Abstract
Background & objectives:
Dengue is one of the most important Arboviral diseases in man with outbreaks in Southeast Asia and India. We report a retrospective analysis of the dengue positivity in the referred samples for three years period (2006 to 2008) at the Department of Virology, King Institute of Preventive Medicine, Chennai, Tamil Nadu, India.
Methods:
Serum samples from 1593 suspected cases (968 male and 625 female) were obtained. Of the 1593 cases screened, 1204 (75.5%) were paediatric cases and 389 (24.4%) adults. The samples were subjected to MAC ELISA and IgG ELISA.
Results:
Of the 968 patients, 686 (43.0%) were positive, of which 579 (84.0%) were in the paediatric age group (<14 yr) and 107 (15.5%) were adults. The IgM positivity being 356 (36.7%) in males and 330 (52.8%) in females. Of the 686 positives, 113 (16.47%) were positive for both IgM and IgG denoting secondary infection. There was a noticeable increased occurrence during the cooler months and during the monsoon and post-monsoon months.
Interpretation & conclusions:
The dengue IgM seropositivity among the suspected cases indicates active dengue virus activity. Increase in the probable secondary infections especially in a country like ours where multiple serotypes are prevalent raises concern over probable increase in the incidence of the more serious DHF/DSS. Studies need to be done to identify circulating serotypes of dengue virus to design preventive strategies.
Keywords: Dengue-arboviral infection, dengue haemorrhagic fever (DHF), dengue shock syndrome (DSS)
Outbreaks of illness clinically resembling dengue fever (DF) have been there ever since 1779 in Java, Indonesia. Similar epidemics of dengue like illness occurred at 10-30 yr interval. Now the spread has been accelerated by the advent of frequent air travel1. Majority of the infections are asymptomatic and the clinical manifestations occur in two forms- classical dengue fever and dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). DHF/DSS are uncommon in individuals above 15 years and are more common in secondary infections2–4. Several virus- and host-specific factors have been suggested to correlate with severe disease outcome, which are mostly associated with secondary infections3,4.
Dengue infection has been known to be endemic in India for over two centuries, as a benign and self-limiting disease. One of the largest outbreaks in north India occurred in Delhi and adjoining areas in the 1996 which was mainly due to dengue-2 virus5. Thereafter, in 2003, another outbreak occurred in Delhi and all four dengue virus serotypes were found to be co-circulating6–8. However, dengue-3 was reported to predominate in certain parts of North India in 20039. In the following years (2004 and 2005), though outbreaks did not occur but a high number of cases of suspected dengue infection were reported during rainy season. The seasonality of transmission of dengue showed increased activity during monsoon and post monsoon. These findings indicate that during epidemic as well as non-epidemic years, dengue infections are mostly seen in monsoon and post-monsoon season.
Dengue has been rampant in parts of Tamil Nadu in the past two decades. The prevalence of dengue vector and silent circulation of dengue viruses have been detected in rural and urban Tamil Nadu, which is ever increasing10.
In this context, a retrospective analysis of data was done on the samples received for dengue testing at King Institute of Preventive Medicine, Guindy, Chennai, Tamil Nadu, during three years from 2006 to 2008. The serum samples from clinically suspected cases referred to Virology laboratory for IgM and IgG testing were included in the study. The results were analysed to investigate whether there is an overall increase in the dengue prevalence over the three years period.
Material & Methods
The study was performed at the King Institute of Preventive Medicine, Department of Virology, Guindy. Samples were received from Government General Hospital, Institute of Child Health, other Government hospitals and private institutions all over Chennai. Since these are tertiary care centres, the samples received were inclusive of referred patients from the various districts in Tamil Nadu. Both adult and paediatric cases were included in this study.
The patients were referred to as suspected DF cases, based on standard diagnostic criteria1. Most of the samples were collected during 5 to 10 days of illness. Approximately 2-5 ml of blood was received, serum separated and subjected to ELISA. MAC ELISA was performed using kit from Panbio, IgM Capture ELISA (Australia) and IgG ELISA was also performed using kits from EUROIMMUNO AG DEUTSCHLAND, SEELEAMP 31. A few representative cases during the year 2006, 2007 and 2008 were subjected to Rapid test, PAN BIO DUO CASETTE (Australia) in which IgG and IgM can both be detected.
