Abstract
Background
An important issue in the transplantation of livers procured from cardiac death donors (CDDs) concerns why some centres report equivalent outcomes and others report inferior outcomes in transplantations using CDD organs compared with standard criteria donor (SCD) organs. Resolving this discrepancy may increase the number of usable organs.
Objectives
This study aimed to test whether differences in cold ischaemic time (CIT) are critical during CDD organ transplantation and whether such differences might explain the disparate outcomes.
Methods
Results of CDD liver transplants in our own centre were compared retrospectively with results in a matched cohort of SCD liver recipients. Endpoints of primary non-function (PNF) and ischaemic cholangiopathy (IC) were used because these outcomes are clearly associated with CDD organ use.
Results
In 22 CDD organ transplants, CIT was a strong predictor of PNF or IC (P = 0.021). Minimising CIT in CDD organ transplants produced outcomes similar to those in a matched SCD organ transplant cohort at our centre and in SCD organ transplant results nationally (1- and 3-year graft and patient survival rates: 90.9% and 73.3% vs. 77.6% and 69.2% in CDD and SCD grafts, respectively. A review of the published literature demonstrated that centres with higher CITs tend to have higher rates of PNF or IC (correlation coefficient: 0.41).
Conclusions
These findings suggest that a targeted effort to minimise CIT might improve outcomes and allow the safer use of CDD organs.
Keywords: cardiac death donors, liver, transplantation, cold ischaemia, cholangiopathy
Introduction
Livers procured from cardiac death donors (CDDs) account for >5% of transplant volume in the USA.1 Although CDDs are an important source of organs, the use of CDD livers is tempered by a number of reports demonstrating worse outcomes and an increased rate of ischaemic cholangiopathy (IC) compared with standard criteria donor (SCD) livers in some centres.2–14 Ischaemic cholangiopathy is a devastating complication that often results in graft loss and significant morbidity and cost; this drives an argument that proposes that CDD liver transplantation should be limited until better outcomes can be achieved.10,13
Nonetheless, reports from other centres demonstrate equivalent outcomes with CDD and SCD livers.15–17 These reports, together with long waiting times and mortality on the liver transplant waiting list provide strong counter-arguments for the continued use of CDD organs. Explaining these disparate results might provide guidance to increase the number of CDD organs used and improve outcomes.
To investigate whether centre-specific patterns of use explain the conflicting reports, the question of whether minimising cold ischaemic time (CIT) can improve outcomes after CDD liver transplantation was addressed. Length of CIT was specifically examined because this is a modifiable parameter that can be adopted universally and is relatively easy to implement. In addition, this pre-study focus minimises potential problems that might arise in a statistical analysis that examines a large number of risk factors for a particular outcome.
The occurrence of primary non-function (PNF) or IC was chosen as the outcome measure that would best reflect an unsatisfactory liver. This outcome, and patient and graft survival, were addressed in three ways. First, CIT was assessed in our programme and correlated with outcomes in transplants using CDD organs. Next, outcomes of transplants in which aggressive attempts were made to limit CIT were compared between CDD grafts and a matched cohort of SCD grafts. Finally, an analysis was undertaken of all the published literature on outcomes after CDD organ transplants for which relevant data were available.
Materials and methods
General considerations
The programme at our institution pursues a policy of CIT minimisation when using CDD livers; two teams are used to accomplish this. When the donor team determines that the liver is useable, the recipient team begins the hepatectomy. Consistent with published guidelines, livers are generally not used if the time between extubation of the donor and flush is >30 min, or the time between profound hypotension (systolic blood pressure [SBP] <50 mmHg) in the donor and flush is >15 min.18,19
Definitions and endpoints
The relevant endpoint was PNF or IC. For a diagnosis of IC, patients needed to have abnormal liver function tests and diffuse intrahepatic strictures on either endoscopic retrograde cholangiography or percutaneous transhepatic cholangiography. This endpoint was chosen to reflect a relevant clinical outcome indicating significant organ damage around the time of procurement. Time to death in CDD livers was defined as minutes from extubation until declaration of death in the donor. Donor total warm ischaemia time was defined as minutes elapsed from extubation to initiation of cold perfusion, which includes phase I (withdrawal) and phase II (circulatory arrest). Cold ischaemia time was defined as hours elapsed from aortic perfusion in the donor to revascularisation of the portal vein in the recipient.
