Abstract
Despite the advances in perinatal and neonatal care and use of newer potent antibiotics, the incidence of neonatal sepsis remains high and the outcome is still severe. For years, investigators have sought a test or panel of tests able to identify septic neonates accurately and rapidly in order to obtain an early diagnosis and develop a specific effective treatment for a successful outcome. In addition to the standard procedures (blood, CSF, and urine cultures), such panels have included a combination of haematological investigations (total, differential and immature cell counts), and levels of acute-phase reactants (principally CRP and procalcitonin), and cytokines (such as IL-6 or neutrophil CD64). Furthermore, the science of proteomics and genomics has been applied to the search for bio-markers, production of protein profiles and genetic polymorphisms that can rapidly help the prediction, early diagnosis, and treatment of human diseases, but, for now, data are as yet insufficient to confirm their validity.
Key words: neonatal sepsis, IgG antibodies.
Introduction
The high incidence and severe outcome of neonatal sepsis, despite the advances in perinatal and neonatal care and use of newer potent antibiotics, is mainly related to the combination of the neonatal reduced immune defence and the complex interactions between the infecting microorganism and the host response.1–3 These factors are only partially mitigated by the transplacental passage of IgG antibodies from mother to fetus during intrauterine life. An additional naturally occurring compensatory mechanism is represented by human milk, that after birth maintains the mother-newborn immunological link by providing a host of protective components.4
Sepsis is a pathogen initiated but a cytokine-mediated condition in which immune, inflammatory, and coagulation homeostasis is disturbed.5 The evolution of disease and clinical symptoms are dependent upon a complex and delicate balance between the pro-inflammatory and anti-inflammatory factors. The inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8, IL-15, IL-18, MIF) and growth factors (IL-3, CSFs), and their secondary mediators, including nitric oxide, thromboxanes, leukotrienes, platelet-activating factor, prostaglandins, and complement, cause activation of the coagulation cascade, the complement cascade, and the production of prostaglandins, leukotrienes, proteases and oxidants. Most of the short (from systemic inflammatory response syndrome - SIRS, and disseminated intravascular coagulation - DIC, to septic shock, and multiple organ dysfunction syndrome - MODS) and long term complication (respiratory, growth and neurological sequelae6) of neonatal sepsis are strictly associated to the effects of these mediators,7–9 not counterbalanced by an adequate synthesis of anti inflammatory cytokines, as TNFsr, IL-1ra, IL-1rII, IL-10, TGF- β2.
Diagnosis of infection
The isolation of microorganisms from blood, cerebrospinal fluid (CSF) or urine remains the gold standard for definitive diagnosis, however, confirmation or exclusion of positive cultures requires days, and more importantly, the sensitivity of the culture methods is frequently low, due to the concomitant antibiotic therapy, or to the combination of small blood sample volume and low colony counts. When a 0.5 mL blood sample is obtained for culture (a likely occurrence in NICUs), the probability to isolate organisms is 0.39 with one CFU/mL, 0.67 with two CFU/mL, 0.87 with four. A count of at least 4 CFU/mL and one ml blood volume are necessary to reach a probability of 0.98.10
In addition, in neonates the clinical signs of sepsis are poor, late and non specific, particularly in preterm infants, in whom the onset of sepsis may be acute and clinical course can quickly deteriorate.
Therefore, early diagnosis of a life-threatening disease like neonatal sepsis, is the mandatory prerequisite for a timely treatment.11
For years, investigators have sought a test or panel of tests able to identify septic neonates accurately and rapidly while awaiting culture results, in order to obtain an early diagnosis and develop a specific effective treatment for a successful outcome.
Diagnostic test characteristics significantly change depending on sensitivity, specificity and predictive value. In relation to neonatal infection, these terms can be defined as follows:12
Sensitivity (positive test/positive diagnosis): if infection is present, how often is the test result abnormal?
Specificity (negative test/negative diagnosis): if infection is absent, how often is the test result normal?
Positive predictive value (positive diagnosis/positive test): if the test result is abnormal, how often is infection present?
Negative predictive value (negative diagnosis/negative test): if the test result is normal, how often is infection absent?
Likelihood ratio, positive test result: if the result is abnormal, how much does that result raise the pretest probability of disease?
Likelihood ratio, negative test result: if the result is normal, how much does that result lower the pretest probability of disease?
Large (>5–10) or small (<0.1–0.2) likelihood ratios significantly raise or minimize the probability of the disease being present.
