Abstract
To stem the rising costs of medications, states have implemented varying generic substitution policies. These policies differ in the extent to which pharmacists or patients can influence medication choice. Using national Medicaid data, we evaluated the relationship between different generic substitution policies and generic simvastatin use after patent expiration of branded Zocor. States implementing policies that require patient consent prior to generic substitution experienced 25% lower rates of generic substitution. By eliminating patient consent requirements, state Medicaid programs could expect to save over $100 million dollars in coverage for 3 top-selling medications nearing patent expiration. The implications of these regulations on national medication spending should be considered.
Keywords: generic substitution, prescription drug costs, patent expiration
Background
In a time of contracting budgets, state governments seek strategies to reduce unnecessary costs of healthcare without compromising quality. Patent expiration represents one particularly appealing opportunity to encourage generic substitution and reduce costs without disrupting established medication regimens. Generic drugs are clinically equivalent, less expensive versions of the identical molecule,1 but sold at a fraction of the cost.2 In 2011, patents will expire for Lipitor, Plavix, and Zyprexa, representing almost $17 billion in annual sales in the U.S. in 2007, and patents for numerous other blockbuster medications are scheduled to expire in the next 4 years.3 Stimulating generic substitution after patents expire may substantially reduce costs without compromising quality.
State governments have relatively few tools available to influence prescription drug use for Medicaid beneficiaries. All states have adopted generic substitution laws, and many require step therapy or prior authorization prior to provide coverage for more expensive medications. Though step therapy and prior authorization have a substantial effect on medication utilization, little is known about what levers are most effective for encouraging generic medication use.4 Studies in the 1980s indicated that generic substitution laws increase the use of generic medications,5,6 however, generic drugs represented only a tiny proportion of filled prescriptions at that time, and the marketplace for medications has changed significantly.7 A more recent study of generic substitution laws in Sweden also found that generic substitution laws increase generic use,8 although Sweden has a very different healthcare delivery system than the US.
Generic substitution laws in the US are determined by individual states and can differ in several important ways. Some state Boards of Pharmacy have adopted mandatory generic substitution laws that require pharmacists to substitute a generic for a branded medication if the prescriber did not otherwise specify that the branded drug should be dispensed as written. More permissive generic substitution laws enacted in other states allow, but do not require, pharmacists to substitute generics, providing them with more discretion with regard to medication utilization. In addition, some states require the patient to provide consent prior to substitution of a generic while others do not. States that require patient consent provide the patient with a greater opportunity to influence medication utilization. These laws are independent and states could adopt one, both, or neither of them. No recent studies have assessed the relationship between these variations in generic substitution laws and rates of generic substitution after patient expiration. Similarly, no studies have explored whether these regulations affect rates of therapeutic interchange, the rate of substituting a generic alternative for a distinct branded molecule.
The end of market exclusivity for branded Zocor (simvastatin) on June 23, 2006, offers an opportunity to study the effect of varying generic substitution laws on generic drug substitution rates. Annual spending on Zocor in the U.S. exceeded $4.6 billion prior to patent expiration, and Zocor was one of the top selling medications in the world for several years.3 We selected Medicaid as the source population to evaluate the effects of different substitution practices because cost-containment is a topic of particular importance to state governments in the current economic climate.
Sources of Data
The Center for Medicare and Medicaid Services (CMS) provides quarterly data on drug use by Medicaid programs.9 These state-level data include the total number of prescriptions filled, the total number of tablets dispensed, and the total Medicaid reimbursement for each product, aggregated by calendar quarter. No data at the level of individual patients are available. Arizona has a decentralized Medicaid program and was not included; we obtained data for the other 49 states and the District of Columbia.
Generic substitution laws were obtained from an annual survey of State Boards of Pharmacy published by the National Association of Boards of Pharmacy.10 This survey characterizes states’ generic substitution laws as 1) mandatory or permissive generic substitution the pharmacist’s role in generic substitution, and 2) patient consent required or not required the patients’ role. We were unable to access legislation data from Oklahoma; Oklahoma was excluded from this analysis.
We contacted all state Medicaid agencies in our sample, from 9–12/2008 to determine whether the state Medicaid programs had specified and implemented any prior-authorization policy regarding brand-name Zocor or Lipitor during the study period.
