Abstract
Background
Ocular syphilis among HIV-infected patients continues to be a problem in the highly active antiretroviral therapy (HAART) era. However, outside of case reports or small case series, little is known about the clinical, laboratory, and treatment outcomes of these patients.
Objective
To examine the literature on HIV-infected patients and determine the results of treatment.
Methods
Systematic review of cases series and case reports among HIV-infected individuals with ocular syphilis. Reviews, languages other than English and pre-1980 reports were excluded. The effect of CD4 count and virological suppression on clinical manifestations and diagnostic laboratory values was evaluated.
Results
A total of 101 HIV-infected individuals in case series and case reports were identified. Ocular syphilis led to the HIV diagnosis in 52% of cases, including patients with CD4 count >200 cells/mm3. Posterior uveitis was significantly more common in individuals with CD4 count <200 cells/mm3 (p=0.002). Three patients with confirmed ocular syphilis had negative non-treponemal tests. Ninety-seven per cent of patients with visual impairment improved following intravenous penicillin or ceftriaxone.
Conclusions
Non-treponemal tests may be negative in HIV-infected patients with ocular syphilis. Ocular syphilis remains an important clinical manifestation that can lead to initial HIV diagnosis.
INTRODUCTION
Unlike the incidence of cytomegalovirus retinitis which decreased after highly active antiretroviral therapy (HAART) became widely available,1 the incidence of ocular syphilis has not decreased in the HAART era.2 While there have been a number of case reports reporting the significance of ocular syphilis in HIV-infected patients,3–15 there have been no studies with more than 13 patients.
Ocular syphilis is a well-described entity that can result from acquired or congenital infection. Studies from the pre-antibiotic era16 as well as more recently2 show that ocular syphilis is one of the most common manifestations of neurosyphilis. In cases of both early and late syphilis, anterior uveitis is the most commonly observed ocular manifestation. 17,18 Several case reports have suggested that posterior uveitis is more common among those with HIV infection,19,20 but few data are available. An enormous variety of clinical manifestations of early syphilis within the eye has been reported, including the following: papulosquamous lesions of the skin of the lids, temporary loss of eyebrows, diffuse papillary conjunctivitis, scleroconjunctivitis, interstitial keratitis, iritis, chorioretinitis and optic neuritis. Studies of ocular syphilisamong HIV-negative individuals have found frequent cerebrospinal fluid (CSF) abnormalities that are consistent with neurosyphilis,21–23 and no substantial differences in CSF characteristics between HIV-infected and uninfected individuals.22,23
This systematic review analysed the clinical manifestations, immunological status, and treatment response of patients with HIV with ocular syphilis. The goal of this study is to better understand the clinical picture and laboratory findings of ocular syphilis in the HIV era, focusing on the effects of CD4 count and antiretroviral therapy.
METHODS
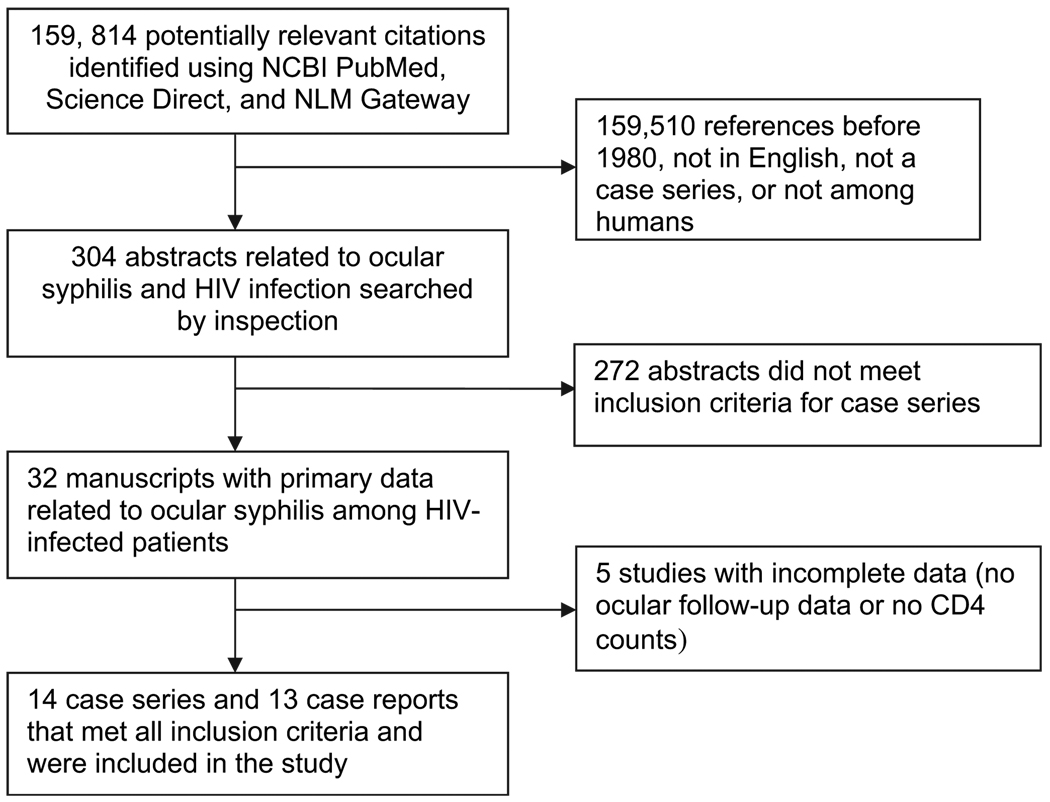
We followed PRISMA guidelines for the search strategy, study selection, data abstraction, analysis and presentation (figure 1). PubMed, Science Direct and NLM Gateway online databases were searched using the following terms: (‘syphilis’ OR ‘Treponema pallidum’) AND (‘HIV’ OR ‘AIDS’) AND (‘eye’ OR ‘ocular’, ‘iridocyclitis’, ‘chorioretinitis’, ‘uveitis’, ‘retinitis’, ‘optic neuritis’, OR ‘conjunctivitis.’) PubMed was searched by titles while Science Direct and NLM Gateway were searched by abstracts, keywords and authors.
