Abstract
Background & objectives :
A large number of cases of undiagnosed fever and joint pain were reported from different parts of the State of Orissa since February 2006. Epidemiological and laboratory investigation were carried out to confirm the cause of emerging illness, which was provisionally suspected as Chikungunya (CHIK) fever.
Methods:
Upon getting the reports of suspected CHIK like illness in different parts of the State, epidemic investigations were carried out in the outbreak affected villages. Case history was recorded, clinical examination undertaken and blood samples collected for seroconfirmation for CHIK IgM antibody using ELISA based kit. Simultaneously vector survey was also carried out.
Results:
With no previous record of CHIK infection in the State, the first outbreak was confirmed during February 2006. Subsequently, the infection spread to 13 of 30 districts in different episodes covering 79 villages till November 2007. Attack rate was 9-43 per cent in the different outbreaks with average seropositivity of 24 per cent to CHIK specific IgM. Morbidity was high though no deaths were recorded. Aedes aegypti and Ae. albopictus were identified as the possible vectors for transmission.
Interpretation & conclusions :
The report confirmed emergence of CHIK infection in the State of Orissa, India, and its spread to a larger geographic zone in a short period which warrants public health measures to control further spread.
Keywords: Aedes, CHIK, epidemic, fever, Orissa
Chikungunya (CHIK) virus is an Arbo virus of the genus Alphavirus and family Togaviridae1,2. Infection due to CHIK virus results in a debilitating but non-fatal illness in form of epidemics in favoured areas with abundance of the vector species i.e., Aedes aegypti and Ae. albopictus. It was suspected to be originated from Africa and was isolated for the first time from the blood of a febrile patient during the Tanzanian outbreak in 19523. Since that time it has been reported to be the cause of many epidemics in Western, Central and Southern Africa and many parts of Asia4.
In India, major epidemics of CHIK fever were reported during 1963-1973 affecting States like West Bengal, Maharastra, Tamil Nadu, Andhra Pradesh, Madhya Pradesh and Puducherry. The infection re-emerged as epidemics after a silence of 32 years i.e., in 2005 affecting many States in the country5.
With no previous record of CHIK infection from Orissa (India), sudden onset of cases with fever and joint pain appeared in large numbers in different parts of the State in 2006-2007. Investigation was carried out to identify the cause of the epidemics, which was suspected as CHIK infection. This report briefly presents about the confirmed CHIK outbreaks from different parts of the State, beginning with the first report from February 2006 until November 2007.
Material & Methods
Study area: The state of Orissa is located on the East coast of India in between 17° 48’ and 22° 34’ North latitude and 81° 24’ and 87° 29’ East longitude. The investigation was carried out in the affected parts situated in both the coastal planes and hilly region of the State. The study covered 33 affected blocks from 13 of 33 districts namely Sundergarh, Gajapati, Bhadrak, Ganjam, Jajpur, Kendrapada, Nayagarh, Khurda, Balasore, Puri, Cuttack, Keonjhar and Jagatsinghpur. This investigation was undertaken in different episodes as per the report of CHIK suspected cases detected during the period from February 2006 to November 2007.
Epidemiological investigation: Field investigation was carried out by the team from RMRC comprising of clinicians, epidemiologist, entomologists, and laboratory and census personnel. Necessary assistance was sought from State Health Epidemic Response Team while investigating the outbreaks for identification of villages, case enumeration and in some areas for sample collection and follow up of the individual cases. Clinical and epidemiological examination was done by house-to-house visit in the affected villages. The individuals with symptoms of suspected CHIK virus infection were enlisted and examined. An individual presenting with sudden onset of fever and/or joint pain with or without associated symptoms like myalgia, rash and swelling of joints, was considered as a clinical case for CHIK fever6. Detailed history and observations were recorded including date of onset of illness, migration and probable exposure, family affection, course of disease, symptoms and signs, recovery of illness and treatment received. Symptomatic treatment was provided to the affected people by the team doctors. Blood sample (4-5 ml) was collected from the willing individuals after obtaining informed written consent for laboratory confirmation. Thick blood smears were collected for examination of malaria parasite. The study was approved by the Institutional Ethics Committees.
