Abstract
Elevated serum phosphorus has been associated with increased mortality from cardiovascular (CV) disease. However, information is scant regarding the influence of serum phosphorus within the normal range on vascular risk in terms of subclinical atherosclerosis in asymptomatic young adults. Serum phosphorus along with other CV risk factor variables were measured in 856 white and 354 black subjects without known CV disease or renal disease. Carotid intima-media thickness (IMT) was measured by B-mode ultrasonography. Significant race and sex differences were noted for serum phosphorus (blacks>whites) and carotid IMT (black females>white females; males>females). In bivariate analyses, serum phosphorus was correlated with carotid IMT (p<0.001); and smokers showed higher phosphorus levels than nonsmokers (p=0.008). In multivariate regression analyses, carotid IMT was significantly associated with serum phosphorus (regression coefficient β=0.028, p<0.001) and smoking (β=0.032, p<0.001), adjusting for other CV risk factors and estimated glomerular filtration rate. In addition, a significant interaction effect of cigarette smoking and serum phosphorus on carotid IMT was noted, with a greater increasing trend of carotid IMT with phosphorus in smokers than that in nonsmokers (p=0.019 for interaction). In conclusion, serum phosphorus within the normal range is an important correlate of carotid IMT in asymptomatic young adults, with smoking potentiating this adverse association.
Keywords: phosphorus, carotid artery, carotid intima-media thickness, cigarette smoking, atherosclerosis
Phosphorus is essential for diverse biological functions and is tightly regulated within the physiological range in healthy subjects. 1, 2 Excess amount of phosphorus has been associated with increased mortality from cardiovascular (CV) disease in subjects with chronic kidney disease, ranging from moderate to end stage renal failure.3–7 Recently, it has been found that phosphorus levels within a normal range are independently associated with the risk of all-cause mortality and CV events in people with previous myocardial infarction.8 Phosphorus levels are also associated with increased risk of chronic kidney disease,9 and may be a potentially modifiable risk factor for stroke and death in a general population.10 However, only a few studies have examined the relationship between phosphorus levels and subclinical atherosclerosis in asymptomatic individuals.11,12 The present study examines the relationship between serum phosphorus and carotid intima-media thickness (IMT), a validated indicator of subclinical atherosclerosis and future CV risk,13–15 in clinically asymptomatic white and black young adults.
Materials and Methods
As part of the Bogalusa Heart Study, a biracial (black-white) community-based investigation of the early nature history of CV disease, A total of 1210 subjects (856 whites and 354 blacks; 43.1% males) aged 24–44 years, residing in the community of Bogalusa, LA, were examined for carotid IMT by ultrasound and CV risk factor variables, including serum phosphorus. All subjects in this study gave informed consent for examination. Study protocols were approved by the Institutional Review Board of the Tulane University Medical Center.
Examinations of study subjects followed the protocols and procedures described elsewhere.16 Subjects were instructed to fast for 12 hours before screening, and the compliance was determined by an interview on the morning of examination. Height and weight were measured twice to ± 0.1 cm and to ± 0.1 kg, respectively. Body mass index (BMI=weight in kilograms divided by the square of the height in meters) was used as a measure of overall adiposity. Blood pressure levels were measured using mercury sphygmomanometers on the right arm of subjects in a relaxed, sitting position by two randomly assigned nurses (three replicates each). The first and fifth Korotkoff phases were used to determine systolic and diastolic blood pressure, respectively. Means of replicate readings were used for analyses. Forced values (140/90mmHg) were assigned for systolic and diastolic blood pressure, respectively, to subjects (n=81) who were on anti-hypertension medication at the time of examination. Mean arterial pressure (MAP=diastolic blood pressure + 1/3 pulse pressure) was used as a measure of hemodynamic status. Information on smoking status was obtained by questionnaires. Those who smoked at least one cigarette per week during the past one year were considered as current smokers.