Figure 1.
Search algorithm outlining strategy for identifying case series of HIV-infected individuals with ocular syphilis.
All manuscripts that reported case series or case reports of HIV-infected patients with ocular syphilis were included. Reviews, languages other than English and pre-1980 reports were excluded. No other restrictions were put on location, study design or type of ocular manifestation. All descriptions of ocular findings were examined by two reviewers, including one ophthalmologist (AML). Data on the following covariates were examined: demographic, clinical and laboratory characteristics (including diagnostic syphilis titres, immunological profiles and ocular manifestations), antibiotic treatment and response to treatment.
The case definition included: (1) HIV-infected patients as demonstrated by ELISA with confirmatory western blot; (2) evidence of syphilis infection defined by a positive serum treponemal test; (3) formal ophthalmological examination documenting ocular inflammation. Eye findings were reviewed by an ophthalmologist and grouped into four general categories as follows: anterior uveitis included keratitis, scleritis, iritis and iridocyclitis with or without keratic precipitates; posterior uveitis included vitritis, chorioretinitis, necrotising (and non-necrotising) retinitis, retinal vasculitis/periphlebitis, serous retinal detachment or papillitis; panuveitis included both anterior and posterior segment inflammation; optic neuritis included retrobulbar optic neuritis and optic perineuritis. CSF white blood cell (WBC) counts were dichotomised into normal and raised based on a threshold of >5 WBCs/mm3. CSF total protein counts were dichotomised based on a threshold of 60 mg/100 ml The following covariates were analysed: CD4 cell count (cells/mm3), HIV-1 RNA (copies/ml), ocular findings (anterior uveitis, posterior uveitis, panuveitis, optic neuritis), clinical manifestations (rash consistent with secondary syphilis, constitutional symptoms), serum rapid plasma reagin (RPR) or Venereal Disease Research Laboratory (VDRL) titres, CSF variables (WBC count, total protein and VDRL test), visual acuity before and after treatment and treatment course.
Differences in the clinical and laboratory findings of patients by CD4 count and viral load suppression were examined using Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables. Data analysis used SPSS 18.0. This study was approved by the Partners Human Research Committee and the Massachusetts Eye and Ear Infirmary Institutional Review Board.
RESULTS
Three hundred and four abstracts were retrieved using our search criteria, and 32 manuscripts met all inclusion criteria and were examined in detail (figure 1). Among these manuscripts, five had incomplete data.24–28 Lack of follow-up ophthalmological examination or CD4 count data were the most common reasons for excluding studies. A total of 101 patients from case series9,19,20,22,26,29–37 and case reports3–15 were included in the final analysis. All selected studies were retrospective case series from the United States and Europe (table 1). For those who had available data, ocular syphilis was the presenting symptom of HIV infection in 28 out of 54 cases (52%). In pre-HAARTera case series, 11/17 (65%) patients had ocular syphilis as the presenting symptom of HIV infection. Among studies done after the advent of HAART, 17/37 (46%) patients had ocular syphilis as the presenting symptom of HIV infection. In the studies with available data, follow-up times ranged from 5 to 45 months.