Statistical Analysis
We evaluated quarterly frequencies of filled prescriptions for all statins, generic simvastatin, brand-name Lipitor, and brand-name Zocor between the first quarter of 2006, approximately six months before generic entry of simvastatin, and the third quarter of 2007, the most recent calendar quarter posted on the CMS website at the time of these analyses.
Our primary outcome was the generic drug use ratio (generic simvastatin prescriptions divided by total prescriptions of generic simvastatin and brand-name Zocor). Secondary outcomes included: 1) the proportion of all statins that were filled for Lipitor, 2) the total Medicaid reimbursement for the sum of generic simvastatin and brand-name Zocor use, and 3) the cost per prescription for generic simvastatin or brand-name Zocor. Since generic simvastatin became available on June 23, 2006, there was only 1 week of minimal generic simvastatin use in the second calendar quarter of 2006. Our study period began after the implementation of Medicare Part D. Patients dually enrolled in Medicare and Medicaid were automatically enrolled in a Part D program on January 1st, 2006. Any changes to the Medicaid population enrollment as a result of Part D should have occurred prior to market entrance of generic simvastatin, limiting potential confounding in this analysis.
We used data describing actual Medicaid reimbursement to assess the relation between patient consent laws and costs to states. We calculated average cost per prescription per calendar quarter by dividing total reimbursement per quarter of generic simvastatin and brand-name Zocor prescriptions by the total number of prescriptions filled per quarter for these medications. In the 5 calendar quarters after patent expiration, we calculated the cost savings per prescription in states that did not require patient consent compared to those who did require consent.
We evaluated bivariate relations independently between the three policies of interest (mandatory generic substitution, patient consent and prior authorization) and the primary outcome, generic fill rate of simvastatin. To assess the comparative effects of different laws on generic drug use, we fit a repeated measures generalized linear regression model in which each state-quarter was an observation. The models included time as a categorical variable to capture the non-linear time-trend in generic prescribing. The effects of the different laws were modeled through the use of time-varying indicator variables as some states changed policies during the study period. We employed an identity link function, so the parameter estimates are appropriately interpreted as the average monthly change in generic prescribing across the study period attributable to the different policies. Standard errors were estimated robustly to account for repeated observations within states.11
To assess the relationship between generic substitution laws and rates of therapeutic interchange from branded Lipitor to generic simvastatin, we conducted a time-series analysis where the outcome was the proportion of all statins filled that were Lipitor. Effects on Lipitor use were estimated in a multivariable adjusted linear regression model with time treated as a class variable. Because Lipitor prior authorization policies may be correlated with other policies and are likely to affect use, we included an indicator for Lipitor prior authorization in the model as well as the other policies. All analyses were conducted with SAS software (Cary, NC).
Results
Mandatory generic substitution regulations, patient consent regulations, and prior authorization requirements for brand-name Zocor are listed, for 2006 and 2007, in Exhibit 1.
Exhibit 1.