Serum phosphorus levels were determined by the ammonium molybdate method, glucose and creatinine by an enzymatic method, as part of multiple chemistry profile (SMA20) by the multichannel Olympus Au-5000 Analyzer (Olympus, Lake Success, NY). Serum cholesterol and triglycerides (TG) were determined enzymatically17 on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN). Serum lipoprotein cholesterols were analyzed by a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures.18 The laboratory is being monitored for precision and accuracy by the Lipid Standardization Program of the Centers for Disease Control and Prevention, Atlanta, GA. Plasma insulin was measured by a commercial radioimmunoassay kit (Padebas insulin kits, Pharmacia, Diagnostics, Piscataway, NJ); Intra-class correlation coefficients, a measure of reproducibility of the entire process from blood collection to data processing, between the blind duplicate values (n=97) were 0.98 for low-density lipoprotein cholesterol (LDL-C), 0.99 for high-density lipoprotein cholesterol (HDL-C), 0.97 for TG, 0.98 for both glucose and insulin, 0.98 for creatinine and 0.99 for serum phosphorus. Glomerular filtration rate (eGFR) was estimated as a function of age, serum creatinine, gender and race using the simplified Modified Diet in Renal Disease (MDRD) equation19: eGFR (mL/min/1.73m2) = 186.3 × creatinine−1.154 × age−0.203 × (0.742 if female) × (1.21 if black), where m2 denotes body surface area in squared meter. An index of insulin resistance (homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the formula:20 fasting insulin μU/mL × fasting glucose (mmol/L)/22.5. Forced values of 160mg/dL for LDL-C and 126 mg/dL for glucose were assigned to subjects who were on medications for hypercholesterolemia (n=26) and diabetes (n=19) at the time of examination, respectively.
Carotid ultrasound measurements were done by an experienced and highly trained technician on a Toshiba Ultrasound instrument (Power Vision Toshiba SSH-380 ultrasound system, Toshiba American Medical Systems, Carrollton, TX), using a 7.5 MHz linear array transducer. Images were recorded at the common carotid, carotid bulb (bifurcation), and internal carotid arteries bilaterally according to previously developed protocols for the Atherosclerosis Risk in Communities study.21 Images were recorded on super VHS video tapes and read by certified readers from the Vascular Ultrasound Research Laboratory in Wake Forest Medical Center, North Carolina, using a semi automatic ultrasound imaging. The maximum carotid IMT readings of left and right far walls were averaged for each segment; if bilateral images could not be obtained, one side was used as the average. Then the averaged carotid IMT of common, bulb, and internal segments was calculated as mean carotid IMT. Common carotid IMT, carotid bulb IMT, internal carotid IMT and the mean carotid IMT were used for analyses. Based on repeated measures of 75 study subjects, the intraclass correlation coefficients for repeat scans were 0.72 for the common carotid, 0.69 for the carotid bulb, and 0.80 for the internal carotid.
All data analyses were performed using SAS version 9.1. HOMA-IR and TG/HDL-C were log-transformed to improve the normality of distribution for correlation and regression analyses; however, their mean values in original scales were presented in Table 1 for description. Differences in mean values of study variables between race-sex groups as well as serum phosphorus between smokers and nonsmokers were tested by analysis of covariance models. The correlation of the serum phosphorus with CV risk factor variables and carotid IMT was assessed using Pearson correlation coefficients. The independent relation of serum phosphorus on carotid IMT was examined by multivariate regression models, adjusting for other CV risk factor variables and eGFR; because cigarette smoking, which contains substantial amounts of nicotine, free radicals and prooxidants to produce oxidative stress22 and higher serum phosphorus,23,24 may confound the results, two separate models without (model I) and with (model II) smoking status were used. Further, the interaction of serum phosphorus and smoking on carotid IMT was tested by including an interaction term.
Table 1.