Table 1.
Characteristics of the ocular syphilis studies among HIV-infected patients
| First author | Years (or duration) | Number of patients |
Location | Concurrent new HIV diagnosis |
Receiving HAART before syphilis |
Median follow-up (months) |
|---|---|---|---|---|---|---|
| Case reports | 1984–2009 | 13 | Multiple centres | 3/8 | 3/7 | NR |
| Balba29 | 1997–2002 | 3 | Single centre, Washington DC, USA | NR | 3/3 | NR |
| Li32 | 1989–2009 | 12 | Two centres—Seattle, USA and Boston USA | 4/12 | 5/12 | 45 |
| Doris30 | 2004 | 2 | Single centre, Manchester, UK | NR | NR | NR |
| McCall26 | 2004 | 2 | Single centre, Nijmegen, Netherlands | 0/2 | 1/2 | NR |
| Parc33 | 2001–4 | 8 | Single centre, Paris, France | 0/8 | NR | 5* |
| Tran36 | 2001–3 | 12 | Single centre, Paris, France | 3/12 | NR | 7.25 |
| Kunkel22 | 1998–2006 | 11 | Single centre, Berlin, Germany | 7/11 | 2/11 | NR |
| Browning19 | 14 years | 5 | Single centre, Charlotte, USA | 3/5 | NR | 8 |
| Shalaby35 | 1983–95 | 13 | Single centre, Baltimore, USA | NR | NA | NR |
| Villanueva37 | 1993–6 | 3 | Single centre, Detroit, USA | NR | NA | 12 |
| Kuo31 | Before 1997 | 3 | Single centre, Los Angeles, USA | 2/3 | NA | NR |
| Levy9 | Before 1989 | 2 | Single centre, Baltimore, US | 2/2 | NA | NR |
| McLeish20 | 1985–8 | 9 | Two centres, Miami and Iowa City, USA | 4/9 | NA | NR |
| Passo34 | 1985 | 3 | Single centre, Baltimore, USA | 3/3 | NA | NR |
All patients had 1 month follow-up, and only four patients were followed up for longer periods. Among those four patients, the median follow-up time was 5 months.
HAART, highly active antiretroviral treatment; NA, not applicable; NR, not reported.
The median age of patients was 38.5 years (range 23–62), and 96.9% of patients were male. The median CD4 cell count was 348 cells/mm3 (range 4–1435), and 17% (17/101) of patients had a CD4 cell count <200 cells/mm3. The median HIV-1 RNA was 1800 copies/ml. A summary of the ocular findings is presented in table 2. Thirty-nine per cent (21/54) of cases had only visual symptoms, and 62% (32/52) of cases had a rash consistent with secondary syphilis infection. Seventy-four per cent (46/62) of ocular syphilis cases had an elevated CSF WBC count, and 75% (33/44) had an elevated total protein. The CSF VDRL test was positive in 57% (37/65) of all cases where a VDRL test was performed on CSF fluid.
Table 2.
Pooled data of HIV-infected patients with ocular syphilis
| Characteristics | Total (n=101) | CD4 count <200 cells/mm3 (n=17) |
p Value <0.1‡ |
|---|---|---|---|
| Median age (range) (years) | 38.5 (23–62) | 39.5 (23–50) | |
| Male (%) | 96.9 | 100 | |
| Median CD4 (range) (cells/mm3) | 348 (4–1435) | 61 (4–181) | |
| CD4>200 (%) | 74 | NA | |
| 200>CD4>50 (%) | 7 | 71 | |
| CD4<50 (%) | 10 | 29 | |
| Median HIV-1 RNA (range)(copies/ml) | 1800 (30 copies—1.9 million copies) | 1.9 million | |
| Ocular findings | n=86 | ||
| Bilateral (%) | 63 | 56 | |
| Anterior uveitis (%) | 17 | 0 | 0.10 |
| Posterior uveitis (%) | 54 | 93 | 0.002 |
| Panuveitis (%) | 20 | 0 | 0.055 |
| Optic neuritis (%) | 20 | 14 | |
| Clinical manifestations | n=54 | ||
| Only visual symptoms (%) | 39 | 60 | |
| Rash of secondary syphilis (%) | 55 | 50 | |
| CSF findings | n=64 | ||
| WBC elevated* (%) | 74 | 90 | |
| Total protein elevated† (%) | 75 | 78 | |
| VDRL positive (%) | 57 | 58 |
χ2 test using dichotomised CD4 counts.