Single-centre retrospective review
A single-centre retrospective review was performed to determine whether CIT correlated with subsequent graft function in 22 patients in whom CDD liver transplants were performed between June 2004 and December 2009 at the Beth Israel Deaconess Medical Center. Approval was obtained from the institutional review board (IRB).
Matched cohort study
Data for a matched group of 22 SCD organ transplant patients were assembled after obtaining approval from the IRB to determine whether CDD organ transplant outcomes were inferior to those in SCD grafts. As recipient cancer is not associated with PNF or IC, laboratory Model for End-stage Liver Disease (MELD) scores were used. The matched group was constructed as follows: each CDD organ recipient was paired with the SCD organ recipient whose surgery was carried out closest in time and whose age was within 5 years (older or younger), MELD score was within 5 points (higher or lower), and graft donor was of similar age. These were chosen as the most relevant parameters consistent with donor risk index and recipient outcome data.20
Literature analysis
To establish the effects of CIT on CDD organ transplant outcomes reported in the world literature, all papers identified by a PubMed search using the terms ‘DCD’[donors after cardiac death], ‘liver’ and ‘transplant’ were reviewed. This search identified nine primary research papers that included interpretable outcome data, including rates of IC and PNF, as well as unambiguous information on CIT.
Donors
The New England Organ Bank (NEOB) maintains a prospectively collected database of all consented cardiac death organ donors. The project was approved by the medical director and the Clinical Policy Board in accordance with the NEOB's research policy. All CDD organ recoveries occurred in patients extubated under controlled conditions after the decision was made to withdraw support (Maastricht Classification III). Withdrawal of support occurred in accordance with individual hospital policy, which governed comfort measures and heparin administration. Data on CDD age, gender, height, weight, co-morbidities, terminal liver function tests, cause of death and biopsy results were collected, as were vital signs in the period between consent and asystole. These included time to death, oxygen saturations, blood pressure and heart rate. After withdrawal of care, patients were monitored by continuous electrocardiogram, blood pressure cuff, finger pulse oximetry and physical examination. Typically, vital parameters were recorded every 1–5 min. In all cases, death was defined as the irreversible cessation of cardiopulmonary activity as evidenced by 5 min of asystole documented on a rhythm strip and absence of spontaneous respirations according to the Uniform Declaration of Death Act (reviewed by Dubois21). Death was pronounced by a doctor not associated with the transplant team. Livers were preserved in University of Wisconsin solution.
Recipients
Information on recipient outcomes was gathered after obtaining permission from the Beth Israel Deaconess Medical Center IRB. All recipient data were collected from prospectively maintained databases and included age, gender, ethnic origin, indication for transplantation, co-morbidities, and post-transplantation variables as outlined above. It was policy to avoid performing transplants in recipients who would be at risk for a difficult or long hepatectomy (e.g. patients with prior surgery or portal vein thrombosis) or long organ travel time (e.g. with out-of-region CDD organs) in order to minimise ischaemia times when the allocation match run permitted. All patients received essentially the same three-drug immunosuppression using tacrolimus or cyclosporine, mycophenolate mofetil and steroids. A minority were converted to rapamycin from prograf at least 1 month after transplantation.
Statistical methods
Continuous variables are presented as mean ± standard deviation; categorical values are presented as percentages. Comparisons between categorical variables were made with the chi-squared test. Comparisons between continuous variables were made with the two-tailed Student's t-test. P-values of <0.05 were considered to indicate statistical significance. The correlation coefficient was calculated using Pearson's formula.
