Abstract
Background
Differentiating between benign and malignant causes of obstructive jaundice can be challenging, even with the advanced imaging and endoscopic techniques currently available. In patients with obstructive jaundice, the predictive accuracy of bilirubin levels at presentation was examined in order to determine whether such data could be used to differentiate between malignant and benign disease.
Methods
A total of 1026 patients with obstructive jaundice were identified. Patients were divided into benign and malignant groups. The benign patients were subgrouped into those with choledocholithiasis and those with inflammatory strictures of the biliary tree. Bilirubin levels at presentation and other demographic data were obtained from case records.
Results
Area under the curve (AUC) values for bilirubin as a predictor of malignancy were highly significant for all benign presentations and for those with benign biliary strictures (AUC: 0.8 for both groups; P < 0.001). A bilirubin level > 100 µmol/l was determined to provide the optimum sensitivity and specificity for malignancy in all patients and in those without choledocholithiasis (71.9% and 86.9%, 71.9% and 88.0%, respectively). The application of a bilirubin level > 250 µmol/l achieved specificities of 97.1% and 98.0% in each subgroup of patients, respectively.
Conclusions
In patients with obstructive jaundice, bilirubin levels in isolation represent an important tool for discriminating between benign and malignant underlying causes.
Keywords: bilirubin, predictor, obstructive jaundice, malignancy, cancer, receiver operating characteristic, ROC, sensitivity, specificity
Introduction
Differentiating between benign and malignant causes of obstructive jaundice can be challenging, even with the advanced imaging and endoscopic techniques currently available. Preoperative histological confirmation of malignancy is not always possible because of the complex regional anatomy of the pancreaticobiliary system. Endoscopic ultrasound (EUS), direct cholangioscopy and endocystoscopy have increased the probability of obtaining preoperative tissue for analysis, but it is widely recognized by hepatobiliary specialists that, out of concern that a potentially resectable lesion may be missed, patients may undergo resection for suspicious lesions without histological confirmation of cancer.1,2 Even when the surgeon seeks to obtain intraoperative tissue confirming malignancy prior to proceeding to resection, the patient is exposed to considerable dissection at the time of surgery in order to allow representative tissue samples to be obtained. The decision to proceed to an explorative operation with or without resection is frequently based on accumulated radiological, clinical and biochemical predictors and subsequent discussion at a multidisciplinary team (MDT) meeting.
Together with radiological imaging, tumour markers, such as CA19-9 (carbohydrate antigen 19-9) levels, are routinely used in many centres to assess patients who present with suspicious pancreaticobiliary lesions; these markers have been shown to be of some value in differentiating between benign and malignant disease.3 The CA19-9 : bilirubin ratio has been previously shown to improve accuracy in attempts to characterize biliary strictures.4 This study examined the predictive accuracy of bilirubin levels at presentation in order to determine whether these values can differentiate between benign and malignant disease.
Materials and methods
The case records of over 1000 patients presenting with obstructive jaundice during the period 2008–2010 were identified from endoscopic retrograde cholangiopancreatography (ERCP) records, interventional radiological procedures and operative logbooks. The selection of patients who had undergone interventional procedures ensured that all selected patients had undergone multimodal assessment of jaundice and that tissue was frequently available to confirm a benign or malignant diagnosis.
Demographic data such as age, final diagnosis and bilirubin level at initial presentation were recorded. The final diagnosis was made using histological tissue (including frozen sections obtained at the time of surgical exploration, formal histology of resected specimens, radiological/laparoscopic biopsies of disseminated or primary disease, and brushings from ERCP), interval radiological imaging (consisting of at least two cross-sectional scans taken ≥6 months apart, each discussed at a specialist hepatobiliary MDT meeting and showing no disease progression in cases of benign disease) and findings at interventions such as ERCP or surgery. Patients were divided into benign and malignant groups according to their underlying pathology. The benign-disease group was subdivided into those with choledocholithiasis and those with inflammatory strictures of the biliary tree. Bilirubin levels were evaluated for accuracy at both the initial presentation with obstructive jaundice and again after initial imaging had excluded stone disease as a cause of the jaundice. Ethical approval was not required for this study. A literature search using PubMed was undertaken for comparative data from previously published manuscripts on the sensitivity and specificity of radiological imaging and tumour markers in determining malignancy. Data were analysed with receiver operating characteristic (ROC) and area under the curve (AUC) values using MedCalc® Version 9.3 (MedCalc Software, Mariakerke, Belgium). Receiver operating characteristics are graphical plots of the sensitivity vs. false positive rate for a binary classifier system (i.e. whether a patient has benign or malignant disease) (Fig. 1). If the AUC value derived from the ROC curve is 1, the predictor is 100% sensitive and specific; if the AUC is 0.5, the predictor is little better than chance alone.