WBC elevated defined as >5 WBC/ml.
Total protein elevated defined as >60 mg/100 ml.
Comparison of individuals with CD4 count <200 and individuals with CD4 count >200.
CSF, cerebrospinal fluid; VDRL, Venereal Disease Research Laboratory; WBC, white blood cells.
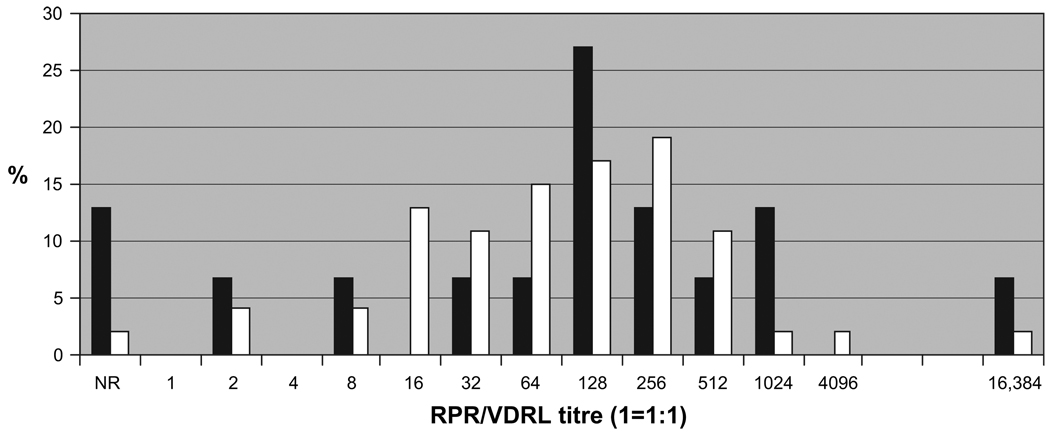
Patients with ocular syphilis with CD4 cell count <200 cells/ mm3 were found to have a significantly greater risk of posterior uveitis (p=0.002, table 2). Patients with CD4 cell count <200 cells/mm3 also tended to have a lower risk of panuveitis and anterior uveitis (p=0.055 and p=0.1, respectively). The majority of patients in both groups had relatively high RPR/VDRL titres (median 1:128), suggesting that they may have been in the early stages of infection when they presented (figure 2).
Figure 2.
Range of RPR/VDRL titres comparing patients with CD4>200 cells/ml (white bars) with those with CD4<200 cells/ml (black bars). NR, non-reactive; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory.
Eleven individuals had HIV-1 RNA levels under the limit of detection. In these patients, there was a trend towards less frequent reactive CSF VDRL titres (20% vs 63%, p=0.051), less frequent leucocytosis (40% vs 76%, p=0.10) and less frequent elevated total protein (29% vs 75%, p=0.13) when compared to patients who had detectable viraemia. No significant differences were seen in the CD4 count or clinical findings between these two groups.
Three cases of ocular syphilis with a negative serum RPR or VDRL test were identified. All three patients were found to have positive treponemal tests and serial assessment following treatment. Of these three cases, two had documented improvement in visual acuity19,20 and one had a post-treatment repeat lumbar puncture showing the CSF WBC count decreasing from 393 to 14 cells/µl (unpublished data). Among these three patients, two had never had been diagnosed with syphilis before, one had been successfully treated for syphilis in the past. One of the patients presented with necrotising retinitis and retinal detachment. Another patient had a rash, bilateral posterior uveitis and a positive CSF VDRL test that became negative after treatment. The CD4 cell counts in these three patients were 56, 158 and 574 cells/µl.
Almost all patients were given 10–21 days of intravenous penicillin or ceftriaxone. Although some patients had poor follow-up, there were no confirmed cases of intravenous penicillin or ceftriaxone failure. Thirty-four out of 35 patients who had follow-up visual acuity formally assessed by an ophthalmologist were found to have either normal visual acuity or an improvement after treatment. One patient had stable visual acuity (20/20) after treatment. However, in the study with the longest follow-up (Li et al), three patients required re-treatment for syphilis, including one for recurrent ocular syphilis. All of these individuals were at risk for reinfection owing to persistent high-risk sexual behaviours.