Entomological investigation: Entomological survey was conducted in the villages by household visit for presence of vector mosquitoes Aedes species known to cause CHIK virus transmission for both adult and larvae. Collection of resting adult was carried out from different locations inside of the house and cow sheds, using sucking tube and mechanical aspirator7. Adults were identified using keys of Barraud8. For collection of larvae, all containers with water were searched inside and outside the houses using dip method or by a Pasteur pipette. Collected larvae were reared to adults and identified. The per man hour density (PMHD) for each species was calculated as number of mosquitoes collected by a man in one hour. Larval collection data were used to calculate house, container and Breteau indices using standard formulae6.
Laboratory diagnosis: Serum samples were tested for CHIK and dengue IgM antibody using IgM antibody capture ELISA kit produced by National Institute of Virology (NIV), Pune, India4. Antigens from African strain of CHIK virus and dengue serotype 2 were used for the respective diagnostic kits. The tests were carried out following the manufacturer instruction. The sample was considered positive for IgM antibody when sample optical density (OD)/negative OD was > 2.1. Both positive and negative controls were used to validate the test. Thick blood smears were examined for presence of malaria parasite in the nearest primary health centre and a part cross-checked at RMRC laboratory.
Statistical analysis: Chi-square test was used to test for association.
Results
Outbreak period, area and population affected: Outbreaks of CHIK were recorded from 13 of the 30 districts of Orissa covering 33 revenue blocks and 78 villages. It was reported for the first time from Panposh town of Sundergarh District. Subsequently it was observed from four more districts during 2006 and 11 districts during 2007 (Table I).The epidemics were reported from both small and big villages/ towns with population ranging from 200>5000. Attack rate ranged from 0.4 to 50.76 per cent in the different villages. The outbreaks were observed to continue for 6 days to 2 months in different areas/episodes. A total of 10867 individuals were recorded to be affected out of 77,866 population at risk in the affected areas. Appearance of CHIK cases in the State, is shown in a weekly epidemic plot in the Fig. It indicated that except the first major outbreak which occurred during spring (February & March), majority of cases were reported in the months of September to November in both the calendar years i.e., 2006 and 2007, which coincided with late or post monsoon period. Population from all ages and both sexes were shown to be affected from CHIK illness (Table II). Comparatively more number of cases (21-31%) were reported from 16-45 yr age group in both the genders while lower numbers (5-6.4%) were seen from elderly people above sixty years (P<0.05).
Table I.
Distribution of Chikungunya outbreaks in different districts of Orissa
| District | Block (No. of Village/Town) | Period of outbreak | Population at risk | No. of clinical cases | Case attack rate (%) |
| Sundergarh | Panposh town (1) | 27.02.06 - 6.3.06 | 10233 | 5194 | 50.76 |
| Gajapati | Parlakhemundi (1) | 22.07.06 - 30.07.06 | 354 | 39 | 11.02 |