State Regulations in 2006 and 2007
| State | Patient Consent Required | Mandatory Generic Substitution | Prior Authorization for Zocor | State | Patient Consent Required | Mandatory Generic Substitution | Prior Authorization for Zocor |
|---|---|---|---|---|---|---|---|
| AL | N | N | N | MO | Y | N | N |
| AK | Y | N | N | MT | Y | N | N |
| AZ | Y | N | N | NE | Y | N | N |
| AR* | Y | N | Y | NV | X | O | N |
| CA | Y | N | Y | NH | Y | N | Y |
| CO | Y | N | N | NJ | N | Y | Y |
| CT | Y | N | Y | NM | N | Y | N |
| DC | Y | N | N | NY | Y | N | Y |
| DE | Y | N | Y | NC | O | N | Y |
| FL | Y | Y | N | ND | Y | N | N |
| GA | Y | N | N | OH | Y | N | Y |
| HI | Y | O | N | OR | N | Y | N |
| ID | Y | N | Y | PA | Y | N | Y |
| IL | Y | N | N | RI | N | Y | N |
| IN | Y | N | Y | SC | Y | N | Y |
| IA | Y | N | Y | SD | Y | N | N |
| KS | Y | N | N | TN | N | Y | N |
| KY | Y | Y | N | TX | Y | N | Y |
| LA | Y | N | N | UT | Y | N | Y |
| ME | Y | N | Y | VT | Y | N | N |
| MD | Y | N | Y | VA | Y | N | N |
| MA | N | Y | N | WA | N | Y | Y |
| MI | Y | N | N | WV | Y | N | Y |
| MN | Y | N | Y | WI | Y | N | Y |
| MS | Y | N | N | WY | N | Y | N |
Y - Required in 2006 and 2007
N - Not required in 2006 and 2007
O - Requirement added in 2007
X - Requirement dropped in 2007
Not used in this analysis due to lack of Medicaid data
Source: Survey of State Boards of Pharmacy published by the National Association of Boards of Pharmacy and contact with individual state Medicaid programs
In 2006, 1,620,797 prescriptions were filled for either generic simvastatin or brand-name Zocor in U.S. Medicaid programs in 48 states and the District of Columbia. In the states that used mandatory generic substitution, 492,443 generic simvastatin and brand-name Zocor prescriptions were filled in 2006, and 146,654 simvastatin and brand-name Zocor prescriptions were filled in the states that did not require patient consent in 2006. The aggregate of generic simvastatin and brand-name Zocor use comprised 25.8% of all statin use in 2006 in Medicaid programs nationally. In the first two calendar quarters after patent expiration, approximately one half of all simvastatin prescriptions were filled for the generic form. (Exhibit 2) This proportion increased to over 90% in the fourth calendar quarter after patent expiration.
Exhibit 2.
Trends in generic simvastatin, Zocor and total statin use by calendar quarter
| Year | Calendar Quarter | Total number of generic simvastatin prescriptions filled in Medicaid programs | Sum of generic simvastatin and branded Zocor prescriptions filled in Medicaid programs | Total number of statins filled | Average state- level proportion (standard deviation) of filled simvastatin prescriptions that were generic |
|---|---|---|---|---|---|
| 2006 | 2 | 9 | 394616 | 1536213 | |
| 2006 | 3 | 106755 | 360118 | 1397266 | 0.47 (0.35) |
| 2006 | 4 | 135196 | 370805 | 1431234 | 0.50 (0.45) |
| 2007 | 1 | 259843 | 386496 | 1450384 | 0.77 (0.29) |
| 2007 | 2 | 360976 | 404923 | 1405863 | 0.92 (0.21) |
| 2007 | 3 | 411012 | 447647 | 1465682 | 0.94 (0.20) |
Source: Aggregate Medicaid prescription medication use data
In the 6 months following patent expiration, the states with laws requiring mandatory generic substitution at the pharmacy filled 48.7% of simvastatin prescriptions with the generic version while states with permissive substitution laws had filled 30.0% with the generic. (Exhibit 3) In the states that did not require patient consent for generic substitution, 98.1% of simvastatin prescriptions were for the generic version 6 months after patent expiration while less than one third were using the generic in states that did require patient consent. (Exhibit 4) In states that required prior authorization for brand-name Zocor, we found inconsistent and small changes in generic substitution rates when compared to states without prior authorization requirements.(Exhibit 1 on-line)
Exhibit 3. Relationships Between Legislation Requiring Mandatory Generic Substitution At The Pharmacy And Generic Fill Rates For Simvastatin. 2006 And 2007.
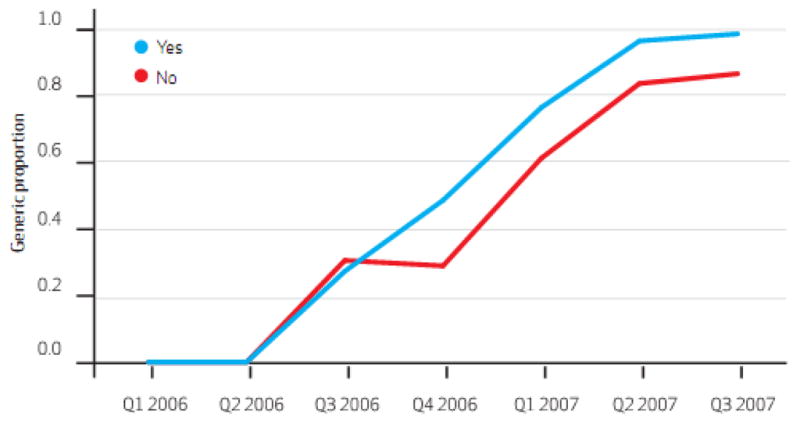
SOURCE Centers for Medicare and Medicaid Services, aggregate Medicaid prescription medication use data. NOTE Yes/no indicates whether or not generic substitution is mandatory.