Levels of study variables by race and sex
| Variable* | White
|
Black
|
P Value for difference
|
|||
|---|---|---|---|---|---|---|
| Male (n=377) | Female (n=479) | Male (n=144) | Female (n=210) | Race | Sex | |
| Age (years) | 36 ± 4 | 36 ± 5 | 36 ± 4 | 35 ± 5 | NS | NS |
| Body mass index (kg/m2) | 29 ±6 | 28 ± 7 | 29 ± 7 | 31 ± 8 | <0.001† | <0.05 |
| Homeostasis model assessment of insulin resistance | 3.0 ± 3.0 | 2.4 ± 2.2 | 2.8 ± 2.6 | 3.6 ± 5.5 | <0.001† | <0.05 |
| Triglycerides/High-density lipoprotein cholesterol | 4.5 ± 4.7 | 2.8 ± 3.6 | 3.1 ± 3.6 | 1.8 ± 1.1 | <0.001 | <0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 130 ±34 | 124 ±33 | 125 ±43 | 114 ±30 | <0.002† | <0.05 |
| Mean arterial pressure (mm Hg) | 93 ± 8 | 87 ±9 | 99 ±13 | 92 ±12 | <0.001 | <0.001 |
| Smoker (%) | 29 | 28 | 42 | 29 | <0.001‡ | <0.05§ |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 97 ±16 | 98 ±22 | 105 ±20 | 116 ±25 | <0.001 | <0.001§ |
| Phosphorus (mg/dL) | 3.35± 0.54 | 3.37± 0.52 | 3.70± 0.59 | 3.61± 0.55 | <0.001 | NS |
| Carotid intima-media thickness (mm) | 0.85± 0.18 | 0.75± 0.12 | 0.87± 0.16 | 0.79± 0.14 | <0.001† | <0.001 |
Means ± SD for continuous variables
NS indicates p>0.05;
females only;
males only;
blacks only
Results
Table 1 shows levels of study variables by race and sex. Significant racial differences were noted for phosphorus (blacks>whites), carotid IMT (black females>white females), BMI (black females>white females), HOMA-IR (black females>white females), TG/HDL-C (whites>blacks), LDL-C (white females>black females), MAP (blacks>whites), eGFR (blacks>whites) and number of smokers (black males>white males). Sex-related difference were significant for carotid IMT (males>females), BMI (white males>white females and black females>black males), HOMI-IR (white males>white females and black females>black males), TG/HDL-C (males>females), LDL-C (males>females), MAP (males>females), eGFR (black females>black males) and number of smokers (black males>black females).
Pearson correlation coefficients of serum phosphorus with carotid IMT and CV risk factor variables are presented in Table 2. Serum phosphorus was significantly correlated with carotid IMT, adjusting for race, sex and age. Importantly, smokers had significantly higher mean levels (±SD) of serum phosphorus (3.52±0.54 mg/dL) than nonsmokers (3.41±0.56 mg/dL), adjusting for race, sex and age (p=0.008). In addition, serum calcium levels did not show significant relationship with carotid IMT (data not shown).
Table 2.
Correlations of serum phosphorus levels with cardiovascular risk factor variables and carotid intima-media thickness
| r | p-value | |
|---|---|---|
| Age* | −0.014 | 0.599 |
| Body mass index † | 0.009 | 0.765 |
| Log-Homeostasis model assessment of insulin resistance † | −0.040 | 0.170 |
| Log-Triglycerides/High-density lipoprotein cholesterol † | −0.017 | 0.559 |
| Low-density lipoprotein cholesterol † | 0.028 | 0.333 |
| Mean arterial pressure † | 0.041 | 0.153 |
| Estimated glomerular filtration rate † | 0.034 | 0.244 |
| Carotid intima-media thickness † | 0.124 | <0.001 |
adjusted for race and sex
adjusted for race, sex and age
Regression coefficients of carotid IMT on serum phosphorus and CV risk factor variables are presented in Table 3. In model I (without smoking status), sex (male>female), age, Log-TG/HDL-C, LDL-C, MAP and phosphorus were significantly and positively associated with carotid IMT. In model II, which included smoking status, serum phosphorus was still significantly associated with carotid IMT along with other variables retained in model I. In addition, smoking was significantly associated with carotid IMT in model II. In addition to the mean carotid IMT, we also examined the relationship of phosphorus to carotid IMT of individual segments. Serum phosphorus was significantly associated with common carotid IMT (p<0.001), carotid bulb IMT (p=0.006) and internal carotid IMT (p=0.010) with CV risk factor variables included in the models.
Table 3.
