Abstract
No chemoprevention strategies have been proven effective for lung cancer.
We evaluated the effect of 13-cis retinoic acid (13-cis RA), with or without α tocopherol, as a lung cancer chemoprevention agent in a phase II randomized controlled clinical trial of adult subjects at high risk for lung cancer as defined by the presence of sputum atypia, history of smoking, and airflow obstruction, or a prior surgically cured nonsmall cell lung cancer (disease free, >3 years). Subjects were randomly assigned to receive either 13-cis RA, 13-cis RA plus α tocopherol (13-cis RA/α toco) or observation for 12 months.
Outcome measures are derived from histologic evaluation of bronchial biopsy specimens obtained by bronchoscopy at baseline and follow-up. The primary outcome measure is treatment “failure” defined as histologic progression (any increase in the maximum histologic score) or failure to return for follow-up bronchoscopy.
Seventy-five subjects were randomized (27/22/26 to obervations/13-cis RA/13-cis RA/α toco); 59 completed the trial; 55 had both baseline and follow-up bronchoscopy. The risk of treatment failure was 55.6% (15 of 27) and 50% (24 of 48) in the observation and combined (13 cis RA plus 13 cis RA/α toco) treatment arms, respectively (odds ratio adjusted for baseline histology, 0.97; 95% confidence interval, 0.36–2.66; P = 0.95). Among subjects with complete histology data, maximum histology score in the observation arm increased by 0.37 units and by 0.03 units in the treated arms (difference adjusted for baseline, −0.18; 95% confidence interval, −1.16 to 0.81; P = 0.72). Similar (nonsignificant) results were observed for treatment effects on endobronchial proliferation as assessed by Ki-67 immunolabeling.
Twelve-month treatment with 13-cis RA produced nonsignificant changes in bronchial histology, consistent with results in other trials. Agents advancing to phase III randomized trials should produce greater histologic changes. The addition of α tocopherol did not affect toxicity.
Lung cancer is the leading cause of cancer deaths worldwide and in the United States where it accounts for 29% of all cancer deaths (1). Exposure to tobacco smoke is the major cause of lung cancer in the United States. Prevention of tobacco exposure is thus a critical factor in reducing lung cancer mortality (2). However, ~50% of new lung cancers arise in former smokers and effective chemoprevention strategies are critical to reduce risk, especially in this large group, representing nearly 25% of the adult U.S. population (3). Chemoprevention is an emerging field whereby drug therapy is used to halt or reverse the carcinogenesis process before the emergence of invasive cancer (4). This approach has been successful to varying degrees with selective estrogen receptor modulators, finasteride, and nonsteroidal anti-inflammatories for subjects at high risk for breast, prostate, and colon cancer, respectively, and is being investigated in many cancers (5–9). Chemoprevention for lung cancer has been explored unsuccessfully for decades (4, 10), during which time, our understanding of the biology of pulmonary carcinogenesis has vastly expanded. The WHO/International Association for the Study of Lung Cancer classification for lung cancer now recognizes distinct histologic lesions, which can be reproducibly graded as precursors of lung cancer (11). The pathway of carcinogenesis in the bronchial epithelium starts with normal epithelium and progresses through hyperplasia, metaplasia, dysplasia, carcinoma in situ to invasive cancer.
Based on epidemiologic data showing that lung cancer patients have lower serum levels of several antioxidant vitamins, several large randomized chemoprevention trials were conducted that evaluated β carotene, α tocopherol, or other vitamin and mineral supplementation (12–14). Unfortunately, none of these showed a reduction in lung cancer incidence or mortality and in several, the lung cancer incidence was increased, especially in smokers (12, 13). Phase III chemoprevention trials in patients with a prior lung or head and neck cancer resected for cure have been completed and require fewer patients but none have shown a reduction in lung cancer incidence (15, 16).
Phase II intermediate end point trials have been proposed as one strategy for prioritizing agents for larger and more expensive phase III chemoprevention trials with lung cancer as an end point. There are no established intermediate biomarkers for lung cancer prevention trials, although intraepithelial neoplasia or dysplasia has been proposed as such (17). Indeed, until an effective chemoprevention treatment is proven, intermediate end point biomarkers cannot be validated and even then uncertainties remain as to whether they will be predictive of outcome (18, 19). However, given the difficulties in choosing agents for phase III chemoprevention trials, modulation of biologically plausible intermediate end point biomarkers is one rational factor, among others, in prioritizing agents for further testing. Metaplasia index and sputum cytologic atypia have been used in some trials but are not validated (10, 20, 21). Metaplasia has been criticized as it can occur as a response to injury and is less specific for tobacco smoke exposure than dysplasia. The histologic grading of dysplastic endobronchial lesions was used in several recent trials, but the best scoring methodology for assessing changes in bronchial histology has not been established (22–24). The use of biological and molecular markers is under investigation (25). Ki-67 is a marker of cell proliferation and has been proposed as a potential intermediate biomarker for lung cancer chemoprevention trials (26, 27). Both celecoxib and 13-cis retinoic acid (13-cis RA) have been reported to decrease Ki67 index in current or former smokers, respectively (28, 29). Ki-67 index is closely related to current smoking, male gender, and the histologic degree of dysplasia, but not to either the presence of lung cancer or chronic obstructive lung disease, raising concerns with regard to its validity as a biomarker of lung cancer risk (27, 30, 31). In this trial, we evaluate the effect of a chemoprevention agent (13-cis RA) on the bronchial epithelium using histologic and molecular biomarkers as a measure of efficacy.