Exhibit 4.
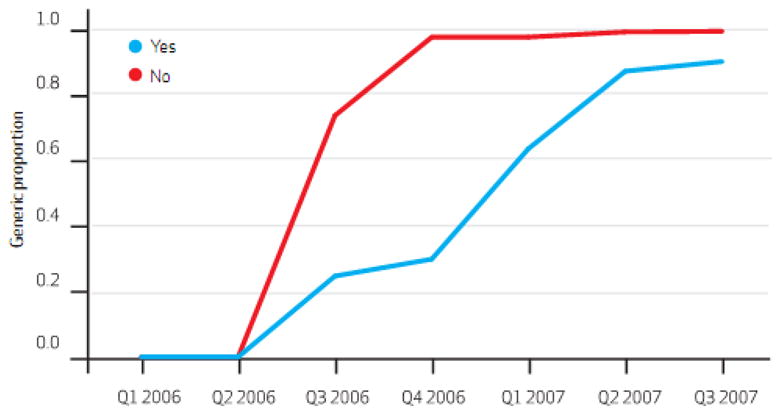
Relationships Between Legislation Requiring Patient Consent For Generic Substitution And Generic Fill Rates For Simvastatin. 2006 And 2007
SOURCE Centers for Medicare and Medicaid Services, aggregate Medicaid prescription medication use data. NOTE Yes/no indicates whether or not patient consent is required for generic substitution.
In multi-variable models controlling for generic substitution policies, prior authorization policies for brand-name Zocor, and repeated observations within states, mandatory generic substitution laws had no statistically significant effect on generic use. (95% C.I (-)0.12 – 0.35, p = 0.33)(Exhibit 2 online) Laws requiring patients to provide consent prior to generic substitution reduced generic use by an average of 24.8% per calendar quarter in the 5 calendar quarters subsequent to generic market entry (95% C.I. (-) 0.43 – (-) 0.05, p = 0.01). We found no significant relationship between prior authorization requirement for brand-name Zocor and the generic substitution rate. (p = 0.63)
Lipitor use declined from 43% of total statin use in Q1 of 2006 to 36% in Q4 of 2007. Adjusting for other policy effects, Lipitor prior authorization requirements were associated with 30.6 percentage point lower Lipitor use. Mandatory generic substitution, patient consent requirements and prior authorization requirements for brand-name Zocor did not affect either overall levels of Lipitor use or changes in rates of Lipitor use following the market entrance of generic simvastatin.
We used actual Medicaid reimbursement levels to assess the cost per prescription of all prescriptions of brand-name Zocor and generic simvastatin filled by calendar quarter after patent expiration. (Exhibit 5.) In the first calendar quarter, states that did not require patient consent for generic substitution paid, on average, $15.35 less per prescription for the sum of brand-name Zocor and generic simvastatin than states that did require patient consent; in the second and third calendar quarters, states that did not require consent paid $16.10 and $18.19 less per prescription. These differences declined to $5.70 and $2.68 per prescription in the 4th and 5th calendar quarters after patent expiration, with costs per prescription remaining lower in states that did not require consent.
Exhibit 5.