DISCUSSION
The literature on ocular syphilis among HIV-infected individuals is limited. In this systematic analysis, we report the clinical and laboratory findings associated with 101 cases of ocular syphilis among HIV-infected individuals. Although this was a systematic review, there was a large amount of heterogeneity in the quality of data and follow-up.
The age, sex, CD4 profile and HIV-1 RNA measurements are consistent with data from US studies of neurosyphilis.27,38 Rash consistent with secondary syphilis was described in a majority of patients, which is similar to a study of neurosyphilis among patients with HIV38 and earlier pre-HIV era studies.39,40 In addition, the high RPR/VDRL titres in the majority of patients suggest that most patients were in the earlier stages of infection. Since the onset of symptoms was not clear in most cases, a more precise discrimination of syphilis stage was not possible. These findings are also consistent with the pre-HIV literature that found a relationship between higher non-treponemal test results and neurosyphilis.41
While a wide range of serum RPR titres have been observed in neurosyphilis studies,27,38 we were able to uncover three cases of ocular syphilis with negative serum RPR/VDRL titres. Although further information about prozone phenomena or the presence of non-syphilis treponeme might explain these cases, there were limited available data on prozone phenomena from the primary manuscripts and follow-up inquiries. Negative non-treponemal tests in the setting of ocular syphilis have been reported among HIV uninfected individuals related to prozone phenomena21 and to other causes,42,43 suggesting that this may not be unique to HIV-infected individuals. These three cases suggest that ocular syphilis may occur in the setting of a negative serum non-treponemal test, emphasising the need for treponemal diagnostic testing and, possibly, lumbar puncture in patients suspected of having ocular syphilis.
This study found a trend towards higher CSF WBC counts in patients with lower CD4 counts (p=0.055). At the same time, seven patients with ocular syphilis and HIV infection had normal CSF white blood cell count, total protein and CSF VDRL titres; all these patients were from the HAART era. This correlated with the finding that those with suppressed viraemia were less likely to have a reactive CSF VDRL test. Syphilis literature from the 1980s suggested that most patients with ocular syphilis had an elevated CSF white blood cell count and total protein,43 consistent with our results. There are conflicting data about the CSF VDRL test among patients who have ocular syphilis. While one study from the pre-HIV era found that none of 50 individuals with ocular syphilis had a reactive CSF VDRL test,43 a greater number of reactive CSF VDRL positive cases were found in an HIV-era study32 and our pooled data.
Several previous case series of ocular syphilis among HIVinfected patients have noted a tendency towards posterior uveitis19,20,31,37 and panuveitis,35 but our analysis supported only the association between posterior uveitis and low CD4 counts. It is unclear why posterior uveitis appears more commonly in HIV-infected patients with low CD4 cell counts. Most patients with uveitis secondary to syphilis have good visual prognosis. Patients with posterior uveitis with central chorioretinal lesions and optic neuritis have poorer prognosis than patients with anterior uveitis and other categories of inflammation.
Persistent symptoms are common among HIV-infected patients with neurosyphilis. A prospective study of 41 HIV-infected patients with neurosyphilis found that patients’ main complaint persisted at 1 year in 38% of cases,27 and another study found that 30% of treated cases had persistent symptoms. 38 However, the patients with ocular syphilis in our analysis had good response to treatment based on repeat examination. Although data are available for only 35 patients, 97% of the cases showed definitive improvement or normal visual acuity on follow-up.
While visual improvement was rapid in most patients after treatment, a small number of patients had persistent visual acuity problems. There were limited data on follow-up and also on the use of steroids which may help prevent visual acuity problems. The importance of completing an adequate course of intravenous antibiotics for all patients with neurosyphilis cannot be overstated.44 The US CDC recommends 10–14 days of intravenous penicillin, although these guidelines are based on limited data.45 A full course of intravenous penicillin may be especially important in patients who may have a compromised immune system.3 Several studies noted early administration of steroids in patients with uveitis in order to prevent an ocular Jarisch–Herxheimer reaction46,47 and prevent further inflammation related to uveitis.48
This study has several limitations worthy of consideration. There was substantial heterogeneity in the quality of the data and follow-up in the analysed literature. Publication bias probably revealed a greater number of severe cases, although based on laboratory abnormalities and clinical course, this group of patients with ocular syphilis and HIV probably has less severe disease than other cohorts with neurosyphilis.27,38 Since RPR and VDRL tests were done at different laboratories, comparison of values must be interpreted with caution. Not all patients underwent lumbar puncture and were followed up for repeat visual acuity examination, but patients who had persistent visual problems would probably be followed up. In addition, the CSF WBC cut-off point of 5 WBC/mm3 was chosen because most studies reported that value. Among HIV-infected people, this cut-off point may have lower specificity when diagnosing neurosyphilis.41
Despite the advent of HAART, ocular syphilis remains an important clinical concern among patients with HIV infection. It can occur in patients with negative serum RPR or VDRL test and clinicians must obtain serum treponemal tests when the suspicion of ocular syphilis is high. Lumbar puncture is recommended for the evaluation of all patients with ocular syphilis as a substantial proportion will have evidence of neurosyphilis. Although the visual recovery of patients who completed 10–21 days of intravenous antibiotic therapy was good, behavioural counselling and close follow-up are advised. More research is needed to better understand ocular syphilis among HIV-infected patients.
