Abstract
Background
Increased visceral fat and pancreatic steatosis promote lymphatic metastases and decreased survival in patients with pancreatic adenocarcinoma after pancreatoduodenectomy (PD).
Objectives
We aim to determine the utility of preoperative computed tomography (CT) measurements of pancreatic steatosis and visceral fat as prognostic indicators in patients with pancreatic adenocarcinoma.
Methods
High-resolution CT scans of 42 patients undergoing PD for pancreatic adenocarcinoma were reviewed. Attenuation in CT of the pancreas, liver and spleen were measured in Hounsfield units and scored by two blinded investigators. Perirenal adipose tissue was measured in mm.
Results
Lymphatic metastases were present in 57% of patients. Age, gender, tumour size and margin status were similar in patients with and without nodal metastases. Node-positive patients had increased visceral but not subcutaneous fat pads compared with node-negative patients and decreased CT attenuation of the pancreatic body and tail and liver. Node-positive patients stratified by visceral adiposity (≥10 mm vs. <10 mm) demonstrated poorer survival (7 ± 1 months vs. 16 ± 2 months; P < 0.01).
Conclusions
In resected pancreatic adenocarcinoma, increased pancreatic steatosis and increased visceral fat stores are associated with lymphatic metastases. Furthermore, increased visceral fat is associated with abbreviated survival in patients with lymphatic metastases. Hence, increased visceral fat may be a causative factor of abbreviated survival and serves a prognostic role in patients with pancreatic malignancies.
Keywords: Pancreatic adenocarcinoma, steatosis, visceral fat
Introduction
Nearly 1.1 billion people worldwide and over a third of the American population are estimated to be overweight.1,2 Further, obesity has been associated with increased risk for the development of multiple cancers.3,4 In 2003, the International Agency for Research on Cancer identified a causal link between obesity and increased risk for colon, breast, endometrial, renal cell, oesophageal, liver and pancreatic cancer.3,4 More recently, evidence supporting this epidemiological relationship has been strengthened with the identification of an altered adipokine milieu in obese patients. For example, levels of serum leptin (a pro-angiogenic, pro-tumorigenic adipokine) are elevated in obesity, whereas serum levels of adiponectin (an anti-tumorigenic adipokine) are decreased.5–9 Furthermore, adipokine receptors have been demonstrated on numerous cancer cells, including in pancreatic adenocarcinoma, which lends mechanistic support to the association of obesity with increased risk for cancer.
Pancreatic cancer has become the fourth leading cause of cancer-related death in the USA and is associated with the shortest stage-for-stage median survival time of all cancer types.10,11 Higher body mass index (BMI) has been shown to increase the relative risk for development of pancreatic cancer.10–14 Importantly, patterns of fat distribution resulting in central adiposity have been shown to represent an independent risk factor in the development of pancreatic cancer.15 Moreover, recent data have shown histologically increased intrapancreatic fat correlates with lymphatic spread and decreased survival in patients undergoing pancreatoduodenectomy (PD) for pancreatic cancer.16 However, a correlation between preoperative adipose measurements assessed by computed tomography (CT) and patient outcomes remains ill defined. Therefore, this study was undertaken to evaluate whether visceral fat and pancreatic steatosis measured by preoperative CT are predictors of nodal metastases and survival after PD for pancreatic adenocarcinoma. Our hypothesis in undertaking this study was that increased visceral fat and pancreatic steatosis would promote nodal metastases and reduced survival after PD for pancreatic adenocarcinoma.
Materials and methods
Approval to conduct this study was obtained from the University of South Florida Independent Research Board. Forty-two patients in whom high-quality, triple-phase CT imaging studies had been taken between January 2002 and December 2008 were identified from our prospectively maintained database. Sample size was limited as a large number of referred patients bring with them CT images obtained at outside diagnostic centres and these cannot be uploaded to obtain the CT measurements described in the methods below. All patients had tumours in the head of the pancreas and underwent pylorus-sparing PD with curative intent. Operative time was measured from the induction of general anaesthesia until discharge from the operating room. Clinicopathological data were extracted and correlated with CT findings. For the purposes of comparison, patients were grouped according to nodal status (N1 vs. N0; American Joint Committee on Cancer [AJCC] criteria, 6th edition).17 Hospital length of stay (LoS) included preoperative hospital days, which were often numerous, reflecting transfers into our hospital. Pancreatic fistulas were categorized according to the International Study Group of Pancreatic Fistula (ISPGF) classification.18
To the best of our knowledge, all patients reported in this study received adjuvant therapy and completed a course of gemcitabine-based chemotherapy. Our tertiary referral centre treats patients from across the state of Florida and a large majority of patients ultimately receive adjuvant chemotherapy administered by their local medical oncologist for convenience.