Figure 1.
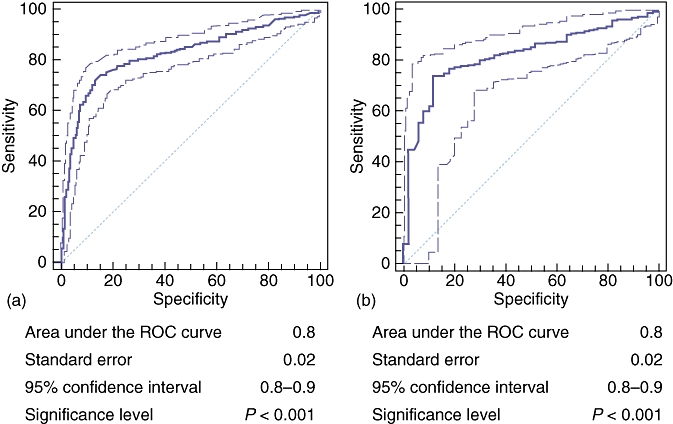
Receiver operating characteristic (ROC) curves for bilirubin and prediction of malignancy with area under the curve values for (a) all patients with either benign or malignant causes of jaundice and (b) patients with common bile duct stones excluded. The bold line represents the ROC curve; faint lines represent the range of values
Results
A total of 1026 patients with obstructive jaundice were identified. Demographic data and bilirubin levels by aetiology are displayed in Table 1. Table 2 shows the final diagnoses in this cohort of patients. Stone disease accounted for the majority of patients with obstructive jaundice caused by benign disease (83.8%). The second most common cause was chronic pancreatitis (4.6%). Pancreatic adenocarcinoma was the most common diagnosis in patients with malignant disease (36.6%).
Table 1.
Demographic data for patients with underlying benign or malignant pathology
| Total | Benign pathology | Malignant pathology | Significance | |
|---|---|---|---|---|
| Number of patients, n | 1026 | 480 | 546 | – |
| Gender, n (%) | 1.000 | |||
| Male | 556 (54.2%) | 254 (53.1%) | 302 (55.1%) | |
| Female | 470 (45.8%) | 224 (46.9%) | 246 (44.9%) | |
| Median age (range), years | 71 (22–98) | 70 (22–98) | 71 (23–86) | 0.331 |
| Median bilirubin (range), µmol/l (including CBD stones) | – | 59 (19–400) | 200 (26–723) | <0.001 |
| Median bilirubin (range), µmol/l (excluding CBD stones) | – | 63 (30–400) | 200 (26–723) | <0.001 |
CBD, common bile duct; SD, standard deviation
Table 2.
Underlying diagnoses of patients presenting with obstructive jaundice
| Diagnosis | Patients, n | Percentage of total |
|---|---|---|
| Benign pathology | ||
| Common bile duct stones | 428 | 89.2 |
| Chronic pancreatitis | 22 | 4.6 |
| Post-cholecystectomy stricture | 17 | 3.5 |
| Autoimmune pancreatitis | 11 | 2.3 |
| Primary sclerosing cholangitis | 2 | 0.4 |
| Malignant pathology | ||
| Pancreatic ductal adenocarcinoma | 200 | 36.6 |
| Gallbladder cancer | 146 | 26.7 |
| Hilar cholangiocarcinoma | 89 | 16.3 |
| Metastatic disease with biliary obstruction | 42 | 7.7 |
| Distal cholangiocarcinoma | 31 | 5.7 |
| Ampullary adenocarcinoma | 31 | 5.7 |
| Duodenal adenocarcinoma | 7 | 1.3 |
Table 3 demonstrates the sensitivity and specificity of bilirubin in predicting malignancy in all patients presenting with obstructive jaundice. Bilirubin levels of 100 µmol/l were found to be 71.9% sensitive and 86.9% specific for malignancy (positive likelihood ratio: 5.5). At higher cut-offs for bilirubin values, specificity increased. Bilirubin levels of >250 µmol/l were 97.1% specific in predicting malignancy (positive likelihood ratio: 11.3). Table 4 demonstrates the accuracy of bilirubin after imaging had excluded choledocholithiasis. The benign-disease group in this table included patients with strictures of the biliary tree caused by various non-malignant aetiologies. Bilirubin levels were found to be consistently accurate in excluding malignancy. Values of 100 µmol/l were 71.9% sensitive and 88.0% specific in detecting malignancy (positive likelihood ratio: 5.5). Values of >250 µmol/l were 98.0% specific in detecting malignancy (positive likelihood ratio: 11.3).