Statistical analysis: The data presented were analyzed using Chi-square test for proportion and the Chi-square test for linear trend using the Graphpad prism 5.02 programmes.
Results
During the study period, the total number of samples screened was 1593 of which 686 (43.0%) were positive for IgM antibodies (Table I). There was an increase in the percentage positivity in 2008 when compared to 2006 (P<0.05).
Table I.
Year-wise distribution of suspected cases of dengue fever and dengue IgM positive cases over a three year period (2006-2008)
| Year | Total no. of suspected dengue cases | Total no. (%) of IgM positives |
| 2006 | 578 | 204 (35.2) |
| 2007 | 517 | 232 (44.8) |
| 2008 | 498 | 250 (50.2) |
| Total | 1593 | 686 (43.0)* |
P<0.05 compared to 2006.
Of the 1593 cases screened (968 males, 625 females), the IgM positivity was 356 (36.7%) in males and 330 (52.8%) in females (Table IIa). The overall increase in the seropositivity among males during the study period was found to be statistically significant (P<0.05).
Table IIa.
Year-wise and gender-wise distribution of suspected cases of dengue fever and dengue IgM positive cases over a three year period (2006-2008)
| Year | Male |
Female |
|||
| Total no. of suspected dengue cases | No. of positives (%) | Total no. of suspected dengue cases | No. of positives (%) | ||
| 2006 | 370 | 110 (29.7) | 208 | 94 (45.1) | |
| 2007 | 300 | 120 (40.0) | 217 | 112 (51.6) | |
| 2008 | 298 | 126 (42.2) | 200 | 124 (62.0) | |
| Total | 968 | 356 (36.77) | 625 | 330 (52.8) | |
Of the 1593 cases screened, 1204 (75.5%) were paediatric cases and 389 (24.4%) were adults. Of the 686 reactive cases, 579 (84.5%) were positive paediatric cases (<14 yr) and 107 (15.5%) were adults (Table IIb). The samples were collected among the age group of 0-89 yr and the mean age was (19 ± 17 yr). The overall increase in the seropositivity among paediatric and adult cases was statistically significant (P<0.05). Among the paediatric age group, positivity was significantly high (P<0.001) in 1-5 and 6-12 yr age group (Table III).
Table IIb.
Year-wise and age-wise (children and adults) distribution of suspected cases of dengue fever and dengue IgM positive cases over a three year period (2006-2008)
| Year | Children |
Adults |
||
| Total no. of suspected dengue cases | No. of positives (%) | Total no. of suspected dengue cases | No. of positives (%) | |
| 2006 | 448 | 182 (40.6) | 130 | 22 (16.9) |
| 2007 | 388 | 195 (50.2) | 129 | 37 (28.6) |
| 2008 | 368 | 202 (54.8) | 130 | 48 (36.9) |
| Total | 1204 | 579 (48.08) | 389 | 107 (27.50) |
Table III.
IgM year-wise positivity in paediatric age group
| Year | Age(yr) |
Total no. of IgM positives | ||||
| <1 | 1-5 | 6-12 | 13-18 | |||
| 2006 | 48 (26.3) | 80 (43.9) | 30 (16.4) | 24 (13.1) | 182 | |
| 2007 | 40 (20.5) | 80 (41.0) | 40 (20.5) | 35 (17.9) | 195 | |
| 2008 | 37 (18.3) | 77 (38.1) | 50 (24.7) | 38 (18.8) | 202 | |
| Total | 125 (21.58) | 237 (40.93) | 120 (20.75) | 97 (16.75) | 579 | |
*values are no. (%)
Of the 686 positives, 113 were positive for both IgM and IgG denoting secondary infection. The percentage of samples positive for both IgM and IgG was found to be more in 2007 and 2008. There could be a co-circulation of different strains causing increased secondary infections. IgM positives are inclusive of both isolated IgM positives and those positive for both IgM and IgG. There was an overall increase in the secondary infections during the three years and was found to be statistically significant (P<0.0001).
The observed dengue IgM seropositivity month wise is illustrated from 2006-2008. (Fig.). The percentage of IgM positivity was found to be high during the months of September and October in all the three years. The observed dengue IgM seropositivity percentage showed an increase with increase in the monthly rainfall. The IgM seropositivity percentage showed temporal relationship with fall in temperature. There was an increased seropositivity during the cooler months.