CT analysis
All CT imaging was evaluated in the non-contrast phase by two blinded radiologists. Fatty infiltration (i.e. steatosis) of the pancreas, liver and spleen was assessed by attenuation, which was measured in Hounsfield units (HU). Attenuation in CT was individually measured in the head, body and tail of the pancreas; CT attenuation in the liver was averaged over three measurements taken in the right, left and caudate lobes of the liver, respectively. Subcutaneous fat thickness was recorded in mm as the distance between the iliac plate and skin at the level of the posterior superior iliac spine. As an indicator of visceral obesity, the perirenal fat pad was measured in mm as the vertical distance between the left posterior renal capsule and the junction of the abdominal wall and paraspinal musculature at the level of the left renal vein (Fig. 1).
Figure 1.
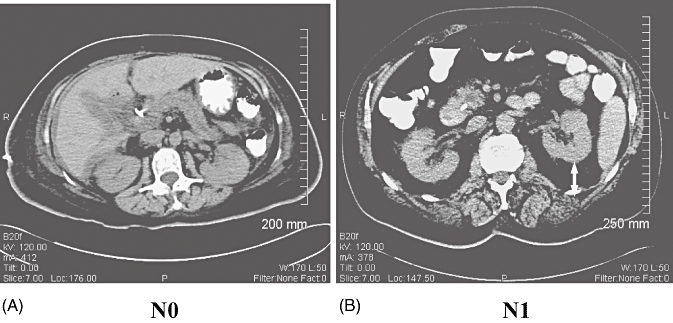
Typical computed tomography scans in (A) a node-negative (N0) and (B) a node-positive (N1) patient. The N0 patient has essentially no visceral fat pad, but the N1 patient has an approximately 3-cm fat pad
Statistical analysis
Statistical analyses were performed using SigmaStat 3.5 (Jandel Corp., San Jose, CA, USA). Data are expressed as mean ± standard deviation (SD). Data that were >2 SD from the mean were determined for each subgroup analysis and excluded from that statistical analysis. Data were analysed using Student's t-test and Fisher's exact test, where appropriate. Survival analyses were performed using Kaplan–Meyer methods. Significance was accepted with 95% confidence.
Results
Patient demographic data are depicted in Table 1, stratified by nodal status. Average patient age was 46 years ± 11, Twenty three patients were men. All patients were overweight (i.e. BMI > 25 kg/m2). Data from the pathology evaluation are depicted in Tables 2 and 3 stratified by nodal status. No in-hospital mortality was noted in the study cohort. Operative time (320 ± 60 min and 382 ± 70 min), estimated blood loss (EBL) (560 ± 120 cc and 620 ± 130 cc) and hospital LoS (15 ± 3 days and 18 ± 2 days) were similar for patients without and with lymph node metastases, respectively. Additionally, no differences were found between patients with perirenal fat pads measuring ≥10 mm and those with perirenal fat pads of <10 mm in operative time (340 ± 60 min and 360 ± 80 min, respectively), EBL (570 ± 110 cc and 630 ± 140 cc, respectively) and hospital LoS (18 ± 4 days and 16 ± 5 days, respectively). Fistula rates (33% and 36%) were similar in patients without and with lymph node metastases, respectively. All six of the fistulas in patients with node-negative disease were Grade A. Seven of the eight fistulas in node-positive patients were Grade A and one was Grade B. Overall survival in patients without and with lymph node metastases was 21 ± 10 months and 11 ± 7 months, respectively (P < 0.01).
Table 1.
Patient demographic data
| N0 | N1 | |
|---|---|---|
| Patients, n | 18 | 24 |
| Age, years | 67 ± 11 | 66 ± 10 |
| Male gender | 61% | 50% |
| Metabolic syndrome | ||
| Body mass index, kg/m2 | 27 ± 4 | 28 ± 6 |
| Hypertension | 44% | 46% |
| Hyperlipidaemia | 33% | 33% |
| Diabetes | 39% | 21% |
N0, node-negative; N1, node-positive
Table 2.