Average cost per prescription of all Zocor and generic simvastatin prescriptions filled per calendar quarter
| Q 2 2006 |
Q 3 2006 |
Q 4 2006 |
Q 1 2007 |
Q 2 2007 |
Q 3 2007 |
|
|---|---|---|---|---|---|---|
| States requiring patient consent | 142.19 | 138.80 | 137.21 | 88.92 | 45.95 | 35.72 |
| States not requiring patient consent | 142.43 | 123.45 | 121.11 | 70.73 | 40.25 | 33.04 |
| Difference, in dollars | −0.24 | 15.35 | 16.10 | 18.19 | 5.70 | 2.68 |
Source: Our analysis of aggregate Medicaid prescription medication use data
We estimated the potential savings of adopting generic substitution laws that do not require patient consent in all those states that did require patient consent during the study period. We multiplied the number of prescriptions for generic simvastatin and brand-name Zocor filled after patent expiration in states that required patient consent by the difference in cost of prescriptions in states that did and did not require consent in that calendar quarter. We found that Medicaid programs nationally could have saved approximately $19.8 million, almost 12% of all expenditures for simvastatin, in the 5 calendar quarters after patent expiration if all states adopted generic substitution policies that did not require patient consent.
Discussion
In this study, we evaluated the relation between three state-level policies and generic substitution rates after the patent expiration of Zocor, one of the world’s top-selling medications. After adjusting for two generic substitution policies that affect either the pharmacist’s or patient’s ability to influence whether a generic is medication is filled, and adjusting for prior authorization policies limiting the use of brand-name Zocor, we found that laws providing patients with greater discretion in determining generic drug substitution were most influential. Requiring patients to provide consent prior to generic substitution led to approximately a 25% reduction in generic substitution, while the other policies had no statistically significant effect in our adjusted model.
It is not surprising that requiring patients to provide consent would limit generic substitution. Recent surveys indicate that most patients believe that generics are safe and effective, that generics offer greater value than branded medications, and that more Americans should use generics.12 However, a majority of patients do not agree when asked if they, personally, prefer to use generics, and poor patients and less educated patients a group more likely to be covered by Medicaid - are least likely to express positive views of generics.12,13 It may be the most vulnerable patients, those for whom cost is the greatest barrier, that refuse generic substitution when offered. This may explain why patients who live in poorer neighborhoods are less likely to use generic medications,14 and may adversely affect patient adherence to essential medications.15 Pharmacists likely are more comfortable with generics than patients, which may explain why we found little effect of mandatory generic substitution on generic fill rates.16 The minimal effect of prior authorization requirements on generic substitution was surprising and suggests that generic substitution regulations are more potent stimuli for generic use upon patent expiration.
While patient consent requirements were strongly associated with generic substitution of simvastatin, we found no relationship between any of the regulations studied here and rates of switching from branded Lipitor, which did not have a generic alternative. Policymakers should be aware that these regulations may have the greatest effect on substitution for the same generic molecule, with little effect on switching among other molecules in the class.
Costs per prescription were substantially lower in states with generic substitution laws that did not require patient consent. However, the differences we observed may be attenuated by a number of factors. The spending reported in the aggregate Medicaid files reflect direct payments to pharmacies, set by each state Medicaid program as a portion of the average wholesale price (AWP) plus a dispensing fee for that pharmacy. States vary in how they set the proportion of AWP that is included in the retail price and the level of dispensing fee. States with more lenient generic substitution laws may be more aggressive about setting lower prescription drug prices for branded medications or negotiating rebates from manufacturers which could reduce the potential savings we estimate from eliminating patient consent policies. In addition, for the first six months after generic entry, the simvastatin market was characterized by an oligopoly, as Teva Pharmaceuticals and Ranbaxy Laboratories shared a period of generic market exclusivity while Merck also introduced an “authorized” generic made through a contract with Dr. Reddy’s Laboratories.17 As a result, during this time, simvastatin prices only fell slightly. The first substantial simvastatin price reductions did not occur until the first quarter of 2007, when the oligopoly period ended and additional competitors entered the market.18
Nonetheless, the prices per prescription are substantially lower in states that do not require patient consent for generic substitution. Laws requiring patient consent likely reflect an attempt to preserve patient autonomy in making decisions about their medical care. While this is an important priority, it is likely that such policy decisions are made in the abstract, without a sense of their opportunity costs. Our findings provide policy-makers with insight into the anticipated costs of these regulations. By simply multiplying the proportion of drug costs for simvastatin saved in the year after patent expiration in states that did not require consent by the annual spending for medications nearing patent expiration, we can roughly estimate potential savings from implementing laws that eliminate patient consent requirements. If the savings experienced by states that do not require patient consent were extended to states that currently do require patient consent, we would expect savings of over $100 million for state Medicaid programs for only 3 medications, Lipitor, Zyprexa, and Plavix, in the year after their respective patents expire. These projected savings would only be for Medicaid, which accounts for about 10% of total drug purchasing nationwide. Additional savings could be expected for private payors and for Medicare part D plans. Policy-makers will need to decide if these foregone savings can be justified in order to provide patients with greater choice in the setting of current economic strains on the healthcare system.