The true positive rate (sensitivity) may be graphically plotted against the false positive rate (1 - specificity) for the different possible cut-off points of a diagnostic test, in order to obtain a Receiver Operating Characteristic (ROC) curve. The area under an ROC curve is a measure of test accuracy, the closer the curve follows the left-hand border and then the top border of the ROC space, the more accurate the test.13
Diagnostic tests with maximal (100%) sensitivity and negative predictive value are desirable for diagnosis of neonatal sepsis. In other words, if infection is present, the result would always be abnormal; if the result is normal, infection would always be absent. The reduced specificity and positive predictive value are usually acceptable because over treatment with antibiotics on the basis of a false-positive result is likely to be of limited harm compared with withholding therapy on the basis of a false-negative result. Although this approach seems reasonable given the dire outcome of a missed diagnosis, improvement in diagnostic accuracy should diminish the exposure of healthy neonates to the risks of unwarranted antibiotic treatment, decrease antibiotic resistances, and reduce the length and cost of hospital stays.12 Nonspecific laboratory investigations for the diagnosis of invasive bacterial infections remain the most important diagnostic aid for the management of septic neonates14 (Table 1).
Table 1. Laboratory investigations for diagnosis of neonatal sepsis.
| Specific laboratory tests |
| Blood, cerebrospinal fluid and urine culture |
| Direct visualisation of bacteria (Gram stain ..…) |
| Detection of bacterial antigens |
| Polymerase chain reaction (amplification of bacterial DNA, i.e. 16S rDNA) |
| Haematological investigations |
| White blood cell counts, total and differential, platelet count |
| CRP, procalcitonin, ESR, serum amyloid, other acute phase reactants: haptoglobin, lactoferrin, |
| neopterin, inter-inhibitor proteins (I Ips), lipopolysaccharide-binding protein (LBP), C5a, C5L2, immunoglobulins |
| Cytokines and receptors |
| IL-1 , IL-6, IL-8, IL-10 |
| IL-1ra, IL-2rs |
| IP-10, RANTES, TNF-α, IFN-γ |
| G-CSF, CSF1, SCF |
| MIP1-a |
| sCD14, sICAM-1, CD11b, CD64, CD69, CD25, CD45RO, CD19, CD33, CD66b |
| Proteomics and genomics |
White blood cell counts and ratios
Total leukocyte count (>20000 or <5000), differential leukocyte count and morphology, total neutrophil count, total nonsegmented neutrophil count, neutrophil ratios, platelet count are the indices most commonly used. These haematological counts and ratios showed a limited accuracy with wide range of sensitivity (17–90%) and specificity (31–100%), due to the relatively long period necessary to become positive and the significant influence of non-specific factors.
However, the band neutrophil:total neutrophil (IT) ratio of >0.2 may reach a sensitivity of 90% and negative predictive value of 98%.15,16 Indeed, the IT ratio is less influenced by non infectious factors, as the method of delivery.17
C-Reactive Protein
C-Reactive Protein (CRP) is a globulin that forms a precipitate when combined with the C-polysaccharide of Streptococcus pneumoniae. It is the most extensively acute phase reactant studied so far.
The sensitivity is low for early diagnosis of sepsis, due to the relatively long time, 6–8 hours following the stimulus, required for synthesis; the peak is observed at 24 hours, and the half life is 19 hours.
The qualitative assay of CRP does not offer significant advantages on the leukocyte indexes. On the other hand, quantitative CRP values, particularly when repeated, are highly specific and have good sensitivity. In addition, serial measurements can be helpful in monitoring the response to treatment. Two serial CRP values <1 mg/dL, excluding the investigation soon after birth, carry a 99% negative predictive value. In spite of the reduced early sensitivity, CRP still remains the preferred index in most NICUs.
Erythrocyte sedimentation rate
Microerythrocyte sedimentation rate (micro-ESR) by the use of a microhematocrit tube has been developed over 50 years ago. Normal values changes significantly during the first two weeks of life, and can be calculated by adding 2 or 3 to the age of the infant in days. Micro-ESR is quite specific; however, sensitivity is low due to the rise delay and to the long time required for normalization after clinical recovery. For these reasons it is considered of little value in either diagnosing or monitoring infection in the neonate.12
Procalcitonin
Procalcitonin, a propeptide of calcitonin, is released into the blood 3 to 6 hours after endotoxin injection and increases up to 24 hours; the increase does not correlate with calcitonin levels and occurs even in subjects who have had thyroidectomy.