Acknowledgements
Special thanks to the MGH Syphilis Working Group. Thanks to Seth Cohen and Kate Mooney for administrative support.
Funding Support from the National Institutes of Health (T32 AI07387, JL), US NIH Fogarty International Clinical Fellowship (R24TW007988, JT) and the American Society of Tropical Medicine and Hygiene (JT).
Footnotes
Competing interests None.
Ethics approval This study was conducted with the approval of the Partners Human Subjects Research Committee and the Massachusetts Eye and Ear Infirmary Institutional Review Board.
Contributors JDT contributed to data interpretation, drafting the article and revising it critically for important intellectual content. JZL contributed to acquisition and interpretation of data, drafting the article and revising it critically for important intellectual content. GKR contributed to the conception and design of the study, interpretation of the data, drafting the article and revising it for important intellectual content. BTD contributed to the conception and design of the study and interpretation of the data. AML contributed to acquisition and interpretation of data, drafting the article and revising it critically for important intellectual content. JL contributed to acquisition and interpretation of data, drafting the article and revising it critically for important intellectual content. GNP contributed to the conception and design of the study and interpretation of the data. MLD contributed to the conception and design of the study, interpretation of the data, drafting the article and revising it for important intellectual content. DF contributed to the conception and design of the study, interpretation of the data, drafting the article and revising it for important intellectual content.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Kedhar SR, Jabs DA. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Herpes. 2007;14:66–71. [PubMed] [Google Scholar]
- 2.Gonzalez-Lopez JJ, Guerrero ML, Lujan R, et al. Factors determining serologic response to treatment in patients with syphilis. Clin Infect Dis. 2009;49:1505–1511. doi: 10.1086/644618. [DOI] [PubMed] [Google Scholar]
- 3.Berry CD, Hooton TM, Collier AC, et al. Neurologic relapse after benzathine penicillin therapy for secondary syphilis in a patient with HIV infection. N Engl J Med. 1987;316:1587–1589. doi: 10.1056/NEJM198706183162507. [DOI] [PubMed] [Google Scholar]
- 4.Booth J, Rodger A, Singh J, et al. Syphilitic panuveitis with retinal necrosis in an HIV positive man confirmed by treponema pallidum PCR. J Infect. 2009;59:373–375. doi: 10.1016/j.jinf.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Carter JB, Hamill RJ, Matoba AY. Bilateral syphilitic optic neuritis in a patient with a positive test for HIV. Case report. Arch Ophthalmol. 1987;105:1485–1486. doi: 10.1001/archopht.1987.01060110031015. [DOI] [PubMed] [Google Scholar]
- 6.Friedman RF, Ganiban GJ, Liss RA, et al. Ocular syphilis and neurosyphilis in a patient with human immunodeficiency virus infection. Md Med J. 1995;44:284–288. [PubMed] [Google Scholar]
- 7.Joyce PW, Haye KR, Ellis ME. Syphilitic retinitis in a homosexual man with concurrent HIV infection: case report. Genitourin Med. 1989;65:244–247. doi: 10.1136/sti.65.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleiner RC, Najarian L, Levenson J, et al. AIDS complicated by syphilis can mimic uveitis and Crohn’s disease. Case report. Arch Ophthalmol. 1987;105:1486–1487. doi: 10.1001/archopht.1987.01060110032016. [DOI] [PubMed] [Google Scholar]
- 9.Levy JH, Liss RA, Maguire AM. Neurosyphilis and ocular syphilis in patients with concurrent human immunodeficiency virus infection. Retina. 1989;9:175–180. [PubMed] [Google Scholar]