Histopathological parameters
| N0 | N1 | |
|---|---|---|
| Tumour size, cm | 3.4 ± 2.34 | 3.6 ± 1.12 |
| Perineural invasion | 67% | 71% |
| Angiovascular invasion | 27% | 29% |
| Poor differentiation | 12% | 52%a |
| Peripancreatic fat invasion | 53% | 90%a |
| R0 resection | 100% | 69% |
P < 0.01 vs. N0
N0, node-negative; N1, node-positive
Table 3.
Comparison of adiposity and survival in patients without and with lymph node metastases stratified by visceral fat
| Lymphatic invasion | Subcutaneous fat pad, mm, mean ± SD | Perirenal fat pad, mm, mean ± SD | Pancreatic head, HU, mean ± SD | Pancreatic body, HU, mean ± SD | Pancreatic tail, HU, mean ± SD | Liver, HU, mean ± SD | Spleen, HU, mean ± SD | Survival, months, mean ± SD |
|---|---|---|---|---|---|---|---|---|
| N0 | 18 ± 9 | 13 ± 7 | 33 ± 15 | 35 ± 15 | 34 ± 15 | 58 ± 14 | 48 ± 15 | 21 ± 10 |
| N1 | 21 ± 13 | 18 ± 5a | 28 ± 10 | 23 ± 9a | 21 ± 8a | 50 ± 8a | 44 ± 7 | 11 ± 7a |
| N1 + fat pad < 10 mm | 20 ± 11 | 9 ± 4 | 26 ± 7 | 27 ± 7 | 27 ± 8 | 53 ± 7 | 48 ± 7 | 16 ± 6 |
| N1 + fat pad ≥ 10 mm | 20 ± 13 | 21 ± 5b | 25 ± 8 | 25 ± 12 | 20 ± 12 | 48 ± 8 | 46 ± 5 | 7 ± 3b |
P < 0.01 vs. N0
P < 0.01 vs. N1 and fat pad < 10 mm
N0, node-negative; N1, node-positive
Data from CT analyses are depicted in Table 3. Attenuation in CT of the pancreatic body (35 ± 15 HU and 23 ± 9 HU; P < 0.01) and pancreatic tail (34 ± 15 HU and 21 ± 8 HU; P < 0.01) were significantly different in patients without and with lymph node metastases, respectively. By contrast, CT attenuation of the pancreatic head (33 ± 15 HU and 28 ± 10 HU) and spleen (48 ± 15 HU and 44 ± 7 HU) did not significantly differ between patients without and with lymph node metastases, respectively. Attenuation in CT of the liver (50 ± 8 HU and 58 ± 14 HU; P < 0.01) differed significantly between patients with and without lymph node metastases, respectively. Attenuation in CT of the pancreas (mean HU) and liver are depicted in Figure 2, stratified by nodal metastases. Subcutaneous fat pads were similar (21 ± 13 mm and 18 ± 9 mm; P = not significant [NS]) in patients with and without lymph node metastases, respectively, although visceral obesity (i.e. perirenal fat) was significantly increased in patients with lymph node metastases compared with patients without lymph node metastases (18 ± 5 mm and 13 ± 7 mm, respectively; P < 0.01) (Fig. 3). Additionally, the percentage of patients with peripancreatic fat invasion was significantly greater in the group with lymph node metastases than in that without (90% and 53%, respectively; P < 0.01) (Fig. 3). Patients with known lymph node metastases were stratified subsequently according to perirenal fat measurements (<10 mm vs. ≥10 mm). Twelve patients had <10 mm of perirenal fat (9 ± 4 mm) and 12 patients had ≥10 mm of perirenal fat (21 ± 5 mm).
Figure 2.
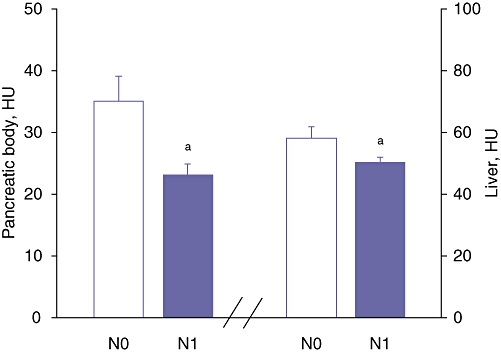
Computed tomography attenuation in Hounsfield units (HU) of the pancreatic body and liver. N0, node-negative patients; N1, node-positive patients; aP < 0.01 vs. N0
Figure 3.
