoduodenectomy. Surg Gynecol Obstet. 1937;65:681–685. doi: 10.3322/canjclin.24.6.363. [DOI] [PubMed] [Google Scholar]
- 21.Trimble IR, Parsons JW, Sherman CP. A one-stage operation for the cure of carcinoma of the ampulla of Vater and of the head of the pancreas. Surg Gynecol Obstet. 1941;73:711–722. [Google Scholar]
- 22.Whipple AO. A reminiscence: pancreaticduodenectomy. Rev Surg. 1963;20:221–225. [PubMed] [Google Scholar]
- 23.Cameron JL. William Stewart Halsted. Our surgical heritage. Ann Surg. 1997;225:445–458. doi: 10.1097/00000658-199705000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutkow IM. William Halsted and Theodor Kocher: ‘an exquisite friendship’. Ann Surg. 1978;188:630–637. doi: 10.1097/00000658-197811000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFadden DW, Nussbaum MS, Fischer JE. Mont R. Reid, M.D. (1889–1943). A centennial tribute. Ann Surg. 1990;211:91–96. doi: 10.1097/00000658-199001000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Specht G, Stinshoff K. [Walther Kausch (1867–1928) and his significance in pancreatic surgery] Zentralbl Chir. 2001;126:479–481. doi: 10.1055/s-2001-14772. [DOI] [PubMed] [Google Scholar]
- 27.Allen Oldfather Whipple JS. A distinguished surgeon and historian. Dig Surg. 2003;20:154–162. [Google Scholar]
- 28.Fuhrman GM. The legacy of Allen Oldfather Whipple. Curr Surg. 2005;62:275–276. doi: 10.1016/j.cursur.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Howard JM. Development and progress in resective surgery for pancreatic cancer. World J Surg. 1999;23:901–906. doi: 10.1007/s002689900597. [DOI] [PubMed] [Google Scholar]
- 30.Bassin A, O'Leary JP. The first report of a successful pancreaticoduodenectomy. Am Surg. 1995;61:845–846. [PubMed] [Google Scholar]
- 31.Peters JH, Carey LC. Historical review of pancreaticoduodenectomy. Am J Surg. 1991;161:219–225. doi: 10.1016/0002-9610(91)91134-5. [DOI] [PubMed] [Google Scholar]