Table 3.
Bilirubin level and likelihood of malignancy in all patients with either benign or malignant causes for obstructive jaundice
| Bilirubin level, µmol/l | Sensitivity, % | 95% confidence interval | Specificity, % | 95% confidence interval | Positive predictive value, % | Negative predictive value, % | Positive likelihood radio |
|---|---|---|---|---|---|---|---|
| >50 | 87.7 | 84.4–90.5 | 38.8 | 34.4–43.4 | 59.0 | 75.9 | 1.4 |
| >100 | 71.9 | 67.6–75.9 | 86.9 | 83.5–89.7 | 84.6 | 75.5 | 5.5 |
| >150 | 60.4 | 55.9–64.8 | 92.7 | 90.0–94.9 | 89.2 | 70.0 | 8.3 |
| >200 | 45.0 | 40.5–49.6 | 95.6 | 93.4–97.3 | 91.1 | 63.4 | 10.3 |
| >250 | 32.9 | 28.7–37.3 | 97.1 | 95.1–98.4 | 91.9 | 59.1 | 11.3 |
| >300 | 21.9 | 18.3–25.8 | 98.5 | 97.0–99.4 | 93.8 | 55.7 | 15.0 |
| >350 | 13.1 | 10.2–16.5 | 99.2 | 97.9–99.8 | 94.0 | 53.3 | 15.7 |
| >400 | 7.1 | 5.0–9.8 | 100 | 99.2–100 | 100 | 51.8 | – |
| >500 | 1.3 | 0.5–2.7 | 100 | 99.2–100 | 100 | 50.3 | – |
Table 4.
Bilirubin and probability of malignancy in patients, excluding those with common bile duct stones
| Bilirubin level, µmol/l | Sensitivity, % | 95% confidence interval | Specificity, % | 95% confidence interval | Positive predictive value, % | Negative predictive value, % | Positive likelihood ratio |
|---|---|---|---|---|---|---|---|
| >50 | 87.7 | 84.4–90.5 | 36.0 | 22.9–50.8 | 92.9 | 23.4 | 1.4 |
| >100 | 71.9 | 67.6–75.9 | 88.0 | 75.7–95.4 | 98.3 | 24.6 | 5.5 |
| >150 | 60.4 | 55.9–64.8 | 90.0 | 78.2–96.6 | 98.3 | 19.1 | 8.3 |
| >200 | 45.0 | 40.5–49.6 | 96.0 | 86.3–99.4 | 99.1 | 15.4 | 10.3 |
| >250 | 32.9 | 28.7–37.3 | 98.0 | 89.3–99.7 | 99.4 | 13.2 | 11.3 |
| >300 | 21.9 | 18.3–25.8 | 98.0 | 89.3–99.7 | 99.1 | 11.6 | 15.0 |
| >350 | 13.1 | 10.2–16.5 | 98.0 | 89.3–99.7 | 98.4 | 10.5 | 15.7 |
| >400 | 7.1 | 5.0–9.8 | 100 | 92.8–100 | 100 | 10.1 | – |
| >500 | 1.3 | 0.5–2.7 | 100 | 92.8–100 | 100 | 9.5 | – |
Area under the curve values for bilirubin as a predictor for malignancy were highly significant, both for all benign presentations (Fig. 1a) and for those with benign biliary strictures only (Fig. 1b) (AUC: 0.8 for both groups; P < 0.0001). Optimum sensitivity and specificity for malignancy was determined to be at a bilirubin level of >100 µmol/l for all patients and for those with biliary strictures only (71.9% and 86.9%, 71.9% and 88.0%, respectively). Specificities of 97.1% and 98.0% were obtained in each group of patients, respectively, at a bilirubin level >250 µmol/l.