13-Cis RA was selected because it had been shown to suppress epithelial carcinogenesis in animals and had reversed the histologically distinct preinvasive lesion, leukoplakia, in clinical trials (32–34). Retinoids act by binding to nuclear retinoid receptors leading to transcriptional activation. 13-Cis RA must undergo stereoisomerization to exert its activity. Furthermore, it has been hypothesized that the 13-cis RA mechanism of action is to restore or increase RAC-β, a retinoic acid–responsive gene that has been shown to be reduced in premalignant epithelium of the aerodigestive tract (35). At high doses of 50 to 100 mg/m2, toxicity was a problem with 13-cis RA, but at doses ranging from 30 to 70 mg/d, it was well-tolerated (36).
A secondary objective of this trial was to determine if α tocopherol could reduce the toxicity from 13-cis RA. This hypothesis was based on data from Dimery and colleagues (37) who conducted a small study involving 39 previously treated patients with head and neck, skin, or lung cancer. Compared with historical controls, the subjects in this study had fewer grade 3 or 4 retinoid toxicities.
Materials and Methods
Subject eligibility and recruitment
The trial enrolled adult subjects at high risk for lung cancer with sputum atypia. High risk was defined as a history of current or former smoking (>30 pack-years) with airflow obstruction (FEV1 of <75% of predicted and an FEV1/FVC ratio of <75% of predicted). Patients with a history of surgically cured stage I and II non–small cell lung cancer who were disease free for >36 mo regardless of smoking history were also eligible. A subject was considered a former smoker if he or she had not smoked for at least 6 mo. Subjects were excluded if they had abnormal hematologic, hepatic, or renal function; a chest radiograph suggestive of lung cancer; or serious concurrent illnesses including insulin dependent diabetes mellitus, uncontrolled hypercholesterolemia, or hypertriglyceridemia. Subjects could not be taking vitamin A or vitamin E supplements. The trial was Institutional Review Board approved, and all participants provided written informed consent in accordance with institutional and federal guidelines.
Trial design
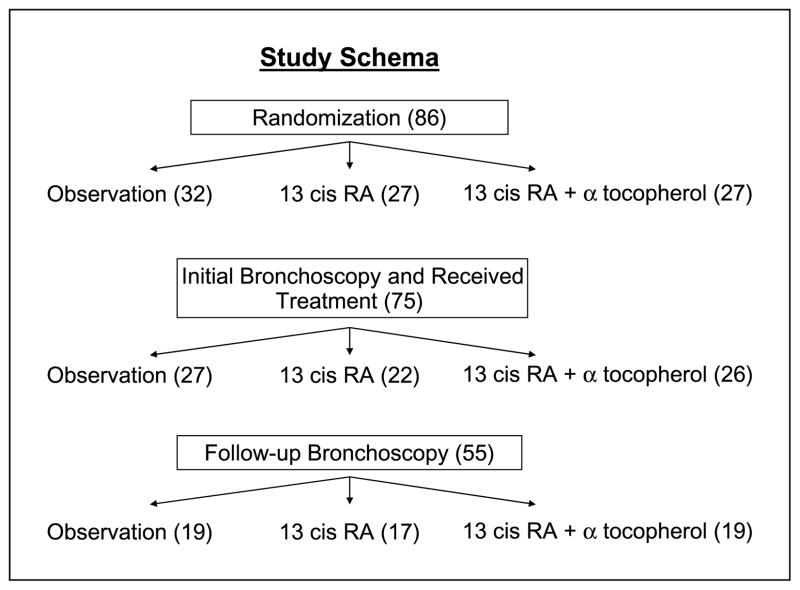
This randomized-controlled phase II trial was designed to evaluate the change in bronchial histology after one year of treatment with 13-cis RA (with or without α tocopherol) compared with usual care in subjects at high risk for lung cancer. In addition to histology, treatment effects on cell proliferation (as measured by the Ki-67 index) were evaluated. The study schema is shown in Fig. 1. Eligible subjects underwent a pretreatment white light and autofluorescence bronchoscopy and were then randomized to receive 13-cis RA at 50 mg/d p.o.; the same dose of 13-cis RA plus α tocopherol 800 mg/d, p.o. or no active treatment (obs). Randomization was blinded and stratified by smoking status (current versus former). Treatment group assignment was not blinded after randomization. Current smokers were encouraged to stop smoking and offered counseling and pharmacologic intervention. Active treatment was given for 1 y followed by 1 y of follow-up. Accrual goal was 100 subjects: 33 in each treatment arm and 34 in the observation arm.
Fig. 1.
Trial schema. Subjects are randomized at the time of bronchoscopy to either 13-cis RA alone or with α tocopherol or to observation. After 12 mo of treatment, bronchoscopy is repeated. The primary outcome is endobronchial histology and Ki67 immunolabeling is the secondary outcome.
Toxicity was graded according to the Southwest Oncology Group toxicity criteria (38). If a subject taking 13-cis RA or 13-cis RA/α toco experienced a toxicity of grade 2 or greater, then treatment was suspended until the grade reduced to 1. If the initial grading was 2, then subjects restarted 13-cis RA at 40 mg/d; if the initial grading was 3, then the restart dose was 30 mg/d. Subjects were removed from study drug if the toxicity recurred (graded, >2), if the toxicity was grade 4, or if the toxicity did not resolve within 2 wk. Dose reductions for α tocopherol were not allowed.
Follow-up
All subjects were evaluated at 4 wk, then every 3 mo during the first year with bronchoscopy at end of treatment. This evaluation consisted of a history and physical examination, performance status, toxicity assessment, and pill count. Laboratory data included a complete blood count with differential and a platelet count, fasting serum chemistries including uric acid, lipids, and triglycerides.
Bronchoscopy protocol
Subjects underwent white light and autofluorescence bronchoscopy using a Xillix LIFE-Lung device. A standardized bronchoscopy procedure was followed (39). The airways were first inspected with white light bronchoscopy followed by autofluorescence exam. Abnormal epithelial sites identified by white light or autofluorescence were photographed and recorded, as were the standard biopsy sites (right upper lobe orifice carina, right middle lobe orifice carina, right lower lobe superior segment orifice carina, left upper lobe orifice carina, carina between lingula and upper division bronchus, superior segment left lower lobe orifice carina). More than one biopsy could be taken at a given site. At least one biopsy was formalin fixed and paraffin embedded for H&E staining and others frozen in ornithine carbamyl transferase compound for immunohistochemical studies.