Fig.
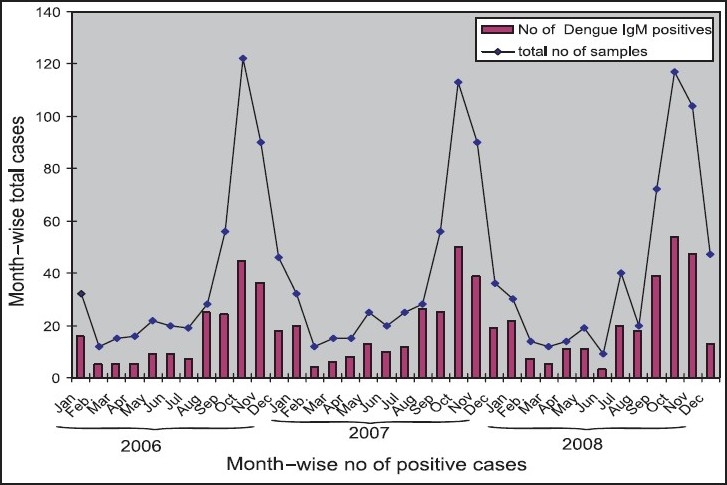
Month-wise distribution of suspected cases of dengue fever and dengue IgM positive cases over a three year period (2006-2008).
The increase in the number of dengue IgM seropositivity among paediatric cases as compared to adults was statistically significant (P<0.005).
Discussion
The first isolation of dengue virus serotypes 1and 4 was reported from India in 196411,12, and dengue virus serotype 3 in 199613. Ever since, intermittent reports of dengue and its sequelae have come from various parts of the country. These include reports from Ludhiana14, Delhi8, Lucknow13, Kolkata15, Chennai16, Mangalore17, Assam/Nagaland18 and Vellore19–21. The present study showed a steady increase in the number of referred cases and increase in the percentage of samples positive for dengue IgM during the study period.
The vulnerability of children to dengue infection was re-established in our study, as has been reported earlier20.
The transmission of dengue increases in monsoon22, as was also observed in our study. This shows that the presence of stagnating water after rainfall favours breeding of the mosquito vector resulting in an increased incidence of dengue. These findings also indicate that preventive measures against dengue infection should probably come into full-swing during the monsoon and post monsoon months.
Molecular studies on the circulating serotypes and their genotypes may be of help in addressing the probabilities of DSS/DHF incidence in future. Involvement of many laboratories in diagnosis of dengue coupled with general awareness among the public and constant vigilance by the health care officials could go a long way in combating dengue.
Acknowledgments
Authors thank Dr J. Revathi, Director, King Institute of Preventive Medicine for facilities provided to carry out the study, the Director, Institute of Child Health and Hospital for Children and Dean, Madras Medical College, Stanley Medical College and Hospital, for the samples and the librarian of Connemara library for providing meteorological data.