Regression coefficients of carotid intima-media thickness on serum phosphorus levels and cardiovascular risk factor variables
| Predictor variable | Model I
|
Model II
|
||
|---|---|---|---|---|
| β | p-value | β | p-value | |
| Black race | 0.017 | 0.080 | 0.016 | 0.115 |
| Male sex | 0.057 | <0.001 | 0.057 | <0.001 |
| Age | 0.010 | <0.001 | 0.010 | <0.001 |
| Body mass index | 0.001 | 0.473 | 0.001 | 0.263 |
| Log-Homeostasis model assessment of insulin resistance | 0.003 | 0.671 | 0.007 | 0.359 |
| Log-Triglycerides/High-density lipoprotein cholesterol | 0.018 | 0.005 | 0.015 | 0.025 |
| Low-density lipoprotein cholesterol | 0.001 | <0.001 | 0.001 | <0.001 |
| Mean arterial pressure | 0.002 | <0.001 | 0.002 | <0.001 |
| Estimated glomerular filtration rate | 0.0003 | 0.104 | 0.0002 | 0.200 |
| Smoking (yes/no) | ----- | ----- | 0.032 | <0.001 |
| Phosphorus | 0.030 | <0.001 | 0.028 | <0.001 |
| R2 | 0.262 | 0.270 | ||
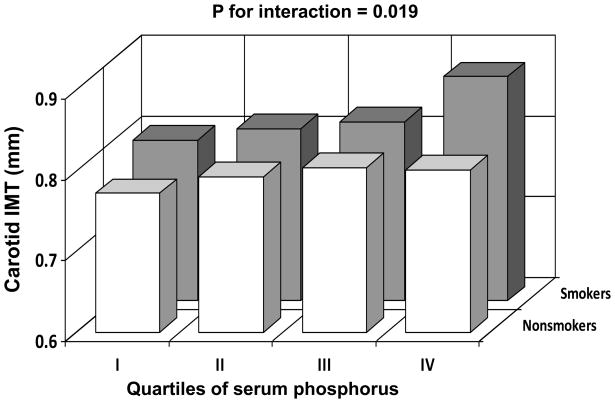
The interaction effect of serum phosphorus with smoking on carotid IMT was tested in an interaction regression model as shown in Figure 1. Carotid IMT increased with increasing quartiles of serum phosphorus in both smokers and nonsmokers; however, the increasing trend in smokers was significantly greater than that in nonsmokers (p=0.019 for interaction). In order to examine the synergistic effect of nicotine intake on the phosphorus-IMT relationship, the interaction effect between number of cigarettes per day and serum phosphorus (as a continuous variable) was also tested. The interaction effect on carotid IMT was significant (p=0.002). Further, in a subset of nonsmokers (n=431) who did not have hypertension, dyslipidemia and diabetes, a similar but nonsignificant trend of association of phosphorus with carotid IMT (β=0.009, p=0.416) was noted.
Figure 1.
The relationship of carotid intima-media thickness (IMT) to smoking status and serum phosphorus quartiles specific for race, sex and age: The Bogalusa Heart Study
Discussion
The present study demonstrates that increasing levels of serum phosphorus, even within the normal range, are positively and independently related to subclinical atherosclerosis, measured as carotid IMT. Furthermore, when estimated glomerular filtration rate derived from the Modification of Diet in Renal Disease study equation19 was added to the multivariate regression model, serum phosphorus, but not estimated glomerular filtration rate, was still significantly related to carotid IMT in these younger adults without kidney disease (data not shown). This is consistent with earlier findings on a relatively older population.11 These findings based on a community-dwelling cohort of younger asymptomatic adults, free of selection bias, are noteworthy in that they underscore the potential value of phosphorus as a risk factor for CV risk in younger population.
Another interesting finding of this study is that the smoking-serum phosphorus interaction was significant and synergetic, with the magnitude of relationship between phosphorus and carotid IMT in smokers vs nonsomkers being significantly stronger. Furthermore, nicotine intake measured as number of cigarettes smoked per day also showed a significant synergistic effect on the phosphorus-IMT relationship. Little comparable data are available in the literature for comparison in this regard. Deleterious effect of smoking on arterial wall properties is attributed to vasoactive substances including carbon monoxide, reactive oxygen species and nicotine related to adverse changes in vascular reactivity, as reflected by impaired basal production of nitric oxide.22 Of note, the current observation showing higher phosphorus levels in smokers vs nonsmokers is consistent with the earlier finding in smokers23 and experimentally with rats treated with nicotine.24 Furthermore, in our study the phosphorus-carotid IMT association became not significant, although the trend was similar, in a subset of nonsmokers without hypertension, dyslipidemia and diabetes, indicating that the effect of phosphorus on carotid IMT may also depend on other CV risk factors.
Although hyperphospheremia is considered a risk factor for CV disease, especially among those with chronic kidney disease, the underlying mechanism(s) linking excess serum phosphorus within normal range to subclinical and overt CV disease in individuals with normal kidney function is not well understood. Based on the research findings on individuals with normal kidney function, the possible mechanisms may include, among others, promotion of vascular calcification by inducing phenotypic alteration of smooth muscle cells into osteoblast/chondrocyte-like cells,25 induction of synthesis of inflammatory cytokine interleukin-6 by increasing circulating parathyroid hormone,26,27 and down regulation of 1,25-dihydroxyvitamin D production and related increase of coronary calcification and risk of myocardial infarction.28,29 It should be emphasized that although excess serum phosphorus and the attendant CV risk may be due to subclinical renal dysfunction and secondary hyperparathyroidism, such a pathogenic pathway may not be likely to explain the observed relationship in this younger adult study cohort with normal kidney function. Further studies in this direction are obviously needed in the general population having serum phosphorus levels within the normal range.