Specimen evaluation
All bronchial biopsies underwent immediate histopathologic evaluation using the WHO classification of preneoplasia of lung tumors, which separates lesions into 8 different grades, each given a number: 1, normal; 2, reserve cell hyperplasia; 3, squamous metaplasia; 4, mild dysplasia; 5, moderate dysplasia; 6, severe dysplasia; 7, carcinoma in situ; and 8, invasive carcinoma (11). A single pathologist (WAF) read all histology for this study.
One biopsy from each site at which evaluable tissue was available was immunostained for Ki-67 using the mib-1 antibody (Dako) as described (31, 40). Photomicrographs of the most histologically advanced lesion within the biopsy were taken, reviewed with a study pathologist (FRH or WAF), and 400 cells within that lesion counted and scored for nuclear Ki-67 expression with percentage Ki-67–positive cells calculated.
Statistical Analysis
The intention-to-treat (ITT) analysis includes all subjects who were randomized and received treatment. Because some subjects did not receive a follow-up bronchoscopy, the ITT analysis used the risk of progression defined as either an increase in histology or failure to obtain a follow-up bronchoscopy. The ITT analysis protects against the possibility that some subjects did not receive follow-up bronchoscopy because of unreported adverse treatment effects. Changes in histology in the subset of subjects who received two bronchoscopies were also analyzed.
Analyses were conducted using three different within-subject summary histology measures: the maximum (or worst) histology, the average histology over all biopsy specimens, and the dysplasia index (proportion of biopsies scored as mild dysplasia or worse). Because α tocopherol was given to reduce toxicity, the two treatment groups were combined for efficacy analysis. The ITT analyses compared the odds of progressing in subjects receiving treatment relative to untreated subjects using logistic regression. The subset histologic analyses compared pretreatment-posttreatment histology changes in the treated and untreated groups using linear regression. To increase precision, all comparisons were adjusted for baseline histology. The results are reported using the standard 2-sided P values and 95% confidence intervals without adjustment for multiple comparisons.
This trial was designed as a pilot study to furnish preliminary data to allow planning for more definitive studies. Preliminary data from repeat bronchoscopies on similar subjects for sample size calculations were not available.
Results
Subject characteristics
Between March 1994 and November, 2002, 96 subjects were consented to the trial. Accrual was discontinued short of the original goal of 100 due to the initiation of a second chemoprevention trial (Iloprost). Between consent and randomization, 10 subjects withdrew. Two had lung cancer diagnosed, two decided not to participate, one died, three were deemed poor candidates by their physicians, one had elevated triglycerides, and one did not have moderate atypia sputum. Eighty-six subjects were randomized to the study. Eleven subjects did not receive any treatment: 4 subjects withdrew before any treatment, 4 subjects were deemed ineligible after completing baseline evaluation but before treatment, 2 subjects died suddenly (1 with a myocardial infarction and the second from unknown cause), and 1 patient was withdrawn by his physician for predicted noncompliance. Table 1 shows the baseline characteristics for all 75 subjects who received treatment. There was no significant difference in baseline characteristics between the three groups. The majority of subjects were White, male, former smokers. Subjects in the treated groups were slightly older than the observation group and the average number of pack-years was higher in the 13-cis RA group. One subject had never smoked but had sputum atypia and a previous history of stage I lung cancer 7 years before enrollment. All subjects had significant airflow obstruction and almost half of the subjects required supplemental oxygen. Clinical characteristics were similar among current and former smokers except that current smokers were more likely to have moderate or severe sputum atypia (20 of 32 for current smokers versus 21 of 41 for former smokers). All subjects had mild or greater sputum atypia. No current smoker quit smoking while on study and no former smoker began smoking.
Table 1.
Baseline characteristics
| Observation | 13-Cis RA | 13-Cis RA + o-toc. | |
|---|---|---|---|
| All subjects | |||
| n | 27 | 22 | 26 |
| Mean age (range) | 59.9 (42–73) | 62.3 (45–80) | 64 (44–79) |
| Female/male (%) | 8:19 (30:70) | 4:18 (18:82) | 7:19 (27:73) |
| Smoking (e-x/current; %) | 15:13 (56:44) | 12:10 (55:45) | 15:11 (58.42) |
| Mean pack years (range) | 56.S (30–105) | 71.7 (30–183) | 58.4 (0–130) |
| Sputum (mild/mod+severe; %) | 8:19 (30:70) | 9:13 (41:59) | 10:14 (42:53) |
| O2 use (no/yes’ %) | 14:13 (52:48) | 14:8 (64:36) | 15:11 (58:42) |
| FEV% (range) | 53.6 (25–103) | 58 (20–99) | 55.3 (24–123) |
| FEV/FVC% (range) | 57.6 (30–82) | 55.7 (25–77) | 53.7 (27–87) |
| Current smokers | |||
| n | 12 | 10 | 11 |
| Mean age (range) | 56.6 (43–68) | 58.4 (45–70) | 59.4 (44–77) |
| Female/male (%) | 4:8 (33:67) | 3:7 (30:70) | 5:6 (45:55) |
| Mean pack years (range) | 58.7 (31–90) | 72.5 (30–168) | 66 (48–130) |
| Sputum (mild/mod+severe; %) | 2:10 (17:83) | 2:8 (20:80) | 2:8 (20:80) |
| O2 use (no/yes; %) | 7:5 (58:42) | 5:5 (50:50) | 6:5 (55:45) |
| FEV% (range) | 52 (27–103) | 51.6 (30–99) | 61.7 (38–98) |
| FEV/FVC% (range) | 56.8 (30–79) | 55.4 (25–70) | 59.2 (27–74) |
| Former smokers | |||
| n | 15 | 12 | 15 |
| Mean age (range) | 62.5 (42–73) | 65.5 (52–80) | 67.4 (54, 79) |
| Female/male (%) | 4:11(27:73) | 1:11 (8:92) | 2:13 (13:87) |
| Mean pack years (range) | 54.4 (30–105) | 71 (30–183) | 52.9 (0–80) |
| Sputum (mild/mod+severe; %) | 6:9 (40:60) | 7:5 (58:42) | 8:6 (57:43) |
| O2 use (no/yes; %) | 7:8 (47:53) | 9:3 (75:25) | 9:6 (60:40) |
| FEV% (range) | 54.7 (25–101) | 63.2 (29–84) | 50.3 (24–123) |
| FEV/FVC% (range) | 58.1 (30–82) | 56 (34–77) | 49 (27–87) |
Abbreviation: 13-cis RA + α-toc, 13-cis RA + α tocopherol.