- 10.Moloney G, Branley M, Kotsiou G, et al. Syphilis presenting as scleritis in an HIV-positive man undergoing immune reconstitution. Clin Experiment Ophthalmol. 2004;32:526–528. doi: 10.1111/j.1442-9071.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 11.Pleimes M, Hartschuh W, Kutzner H, et al. Malignant syphilis with ocular involvement and organism-depleted lesions. Clin Infect Dis. 2009;48:83–85. doi: 10.1086/594127. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Diaz E, Moran-Estefania M, Lopez-Avila A, et al. Clinical expression of secondary syphilis in a patient with HIV infection. J Dermatol. 1994;21:111–116. doi: 10.1111/j.1346-8138.1994.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 13.Thami GP, Kaur S, Gupta R, et al. Syphilitic panuveitis and asymptomatic neurosyphilis: a marker of HIV infection. Int J STD AIDS. 2001;12:754–756. doi: 10.1258/0956462011924146. [DOI] [PubMed] [Google Scholar]
- 14.Zaidman GW. Neurosyphilis and retrobulbar neuritis in a patient with AIDS. Ann Ophthalmol. 1986;18:260–261. [PubMed] [Google Scholar]
- 15.Zambrano W, Perez GM, Smith JL. Acute syphilitic blindness in AIDS. J Clin Neuroophthalmol. 1987;7:1–5. doi: 10.3109/01658108709007423. [DOI] [PubMed] [Google Scholar]
- 16.Woods AC. Syphilis of the eye. Am J Syph Gonor Vener Dis. 1943;27:133. [Google Scholar]
- 17.Hogan MJ, Zimmerman LE. Opthalmic pathology. 3rd edn. Philadelphia: WB Saunders; 1968. [Google Scholar]
- 18.Moore JE. Syphilitic iritis. Am J Ophthalmol. 1931;14:110–126. [Google Scholar]
- 19.Browning DJ. Posterior segment manifestations of active ocular syphilis, their response to a neurosyphilis regimen of penicillin therapy, and the influence of human immunodeficiency virus status on response. Ophthalmology. 2000;107:2015–2023. doi: 10.1016/s0161-6420(00)00457-7. [DOI] [PubMed] [Google Scholar]
- 20.McLeish WM, Pulido JS, Holland S, et al. The ocular manifestations of syphilis in the human immunodeficiency virus type 1-infected host. Ophthalmology. 1990;97:196–203. doi: 10.1016/s0161-6420(90)32605-2. [DOI] [PubMed] [Google Scholar]
- 21.Maves RC, Cachay ER, Young MA, et al. Secondary syphilis with ocular manifestations in older adults. Clin Infect Dis. 2008;46:e142–e145. doi: 10.1086/588483. [DOI] [PubMed] [Google Scholar]
- 22.Kunkel J, Schurmann D, Pleyer U, et al. Ocular syphilis–indicator of previously unknown HIV-infection. J Infect. 2009;58:32–36. doi: 10.1016/j.jinf.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Ormerod LD, Puklin JE, Sobel JD. Syphilitic posterior uveitis: correlative findings and significance. Clin Infect Dis. 2001;32:1661–1673. doi: 10.1086/320766. [DOI] [PubMed] [Google Scholar]
- 24.Becerra LI, Ksiazek SM, Savino PJ, et al. Syphilitic uveitis in human immunodeficiency virus-infected and noninfected patients. Ophthalmology. 1989;96:1727–1730. doi: 10.1016/s0161-6420(89)32657-1. [DOI] [PubMed] [Google Scholar]
- 25.Tamesis RR, Foster CS. Ocular syphilis. Ophthalmology. 1990;97:1281–1287. doi: 10.1016/s0161-6420(90)32419-3. [DOI] [PubMed] [Google Scholar]
- 26.McCall MB, van Lith-Verhoeven JJ, van Crevel R, et al. Ocular syphilis acquired through oral sex in two HIV-infected patients. Neth J Med. 2004;62:206–208. [PubMed] [Google Scholar]
- 27.Ghanem KG, Moore RD, Rompalo AM, et al. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS. 2008;22:1145–1151. doi: 10.1097/QAD.0b013e32830184df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonollosa A, Giralt J, Pelegrin L, et al. Ocular syphilis–back again: understanding recent increases in the incidence of ocular syphilitic disease. Ocul Immunol Inflamm. 2009;17:207–212. doi: 10.1080/09273940902741709. [DOI] [PubMed] [Google Scholar]