Results
CDD and recipient characteristics and outcomes
Donor and recipient characteristics are summarised in Table 1. Donors tended to be young, with an average age of 35 years, mostly male (64%) and non-obese. Common causes of death included head trauma and stroke. Cold times were generally short (<6 h) and donors expired quickly after extubation. Times between extubation or profound haemodynamic instability and flush were similarly short, consistent with CDD organ practice at our institution. All CDDs were extubated and death declared after 5 min of cardiac standstill by a doctor not associated with the recovery team. Hence, all donors were extubated. All developed profound hypotension (SBP < 50 mmHg) prior to asystolic arrest.
Table 1.
Characteristics of transplants using livers from cardiac death donors
| Donors (n=22) | |
|---|---|
| Age, years, mean ± SD | 35.3 ± 12.4 |
| Gender, n (%) | |
| Male | 14 (64%) |
| Female | 8 (36%) |
| Body mass index, mean ± SD | 23.4 ± 5.5 |
| Cause of death, n (%) | |
| Head trauma | 6 (27%) |
| Stroke | 8 (36%) |
| Drug overdose | 2 (9%) |
| Cold time, h, mean ± SD | 5.9 ± 2.0 |
| Time from asystole to flush, min, mean ± SD | 8.0 ± 2.8 |
| Time from SBP < 50 mmHg to flush, min, mean ± SD | 13.9 ± 6.7 |
| Time from extubation to flush, min, mean ± SD | 24.0 ± 7.1 |
| Recipients (n=22) | |
|---|---|
| Age, years, mean ± SD | 54.4 ± 7.5 |
| Gender, n (%) | |
| Male | 16 (72%) |
| Female | 6 (27%) |
| Cause of disease, n (%) | |
| Idiopathic | 1 (5%) |
| Hepatitis C virus | 15 (68%) |
| Alcohol-related | 5 (23%) |
| Hepatoma | 12 (55%) |
| Laboratory MELD score, mean ± SD | 18.1 ± 7.6 |
SD, standard deviation; SBP, systolic blood pressure; MELD, Model for End-stage Liver Disease
Recipients had an average age of 54 years and most were men (72%). In 55%, hepatocellular carcinoma (HCC) was the primary diagnosis; other common diagnoses included hepatitis C and alcohol abuse. Laboratory MELD scores averaged 18.1, reflecting the high percentage of patients with exception points for HCC.
Average follow-up was 35 months. Patient and graft survival was 90.9% at 1 year and 73.3% at 3 years.
Parameters that affect outcomes after CDD organ transplantation
To determine whether CIT was a risk factor for the development of IC or PNF, a composite endpoint incorporating both outcomes was chosen. Results are provided in Table 2. This composite outcome was reported in five of 22 patients and included three cases of IC and two of PNF. Of the three patients with IC, one is alive and on the transplant waiting list 5 years after transplant and the other two died at 13 months and 3 years after transplant. Body mass index (BMI) was similar in patients who reached the composite endpoint and those who did not. Donor age and MELD score were higher in the poor outcome group, but these differences did not reach statistical significance. By contrast, the poor outcome group had longer CIT (P = 0.021), longer time from extubation to flush (P = 0.027) and longer time of profound hypotension (P = 0.043) compared with the good outcome group.
Table 2.