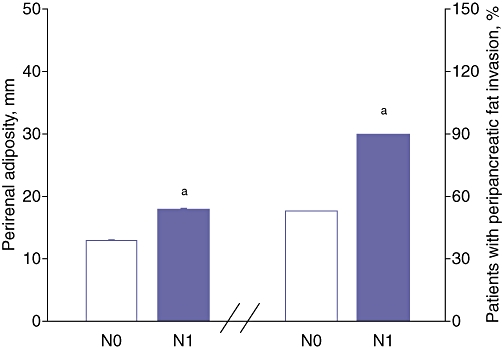
Perirenal adiposity in mm and percentage of patients with peripancreatic fat invasion. N0, node-negative patients; N1, node-positive patients; aP < 0.01 vs. N0
Overall survival was significantly shorter in patients with lymph node metastases and perirenal fat ≥10 mm (7 ± 3 months) compared with patients with lymph node metastases and perirenal fat of < 10 mm (16 ± 6 months) (P < 0.01) despite similar pancreatic CT attenuation measurements (Figs 4, 5, Table 3). No differences in survival were noted between patients with perirenal fat of ≥10 mm (n = 30; 12 ± 14 months) and those with perirenal fat of <10 mm (n = 12; 16 ± 12 months) (P = 0.3). Similarly, no differences were noted in the percentage of patients with lymph node metastases among patients with perirenal fat of ≥10 mm and those with perirenal fat of <10 mm (50% and 58%, respectively; P = 0.37).
Figure 4.
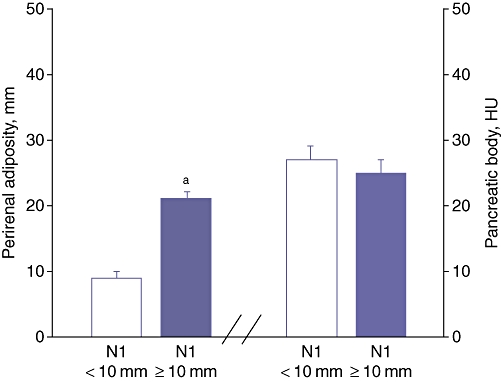
Node-positive patients stratified by perirenal adiposity in mm and computed tomography attenuation of the pancreatic body in Hounsfield units (HU). N0, node-negative patients; N1, node-positive patients; aP < 0.01 vs. N1 + fat pad of <10 mm
Figure 5.
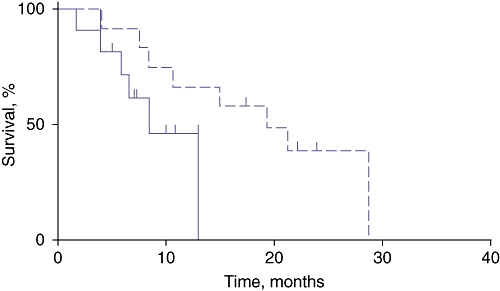
Survival rates in node-positive patients with perirenal adiposity of ≥10 mm (solid line) and <10 mm (broken line)
No differences in survival were noted between patients with lymph node metastases and pancreatic body CT attenuation of ≥30 HU (n = 9; 15 ± 6 months) and patients with lymph node metastases and pancreatic body CT attenuation of <30 HU (n = 15; 10 ± 7 months) (P = 0.08). No differences in survival were noted between patients with pancreatic body CT attenuation of ≥30 HU (n = 19; 18 ± 11 months) and patients with pancreatic body CT attenuation of <30 HU (n = 23; 16 ± 15 months) (P = 0.7). However, a statistically greater percentage of patients with a fatty pancreas (<30 HU vs. ≥30 HU) had positive lymph nodes (70% vs. 42%; P < 0.002).
The average BMI of all patients was 27 ± 6 kg/m2. No statistically significant correlation was noted between BMI and visceral fat (R = 0.375, P = 0.4), BMI and subcutaneous fat (R = 0.53, P = 0.08) or perirenal fat and subcutaneous fat (R = 0.18, P = 0.7).