| Ganjam | Keluapali (1) | 21.08.06 - 30.08.06 | 401 | 70 | 17.46 |
| Kendrapada | Mahakalapada (2) | 08.09.06 - 25.10.06 | 2495 | 752 | 30.14 |
| Cuttack | Mahanga (4) | 13.09.06 - 14.10.06 | 1832 | 537 | 29.31 |
| Nayagarh | Odagaon (4) | 2.05.07 - 7.06.07 | 8820 | 932 | 10.57 |
| Bhadrak | Barpada (1) | 7.06.07 - 15.06.07 | 300 | 88 | 29.33 |
| Ganjam | Beguniapada (1) Buguda (1) Polasara (3) | 21.07.07 - 17.09.07 | 3000 | 50 | 1.67 |
| Nayagarh | Bhapur (1) Khandapada (3) | 20.08.07 - 25.09.07 | 1682 | 180 | 10.70 |
| Cuttack | Niali (1) | 27.08.07 - 4.10.07 | 500 | 2 | 0.40 |
| Jajpur | Madhuban (1) Badachana (1) Bari (1) Rasulpur (4) | 29.08.07 - 2.10.07 | 4890 | 462 | 9.45 |
| Bhadrak | Dhamnagar (1) | 1.09.07 - 12.09.07 | 3500 | 226 | 6.46 |
| Kendrapada | Mahakalapada (7) Garadpur (10) Rajkanika Deabish (1) (3) Kendrapada (4) | 1.09.07 - 6.11.07 | 26117 | 655 | 2.51 |
| Puri | Nimapara (3) Satyabadi (1) Puri sadar (4) Gop (1) Astaranga (1) | 4.09.07 - 3.11.07 | 4691 | 826 | 17.61 |
| Jagatsinghpur | Nuagaon (3) Mandasahi (3) | 20.09.07 - 10.10.07 | 6451 | 684 | 10.60 |
| Khurda | Bankoi (2) | 22.09.07 - 31.10.07 | 1024 | 80 | 7.81 |
| Balesore | Bhogarai (2) | 8.10.07 - 5.11.07 | 776 | 50 | 6.44 |
| Keonjhar | Ananadpur (1) | 10.10.07 - 3.11.07 | 800 | 40 | 5.00 |
| Total district - 13 | 33 blocks (78) | Feb 06 - Nov 07 | 77866 | 10867 | 13.96 |
Fig.
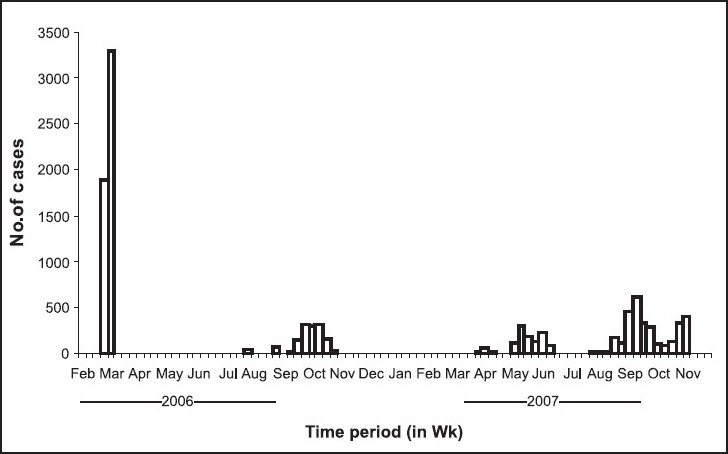
Epidemic curve showing week-wise onset of CHIK cases in Orissa (2006-2007).
Table II.
Age and sex distribution of CHIK cases in the State (2006-2007)
| Age group (yr) | Number of Chikungunya cases |
||
| Male (%) | Female (%) | Total (%) | |
| 0-15 | 1111 (22.5) | 1246 (20.9) | 2357 (21.6) |
| 16-30 | 1348 (27.3) | 1876 (31.5) | 3224 (29.6) |
| 31-45 | 1255 (25.4) | 1257 (21.1) | 2512 (23.1) |
| 46-60 | 18 (896.1) | 1255 (21.1) | 2151 (19.7) |
| >60 | 316 (6.4) | 307 (5.1) | 623 (5.7) |
| Total | 4926 (45.3) | 5941 (54.6) | 10867 |
Statistical significance not shown; X2 P< 0.05 – as given in Results
Signs and symptoms: Acute onset of fever or joint pain was the initial symptom to present with. Common symptoms complained were fatigue (89.2%) and fever (87%). Fever was also associated with chills (37%), headache (63%) and head reeling (68%). Joint pain with/without swelling was present in 68.5 per cent individuals and it was with fever in 64 per cent cases. Joint pain started on first or second day of fever and involved both small and big joints of extremity and spine. Maculopapular rash often with itching was seen in both trunk and extremities in 35 per cent cases and haemorrhagic manifestations (nasal and gum bleeding) were observed in a few individuals (0.9%). Anorexia, nausea and pain abdomen were the other associated minor symptoms. Majority of the symptoms subsided within 3-5 days whereas joint pain and swelling persisted for more than 7 days. All the individuals were successfully treated symptomatically with common analgesics and antihistaminics. No death was observed due to the above illness. Average work days lost (expressed as numbers of days an individual was unable to perform his usual activity and remained confined to home) was up to 4 days(ranging from 2 - 7 days) in a majority of individuals.