Comparison of groups with and without primary non-function (PNF) or ischaemic cholangiopathy (IC)
| Endpoint | Patients, n | Age, years, mean±SD | Donor BMI, mean±SD | Cold time, h, mean±SD | Extubation to flush, min, mean±SD | SBP < 50 mmHg to flush, min, mean±SD | MELD score, mean±SD |
|---|---|---|---|---|---|---|---|
| PNF/IC (+) | 5 | 43 ± 4.1 | 23.4 ± 4.5 | 7.72 ± 2.6 | 30.2 ± 9.0 | 18.8 ± 8.2 | 22.6 ± 5.7 |
| PNF/IC (−) | 17 | 32.6 ± 13.0 | 23.3 ± 2.4 | 5.4 ± 2.9 | 22.1 ± 5.1 | 12.4 ± 8.5 | 17.0 ± 7.4 |
| P-value | 0.112 | NS | 0.021 | 0.027 | 0.043 | 0.12 | |
SD, standard deviation; BMI, body mass index; SBP, systolic blood pressure; MELD, Model for End-stage Liver Disease; NS, not significant
Outcomes in a matched cohort of SCD liver recipients
To further evaluate outcomes in CDD liver transplants, a matched cohort of non-CDD organ recipients was created (Table 3). Donor factors compared between groups included age, BMI, gender and trauma as the cause of death. There were no significant differences between the groups in these parameters except that trauma was a more common cause of death in CDDs (32% vs. 9%). Recipient age and disease processes were also similar between the groups. There were no statistically significant differences between the groups in 1- or 3-year patient or graft survival rates. To determine whether CDD organ use affected hospital costs, we examined average length of stay (LoS) and number of readmissions in the first year. Recipients of CDD organs had a shorter average stay than SCD organ recipients (13.3 days vs. 19.0 days), although this did not reach statistical significance (P = 0.14). Numbers of readmissions did not vary between CDD and SCD organ recipients.
Table 3.
Donor and recipient characteristics and outcomes in matched cohorts of patients transplanted with cardiac death donor (CDD) and standard criteria donor (SCD) organs, respectively
| CDD organs | SCD organs | P-value | |
|---|---|---|---|
| Transplant cases, n | 22 | 22 | |
| Donor characteristics | |||
| Donor age, years, mean ± SD | 35.3 ± 7.7 | 38.9 ± 12.4 | NS |
| Trauma as cause of death, % | 32% | 9% | |
| Body mass index, mean ± SD | 23.6 ± 5.4 | 24.7 ± 5.1 | NS |
| Donor male gender, % | 55% | 59% | |
| Recipient characteristics | |||
| Age, years, mean ± SD | 56.2 ± 7.5 | 56.4 ± 6.1 | NS |
| Disease process, n (%) | |||
| Alcohol-related | 5 (23%) | 6 (27%) | |
| Hepatitis C virus | 11 (50%) | 14 (64%) | |
| Malignancy | 2 (9%) | 1 (5%) | |
| Unknown/other | 4 (18%) | 1 (5%) | |
| MELD score, mean ± SD | 18.1 ± 7.7 | 17.8 ± 6.9 | NS |
| Outcomes | |||
| PNF or IC, n (%) | 5 (23%) | 3 (14%) | NS |
| 1-year survival, | 90.9% | 77.6% | 0.24 |
| 3-year survival | 73.3% | 69.2% | NS |
| Length of stay, days, mean ± SD | 13.3 ± 9.5 | 19 ± 14.5 | 0.12 |
| Readmissions in the first year, mean | 3.5 | 3.5 | NS |
SD, standard deviation; MELD, Model for End-stage Liver Disease; PNF, primary non-function; IC, ischaemic cholangiopathy
Summary of CIT and outcomes across the literature
To evaluate the entire international experience in CDD liver transplantation, the nine publications reporting outcomes and cold time data in such transplants were examined.12,13,15,16,22–25 These are presented in Table 4. Sample sizes ranged from 13 to 141 patients. The relevant endpoint of PNF or IC occurred in 0–55% of patients. Cold ischaemia time varied between 295 min and 657 min.
Table 4.