Bronchial histology at baseline is described in Table 2. On average, eight biopsies were obtained per subject. There were no significant differences between treatment groups (either overall or in the current and former smoker subgroups). Overall, 162 of 561 (29%) of biopsies showed some degree of dysplasia and 51 of 75 (68%) of subjects had at least 1 dysplastic site at baseline. At baseline, current smokers had significantly worse histology than former smokers, as previously described (P < 0.001 regardless of histology measure; ref. 31).
Table 2.
Baseline histology
| Observation | Treated | Difference (treated – observed)
|
|||
|---|---|---|---|---|---|
| Difference | 95% CI | P | |||
| All subjects | |||||
| Maximum | 3.63 (1.80) | 4.19 (1.58) | 0.56 | (−0.28 to 1.39) | 0.18 |
| Average | 2.33 (1.15) | 2.53 (1.10) | 0.19 | (−0.35 to 0.74) | 0.48 |
| Dysplasia index | 0.24 (0.26) | 0.28 (0.27) | 0.04 | (−0.09 to 0.16) | 0.57 |
| Current smokers | |||||
| Maximum | 4.58 (1.31) | 4.81 (0–81) | 0.23 | (−0.66 to 1.11) | 0.60 |
| Average | 3.12 (0.94) | 3.17 (0.93) | 0.05 | (−0.65 to 0.75) | 0.89 |
| Dysplasia index | 0.39 (0.25) | 0.40 (0.28) | 0.01 | (−0.18 to 0.21) | 0.88 |
| Former smokers | |||||
| Maximum | 2.87 (1.81) | 3.70 (1.86) | 0.84 | (−0.36 to 2.04) | 0.16 |
| Average | 1.70 (0.89) | 2.03 (0.97) | 0.33 | (−0.27 to 0.93) | 0.28 |
| Dysplasia index | 0.12 (0.20) | 0.18 (0.22) | 0.06 | (−0.08 to 0.20) | 0.41 |
NOTE: Values are mean (SD).
Abbreviation: 95% CI, 95% confidence interval.
Treatment effects on bronchial histology
Fifty five subjects had a posttreatment bronchoscopy. Reasons for not undergoing a repeat bronchoscopy were as follows: subject refusal (7), deemed too ill by investigators due to comorbid conditions (9), physician decision (1), and toxicity (1). On average, there were 9 biopsies per subject at follow-up bronchoscopy.
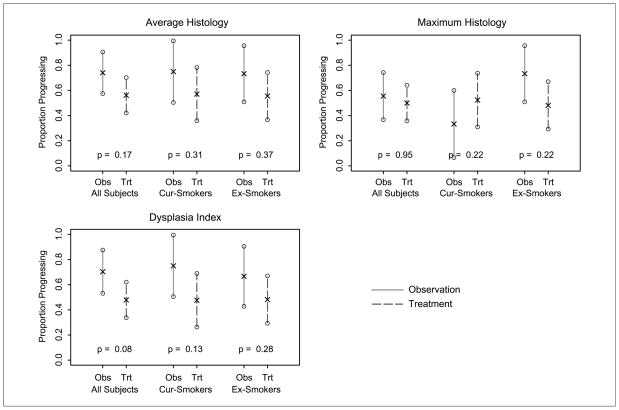
The results of the ITT analysis (Table 3; Fig. 2) show that although the risk of failure (progression in terms of maximum histology or failure to receive follow-up bronchoscopy) is 55.6% in the observation group and 50% in the treatment group, the decrease in the risk of progression is not statistically significant (P values ranging from 0.08–0.95, depending on histology parameter). The primary histology measure (maximum histology) indicates that odds of failure with treatment are approximately half the odds in the observation group; however, the confidence intervals are wide.
Table 3.
Risk of histology progression
| Risk of progression* |
Comparison† (treated/observed)
|
|||
|---|---|---|---|---|
| Observation | Treated | Odds ratio (95% CI) | P | |
| All subjects | ||||
| n | 27 | 48 | ||
| Maximum | 15 (55.6%) | 24 (50.0%) | 0.97 (0.36–2.66) | 0.95 |
| Average | 20 (74.1%) | 27 (56.2%) | 0.48 (0.17–1.37) | 0.17 |
| Dysplasia index | 19 (70.4%) | 23 (47.9%) | 0.39 (0.14–1.10) | 0.08 |
| Current smokers | ||||
| n | 12 | 21 | ||
| Maximum | 4 (33.3%) | 11 (52.4%) | 2.67 (0.55–12.98) | 0.22 |
| Average | 9 (75.0%) | 12 (57.1%) | 0.44 (0.09–2.16) | 0.31 |
| Dysplasia Index | 9 (75.0%) | 10 (47.6%) | 0.28 (0.05–1.45) | 0.13 |
| Former smokers | ||||
| n | 15 | 27 | ||
| Maximum | 11 (73.3%.) | 13 (48.1%) | 0.41 (0.10–1.71) | 0.22 |
| Average | 11 (73.3%,) | 15 (55.6%) | 0.52 (0.13–2.15) | 0.37 |
| Dysplasia index | 10 (66.7%) | 13 (48.1%,) | 0.48 (0.13–1.83) | 0.28 |
Progression defined as worsening of a subject’s histologic score or failure to obtain a follow-up bronchoscopy.