- 29.Balba GP, Kumar PN, James AN, et al. Ocular syphilis in HIV-positive patients receiving highly active antiretroviral therapy. Am J Med. 2006;119(448):e21–e25. doi: 10.1016/j.amjmed.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Doris JP, Saha K, Jones NP, et al. Ocular syphilis: the new epidemic. Eye. 2006;20:703–705. doi: 10.1038/sj.eye.6701954. [DOI] [PubMed] [Google Scholar]
- 31.Kuo IC, Kapusta MA, Rao NA. Vitritis as the primary manifestation of ocular syphilis in patients with HIV infection. Am J Ophthalmol. 1998;125:306–311. doi: 10.1016/s0002-9394(99)80136-6. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Tucker JD, Lobo A, et al. Ocular syphilis among HIV-infected individuals. Clin Infect Dis. 2004;62:206–208. doi: 10.1086/654797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parc CE, Chahed S, Patel SV, et al. Manifestations and treatment of ocular syphilis during an epidemic in France. Sex Transm Dis. 2007;34:553–556. doi: 10.1097/01.olq.0000253385.49373.1a. [DOI] [PubMed] [Google Scholar]
- 34.Passo MS, Rosenbaum JT. Ocular syphilis in patients with human immunodeficiency virus infection. Am J Ophthalmol. 1988;106:1–6. doi: 10.1016/s0002-9394(14)76378-0. [DOI] [PubMed] [Google Scholar]
- 35.Shalaby IA, Dunn JP, Semba RD, et al. Syphilitic uveitis in human immunodeficiency virus-infected patients. Arch Ophthalmol. 1997;115:469–473. doi: 10.1001/archopht.1997.01100150471003. [DOI] [PubMed] [Google Scholar]
- 36.Tran TH, Cassoux N, Bodaghi B, et al. Syphilitic uveitis in patients infected with human immunodeficiency virus. Graefes Arch Clin Exp Ophthalmol. 2005;243:863–869. doi: 10.1007/s00417-005-1137-6. [DOI] [PubMed] [Google Scholar]
- 37.Villanueva AV, Sahouri MJ, Ormerod LD, et al. Posterior uveitis in patients with positive serology for syphilis. Clin Infect Dis. 2000;30:479–485. doi: 10.1086/313689. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) Symptomatic early neurosyphilis among HIV-positive men who have sex with men–four cities, United States, January 2002–June 2004. MMWR Morb Mortal Wkly Rep. 2007;56:625–628. [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh FB, Hoyt WF. Clinical neuro-ophthalmology. Baltimore: Williams and Wilkins; 1969. [Google Scholar]
- 40.Whitfield R, Wirostko E. Ocular syphilis. In: Locatcher-Khorazo D, editor. Microbiology of the eye. St. Louis: Mosby Co; 1972. pp. 322–350. [Google Scholar]
- 41.Marra CM, Maxwell CL, Smith SL, et al. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis. 2004;189:369–376. doi: 10.1086/381227. [DOI] [PubMed] [Google Scholar]
- 42.Spoor TC, Wynn P, Hartel WC, et al. Ocular syphilis. Acute and chronic. J Clin Neuroophthalmol. 1983;3:197–203. [PubMed] [Google Scholar]
- 43.Spoor TC, Ramocki JM, Nesi FA, et al. Prevalence of FTA-ABS reactivity and cerebrospinal fluid findings. J Clin Neuroophthalmol. 1987;7:191–195. [PubMed] [Google Scholar]
- 44.Holmes KK. Sexually transmitted diseases. 4th edn. New York: McGraw-Hill Medical; 2008. [Google Scholar]
- 45.CDC. Sexually transmitted disease treatment guidelines. MMWR Morb Mortal Wkly Rep. 2006;55:R-7. [Google Scholar]
- 46.Fathilah J, Choo MM. The Jarisch-Herxheimer reaction in ocular syphilis. Med J Malaysia. 2003;58:437–439. [PubMed] [Google Scholar]
- 47.Danesh-Meyer H, Kubis KC, Sergott RC. Not so slowly progressive visual loss. Surv Ophthalmol. 1999;44:247–252. doi: 10.1016/s0039-6257(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 48.Wilhelmus K, Lukehart SA. Syphilis. In: Pepose J, Holland G, Wilhelmus K, editors. Ocular infection and immunity. Philadelphia: Mosby; 1996. pp. 1437–146. [Google Scholar]