Data reported in the published literature
| Authors | CIT, min | Transplants, n | PNF, n | DBI, n | Composite endpoint |
|---|---|---|---|---|---|
| Dubbeld et al.22 | 456 | 55 | 1 | 13 | 25% |
| Detry et al.23 | 451 | 58 | 2 | 7 | 15% |
| Grewal et al.16 | 378 | 108 | 4 | 9 | 12% |
| Pine et al.14 | 397 | 39 | 2 | 8 | 26% |
| de Vera et al.13 | 657 | 141 | 17 | 24 | 29% |
| Detry et al.15 | 295 | 13 | 0 | 0 | 0% |
| Chan et al.12 | 437 | 52 | 0 | 7 | 14% |
| Maheshwari et al.24 | 522 | 20 | 1 | 10 | 55% |
| Skaro et al.25 | 330 | 32 | 1 | 12 | 41% |
| Present study (BIDMC) data | 385 | 22 | 2 | 3 | 23% |
CIT, cold ischaemic time; PNF, primary non-function; DBI, diffuse biliary ischaemia; BIDMC, Beth Israel Deaconess Medical Center
Outcomes after CDD organ transplants correlate with CIT
To determine if disparate outcomes reported by different centres could be explained by CIT, IC and PNF were plotted against CIT (correlation coefficient: 0.41) (Fig. 1).
Figure 1.
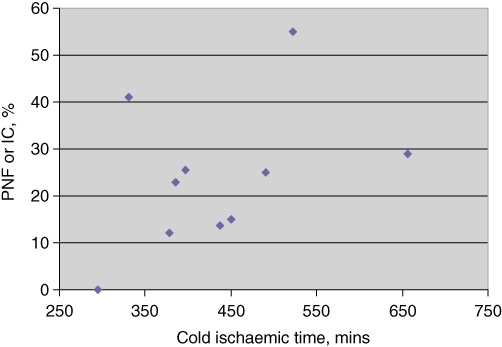
Analysis of the literature comparing primary non-function (PNF) and ischaemic cholangiopathy (IC) with cold time when using cardiac death donor livers for transplantation. There is a correlation between the rate of significant complications and increasing cold time (correlation coefficient: 0.41)
Discussion
Our analysis offers three major findings.
Cold ischaemia time is a risk factor for poor outcomes after CDD liver transplantation in our centre.
Matched cohort analysis demonstrates that outcomes in our centre, which follows an aggressive policy to minimise CIT, are equal between CDD and SCD organ transplantations.
In the published literature, CIT correlates with outcomes.
These findings strongly suggest that increased CIT is a causative factor in poor outcomes after CDD liver transplantation. This is an important result as it suggests that a policy to minimise CIT can improve outcomes.
One-year patient survival after CDD liver transplantation appears better than after SCD organ transplantation, although this difference did not reach statistical significance. This finding is almost certainly the result of donor selection, which we believe is critically important in CDD liver transplantation.
The correlation of CIT with poor outcomes after CDD transplantation is not new. Chan and colleagues found an increased risk for IC when CIT exceeded 9 h, and de Vera et al. found CIT of 8 h to be a significant risk factor.12,13 Additionally, Dubbeld and colleagues found increased CIT correlated with worse outcomes.22 By contrast, other groups15–17,23 have demonstrated similar outcomes in SCD and CDD organ transplantation. The significance of our findings places all of these reports into a larger context that potentially explains these disparate results on the basis of overall CIT at a centre, which is a modifiable factor.
These findings have important clinical relevance. There is considerable belief in the transplant community that CDD livers are inferior to SCD organs. A consensus statement from the American Society of Transplant Surgeons (ASTS) states that CDD outcomes are less favourable20 and a recent editorial stated: ‘Fundamental change is required to re-ignite interest in utilisation of DCD [organs donated after cardiac death] for liver transplantation. Until scientific or technologic advances are available, our current strategies, techniques and policies are insufficient to maximise the donor-pool potential.’11 Furthermore, costs and resource utilisation may be significantly higher in CDD liver transplantions,25,26 although, in our study, LoS and number of readmissions were similar between the CDD and SCD organ transplant groups.