Adjusted for baseline histology (either maximum, average, or dysplasia index). All subject analysis is also adjusted for smoking status.
Fig. 2.
Risk of progression in intention to treat analysis.
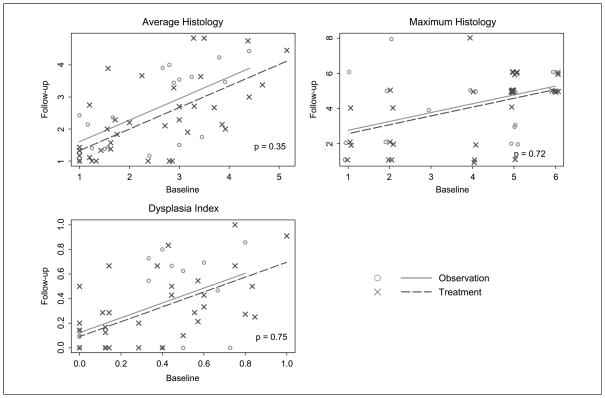
The ITT results are confirmed in the subset analysis of histologic change (Table 4; Fig. 3). Although the improvements in histology measures are greater in the treated group, the differences are not statistically significant. We note that some of the differences between groups are quite large (e.g., a 0.92 between-group difference in maximum histology among former smokers) and the confidence intervals are wide. Thus, there are meaningful differences between treatment groups, but variability is high. The results indicate that 13-cis RA treatment might have greater benefits for former smokers than current smokers, although the statistical test for differences between the treatment effects in current and former smokers is not significant.
Table 4.
Change in histology measures
| Observation | Treated | Difference* (treated – observed)
|
|||
|---|---|---|---|---|---|
| Difference | 95% CI | P | |||
| All subjects | |||||
| n | 19 | 36 | |||
| Maximum | 0.37 (2.34) | 0.03 (1.61) | −0.18 | (−1.16 to 0.81) | 0.72 |
| Average | 0.14 (0.88) | −0.17 (1.04) | −0.25 | (−0.77 to 0.28) | 0.35 |
| Dysplasia index | 0.00 (0.29) | −0.04 (0.26) | −0.02 | (−0.16 to 0.12) | 0.75 |
| Current smokers | |||||
| n | 9 | 15 | |||
| Maximum | −0.56 (1.24) | 0.00 (1.81) | 0.69 | (−0.77 to 2.15) | 0.34 |
| Average | 0.01 (0.93) | −0.32 (1.34) | −0.19 | (−1.27 to 0.88) | 0.71 |
| Dysplasia index | 0.00 (0.35) | −0.11 (0.32) | −0.04 | (−0.33 to 0.24) | 0.76 |
| Former smokers | |||||
| n | 10 | 21 | |||
| Maximum | 1.20 (2.82) | 0.05 (1.50) | −0.92 | (−2.31 to 0.47) | 0.18 |
| Average | 0.26 (0.87) | −0.07 (0.78) | −0.31 | (−0.84 to 0.23) | 0.25 |
| Dysplasia index | 0.01 (0.24) | 0.01 (0.20) | 0.00 | (−0.15 to 0.14) | 0.97 |
Adjusted for baseline histology (either maximum, average, or index). All subject analysis is also adjusted for smoking status.
Fig. 3.
Comparison of follow-up histology measures as a function of baseline histology in those subjects completing treatment and both bronchoscopies. Numbers, WHO histologic grading criteria.
Ki-67 Analysis
Cell proliferation as measured by Ki-67 immunostaining was determined on all biopsy sites as the percentage of cells (of 400) that stained positive for Ki-67. Three measures of Ki67 proliferation were then calculated for each subject: maximum Ki-67 (largest of all Ki-67 scores for that subject’s biopsies), average Ki-67 (average Ki-67 score over all biopsies), and the Ki-67 Fraction (the fraction of biopsies for which the Ki-67 score exceeded 15%). The baseline levels are shown in Table 5. There were no significant differences between observation and treatment groups for any of these parameters. Current smokers had higher Ki-67 immunostaining than former smokers, as previously described (31). Changes in Ki-67 positivity with treatment or observation are shown in Table 6. There were no statistically significant differences between observation and treatment groups in Ki-67 for any of the three parameters analyzed.
Table 5.
Baseline Ki67 levels
| Observation | Treated | Difference (treated – observed)
|
|||
|---|---|---|---|---|---|
| Difference | 95% CI | P | |||
| All subjects | |||||
| n | 27 | 48 | |||
| Maximum | 38.42 (24.28) | 45.14 (26.65) | 6.89 | (−4.22 to 17.90) | 0.22 |
| Average | 18.51 (12.39) | 21.82 (13.60) | 3 41 | (−2.04 to 8.85) | 0.22 |
| Ki67 fraction | 0.40 (0.35) | 0.44 (0.31) | 0.04 | (−0.10 to 0.17) | 0.59 |
| Current smokers | |||||
| n | 12 | 21 | |||
| Maximum | 53.17 (21.33) | 57.29 (20.93) | 4.12 | (−11.43 to 19.08) | 0.59 |
| Average | 27.52 (10.53) | 28.62 (13.20) | 1.11 | (−7.99 to 10.20) | 0.81 |
| Ki67 fraction | 0.06 (0.30) | 0.59 (0.28) | −0.06 | (−0.27 to 0.14) | 0.53 |
| Former smokers | |||||
| n | 15 | 27 | |||
| Maximum | 26.62 (20.04) | 35.70 (27.12) | 9.07 | (−7.11 to 25.26) | 0.26 |
| Average | 11.31 (8.53) | 16.54 (11.57) | 5.23 | (−1.68 to 12.13) | 0.13 |
| Ki67 fraction | 0.20 (0.25) | 0.32 (0.28) | 0.12 | (−0.06 to 0.30) | 0.19 |
NOTE: “All subject” analysis is adjusted for smoking status. Ki67 fraction is proportion of all samples within the individuals in which the Ki67 percentage exceeds 15%.