Our study is limited by the population that we studied. Generic substitution rates in this Medicaid population were lower than was seen in commercially-insured patients.7 Similar studies in a commercially-insured population are needed before generalizing more broadly. We only evaluated generic substitution after patent expiration of a single medication; results should be confirmed for other medications. We also did not control for different copayment requirements in the states we studied, we could not fully control for all formulary coverage policies within states or the precise levels of prices and rebates worked out by each state (such as policies where reimbursement is based on Maximum Allowable Cost pricing) and we could not measure the intensity with which each state enforces its coverage preferences. We hoped to capture a proxy for formulary management by assessing prior authorization rules, which have been shown to influence drug use,4 but additional policies may have been influential as well. However, it is unlikely that copayment requirements or other formulary procedures closely track with consent laws and not the other two laws studied here; we think it is unlikely that our findings could be entirely due to unmeasured confounding.
As states and other payors continue to experience the strain of reduced revenue to support healthcare expenses, those that require patient consent prior to generic substitution may consider altering this legislation. While it is generally appealing to provide patients with more choice in their medical care, a more restrictive approach to generic substitution may lead to cost savings without compromising quality, providing greater opportunities to invest healthcare dollars more cost-effectively.
Exhibit 6.
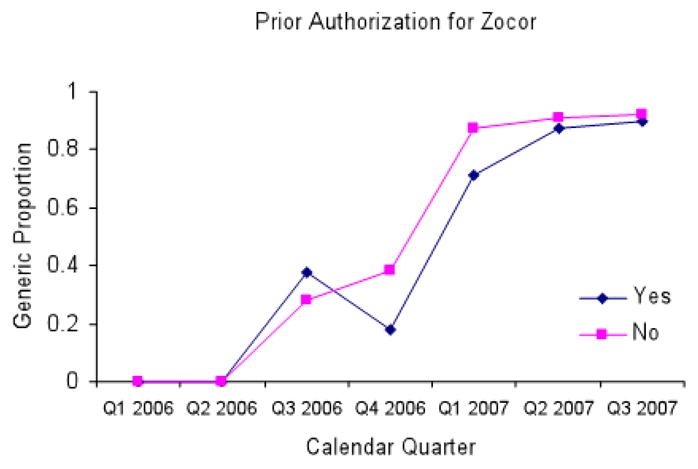
Relationship between legislation requiring prior authorization for Zocor and generic fill rates for simvastatin
Source: Aggregate Medicaid prescription medication use data
Exhibit 7.
Effect of three state policies on generic substitution rates*
| Parameter | Estimate | Standard Error | 95% Confidence Limits | Pr > |Z| | |
|---|---|---|---|---|---|
| Intercept | 1.0888 | 0.0933 | 0.9059 | 1.2718 | <.0001 |
| Mandatory Substitution | 0.1187 | 0.1222 | −0.1207 | 0.3582 | 0.3311 |
| Patient Consent Required | −0.2478 | 0.0974 | −0.4387 | −0.0569 | 0.0109** |
| Prior Authorization Required | −0.0406 | 0.0852 | −0.2075 | 0.1263 | 0.6335 |
Source: Our analysis of aggregate Medicaid prescription medication use data
Multivariable linear regression using generalized estimating equations to control for clustering at the state level
A time variable was included in the model but not shown
p < 0.05
Contributor Information
William H. Shrank, Email: wshrank@partners.org, Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School, in Boston, Massachusetts.
Niteesh K. Choudhry, Division of Pharmacoepidemiology and Pharmacoeconomics.
Jessica Agnew-Blais, Division of Pharmacoepidemiology and Pharmacoeconomics..
Alex D. Federman, Mount Sinai School of Medicine, in New York City.