Very high serum procalcitonin levels are present in neonates with proven or clinically diagnosed bacterial infection; early decrease of these concentrations reflects appropriate antibiotic therapy.18
Daily variations of procalcitonin levels in noninfected neonates have been reported, with a peak on day 1–2 of life, followed by a regular decrease to normal levels.19,20
Compared with CRP, procalcitonin has the advantage that it increases more rapidly; however, the significant rapid variations of basal levels after birth, and the need for several different cut-off values with changing neonatal age, have limited the diffusion of this marker in comparison to the CRP.21 Nevertheless, a recent meta-analysis suggested that procalcitonin showed better accuracy than the CRP test for the diagnosis of late-onset sepsis.22
In our opinion, procalcitonin should be used as an adjunct, rather than a replacement, to CRP to improve the diagnostic accuracy.
Increases of the above indices have been also associated with several non-infectious perinatal factors causing tissue injury or inflammation, such as maternal hypertension, mode of delivery, asphyxia, respiratory distress syndrome, intracerebral hemorrhage, meconium aspiration syndrome, prolonged crying, haemolytic disease, surgery,12 or post-natal age.23
Therefore, haematological indices for diagnosis of neonatal sepsis are of limited value in the early diagnosis of infection in this population.24 Clinicians faced with a neonate with suspected sepsis cannot rely on either C-reactive protein or leukocyte indices or procalcitonin alone to make a decision, given that the results vary significantly depending on the methods of measurement used and the target population.
Cytokines (and receptors) in the diagnosis of infection in neonates
An important limit of haematological indices for early diagnosis of sepsis is the time required for the test to become positive. It takes several hours for leukocyte indices and acute-phase reactants to change significantly after the onset of reaction.
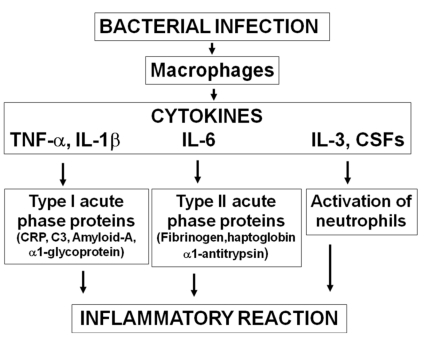
The cascade of events initiated by the bacterial infection usually begins with the activation of macrophages and release of inflammatory cytokines (TNF-α, IL-1β, IL-6) and growth factors (IL-3, CSFs). These trigger the inflammatory reaction, with acute phase reactants synthesis by the liver and activation of neutrophils. The increase of cytokines levels is therefore observed much earlier than that of haematological indices (Figure 1).
Figure 1.
Inflammatory reaction during sepsis.
Several cytokines and receptors have been evaluated for the early diagnosis of infection in neonates (Table 1).25–33
lnterleukin-6 (IL-6) is a pleiotropic cytokine involved in many aspects of the immune system. It is synthesized by a number of cells, such as monocytes, endothelial cells, and fibroblasts, after TNF and IL-1 stimulation. IL-6 is the major inducer of hepatic acute-phase protein synthesis, including CRP and fibrinogen. In most cases of neonatal sepsis, IL-6 increases rapidly, several hours before the increase in the concentration of CRP, and decreases within 24 hours to undetectable levels. The short half-life of IL-6 is caused by binding to plasma proteins such as α2-macroglobulin, early clearance in the liver, and inhibition by other cytokines.
When used as a very early marker of infection, IL-6 has good sensitivity and good specificity.
A study showed that IL-6 and interleukin-1 receptor antagonist (IL-1ra) increased significantly two days before clinical diagnosis of sepsis. The diagnostic sensitivities of IL-1ra, IL-6, and CRP concentrations on day 0 of diagnosis were 93%, 86%, and 43%, respectively; corresponding values one day before diagnosis of infection were 64%, 57%, and 18%. The specificities of IL-1ra, IL-6, and CRP concentrations were 92%, 83%, and 93%.26
In contrast to CRP, IL-6 is a very early marker, but levels can become normal even if infection continues. This leads to an increasing proportion of false-negative findings when sampling is performed later in the course. The simultaneous determination of CRP can obviate this problem, because the rise in plasma CRP levels occurs 12 to 48 hours after the onset of infection, at a time when IL-6 levels probably would have fallen. When these two markers are combined, the sensitivity is high for infected infants at any postnatal age. The specificity of IL-6 is reduced in infants with perinatal asphyxia.