Table 6.
Change in Ki67
| Observation | Treated | Difference* (treated – observed)
|
|||
|---|---|---|---|---|---|
| Difference | 95% CI | P | |||
| All subjects | |||||
| n | 17 | 32 | |||
| Maximum | −5.14 (34.55) | −8.09 (33.04) | −1.48 | (−18.24 to 15.29) | 0.86 |
| Average | 5.63 (21.22) | −1.68 (19.20) | −4.69 | (−15.94 to 6.57) | 0.41 |
| Ki67 fraction | 0.04 (0.40) | 0.03 (0.38) | −0.02 | (−0.23 to 0.20) | 0.88 |
| Current smokers | |||||
| n | 9 | 14 | |||
| Maximum | −10.00 (36.92) | −13.77 (38.83) | 3.58 | (−22.47 to 29.62) | 0.78 |
| Average | 5.31 (27.29) | −1.63 (22.54) | −3.54 | (−23.64 to 16.56) | 0.72 |
| Ki67 Fraction | −0.01 (0.49) | 0.07 (0.38) | 0.04 | (−0.32 to 0.40) | 0.81 |
| Former smokers | |||||
| n | 8 | 18 | |||
| Maximum | 0.34 (33.27) | −3.67 (28.12) | −3.93 | (−26.28 to 18.42) | 0.72 |
| Average | 5.99 (13.31) | −1.73 (16.84) | −5.76 | (−18.87 to 7.35) | |
| Ki67 fraction | 0.09 (0.30) | −0.01 (0.38) | −0.08 | (−0.36 to 0.20) | 0.57 |
Adjusted for baseline Ki67 level (either maximum, average, or fraction) “All subject” analysis is also adjusted for smoking status. Ki67 fraction is proportion of all samples within the individuals in which the Ki67 percentage exceeds 15%.
Adverse events
Seventy three percent (55 of 75) of subjects completed the first year of the study. Fourteen of the 16 subjects who stopped therapy were receiving 13-cis RA with or without α tocopherol. Treatment was primarily stopped because of toxicity: grade 1 (3), grade 2 (5), and grade 3 (2), and the remaining 4 subjects discontinued therapy for other reasons. Eight subjects received <3 months of therapy. Skin toxicities including dry skin and eyes were the most frequent toxicity seen with treatment but were mild. Elevated uric acid, triglycerides, and cholesterol were common in both the treated and untreated groups. Events above grade 2 were uncommon. α Tocopherol did not reduce the side effects of 13-cis RA. (Table 7). One patient who had received 7 months of both agents died unexpectedly from heart failure. Two additional subjects died from cardiopulmonary causes during the follow-up period. Bronchoscopy was well-tolerated without pneumothorax, clinically significant bleeding, or respiratory failure requiring intubation or assisted ventilation.
Table 7.
Toxicities
| Toxicity | Observation (25 subjects): subjects with toxicity (grade toxicity) | 13-Cis RA (22 subjects): subjects with toxicity (grade toxicity) | 13-Cis RA/α toco (25 subjects): subjects with toxicity (grade toxicity) |
|---|---|---|---|
| Gastrointestinal | 1 (1) | 1 (1) | 8 (1) |
| Musculoskeletal | 4 (1) | 6 (1). 2 (2) | 3 (1). 3 (2) |
| Eye | 1 (1) | 7 (1), 4 (2) | 7 (1), 2 (2) |
| Skin | 6 (1). 2 (2) | 7 (1). 5 (2) | 18 (1), 6 (2) |
| Triglyceride | 10 (1) | 8 (1), 4 (2), 2 (3) | 10 (1), 2 (2) |
| Cholesterol | 4 (1) | 1 (1) | 9 (1), 1 (2) |
| Glucose | 7 (1), 2 (2) | 1 (1), 2 (2). 1 (3) | 6 (1) |
| Liver function | 9 (1), 2 (2) | 10 (1), 2 (2) | 17 (1), 1 (2) |
| Uric acid | 6 (1), 1 (3) | 5 (1), 4 (2), 1 (4) | 4(1), 1 (2). 1 (4) |
One patient receiving 13-cis RA was diagnosed with stage I microinvasive squamous cell lung cancer on the 1-year posttreatment bronchoscopy. She was treated with brachytherapy and died 11 months after the 1-year posttreatment bronchoscopy from pneumonia and chronic obstructive lung disease.
Discussion
Phase III trials with lung cancer as the end point are needed before potential chemoprevention agents can be recommended for widespread use in high-risk populations. These trials are difficult, expensive, and time-consuming, so strategies to prioritize agents for phase III trials are needed. Information from epidemiology, cell biology, preclinical models, and phase II intermediate end point trials all may be useful to inform choices of agents for definitive phase III chemoprevention trials.
In this phase II study, we show that 13-cis RA–treated subjects had minor but nonstatistically significant improvement in the histologic appearance of their bronchial biopsies assessed by maximum or average score or by the dysplasia index (Table 4). Similarly, in the ITT analysis the risk of failure (progression in terms of maximum histologic score or failure to receive follow-up bronchoscopy) was 55.6% of subjects in the observation arm but only 50% of the treated group (P = 0.95; Table 3). The possible small effect of the 13-cis RA on histologic progression was not statistically different between current and former smokers. In a similar fashion, this trial showed no statistically significant differences in the changes between treatment and observation groups in bronchial epithelial proliferation measured by Ki-67 immunostaining. Although disappointing, these data are similar to what is known about the effectiveness of 13-cis RA from a large randomized trial where it failed to reduce the incidence of second primary lung cancers (15). The data from our trial would not have supported moving 13-cis RA to a large phase III trial. Our results support the use of phase II trials with histology as an end point in prioritizing agents for phase III chemoprevention trials as similar negative results have been now been obtained for 13-cis RA in both.