Discussion
Outcomes for patients with pancreatic adenocarcinoma are extremely poor for a myriad of reasons, including difficulties in early detection, aggressive tumour biology with a propensity for early systemic metastases, and the unavailability of efficacious chemotherapeutic agents. Recently, the negative impact of obesity on cancer-specific outcomes has been elucidated in pancreatic adenocarcinoma, as well as in other malignancies, although accurate preoperative identification of patients with clinically significant changes in adiposity remains challenging. Computed tomography has been recently validated as a measure of evaluating solid organ and intra-abdominal fat.19,20 Therefore, we chose to use preoperative CT to measure visceral steatosis. We have demonstrated a correlation between CT attenuation of the pancreas and liver, as well as increased perirenal fat, and the development of lymph node metastases. More importantly, we have documented that organ steatosis as measured by CT attenuation portends poor survival and that increased perirenal fat is an independent predictor of abbreviated survival in patients with lymph node metastases, which suggests that the negative physiological impacts of visceral adiposity can be inferred from routine radiographic evaluations.
Our study population is representative of patients with pancreatic adenocarcinoma undergoing PD both at our institution and around the country.21,22 The patients were generally older than middle age and more than half were men. All patients in the study population were overweight. Similarly, survival data for the study population concur with survival data reported in other large-institution studies.20,21 Unfortunately, our sample size was limited by the availability of high-quality, triple-phase CT in our radiological database. Our small sample size has made our statistics vulnerable to type II error and this may account for the lack of statistical significance with respect to increased pancreatic steatosis and survival.
Pancreatoduodenectomy represents the only chance for longterm cure in pancreatic cancer. However, pancreatic cancer continues to remain a lethal disease with a mean 5-year survival of <5% because most patients are not candidates for surgery.14,22 Recent epidemiological evidence has implicated obesity in the development of and mortality from pancreatic cancer.13,14 In one large-cohort study, Stolzenberg-Solomon et al. showed that people with a BMI > 35 have a 45% greater risk for developing pancreatic cancer.13 This association has been further strengthened by a recent meta-analysis which demonstrated that the relative risk for pancreatic cancer increases by 1.12 for every 5-point increase in BMI.14 Moreover, Calle et al. have shown that mortality from pancreatic cancer rises with increasing BMI.10
Fatty infiltration of the pancreas secondary to obesity was first described by Ogilve in 1933.23 Multiple radiological studies have validated this correlation between obesity and pancreatic fat.24 However, pancreatic steatosis is no longer believed to represent a benign fallout of the obesity epidemic. Accumulating evidence has now implicated pancreatic steatosis in the development of pancreatic pathology and morbidity from PD.16,25 This paradigm shift is supported by this report and by a recent study demonstrating that increased pancreatic steatosis correlates with increased lymphatic invasion, positive lymph nodes, and decreased survival after PD.16 Our study provides further evidence of the deleterious oncological outcomes of visceral steatosis by providing radiological evidence that a statistically significant decrease in HU in the pancreatic body, which corresponds to increased fatty infiltration, is associated with a greater frequency of positive lymph nodes. Moreover, the corollary is also true: patients with node-positive disease have a more fatty pancreas. Interestingly, CT HU in the pancreatic head did not differ between patients with node-positive and node-negative disease. Differential distribution of fat in the pancreas has been described anecdotally by radiological imaging and may explain this finding. Another potential explanation may lie in the fact that all the pancreatic cancers in this report were located in the head of the pancreas and it is well known that pancreatic cancer results in an intense desmoplastic response in the surrounding tissue, which alters the texture of the peritumoral gland.
The molecular mechanisms underlying the adverse impact of pancreatic steatosis are likely to derive from an alteration of the tumour microenvironment. Tumour–stroma interaction has been established as playing a critical role in tumour proliferation and metastases. Peritumoral inflammatory cell invasion and chemokine expression patterns orchestrate these phenomena.26,27 Tissue-associated macrophages and the subsequent production of tumour necrosis factor (TNF), and interleukin-1 (IL-1) and IL-6 have been shown to stimulate angiogenic factors including vascular endothelial growth factor (VEGF).26,27 Additionally, macrophage-proinflammatory chemokine-3α (CCL) is overexpressed in peritumoral tissue in pancreatic cancer and stimulates the growth of neoplastic cells in vitro.26,27 Animal data have shown that obese mice have increased pancreatic fat content and increased baseline levels of proinflammatory cytokines, including TNF and IL-6.28 Moreover, TNF and CCL induce the production of proteases and tumour-adhesion molecules that induce the invasion and migration of tumour cells, thus promoting tumour metastases.26 Our study supports this concept by showing that elevated pancreatic steatosis is associated with lymph nodal metastases.