Screening panels
None of the adjunctive tests considered earlier is sufficiently sensitive and specific to exclude or confirm the diagnosis of neonatal sepsis. Clinicians have considered screening panels or a sepsis screen that combine data from several individual analyses. In addition to the standard procedures (blood, CSF, and urine cultures, chest film), such panels have included a combination of total and differential cell counts, total immature neutrophil counts, immature to total neutrophil ratio, platelet counts, and levels of acute-phase reactants and cytokines.
At present, the best combination of markers for diagnosing sepsis consists of performing IL-6 and IL-1ra one-two days before the onset of symptoms, IL-6 (or IL1-ra, IL-8, CD11b, CD64, G-CSF, TNF-α), CRP, procalcitonin, and haematological indices on day 0, CRP, haematological indices, and procalcitonin on the following days to monitor the response to therapy.
Overall, the results of these studies have shown little increase in positive predictive value compared with most of the individual screening tests, although negative predictive value is remarkably good, approaching 100% in some studies. Because of the high negative predictive value, screening panels may produce a significant decrease in the use of antimicrobial agents. With their use, fewer neonates would receive antimicrobial agents, and antibiotic treatment could be more confidently discontinued earlier.12,14
It should be emphasized, however, that screening tests are intended only to augment clinical evaluation. When the evidence obtained by history or physical examination conflicts with a negative screen, antimicrobial therapy should be started. Thus, clinical signs of sepsis remain the most important criteria for use of antimicrobial agents.
Proteomics and genomics
Modern applications of molecular pathology embrace various disciplines: genomics (sequence DNA), transcriptomics (mRNA identification), proteomics (protein identification) and pharmacogenomics (genes that define the behavior of drugs).
Proteomics is the study of expressed proteins in a tissue, cell, or organism at a given moment. It is more clinically significant and easier to translate into diagnostic tools and therapeutical strategies than genomics, which studies DNA. In fact, proteomics examine directly functional molecules and not the source code. Evidence to support this statement is that protein quantity and activity do not show a relationship with mRNA amount.
The science of proteomics has been applied to the search for biomarkers and production of protein profiles that can rapidly help the prediction, early diagnosis, and treatment of human diseases.27
Several informations can be obtained from proteomics study, although only part of these are biologically significant. To analyze proteomics data from SELDI-TOF(enhanced laser desorption/ionization time of flight) outputs, Buhimschi et al. developed a strategy to extract significant proteomic biomarkers characteristic for intra-amniotic inflammation, based on sequentially applied filter preferences. This strategy was called mass restricted (MR) scoring. The MR score indicate the number of identifiable markers among the following factors of innate immunity: neutrophil defensin-2, neutrophil defensin-1, S100A12 (calgranulin C) and S100A8 (calgranulin A).34
An MR score of 3–4 indicated the presence of inflammation, while a score of 0–2 excluded it.
The same authors prospectively validated the clinical utility of the MR score in predicting preterm births and neonatal sepsis in a cohort of 169 consecutive women with single pregnancies. An MR score of 3–4 had the highest accuracy (92.6%) in diagnosing intra-amniotic inflammation, and was significantly better than white blood cell (WBC) count or IL-6. Therefore, high MR scores are associated with preterm delivery, histological chorioamnionitis, and early-onset neonatal sepsis (EONS).
Detection of monomeric S100A8 (calgranulin A) in the amniotic fluid was closely associated with EONS. The gestational age and the presence of S100A12 and S100A8 in amniotic fluid tightly correlated with neonatal neuro-developmental impairment.
In conclusion, the amniotic fluid proteome closely reflects the fetal inflammatory response to intra-amniotic infection and SELDI-TOF technology carries the attributes to allow for a direct, rapid and reliable identification of proteomic biomarkers of intrauterine inflammation.
There are also molecular-based approaches for diagnosis of neonatal infection:
i) whole blood directly tested by target amplification; ii) whole blood pre-enrichment before target amplification; iii) fluids from positive blood culture bottles tested by polymerase chain reaction (PCR); iv) Nucleic Acid Sequence-Based Amplification (NASBA); v) Nucleic Acid Amplification Tests (NAAT); vi) PCR in conjunction with sequencing or microarray analysis; vii) non amplification-based fluorescence in situ hybridization (FISH).