Joshua N. Liberman, CVS Caremark, in Hunt Valley, Maryland.
Jun Liu, Division of Pharmacoepidemiology and Pharmacoeconomics..
Aaron S. Kesselheim, Division of Pharmacoepidemiology and Pharmacoeconomics.
M. Alan Brookhart, Division of Pharmacoepidemiology and Pharmacoeconomics..
Michael A. Fischer, Division of Pharmacoepidemiology and Pharmacoeconomics.
References
- 1.Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, Shrank WH. The Clinical Equivalence of Generic and Brand-Name Drugs Used in Cardiovascular Disease: A Systematic Review and Meta-Analysis. JAMA. 2008;300(21):2514–26. doi: 10.1001/jama.2008.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Facts and Figures [Internet] Alexandria (VA): National Association of Chain Drug Stores; c2009. [cited 2009 May 20]. Available from: http://www.nacds.org/wmspage.cfm?parm1=507#pharmpricing. [Google Scholar]
- 3.Top-line Industry Data [Internet] Norwalk (CT): IMS Health Incorporated; c2009. [cited 2009 May 21]. Available from: http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=936d9df4609e9110VgnVCM10000071812ca2RCRD&cpsextcurrchannel=1. [Google Scholar]
- 4.Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Engl J Med. 2004;351(21):2187–94. doi: 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg T, DeVito CA, Smith D, Stano M, Vidis JS, Moore WE, Dickson WM. Evaluation of economic effects of drug product selection legislation. Med Care. 1979;17(4):411–9. doi: 10.1097/00005650-197904000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Carroll NV, Fincham JE, Cox FM. The effects of differences in state drug product selection laws on pharmacists’ substitution behavior. Med Care. 1987;25(11):1069–77. doi: 10.1097/00005650-198711000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Aitken M, Berndt ER, Cutler DM. Prescription drug spending trends in the United States: looking beyond the turning point. Health Aff (Millwood) 2009;28(1):w151–60. doi: 10.1377/hlthaff.28.1.w151. [DOI] [PubMed] [Google Scholar]
- 8.Andersson K, Bergström G, Petzold MG, Carlsten A. Impact of a generic substitution reform on patients’ and society’s expenditure for pharmaceuticals. Health Policy. 2007;81(2–3):376–84. doi: 10.1016/j.healthpol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.State drug utilization data [Internet] Baltimore (MD): Health Care Financing Administration; c2000. [cited 2009 May 22]. Available from http://www.cms.hhs.gov/medicaid/drugs/drug5.asp. [Google Scholar]
- 10.National Association of Boards of Pharmacy. State Pharmacy Law and Rules Database. 2008. [Google Scholar]
- 11.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 12.Shrank WH, Cox E, Fischer MA, Mehta J, Choudhry NK. Patient perceptions of generic medications. Health Aff (Millwood) 2009;28(2):546–556. doi: 10.1377/hlthaff.28.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iosifescu A, Halm EA, McGinn T, Siu AL, Federman AD. Beliefs about generic drugs among elderly adults in hospital-based primary care practices. Patient Educ Couns. 2008;73(2):377–83. doi: 10.1016/j.pec.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrank WH, Stedman M, Ettner SL, DeLapp D, Dirstine J, Brookhart MA, et al. Patient, Physician, Pharmacy and Pharmacy Benefit Design Factors Related to Generic Medication Use. J Gen Intern Med. 2007;22(9):1298–304. doi: 10.1007/s11606-007-0284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166:332–7. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 16.Kirking DM, Gaither CA, Ascione FJ, Welage LS. Pharmacists’ individual and organizational views on generic medications. J Am Pharm Assoc. 2001;41:723–8. [Google Scholar]
- 17.Glass G. Authorized generics. Nature Reviews Drug Discovery. 2005;4:952–954. doi: 10.1038/nrd1906. [DOI] [PubMed] [Google Scholar]
- 18.Bloomberg News. Regulators clear 6 more companies to sell generic Zocor. The New York Times [Internet]; 2006. Dec 28, [cited 2009 Nov 13]. Available from http://www.nytimes.com/2006/12/28/business/28generic.html?_r=1#. [Google Scholar]