Laboratory confirmation:Blood samples were collected from 725 individuals who volunteered for the investigation from different age and sex groups in the studied districts (Table III). The results confirmed that the outbreaks were due to CHIK virus infection. Seropositivity of the tested samples for CHIK IgM ranged from 3 to 44 per cent in the different outbreaks (Table IV). Dengue IgM test revealed positivity in a few samples from Nayagarh (n=3; 2.8%) and Puri (n=1; 0.8%) districts. Thick blood smears were examined for malaria parasiteand none of the blood smears were positive.
Table III.
Investigation for CHIK antibody (IgM) on blood samples collected from the outbreaks
| Age group (yr) | Male |
Female |
Total |
|||
| Samples tested | Chik IgM positive N (%) | Samples tested | Chik IgM positive N (%) | Samples tested | Chik IgM positive N (%) | |
| 0-15 | 44 | 9 (20.4) | 65 | 16 (24.6) | 109 | 25 (23) |
| 16-30 | 65 | 18 (27.7) | 68 | 14 (20.5) | 133 | 32 (24) |
| 31-45 | 80 | 23 (28.7) | 74 | 17 (23) | 154 | 40 (26) |
| 46-60 | 100 | 16 (16) | 119 | 32 (26.8) | 219 | 48 (22) |
| >60 | 60 | 14 (23.3) | 50 | 13 (26) | 110 | 27 (24.5) |
| Total | 349 | 80 (23) | 376 | 92 (24.4) | 725 | 172 (23.7) |
Table IV.
Seropositivity for CHIK and dengue IgM in the tested samples from different districts
| Name of the district | No. of samples tested | No. of Chik IgM positives (%) | No. of dengue IgM positives (%) |
| Bhadrak | 26 | 4 (15.38) | 0 |
| Ganjam | 16 | 7 (43.75) | 0 |
| Jajpur | 44 | 5 (11.36) | 0 |
| Kendrapada | 153 | 40 (26.14) | 0 |
| Nayagarh | 105 | 44 (41.9) | 3 (2.8) |
| Khurda | 18 | 5 (27.77) | 0 |
| Balesore | 12 | 2 (16.66) | 0 |
| Puri | 116 | 41 (35.34) | 1 (0.8) |
| Keonjhar | 10 | 3 (30) | 0 |
| Cuttack | 163 | 16 (9.81) | 0 |
| Jagatsinghpur | 32 | 1 (3.12) | 0 |
| Sundergarh | 20 | 2(10) | 0 |
| Gajapati | 10 | 2(20) | 0 |
| Total districts (13) | 725 | 172 (23.7) | 4 (0.5) |
Mosquito vector in the outbreak areas:Adult mosquito and larval survey were conducted in outbreak affected villages of Cuttack, Nayagarh and Kendrapara districts (Table V). The density (PMHD) of Ae. aegypti, Ae. albopictus and Ae. vittatus were 4.2, 7.6 and 1.28 in Kendrapara, 3.7, 6.2 and 0.8 in Cuttack and 2.3, 8.0 and 1.6 in Nayagarh districts respectively. Adult mosquitoes were found both in the living rooms and Cattle sheds. The house, container and Breteau indices calculated were 19, 18.9 and 76 in Kendrapara, 17.2, 16.9 and 73.6 in Cuttack and 21.9, 20.1 and 84.7 in Nayagarh respectively.