The sensitivity and specificity of real-time PCR assay was 96.2% and 100% respectively,35 with a limit of recognition for E.Coli and group B Streptococcus, while in another study that targeted several larger 16S rDNA, was 66.7% and 87.5%.36
Makhoul et al. showed that sensitivity, specificity, positive predictive value and negative predictive value of Staphylococcus-specific PCR, used for detection of staphylococcal bacteremia, was 57.1%, 94.7%, 53.3% and 95.4%, respectively.37
A technique based on PCR to amplify the gene cfb directly from swabs to accurately identify the colonization by group B streptococcus, with results within 1–2 hours, was recently approved for use in both prepartum and intra-partum by the Food and Drug Administration (FDA), while NAAT for Perinatal Group B Streptococcus identification has been considered in the most recent Guidelines from CDC.38 Despite the cfb-PCR has been approved by the FDA, Atkins39 showed, by comparing the PCR and double (direct inoculation on selective agar and broth) culture methods in a study of 233 samples, that PCR had sensitivity, specificity, positive and negative predictive value of 86.8%, 95.2%, 88.1% and 94.6%, respectively, with a false negative rate of 13.2%.
Using whole blood directly in a target amplification-based assay for detecting bacteria has the advantage of rapid diagnosis, but the challenges of suboptimal sensitivity and specificity. Several strategies are under investigations to improve this problem, as an increase of blood volume (but it is not always possible, particularly in small infants), improvement of extraction procedures to obtain greater recovery of bacterial nucleic acid over human genomic DNA, or whole blood pre-enrichment.
Bacterial detection platforms such as DNA microarray, FISH, and mass spectrometry do not require target amplification. Several studies reported that the sensitivity and the specificity of this molecular-based approaches was from 98–100% and from 99–100%, respectively.
The major benefit of a molecular test is speed; however, so far, none of the rapid test has been shown to have a sensitivity and specificity sufficient to replace standard blood culture techniques, that carries the additional important advantage of the ability of testing pathogens antibiotics susceptibility.
Genetic polymorphisms
The possibility of understanding the genetic contribution to response to microbial pathogens remains one of the most stimulating prospects of the unravelling of the human genome. The identification of strong associations between certain genetic polymorphisms and susceptibility to severe sepsis supports further research using appropriate association studies.40
Recent evidence that the genetic background of the host affects the systemic response to infection has stimulated considerable interest in the evaluation of genetic susceptibility to sepsis, concerning in particular factors of the initial immune response of the innate immunity, as Toll-like receptors (TLRs), mannose-binding lectin (MBL), nucleotide-binding oligomerization domain (NODs) and cytokines.41–43
Genes involved in the regulation of immune function, particularly the systemic inflammatory response, have been evaluated in an attempt to identify markers of infection in neonates.44
A research performed on two polymorphisms of TNF did not provide evidence that these can influence the incidence of early-onset sepsis in premature infants.45
Other studies about IL-6 failed to identify a strong genetic correlation.46,47 A recent meta-analysis assessed the evidence for the association of the IL-6 (−174C) polymorphism (guani-dine to cytosine transition at position −174 nucleotides relative to the transcription start site in the interleukin-6 gene) with the risk of sepsis in Very Light Birth Weight (VLBW) newborn infants. The results of six cohort studies including a total of 1323 VLBW infants found no significant association between carriage of the IL-6 (−174C) polymorphism and sepsis: pooled relative risk 0.90 (95% CI 0.62 to 1.31). These data did not support screening infants for this allele in order to guide selective antimicrobial prophylaxis.48