We propose that CDD organ transplant outcomes can be made equal or to at least approach those of SCD organ transplants by minimising CIT in the setting of careful donor and recipient selection. We would further propose that centres put in place procedures to foster this. One strategy would be to use two teams in CDD organ transplants so that the recipient team can commence surgery as soon as the donor team confirms that the liver is useable. Our analysis suggests that predicted but unavoidable delays, such as in a difficult hepatectomy, may represent a relative contraindication for the use of a CDD liver. Other considerations should include the presence of portal vein thrombosis or hepatic arterial problems in recipients. These patients may need more extensive vascular reconstruction. We do not believe these patients should be excluded, but the extra operative time required should be regarded as a factor in the decision for surgery.
This study is limited by a number of factors. Our data are limited by small sample size and a low rate of complications, which admit the possibility of type II statistical error. The matched cohort data are also limited by sample size. Importantly, we note that absolute outcomes in CDD organ transplants at our centre, where 1-year graft survival is >90%, would be considered acceptable regardless of the control group. Ideally, a multivariate analysis is required, but this is not possible with this small sample. Our analysis of the relevant literature is complicated by differences in practices at the various centres, including, but not limited to, the choice of immunosuppressants, donor and recipient characteristics, and surgeon and centre experience with the technique. In addition, the correlation is not particularly strong. In particular, the study by Skaro et al. seems to be an outlier.25 These confounding variables cannot be controlled for in this type of study.
Notwithstanding these limitations, we feel that the results presented, along with the well-known deterioration of organ physiology during cold storage, present a compelling case for the minimising of CIT in CDD organ transplantation. As a randomised trial would not be ethical, this report presents an argument for the implementation of practices intended to keep CIT as short as possible and always <7 h. Future research should focus on investigating these short CIT transplants using a case–control study design to determine whether outcomes in CDD and SCD organ transplants can be made equivalent across centres.
Acknowledgments
We gratefully appreciate the participation of the New England Organ Bank in recording the data for this report. This work was supported in part by the Julie Henry Fund at the Beth Israel Deaconess Medical Center.
Conflicts of interest
None declared.
References
- 1.Berga CL, Steffick DE, Edwards EB, Heimbache JK, Magee JC, Washburn WK, et al. Liver and intestine transplantation in the United States 1998–2007. Am J Transplant. 2009;9(Part 2):907–931. doi: 10.1111/j.1600-6143.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Fructuoso AI, Prats D, Torrente J, Perez-Contin MJ, Fernandez C, Sanchez-Urdazpal L, et al. Ischaemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 3.Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659–1663. doi: 10.1097/01.TP.0000062574.18648.7C. [DOI] [PubMed] [Google Scholar]
- 4.D'Alessandro AM, Fernandez LA, Chin LT, Shames BD, Turgeon NA, Scott DL, et al. Donation after cardiac death: the University of Wisconsin experience. Ann Transplant. 2004;9:68–71. [PubMed] [Google Scholar]
- 5.Manzarbeitia CY, Ortiz JA, Jeon H, Rothstein KD, Martinez O, Araya VR, et al. Longterm outcome of controlled, non-heart-beating donor liver transplantation. Transplantation. 2004;78:211–215. doi: 10.1097/01.tp.0000128327.95311.e3. [DOI] [PubMed] [Google Scholar]
- 6.Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242:724–731. doi: 10.1097/01.sla.0000186178.07110.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mateo R, Cho Y, Singh G, Stapfer M, Donovan J, Kahn J, et al. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2006;6:791–796. doi: 10.1111/j.1600-6143.2006.01243.x. [DOI] [PubMed] [Google Scholar]