Table V.
Results of survey for Aedes larvae
| Districts | No. of HH searched for Aedes larvae | No of HH +ve for Aedes larvae | No of containers searched | No of containers +ve for Aedes larvae |
| Kendrapara | 121 | 23 | 488 | 92 |
| Cuttack | 87 | 15 | 377 | 64 |
| Nayagarh | 105 | 23 | 442 | 89 |
Discussion
India experienced outbreaks due to CHIK infection in 2005 after a long gap9 whereas Orissa with no history of CHIK infection experienced its first epidemic in 2006. After emergence of CHIK virus infection in the State, it spread widely affecting mostly rural areas from about half of the administrative districts. These villages were from different physiographic divisions of the State like coastal plane (Eastern Orissa), Northern plateau and Eastern Ghat (southern Orissa) which included both plane and hilly areas and it occurred within a period of one year and nine months. Clinical attack rate was up to 50 per cent in the different outbreak sites, which was also similar to reports from other States of India showing attack rate up to 45 per cent10. The seropositivity rate would have been increased if paired sera could be tested from the individual cases. The observed seroprevalence for CHIK IgM antibody varied between 3 to 44 per cent in tested samples from different districts and the overall prevalence rate was 13.9 per cent. Similar seropositivity rates were also noted from other affected States of India10,11
Though the illness was non fatal in all the outbreak sites, morbidity was high with loss of work and population affected rate as high as 50 per cent in the major outbreaks.
Three species of Aedes, i.e., aegypti, albopictus and vitattus were identified in the affected areas which are the possible vectors for transmission. The house and Breateau indices were observed to be much higher than the recommended indices of 5 and 20 respectively, which might have favoured transmission of the virus within the population12. Earlier reports had also shown the presence of Aedes species in varying densities in other regions of the State13–16 which indicated potential for spread to other areas. The CHIK virus is also known to spread through transovarial transmission in the mosquitoes and possible sylvatic cycles17.All these indicate possibility of spread of the infection to other areas of the region.
Though no community study on seroprevalence of CHIK antibody is available from the State, a cross-sectional survey carried out in Kolkota 10 years ago indicated low level (4.37% antibody positivity) of herd immunity to CHIK infection18. Hence the reasons for re-emergence of CHIK in the Indian subcontinent though not precisely known, might be due to a variety of social, environmental, behavioural and biological changes19,20. Alternatively, it was expected that the lack of herd immunity probably led to its rapid spread21.
In conclusion, the present report confirmed the emergence of CHIK virus infection in Orissa, India, in 2006 and its spread in the s0 tate affecting a wider geographic zone in a shorter period which was facilitated by presence of the Aedes vector species. The present findings will help the health authorities and the community physicians to keep vigil over the problem and taking steps for early diagnosis of the illness and undertaking preventive measures to curtail morbidity and spread.
Acknowledgments
The authors wish to acknowledge the team members Shriyut K. Dhala, T. Moharana, R.N. Nayak, Lal Mohan Ho and Shri S.K. Mishra of Clinical and Epidemiology Divisions and Shriyut G.D Mansingh, S.S. Beuria, C.S. Tripathy, B. Pradhan and G. Simachallam of Entomology division for their support and activities during the field survey and laboratory investigation. Authors are also Director, Health Services, Govt. of Orissa for outbreak information and support for investigation.