We used three different within subject histology measures: maximum histology, average histology, and dysplasia index. It is not known which if any of these will be most highly predictive of chemoprevention in phase III trials. Maximum histology is likely the most clinically relevant, whereas average histology or dysplasia index might be most indicative of a field effect. Average histology and dysplasia index are dependent on the number of biopsies taken, as well as protocol details, such as whether only areas suspicious for dysplasia are biopsied or whether specific biopsy sites are mandated, in addition to suspicious areas.
We did not find a treatment effect of 13-cis RA on Ki-67 labeling index. Hittelman and colleagues (29) have recently reported that in both per subject and per site analysis, Ki-67 labeling decreased with 13-cis RA treatment in the parabasal but not the basal or superficial layers of the bronchial epithelium of former smokers. We do not fully understand the basis for this discrepancy, but it may be related to technical issues. Our study pathologists find that in the presence of dysplasia, definition of the basal and parabasal layers of the bronchial epithelium is not clear and we therefore determine Ki-67 labeling on the entire thickness of the bronchial epithelium. In fact, in the presence of dysplasia, Ki67 immunolabeling often extends to the most superficial layers of the epithelium. Although distinguishing between Ki-67 immunolabeling of various layers of the bronchial epithelium may be useful in studies on biopsies of lower grade premalignancy, such as metaplasia, this would be technically difficult in higher grades of atypia. A trial reporting decreased Ki-67 index with celecoxib did not discriminate between basal, parabasal, and superficial layers of the bronchial epithelium (28).
This study also failed to confirm the ability of α tocopherol to reduce the toxicity associated with 13-cis RA. The severity of drug-associated toxicities was similar in each group and the fraction of subjects who discontinued therapy due to subject choice was also similar.
Recruitment of subjects to phase II chemoprevention trials with histology as an end point has proved difficult, partly due to the requirement for bronchoscopy. Rates of dysplasia have often been low (21). We selected high-risk subjects based on smoking history, airflow obstruction, and/or history of prior lung cancer without evidence of reccurrence, all with sputum atypia. At the baseline bronchoscopy, 29% (162 of 561) of bronchial biopsies had dysplasia and 68% (51 of 75) of subjects had 1 or more biopsy with dysplasia. Other investigators have required dysplasia on a baseline bronchoscopy as an entry criterion, but this might be a disincentive to patient enrollment and increases cost. We do not know if modification of the entry criteria further would improve recruitment of subjects with dysplasia. The requirement for sputum atypia presents a significant barrier to enrollment, as not all subjects with a >30 pack-year smoking history and airflow obstruction can provide adequate expectorated samples. The methodology used in this trial can easily be adapted to the study of other novel agents and similar designs have been applied to the study of celecoxib, inhaled corticosteroids, iloprost, and green tea extract (22, 23, 28).
Using the entry criteria of a 30 pack-year smoking history, airflow obstruction and sputum atypia or a lung cancer resected >36 months before enrollment and sputum atypia, we were successful in recruiting subjects with a high incidence of endobronchial dysplasia for a phase II chemoprevention trial of 13-cis RA with or without α tocopherol compared with observation alone. α Tocopherol supplementation had no effect on the frequency of adverse events with 13-cis RA treatment. Although 13-cis RA treatment was associated with trends toward lower rates of progression and improvement in endobronchial histology, no statistically significant treatment effects were seen. There was no effect of 13-cis RA on maximal or average Ki-67 labeling index, nor on the proportion of biopsies within individuals with >15% Ki-67 labeling. The results of this phase II chemoprevention trial are congruent with those of a larger phase III trial of 13-cis RA for chemoprevention of lung cancer. We conclude that there may be small effects of 13-cis RA on bronchial histology, but a new agent would have to show much greater and statistically significant effects to justify moving to a phase III trial. The basic study design of this trial is suitable for phase II trials and the results may be used to inform sample size calculations for future trials.
Acknowledgments
Grant support: NIH/National Cancer Institute P50 CA058187 Specialized Programs of Research Excellence in Lung Cancer NIH/National Cancer Institute P30 CA46934 Cancer Center Core Grant.