- 8.Merion RM, Pelletier SJ, Goodrich N, Englesbe MJ, Delmonico FL. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244:555–562. doi: 10.1097/01.sla.0000239006.33633.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doshi MD, Hunsicker LG. Short- and longterm outcomes with the use of kidneys and livers donated after cardiac death. Am J Transplant. 2007;7:122–129. doi: 10.1111/j.1600-6143.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- 10.Fujita S, Mizuno S, Fujikawa T, Reed AI, Kim RD, Howard RJ, et al. Liver transplantation from donation after cardiac death: a single-centre experience. Transplantation. 2007;84:46–49. doi: 10.1097/01.tp.0000267424.88023.7b. [DOI] [PubMed] [Google Scholar]
- 11.Renz JF. Is DCD for liver transplantation DNR? Am J Transplant. 2008;8:485–488. doi: 10.1111/j.1600-6143.2007.02111.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, et al. Ischaemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604–610. doi: 10.1002/lt.21361. [DOI] [PubMed] [Google Scholar]
- 13.de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, et al. Liver transplantation using donation after cardiac death donors: longterm follow-up from a single centre. Am J Transplant. 2009;9:773–781. doi: 10.1111/j.1600-6143.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 14.Pine JK, Aldouri A, Young AL, Davies MH, Attia M, Toogood GJ, et al. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl. 2009;15:1072–1082. doi: 10.1002/lt.21853. [DOI] [PubMed] [Google Scholar]
- 15.Detry O, Seydel B, Delbouille MH, Monard J, Hans MF, De Roover A, et al. Liver transplant donation after cardiac death: experience at the University of Liege. Transplant Proc. 2009;41:582–584. doi: 10.1016/j.transproceed.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Grewal HP, Willingham DL, Nguyen J, Hewitt WR, Taner BC, Cornell D, et al. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-centre experience. Liver Transpl. 2009;15:1028–1035. doi: 10.1002/lt.21811. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto S, Wilczek HE, Duraj FF, Groth CG, Ericzon BG. Liver transplantation with grafts from controlled donors after cardiac death: a 20-year follow-up at a single centre. Am J Transplant. 2010;10:602–611. doi: 10.1111/j.1600-6143.2009.02965.x. [DOI] [PubMed] [Google Scholar]
- 18.Ho KJ, Owens CD, Johnson SR, Khwaja K, Curry MP, Pavlakis M, et al. Donor post-extubation hypotension and age correlate with outcome after donation after cardiac death transplantation. Transplantation. 2008;85:1588–1594. doi: 10.1097/TP.0b013e318170b6bb. [DOI] [PubMed] [Google Scholar]
- 19.Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D'Alessandro A, et al. ASTS Standards on Organ Transplantation Committee. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004–2011. doi: 10.1111/j.1600-6143.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- 20.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 21.DuBois J. Non-heart beating organ donation: a defence of the required determination of death. J Law Med Ethics. 1999;27:126–136. doi: 10.1111/j.1748-720x.1999.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 22.Dubbeld J, Hoekstra H, Farid W, Ringers J, Porte RJ, Metselaar HJ, et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97:744–753. doi: 10.1002/bjs.7043. [DOI] [PubMed] [Google Scholar]
- 23.Detry O, Donckier V, Lucidi V, Ysebaert D, Chapelle T, Lerut J, et al. Liver transplantation from donation after cardiac death donors: initial Belgian experience 2003–2007. Transpl Int. 2010;23:611–618. doi: 10.1111/j.1432-2277.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 24.Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645–1653. doi: 10.1002/lt.21212. [DOI] [PubMed] [Google Scholar]
- 25.Skaro AI, Jay CL, Baker TB, Wang E, Pasricha S, Lyuksemburg V, et al. The impact of ischaemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146:543–552. doi: 10.1016/j.surg.2009.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jay CL, Lyuksemburg V, Kang R, Preczewski L, Stroupe K, Holl JL, et al. The increased costs of donation after cardiac death liver transplantation: caveat emptor. Ann Surg. 2010;251:743–748. doi: 10.1097/SLA.0b013e3181d3d3da. [DOI] [PubMed] [Google Scholar]