References
- 1.Strauss EG, Strauss JH. Structure and replication of the alphavirus genome. In: Schlesinger S, Schlesinger MJ, editors. The togaviridae and flaviviridae. New York: Plenum Press; 1986. pp. 35–90. [Google Scholar]
- 2.Porterfield JH. Schlesinger R, editor. Antigenic characteristics and classification of the Togaviridae. The togaviruses. 1980:13–46. [Google Scholar]
- 3.Ross RW. The Newala epidemic III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J Hyg. 1956;54:177–91. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Gandhe SS, et al. CHIK outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–3. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalantri SP, Joshi R, Riley LW. CHIK epidemic: An Indian perspective. Natl Med J India. 2006;19:315–22. [PubMed] [Google Scholar]
- 6.Guidelines for Prevention and Control of Chikungunya Fever, WHO-SEARO. 2007. Available from: www.searo.who.int/LinkFiles/ Publication_SEA-CD-182.pdfaccessed on July 25, 2007.
- 7.Christophers SR. Aedes aegypti(L) The yellow fever mosquito. Cambridge University Press; 1960. pp. IX–739. [Google Scholar]
- 8.Barraud PJ. The fauna of British India. vol. V. London: Taylor and Francis; 1934. Tribes Megarhinini and Culicini; p. 463. [Google Scholar]
- 9.Lahariya C, Pradhan SK. Emergence of CHIK virus in Indian subcontinent after 32 years: a review. J Vector Borne Dis. 2006;43:151–60. [PubMed] [Google Scholar]
- 10.WHO (SEARO) Epidemic and Pandemic Alert and Response, Diseases outbreak news Chikungunya in India; 2006, October 17. 2007. Available from: http://wwwwhoint/csr/don/2006_10_17/en/indexhtml, accessed on July 23, 2007.
- 11.Epidemic and pandemic alerts and response (EPR) WHO 2007. 2007. Available from: http://wwwsearowhoint/en/Section10/Section2246_13975html, accessed on July 24, 2007.
- 12.Geneva: WHO; 1995. WHO guidelines for dengue surveillance and mosquito control. VIII+103. [Google Scholar]
- 13.Yadav RS, Sharma VP, Chand SK. Mosquito breeding and resting in tree holes in the forest ecosystem in Orissa. Indian J Maleriol. 1997;34:8–16. [PubMed] [Google Scholar]
- 14.Rajavel AR, Natrajan R, Vidyanathan K. Mosquitoes of the mangrove forests of India: Part 1- Bhiterkanika, Orissa. J Am Mosq Control Assoc. 2005;21:131–5. doi: 10.2987/8756-971X(2005)21[131:MOTMFO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Sharma SK, Padhan K, Rath Y, Rao SK. Observations on the breeding habitat of Aedes species in the steel township, Rourkela. J Commun Dis. 2001;33:28–35. [PubMed] [Google Scholar]
- 16.Hazra RK, Mahapatra N, Parida SK, Marai NS, Tripathy HK. Vector mapping with its susceptibility status to insecticides in high risk districts of Orissa. Annual Report, Regional Medical Research Centre, Bhubaneswar, Orissa. Bhubaneswar: RMRC; 2006. pp. 25–32. [Google Scholar]
- 17.Diallo M, Thonnon J, Lamizana TL, Fontenille D. Vectors of CHIK virus in Senegal: Current data and transmission cycle. Am J Trop Med Hyg. 1999;60:281–6. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- 18.Neogi DK, Bhattacharya N, Mukherjee KK, Chakraborty MS, Banerjee P, Mitra K. Serosurvey of CHIK antibody in Calcutta metropolis. J Commun Dis. 1995;27:19–22. [PubMed] [Google Scholar]
- 19.Power AM, Brault AC, Tesh RB, Weaver SC. Re emergence of CHIK and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–9. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 20.Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim R. Tracking the re-emergence of epidemic CHIK virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99:128–41. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Ravi V. Re-emergence of CHIK virus in India. Indian J Med Microbiol. 2006;24:83–4. doi: 10.4103/0255-0857.25175. [DOI] [PubMed] [Google Scholar]