Discussion
The results suggest that, even in isolation, bilirubin levels are an important discriminator in patients with obstructive jaundice. Although sensitivity inevitably drops with increasing levels of bilirubin, a markedly raised bilirubin (≥100 µmol/l) has an important positive predictive value for the presence of malignancy. These findings are supported by other papers which have examined bilirubin levels in malignant disease. Previous studies have also found bilirubin to predict malignancy with AUC values of 0.85 and quoted bilirubin levels of 84 µmol/l (sensitivity and specificity of 98.6% and 59.3%, respectively) and 75 µmol/l.6,7 These values are commensurate with the results from the current data. A previous study has reported that patients presenting with malignant obstructive jaundice have a median bilirubin level of 160 µmol/l and that only 23% of patients have a bilirubin level <100 µmol/l and a rate of rise of bilirubin of 100 µmol/l per week.8 To the authors' knowledge, the data presented here are sourced from the largest cohort of such patients (>1000 patients) reported in the literature.
Although we do not suggest that bilirubin alone can be used to characterize biliary strictures, it is useful to compare these results with those from more generally accepted modalities used for assessment, such as tumour markers and cross-sectional imaging. The data presented in Table 53,9–17 are not intended to represent an exhaustive review of the literature, but, rather, to demonstrate data from individual studies and systematic reviews that have examined the accuracy of CA19-9, magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET-CT) and EUS in diagnosing the cause of obstructive jaundice. However, although the meta-analysis investigating MRI found this modality to be very sensitive and specific in diagnosing the aetiology of obstructive jaundice, it included patients with stone disease.12
Table 5.
Sensitivity and specificity of bilirubin levels compared with other assessment modalities
| Modality | Patients, n | Sensitivity, % | Specificity, % | AUC |
|---|---|---|---|---|
| Bilirubin, µmol/l | 1026 | 0.82 | ||
| >100 | 71.9 | 88.0 | ||
| >250 | 31.9 | 98.0 | ||
| CA19-93,9–11 | 776 | 68.4 | 72.5 | 0.75 |
| MRCP12a | 4711 | 95.0 | 97.0 | 0.96 |
| ERCP13–15 | 220 | 74.0 | 94.0 | – |
| EUS16 | 3532 | 78.0 | 84.0 | – |
| CT17 | 92 | – | – | 0.80 |
| PET-CT15 | 46 | 89.0 | 74.0 | – |
Includes patients with jaundice secondary to stone disease
MRCP, magnetic resonance cholangiopancreatography; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasonography; CT, computed tomography; PET-CT, positron emission tomography
The comparison indicates that bilirubin levels may be a useful adjunct when used together with these other modalities. Bilirubin levels at presentation may be of use to the clinician in several ways. A very high bilirubin level may prompt a more detailed evaluation (including for tumour markers) to exclude underlying malignancy and may lower the threshold for referring a patient to a tertiary unit. For the hepatopancreatobiliary specialist faced with a patient who has a stricture of unknown aetiology, bilirubin level may represent another factor within a multimodal assessment that prompts an exploration with or without resection.
Possible reasons why bilirubin levels are lower in patients with benign disease may refer to the fact that patients with stone disease present earlier as a result of associated pain or sepsis from bacteraemia within the biliary tree. As a consequence, bilirubin levels in these patients at presentation are lower than those in patients with malignant disease, in whom jaundice (which is not always immediately apparent to the patient) is not infrequently the sole presenting symptom. In addition, increasing degrees of biliary tract dilatation may allow stones to disimpact from the distal common bile duct, leading to a ‘ball–valve’ effect that prevents any further increase in bilirubin levels. In biliary tract strictures without choledocholithiasis, the pathophysiology of an inflammatory stricture (associated with improving and relapsing degrees of obstruction) differs markedly from the inexorable progression of a malignant process in the biliary tree.
Conclusions
In the clinical setting of the jaundiced patient, bilirubin levels in isolation may represent an important early indicator of the likely underlying cause of obstructive jaundice. Bilirubin levels are always measured as a first test in any patient presenting with jaundice. Clearly, a patient's bilirubin level in isolation cannot replace a careful clinical history and cross-sectional imaging. However, bilirubin values can influence the subsequent work-up of the patient. Optimum sensitivities and specificities are obtained with bilirubin levels of ≥100 µmol/l. These findings highlight the need to take a multimodal approach in the assessment of these patients.
Conflicts of interest
None declared.