Body mass index has historically been used as a surrogate method for measuring an individual's degree of obesity. Patients with pancreatic cancer and a BMI > 35 have been shown to have a 12-fold increase in risk for nodal metastases and a two-fold increase in risk for recurrence.29 However, BMI does not differentiate among differing body fat distributions. Relative to subcutaneous fat, visceral fat has been established as the metabolically active component of fat-producing adipocytokines. Central adiposity modulates the deranged production of cytokines, which results in a systemic low-grade pro-tumorigenic, inflammatory state. Thus, BMI represents, at best, a poor index for the deleterious effects of obesity. We did not find any differences in BMI between patients with and without nodal metastases. To further elucidate the impact of the type of obesity, we measured both the metabolically active component (visceral fat) and the inactive component (subcutaneous fat). Visceral fat pad was increased by 38% in patients with nodal metastases. By stark contrast, no difference was noted in the subcutaneous fat pad in patients with and without nodal metastases. Importantly, we did not find any statistically significant correlation between BMI and visceral fat, or visceral fat and subcutaneous fat. These findings underscore the importance of distinguishing the metabolically active component of fat to provide greater sensitivity and relevance when examining the effects of obesity on pancreatic pathology.
Multiple factors are known to influence prognosis following PD, including age, tumour size, differentiation, resection margins and lymph node status.30–32 We found no differences in our study between node-positive and node-negative patients with respect to age, tumour size and resection margins. The percentage of patients with poor tumour differentiation was greater in node-positive disease. This may be secondary to the pro-tumorigenic environment provided by visceral and pancreatic steatosis. Importantly, a recent multivariate analysis identified lymph node status as the only independent prognostic factor for longterm survival.32 Therefore, we further evaluated the survival of node-positive patients after stratifying for a visceral fat pad of ≥10 mm. Doubling the visceral fat in node-positive patients resulted in a dramatic 50% decrease in median survival. Interestingly, stratifying the node-positive patients by pancreatic steatosis (pancreatic body ≥30 HU vs. < 30 HU) did not yield a statistically significant difference in survival, although there was a trend towards decreased survival in patients with increased pancreatic steatosis. This may represent a type II statistical error secondary to a small sample size.
Intuitively, we would expect increased BMI and visceral adiposity to result in a technically more difficult operation. However, Fleming et al. have shown that surrogate measures of surgical complexity such as EBL and operative time are not affected by increased BMI.29 Our findings concur with these results in that no differences in EBL or operative time were found in node-positive patients with increased visceral adiposity. Moreover, there were no differences in LoS and in-hospital mortality. This suggests that visceral adiposity is not a deterrent to safe PD. However, studies with larger sample sizes are needed to specifically address this issue.
Since Kausch33 performed the first pancreatosuodenectomy, mortality arising from the procedure has decreased to <5% at most high-volume centres. However, morbidity continues to range from 40% to 50%.30,31 Pancreatic fistula represents the most important cause of morbidity. The risk factors identified for fistula formation include a soft gland, small pancreatic duct, underlying pathology, local blood flow and surgeon experience.34,35 Recent data have shown that increased pancreatic steatosis is an independent risk factor for fistula formation.36 The reasons postulated are that a fatty gland would account for a technically more difficult anastomosis and that elevated local adipose tissue cytokines may result in a worsening of perianastomotic inflammation after surgical stress, which may enhance fistula formation. In our study, node-positive patients had a more fatty gland; however, although the fistula rate was increased in node-positive vs. node-negative patients (36% vs. 33%), the difference was not statistically significant. This may be because our study was not designed to evaluate fistula occurrence and was underpowered to do so.
In summary, in resected pancreatic adenocarcinoma, increased pancreatic steatosis and increased visceral fat stores are associated with lymphatic metastases. Moreover, increased visceral fat is associated with abbreviated survival in patients with lymphatic metastases. Therefore, we conclude that visceral fat and pancreatic steatosis play a role in disseminating cancer to lymph nodes (and beyond) and that CT measurements of visceral fat predict the dissemination and lethality of pancreatic adenocarcinoma.