As the inflammatory cytokine cascade is implicated in the pathogenesis of necrotising enterocolitis (NEC), the associations between the common genetic variants in candidate inflammatory cytokine genes and NEC in preterm infants was examined in a meta-analysis. Ten single-nucleotide polymorphisms in cytokines previously associated with infectious or inflammatory diseases were genotyped: TNF (2308A), TNF (2238A), IL1 (231G), IL1 (2511T), IL4R (+1902G), IL6 (2174C), IL8 (2251A), IL10 (21082G), IL18 (2137C), IL18 (2607A). The results suggested that available data were not consistent with more than modest associations between these candidate cytokine variant alleles and NEC in preterm infants.49
Del Vecchio et al compared the prevalence of TNF-α and IL-10 polymorphisms in preterm neonates with late-onset sepsis with a noninfected reference group. In the septic neonates, 308G-TNF-α and 1082-IL-10 polymorphisms resulted, in homozygous and heterozygous forms, more frequent with statistical significance. The authors, however, concluded that the analysis of a larger group of subjects was needed to confirm these data.50
Recently, a significant association of IL-6-174CC and IL-10-1082GG genetic polymorphisms in homozygosis with increased risk of mortality was reported. Moreover, the studied genotypes were significantly higher in neonates who required inotropic support and those who developed disseminated intravascular coagulopathy.51
Conclusions
On the whole, these preliminary studies tend to confirm that genetic variations and single nucleotide polymorphisms of factors involved in the immune defence against infection are significantly involved in the pathogenesis of sepsis, and may influence the outcome. Data, however, are as yet insufficient to consider these factor useful for the evaluation of the risk to develop sepsis, or to include any of these markers in the screening panels currently used for the early diagnosis or to guide the treatment of infection in the neonate.52
References
- 1.Chirico G, Cortinovis S, Fonte C, Giudici G. Bacterial sepsis. J Chemother. 2007;19:28–30. doi: 10.1080/1120009x.2007.11782440. [DOI] [PubMed] [Google Scholar]
- 2.Chirico G. Development of the Immune System in Neonates. J Arab Neonatal Forum. 2005;2:5–11. [Google Scholar]
- 3.Lewis DB, Tu W. The physiologic immunodeficiency of immaturity. In Immunologic disorders in infants and children. In: Stiehm ER, Ochs HD, Winkelstein JA, editors. fifth Edition. Philadelphia: Elsevier Saunders Company; 2004. [Google Scholar]
- 4.Chirico G, Marzollo R, Cortinovis S, et al. Antiinfective properties of human milk. J Nutr. 2008;138:1801S–1806S. doi: 10.1093/jn/138.9.1801S. [DOI] [PubMed] [Google Scholar]
- 5.Russell JA. Management of Sepsis. N Engl J Med. 2006;355:1699–713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and Growth Impairment Among Extremely Low-Birth-Weight Infants With Neonatal Infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 7.Hermansen MC, Hermansen MG. Perinatal Infections and Cerebral Palsy. Clin Perinatol. 2006;33:315–33. doi: 10.1016/j.clp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Dammann O, Leviton A. Inflammation, brain damage and visual dysfunction in preterm infants. Semin Fetal Neonatal Med. 2006;11:363–8. doi: 10.1016/j.siny.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.La Pine TR, Hill HR. Host defense mechanisms against bacteria. In: Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. 3th ed. Philadelphia: Saunders; 2004. pp. 1475–1486. [Google Scholar]
- 10.Schelonka RL, Chai MK, Yoder BA, et al. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129:275–8. doi: 10.1016/s0022-3476(96)70254-8. [DOI] [PubMed] [Google Scholar]
- 11.Mishra UK, Jacobs SE, Doyle LW, Garland SM. Newer approaches to the diagnosis of early onset neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2006;91:208–212. doi: 10.1136/adc.2004.064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg GA, D’Angio CT. Laboratory Aids for Diagnosis of Neonatal Sepsis. In: Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. Sixth ed. Philadelphia: W.B. Saunders Company; 2006. pp. 1207–1222. [Google Scholar]