References
- 1.Hurtuk MG, Shoup M, Oshima K, Yong S, Aranha GV. Pancreaticoduodenectomis in patients without periampullary lesions: lesions that masquerade as cancer. Am J Surg. 2010;199:372–376. doi: 10.1016/j.amjsurg.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Kassahun WT, Jonas S. Spectrum of benign lesions mimicking a malignant stricture at the liver hilum. Rev Recent Clin Trials. 2009;4:185–194. doi: 10.2174/157488709789957547. [DOI] [PubMed] [Google Scholar]
- 3.Morris-Stiff G, Teli M, Jardine N, Puntis MC. CA19-9 antigen levels can distinguish between benign and malignant pancreaticobiliary disease. Hepatobiliary Pancreat Dis Int. 2009;8:620–626. [PubMed] [Google Scholar]
- 4.Ong SL, Sachdeva A, Garcea G, Gravante G, Metcalfe MS, Lloyd DM, et al. Elevation of carbohydrate antigen 19-9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig Dis Sci. 2008;53:3213–3217. doi: 10.1007/s10620-008-0289-8. [DOI] [PubMed] [Google Scholar]
- 5.Sundeep S, Sharma R, Pal S, Sahni S, Chattopadyay TK. Differentiation between benign and malignant hilar obstructions using laboratory and radiological investigations: a prospective study. HPB. 2007;9:373–382. doi: 10.1080/13651820701504207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Mofleh IA, Aljebreen AM, Al-Amri SM, Al-Rashed RS, Al-Faleh FZ, Al-Freihi HM, et al. Biochemical and radiological predictors of malignant biliary strictures. World J Gastroenterol. 2004;10:1504–1507. doi: 10.3748/wjg.v10.i10.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bain VG, Abraham N, Janghri GS. Prospective study of biliary strictures to determine predictors of malignancy. Can J Gastroenterol. 2000;14:397–402. doi: 10.1155/2000/467567. [DOI] [PubMed] [Google Scholar]
- 8.Mansfield SD, Sen G, Oppong K, Jacques BC, O'Suillebhain CB, Manas DM, et al. Increase in serum bilirubin levels in obstructive jaundice secondary to pancreatic and periampullary malignancy – implications for timing of resectional surgery and use of biliary drainage. HPB. 2006;8:442–445. doi: 10.1080/13651820600919860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leelawat K, Sakchinabut S, Narong S, Wannaprasert J. Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC Gastroenterol. 2009;9:30. doi: 10.1186/1471-230X-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333–339. doi: 10.1016/j.amjsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Li YG, Zhang N. Clinical significance of serum tumour M2-PK and CA19-9 detection in the diagnosis of cholangiocarcinoma. Dig Liver Dis. 2009;41:605–608. doi: 10.1016/j.dld.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;7:547–557. doi: 10.7326/0003-4819-139-7-200310070-00006. [DOI] [PubMed] [Google Scholar]
- 13.Park MS, Kim TK, Kim KW, Park SW, Lee JK, Kim JS, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234–240. doi: 10.1148/radiol.2331031446. [DOI] [PubMed] [Google Scholar]
- 14.Adamek HE, Albert J, Breer H, Weitz M, Schilling D, Riemann JF. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;15:190–193. doi: 10.1016/S0140-6736(00)02479-X. [DOI] [PubMed] [Google Scholar]
- 15.Schick V, Franzius C, Beyna T, Oei ML, Schnekenburger J, Weckesser M, et al. Diagnostic impact of 18F-FDG PET-CT evaluating solid pancreatic lesions versus endosonography, endoscopic retrograde cholangiopancreatography with intraductal ultrasonography and abdominal ultrasound. Eur J Nucl Med Mol Imaging. 2008;35:1775–1785. doi: 10.1007/s00259-008-0818-x. [DOI] [PubMed] [Google Scholar]
- 16.Garrow D, Miller S, Sinha D, Conway J, Hoffman BJ, Hawes RH, et al. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616–623. doi: 10.1016/j.cgh.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Andersson M, Kostic S, Johansson M, Lundell L, Asztély M, Hellström M. MRI combined with MR cholangiopancreatography versus helical CT in the evaluation of patients with suspected periampullary tumours: a prospective comparative study. Acta Radiol. 2005;46:16–27. doi: 10.1080/02841850510016018. [DOI] [PubMed] [Google Scholar]