Conflicts of interest
None declared.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer Working Group on Evaluation of Cancer-Preventive Strategies. Weight Control and Physical Activity. Lyon: IARC Press; 2002. [Google Scholar]
- 4.Joint World Health Organization/Food and Agriculture Organization Expert Consultation. Geneva: WHO/FAO; 2003. Diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series no. 916. [Google Scholar]
- 5.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 6.Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. J Surg Res. 2003;113:50–55. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 7.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Dalamaga M, Migdalis I, Fargnoli JL, Papadavid E, Bloom E, Mitsiades N, et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinaemia and hyperadiponectinaemia: a case–control study. Cancer Causes Control. 2009;20:625–633. doi: 10.1007/s10552-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 11.Michaud DS. Epidemiology of pancreatic cancer. Minerva Chir. 2004;59:99–111. [PubMed] [Google Scholar]
- 12.Silverman DT, Swanson CA, Gridley G, Wacholder S, Greenburg RS, Brown L, et al. Dietary and nutritional factors and pancreatic cancer: a case–control study based on direct interviews. J Natl Cancer Inst. 1998;90:1710–1719. doi: 10.1093/jnci/90.22.1710. [DOI] [PubMed] [Google Scholar]
- 13.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, Schairer C, Michaud DS, Hollenbeck A, et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2007;167:586–597. doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 14.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer. 2007;120:1993–1998. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 15.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun M, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large US cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:459–466. doi: 10.1158/1055-9965.EPI-04-0583. [DOI] [PubMed] [Google Scholar]
- 16.Mathur A, Zyromski NJ, Pitt HA, Al-Azzawi H, Walker JJ, Saxena R, et al. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg. 2009;208:989–994. doi: 10.1016/j.jamcollsurg.2008.12.026. discussion 994–996. [DOI] [PubMed] [Google Scholar]
- 17.Greene F, Page D, Fleming I, Fritz A, Balch C, Haller D, et al. AJCC Cancer Staging Manual. 7th Edition. New York: Springer Publishing Company; 2009. [Google Scholar]
- 18.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Speliotes EK, Massaro JM, Hoffmann U, Foster MC, Sahani DV, Hirschhorn JN, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–899. doi: 10.1111/j.1440-1746.2008.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–278. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 21.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–435. doi: 10.1097/00000658-199305010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogilvie RF. The islands of Langerhans in 19 cases of obesity. J Pathol Bacteriol. 1933;37:473–481. [Google Scholar]
- 24.Kovanlikaya A, Mittelman SD, Ward A, Geffner ME, Dorey F, Gilsanz V. Obesity and fat quantification in lean tissues using three-point Dixon MR imaging. Pediatr Radiol. 2005;35:601–607. doi: 10.1007/s00247-005-1413-y. [DOI] [PubMed] [Google Scholar]
- 25.Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:19–26. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 28.Mathur A, Marine M, Lu D, Swartz-Basile DA, Saxena R, Zyromski NJ, et al. Non-alcoholic fatty pancreas disease. HPB. 2007;9:312–318. doi: 10.1080/13651820701504157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleming JB, Gonzalez RJ, Petzel MQ, Lin E, Morris JS, Gomez H, et al. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg. 2009;144:216–221. doi: 10.1001/archsurg.2008.580. [DOI] [PubMed] [Google Scholar]
- 30.Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Longterm survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Tomassetti Fernandez EM, Luis HD, Malagon AM, Gonzalez IA, Pallares AC. Recurrence of inflammatory pseudotumor in the distal bile duct: lessons learned from a single case and reported cases. World J Gastroenterol. 2006;28;12:3938–3943. doi: 10.3748/wjg.v12.i24.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh TS, Jan YY, Jeng LB, Hwang TL, Wang CS, Chen SC, et al. Pancreaticojejunal anastomotic leak after pancreaticoduodenectomy – multivariate analysis of perioperative risk factors. J Surg Res. 1997;1:119–125. doi: 10.1006/jsre.1996.4974. [DOI] [PubMed] [Google Scholar]
- 35.Lillemoe KD, Cameron JL, Kim MP, Campbell KA, Sauter PK, Coleman JA, et al. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2004;8:766–772. doi: 10.1016/j.gassur.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Mathur A, Pitt HA, Marine M, Saxena R, Max Schmidt C, Howard TJ, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Annals Surgery. 2007;246:1058–1064. doi: 10.1097/SLA.0b013e31814a6906. [DOI] [PubMed] [Google Scholar]