- 13.Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157–62. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benitz WE. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol. 2010;37:421–38. doi: 10.1016/j.clp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Da Silva O, Ohlsson A, Kenyon C. Accuracy of leukocyte indices and C-reactive protein for diagnosis of neonatal sepsis: a critical review. Pediatr Infect Dis J. 1995;14:362–6. doi: 10.1097/00006454-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Hatherill M, Tibby SM, Sykes K, et al. Diagnostic markers of infection: comparison of procalcitonin with C reactive protein and leucocyte count. Arch Dis Child. 1999;81:417–21. doi: 10.1136/adc.81.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirico G, Gasparoni A, Ciardelli L, et al. Leukocyte counts in relation to the method of delivery during the first five days of life. Biol Neonate. 1999;75:294–9. doi: 10.1159/000014107. [DOI] [PubMed] [Google Scholar]
- 18.Mussap M, Degrandi R, Cataldi L, et al. Biochemical markers for the early assessment of neonatal sepsis: the role of procalcitonin. J Chemother. 2007;19:35–8. doi: 10.1080/1120009x.2007.11782442. [DOI] [PubMed] [Google Scholar]
- 19.Assumma M, Signore F, Pacifico L, et al. Serum procalcitonin concentrations in term delivering mothers and their healthy offspring: a longitudinal study. Clin Chem. 2000;46:1583–7. [PubMed] [Google Scholar]
- 20.Chiesa C, Signore F, Assumma M, et al. Serial measurements of C-reactive protein and interleukin-6 in the immediate postnatal period: reference intervals and analysis of maternal and perinatal confounders. Clin Chem. 2001;47:1016–22. [PubMed] [Google Scholar]
- 21.Lapillonne A, Basson E, Monneret G, et al. Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet. 1998;351:1211–2. doi: 10.1016/S0140-6736(05)79165-0. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z, Liu J, Sun Q, et al. The accuracy of the procalcitonin test for the diagnosis of neonatal sepsis: a meta-analysis. Scand J Infect Dis. 2010;42:723–33. doi: 10.3109/00365548.2010.489906. [DOI] [PubMed] [Google Scholar]
- 23.Chirico G, Motta M, Villani P, et al. Late-onset neutropenia in very low birthweight infants. Acta Paediatr Suppl. 2002;91:104–8. doi: 10.1111/j.1651-2227.2002.tb02913.x. [DOI] [PubMed] [Google Scholar]
- 24.Fowlie PW, Schmidt B. Diagnostic tests for bacterial infection from birth to 90 days a systematic review. Arch Dis Child. 1998;78:F92–F98. doi: 10.1136/fn.78.2.f92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berner R, Niemeyer CM, Leititis JU, Funke A, Schwab C, Rau U, Richter K, Tawfeek MS, Clad A, Brandis M. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, inter-leukin (IL)-1beta, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res. 1998;44:469–77. doi: 10.1203/00006450-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Buscher U, Chen FC, Pitzen A, Menon R, Vogel M, Obladen M, Dudenhausen JW. Il-1 beta, Il-6, Il-8 and G-CSF in the diagnosis of early-onset neonatal infections. J Perinat Med. 2000;28:383–8. doi: 10.1515/JPM.2000.049. [DOI] [PubMed] [Google Scholar]
- 27.Ng PC, Lam HS. Biomarkers for late-onset neonatal sepsis: cytokines and beyond. Clin Perinatol. 2010;37:599–610. doi: 10.1016/j.clp.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantar M, Kultursay N, Kutukculer N, Akisu M, Cetingul N, Caglayan S. Plasma concentrations of granulocyte-macrophage colony-stimulating factor and interleukin-6 in septic and healthy preterms. Eur J Pediatr. 2000;159:156–7. doi: 10.1007/s004310050041. [DOI] [PubMed] [Google Scholar]
- 29.Chirico G, Ciardelli L, Cecchi P, De Amici M, Gasparoni A, Rondini G. Serum concentration of granulocyte colony stimulating factor in term and preterm infants. Eur J Pediatr. 1997;156:269–71. doi: 10.1007/s004310050598. [DOI] [PubMed] [Google Scholar]
- 30.Krueger M, Nauck MS, Sang S, Hentschel R, Wieland H, Berner R. Cord blood levels of interleukin-6 and interleukin-8 for the immediate diagnosis of early-onset infection in premature infants. Biol Neonate. 2001;80:118–23. doi: 10.1159/000047130. [DOI] [PubMed] [Google Scholar]
- 31.Romagnoli C, Frezza S, Cingolani A, De Luca A, Puopolo M, De Carolis MP, Vento G, Antinori A, Tortorolo G. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr. 2001;160:345–50. doi: 10.1007/pl00008445. [DOI] [PubMed] [Google Scholar]
- 32.Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121:129–34. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 33.Küster H, Weiss M, Willeitner AE, Detlefsen S, Jeremias I, Zbojan J, Geiger R, Lipowsky G, Simbruner G. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet. 1998;352:1271–7. doi: 10.1016/S0140-6736(98)08148-3. [DOI] [PubMed] [Google Scholar]