As a limitation, the cross-sectional nature of this observational study cannot address the issue of causality but only suggest putative mechanism(s) as discussed above. Further, the present study did not assess the determinants of serum phosphorus levels including the parathyroid hormone, vitamin D levels and dietary phosphorus intake.
Acknowledgments
This study was supported by grants HD-061437 and HD-062783 from the National Institute of Child Health and Human Development, 0855082E from American Heart Association, and AG-16592 from the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weisinger JR, Bellorín-Font E. Magnesium and phosphorus. Lancet. 1998;352:391–396. doi: 10.1016/S0140-6736(97)10535-9. [DOI] [PubMed] [Google Scholar]
- 2.Blumsohn A. What have we learnt about the regulation of phosphate metabolism? Curr Opin Nephrol Hypertens. 2004;13:397–401. doi: 10.1097/01.mnh.0000133983.40182.c3. [DOI] [PubMed] [Google Scholar]
- 3.Vassalotti JA, Uribarri J, Chen SC, Li S, Wang C, Collins AJ, Calvo MS, Whaley-Connell AT, McCullough PA, Norris KC Kidney Early Evaluation Program Investigators. Trends in mineral metabolism: Kidney Early Evaluation Program (KEEP) and the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008;51(Suppl 2):S56–68. doi: 10.1053/j.ajkd.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 5.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, Klag MJ, Powe NR. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 6.Phelan PJ, O’Kelly P, Walshe JJ, Conlon PJ. The importance of serum albumin and phosphorous as predictors of mortality in ESRD patients. Ren Fai. 2008;30:423–429. doi: 10.1080/08860220801964236. [DOI] [PubMed] [Google Scholar]
- 7.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephro. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 9.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 10.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199:424–431. doi: 10.1016/j.atherosclerosis.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum Phosphorus Levels Associate with Coronary Atherosclerosis in Young Adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 15.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 16.Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC, Foster TA, Blonde CV. In: Cardiovascular Risk Factors in Children--The Early Natural History of Atherosclerosis and Essential Hypertension. Andrews C, Hester HE, editors. New York: Oxford University Press; 1980. pp. 47–123. [Google Scholar]
- 17.Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–474. [PubMed] [Google Scholar]
- 18.Srinivasan SR, Berenson GS. Serum lipoproteins in children and methods for study. In: Lewis LA, editor. CRC Handbook of Electrophoresis Vol III: Lipoprotein Methodology and Human Studies. Boca Raton, FL: CRC Press; 1983. pp. 185–203. [Google Scholar]
- 19.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Bond MG, Barnes RW, Wiley WA, Wilmoth SK, Chambless LE, Howard G. High-resolution B-mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. The ARIC Study Group. J Neuroimmaging. 1991;1:168–172. [PubMed] [Google Scholar]
- 22.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto N, Sakamoto K, Ando S. Mineral concentrations in the blood of male smokers and non-smoking males and females measured with fluoro-X-ray analyzer. Cent Eur J Public Health. 1998;6:284–287. [PubMed] [Google Scholar]
- 24.Iwaniec UT, Fung YK, Akhter MP, Haven MC, Nespor S, Haynatzki GR, Cullen DM. Effects of nicotine on bone mass, turnover, and strength in adult female rats. Calcif Tissue Int. 2001;68:358–364. doi: 10.1007/s00223-001-0011-8. [DOI] [PubMed] [Google Scholar]
- 25.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 26.Grey A, Mitnick MA, Masiukiewicz U, Sun BH, Rudikoff S, Jilka RL, Manolagas SC, Insogna K. A role for interleukin-6 in parathyroid hormone-induced bone resorption in vivo. Endocrinology. 1999;140:4683–4690. doi: 10.1210/endo.140.10.7036. [DOI] [PubMed] [Google Scholar]
- 27.Mitnick MA, Grey A, Masiukiewicz U, Bartkiewicz M, Rios-Velez L, Friedman S, Xu L, Horowitz MC, Insogna K. Parathyroid hormone induces hepatic production of bioactive interleukin-6 and its soluble receptor. Am J Physiol Endocrinol Metab. 2001;280:E405–412. doi: 10.1152/ajpendo.2001.280.3.E405. [DOI] [PubMed] [Google Scholar]
- 28.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 29.Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–563. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]