Footnotes
Disclosure of Potential Conflicts of Interest
Drs. Miller and Keith have applied for a patent regarding the use of prostacyclin analogs for the chemoprevention of cancer. The other authors disclosed no potential conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Tong L, Spitz MR, Fueger JJ, Amos CA. Lung carcinoma in former smokers. Cancer. 1996;78:1004–10. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1004::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 5.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 6.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 8.Giardiello FM, Yang VW, Hylind LM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346:1054–59. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 10.Saccomanno G, Moran PG, Schmidt R, et al. Effects of 13-CIS retinoids on premalignant and malignant cells of lung origin. Acta Cytol. 1982;26:78–85. [PubMed] [Google Scholar]
- 11.Nicholson AG, Perry LJ, Cury PM, et al. Reproducibility of the WHO/IASLC grading system for pre-invasive squamous lesions of the bronchus: a study of inter-observer and intra-observer variation. Histopathology. 2001;38:202–8. doi: 10.1046/j.1365-2559.2001.01078.x. [DOI] [PubMed] [Google Scholar]
- 12.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of β carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 13.The α-Tocopherol, β Carotene Cancer Prevention Study Group. The effect of vitamin E and β carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 14.Kamangar F, Qiao YL, Yu B, et al. Lung cancer chemoprevention: a randomized, double-blind trial in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2006;15:1562–1564. doi: 10.1158/1055-9965.EPI-06-0316. [DOI] [PubMed] [Google Scholar]
- 15.Lippman SM, Lee JJ, Karp DD, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001;93:605–18. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 16.van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EU-ropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–86. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 17.O’Shaughnessy JA, Kelloff GJ, Gordon GB, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 1989;8:314–46. [PubMed] [Google Scholar]
- 18.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 2002;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 19.Berger VW. Does the Prentice criterion validate surrogate endpoints? Stat Med. 2004;23:1571–8. doi: 10.1002/sim.1780. [DOI] [PubMed] [Google Scholar]
- 20.McLarty JW, Holiday DB, Girard WM, Yanagihara RH, Kummet TD, Greenberg SD. β-Carotene, vitamin A, lung cancer chemoprevention: results of an intermediate endpoint study. Am J Clin Nutr. 1995;62:1431S–8S. doi: 10.1093/ajcn/62.6.1431S. [DOI] [PubMed] [Google Scholar]
- 21.Kurie JM, Lee JS, Khuri FR, et al. N-(4-hydroxyphenyl)retinamide in the chemoprevention of squamous metaplasia and dysplasia of the bronchial epithelium. Clin Cancer Res. 2000;6:2973–9. [PubMed] [Google Scholar]
- 22.Lam S, MacAulay C, Le Riche JC, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J Natl Cancer Inst. 2002;94:1001–9. doi: 10.1093/jnci/94.13.1001. [DOI] [PubMed] [Google Scholar]
- 23.Lam S, leRiche JC, McWilliams A, et al. A randomized phase IIb trial of pulmicort turbuhaler (bu-desonide) in people with dysplasia of the bronchial epithelium. Clin Cancer Res. 2004;10:6502–11. doi: 10.1158/1078-0432.CCR-04-0686. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg RM, van TH, van ZN, et al. The influence of fluticasone inhalation on markers of carcinogenesis in bronchial epithelium. Am J Respir Crit Care Med. 2007;175:1061–65. doi: 10.1164/rccm.200612-1770OC. [DOI] [PubMed] [Google Scholar]
- 25.Kelloff GJ, Lippman SM, Dannenberg AJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer-a plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 26.Szabo E. Lung epithelial proliferation: a biomarker for chemoprevention trials? J Natl Cancer Inst. 2001;93:1042–43. doi: 10.1093/jnci/93.14.1042. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Liu D, Lee JS, et al. Long-term impact of smoking on lung epithelial proliferation in current and former smokers. J Natl Cancer Inst. 2001;93:1081–88. doi: 10.1093/jnci/93.14.1081. [DOI] [PubMed] [Google Scholar]
- 28.Mao JT, Fishbein MC, Adams B, et al. Celecoxib decreases Ki-67 proliferative index in active smokers. Clin Cancer Res. 2006;12:314–20. doi: 10.1158/1078-0432.CCR-05-1440. [DOI] [PubMed] [Google Scholar]
- 29.Hittelman WN, Liu DD, Kurie JM, et al. Proliferative changes in the bronchial epithelium of former smokers treated with retinoids. J Natl Cancer Inst. 2007;99:1603–12. doi: 10.1093/jnci/djm205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam S, leRiche JC, Zheng Y, et al. Sex-related differences in bronchial epithelial changes associated with tobacco smoking. J Natl Cancer Inst. 1999;91:691–6. doi: 10.1093/jnci/91.8.691. [DOI] [PubMed] [Google Scholar]
- 31.Miller YE, Blatchford P, Hyun DS, et al. Bronchial epithelial Ki-67 index is related to histology, smoking, and gender, but not lung cancer or chronic obstructive pulmonary disease. Cancer Epidemiol Biomarkers Prev. 2007;16:2425–31. doi: 10.1158/1055-9965.EPI-07-0220. [DOI] [PubMed] [Google Scholar]
- 32.Shklar G, Flynn E, Szabo G, Marefat P. Retinoid inhibition of experimental lingual carcinogenesis: ultrastructural observations. J Natl Cancer Inst. 1980;65:1307–16. [PubMed] [Google Scholar]
- 33.Lippman SM, Batsakis JG, Toth BB, et al. Comparison of low-dose isotretinoin with β carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 34.Hong WK, Endicott J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 35.Xu XC, Lee JS, Lee JJ, et al. Nuclear retinoid acid receptor β in bronchial epithelium of smokers before and during chemoprevention. J Natl Cancer Inst. 1999;91:1317–21. doi: 10.1093/jnci/91.15.1317. [DOI] [PubMed] [Google Scholar]
- 36.Kurie JM, Lotan R, Lee JJ, et al. Treatment of former smokers with 9-cis-retinoic acid reverses loss of retinoic acid receptor-β expression in the bronchial epithelium: results from a randomized placebo-controlled trial. J Natl Cancer Inst. 2003;95:206–14. doi: 10.1093/jnci/95.3.206. [DOI] [PubMed] [Google Scholar]
- 37.Dimery IW, Hong WK, Lee JJ, et al. Phase I trial of α-tocopherol effects on 13-cis-retinoic acid toxicity. Ann Oncol. 1997;8:85–9. doi: 10.1023/a:1008209525671. [DOI] [PubMed] [Google Scholar]
- 38.Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239–53. doi: 10.1007/BF00944177. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch FR, Prindiville SA, Miller YE, et al. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst. 2001;93:1385–91. doi: 10.1093/jnci/93.18.1385. [DOI] [PubMed] [Google Scholar]
- 40.Merrick DT, Kittelson J, Winterhalder R, et al. Analysis of c-ErbB1/epidermal growth factor receptor and c-ErbB2/HER-2 expression in bronchial dysplasia: evaluation of potential targets for chemoprevention of lung cancer. Clin Cancer Res. 2006;12:2281–8. doi: 10.1158/1078-0432.CCR-05-2291. [DOI] [PubMed] [Google Scholar]