- 34.Buhimschi IA, Buhimschi CS. The role of proteomics in the diagnosis of chorioamnionitis and early-onset neonatal sepsis. Clin Perinatol. 2010;37:355–74. doi: 10.1016/j.clp.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan JA, Durso MB, Butchko AR, Jones JG, Brozanski BS. Evaluating the near-term infant for early onset sepsis: progress and challenges to consider with 16S rDNA polymerase chain reaction testing. J Mol Diagn. 2006;8:357–63. doi: 10.2353/jmoldx.2006.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reier-Nilsen T, Farstad T, Nakstad B, Lauvrak V, Steinbakk M. Comparison of broad range 16S rDNA PCR and conventional blood culture for diagnosis of sepsis in the newborn: a case control study. BMC Pediatr. 2009;9:5–5. doi: 10.1186/1471-2431-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makhoul IR, Yacoub A, Smolkin T, Sujov P, Kassis I, Sprecher H. Values of C-reactive protein, procalcitonin, and Staphylococcus-specific PCR in neonatal late-onset sepsis. Acta Paediatr. 2006;95:1218–23. doi: 10.1080/08035250600554250. [DOI] [PubMed] [Google Scholar]
- 38.Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases. Prevention of Perinatal Group B Streptococcal Disease - Revised Guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59((RR-10)):1–36. [PubMed] [Google Scholar]
- 39.Atkins KL, Atkinson RM, Shanks A, et al. Evaluation of polymerase chain reaction for group B streptococcus detection using a improved culture method. Obstet Gynecol. 2006;108:488–91. doi: 10.1097/01.AOG.0000228961.42272.31. [DOI] [PubMed] [Google Scholar]
- 40.Kwiatkowski D. Science, medicine, and the future: susceptibility to infection. BMJ. 2000;321:1061–5. doi: 10.1136/bmj.321.7268.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroder J, Kahlke V, Book M, Stuber F. Gender differences in sepsis: genetically determined? Shock. 2000;14:307–10. [PubMed] [Google Scholar]
- 42.Fleer A, Krediet TG. Innate immunity: toll-like receptors and some more. A brief history, basic organization and relevance for the human newborn. Neonatology. 2007;92:145–57. doi: 10.1159/000102054. [DOI] [PubMed] [Google Scholar]
- 43.Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114:347–60. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- 44.Del Vecchio A, Ladisa G, Laforgia N, De Felice C, Presta G, Latini G. Genetic polymorphisms in neonatal sepsis. Haematologica Reports. 2006;2:31–37. (available at http://www.pagepress.org/ journals/index.php/hmr/article/viewFile/ 449/464) [Google Scholar]
- 45.Schueller AC, Heep A, Kattner E, Kroll M, Wisbauer M, Sander J, Bartmann P, Stuber F. Prevalence of Two Tumor Necrosis Factor Gene Polymorphisms in Premature Infants with Early Onset Sepsis. Biol Neonate. 2006;90:229–32. doi: 10.1159/000093605. [DOI] [PubMed] [Google Scholar]
- 46.Levy O. Genetic screening for susceptibility to infection in the NICU setting. Pediatr Res. 2004;55:546–8. doi: 10.1203/01.PDR.0000112161.48023.86. [DOI] [PubMed] [Google Scholar]
- 47.Michalek J, Svetlikova P, Fedora M, Klimovic M, Klapacova L, Bartosova D, Hrstkova H, Hubacek JA. Interleukin-6 gene variants and the risk of sepsis development in children. Hum Immunol. 2007;68:756–60. doi: 10.1016/j.humimm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Chauhan M, McGuire W. Interleukin-6 (−174C) polymorphism and the risk of sepsis in very low birth weight infants: meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008;93:F427–9. doi: 10.1136/adc.2007.134205. [DOI] [PubMed] [Google Scholar]
- 49.Henderson G, Craig S, Baier RJ, Helps N, Brocklehurst P, McGuire W. Cytokine gene polymorphisms in preterm infants with necrotising enterocolitis: genetic association study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F124–8. doi: 10.1136/adc.2007.119933. [DOI] [PubMed] [Google Scholar]
- 50.Del Vecchio A, Laforgia N, Capasso M, Iolascon A, Latini G. The role of molecular genetics in the pathogenesis and diagnosis of neonatal sepsis. Clin Perinatol. 2004;31:53–67. doi: 10.1016/j.clp.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Hady H, El-Naggar M, El-Nady G, Badr R, El-Daker M. Genetic Polymorphisms of IL-6-174 and IL-10-1082 in Neonatal Blood Stream Infections. JPID. 2009;4:357–65. [Google Scholar]
- 52.Chirico G, Chiappa S. Genetic polymorphisms and susceptibility to infection in neonates. JPID. 2009;4:317–20. [Google Scholar]