References
- 1.Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuckerman AJ, Banatvala JE, Pattison JR, Griffiths PP, Schoub BD, editors. Principles and practice of clinical virology. 5th ed. West Sussex, England: John Wiley & Sons, Ltd; 2004. [Google Scholar]
- 3.Gregson A, Edelman R. Dengue virus infection. Pediatr Infect Dis J. 2003;22:179–81. doi: 10.1097/01.inf.0000053016.43256.c7. [DOI] [PubMed] [Google Scholar]
- 4.Nimmannitya S. Dengue and dengue hemorrhagic fever. In: Cook GC, Zumla AI, editors. Manson’s tropical diseases. 21st ed. Philadelphia, USA: Saunders; 2003. p. 7672. [Google Scholar]
- 5.Dar L, Gupta E, Narang P, Broor S. Cocirculation of dengue serotypes, Delhi, India 2003. J Emerg Infect Dis. 2006;12:352–3. doi: 10.3201/eid1202.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dar L, Broor S, Sengupta S, Xess I, Seth P. The first major outbreak of dengue hemorrhagic fever in Delhi, India. Emerg Infect Dis. 1999;5:589–90. doi: 10.3201/eid0504.990427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurukumbi M, Wali JP, Broor S, Aggarwal P, Seth P, Handa R, et al. Seroepidemiology and active surveillance of dengue fever/dengue haemorrhagic fever in Delhi. Indian J Med Sci. 2001;55:149–56. [PubMed] [Google Scholar]
- 8.Vajpayee M, Mohankumar K, Wali JP, Dar L, Seth P, Broor S. Dengue virus infection during post-epidemic period in Delhi, India. Southeast Asian J Trop Med Public Health. 1999;30:507–10. [PubMed] [Google Scholar]
- 9.Dash PK, Saxena P, Abhyankar A, Bhargava R, Jana AM. Emergence of dengue virus type-3 in northern India. Southeast Asian J Trop Med Public Health. 2005;36:370–7. [PubMed] [Google Scholar]
- 10.Tewari SC, Thenmozhil V, Katholi CR, Manavalan1 R, Munirathinam A, Gajanana A. Dengue vector prevalence and virus infection in a rural area in south India. Trop Med Int Health. 2004;9:499–507. doi: 10.1111/j.1365-3156.2004.01103.x. [DOI] [PubMed] [Google Scholar]
- 11.Carey DE, Myers RM, Reuben R. Dengue types 1 and 4 viruses in wild-caught mosquitoes in south India. Science. 1964;143:131–2. doi: 10.1126/science.143.3602.131. [DOI] [PubMed] [Google Scholar]
- 12.Myers RM, Carey DE, Rodrigues FM, Klontz CE. The isolation of dengue type 4 virus from human sera in south India. Indian J Med Res. 1964;52:559–65. [PubMed] [Google Scholar]
- 13.Agarwal R, Kapoor S, Nagar R, Misra A, Tandon R, Mathur A, et al. A clinical study of the patients with dengue hemorrhagic fever during the epidemic of 1996 at Lucknow, India. Southeast Asian J Trop Med Public Health. 1999;30:735–40. [PubMed] [Google Scholar]
- 14.Kaur H, Prabhakar H, Mathew P, Marshalla R, Arya M. Dengue haemorrhagic fever outbreak in October- November 1996 in Ludhiana, Punjab, India. Indian J Med Res. 1997;106:1–3. [PubMed] [Google Scholar]
- 15.Chatterjee SN, Chakravarty SK, Sarkar JK. Isolation of dengue virus from human blood in Calcutta. Bull Calcutta Sch Trop Med. 1966;14:121–2. [PubMed] [Google Scholar]
- 16.Kabilan L, Balasubramanian S, Keshava SM, Thenmozhi V, Sekar G, Tewari SC, et al. Dengue disease spectrum among infants in the 2001 dengue epidemic in Chennai, Tamil Nadu, India. J Clin Microbiol. 2003;41:3919–21. doi: 10.1128/JCM.41.8.3919-3921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padbidri VS, Adhikari P, Thakare JP, Ilkal MA, Joshi GD, Pereira P, et al. The 1993 epidemic of dengue fever in Mangalore, Karnataka state, India. Southeast Asian J Trop Med Public Health. 1995;26:699–704. [PubMed] [Google Scholar]
- 18.Barua HC, Mahanta J. Serological evidence of DEN-2 activity in Assam and Nagaland. J Commun Dis. 1996;28:56–8. [PubMed] [Google Scholar]
- 19.Myers RM, Varkey MJ, Reuben R, Jesudass ES. Dengue outbreak in Vellore, southern India, in 1968, with isolation of four dengue types from man and mosquitoes. Indian J Med Res. 1970;58:24–30. [PubMed] [Google Scholar]
- 20.Cherian T, Ponnuraj E, Kuruvilla T, Kirubakaran C, John TJ, Raghupathy P. An epidemic of dengue haemorrhagic fever & dengue shock syndrome in & around Vellore. Indian J Med Res. 1994;100:51–6. [PubMed] [Google Scholar]
- 21.Myers RM, Varkey MJ. Detection of sporadic cases of dengue hemorrhagic fever. Indian J Med Res. 1970;58:1301–6. [PubMed] [Google Scholar]
- 22.Reiter P. Climate change and mosquito-borne disease. Environ Health Perspect. 2001;109(Suppl 1):141–61. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]


