Abstract
Background
Patients with acute myocardial infarctions (AMI) who are admitted to hospitals without coronary revascularization are frequently transferred to hospitals with this capability, yet we know little about the basis for how such revascularization hospitals are selected.
Methods and Results
We examined interhospital transfer patterns in 71,336 AMI patients admitted to hospitals without revascularization capabilities in the 2006 Medicare claims using network analysis and regression models. A total of 31,607 (44.3%) AMI patients were transferred from 1,684 non-revascularization hospitals to 1,104 revascularization hospitals. Median time to transfer was 2 days. Median transfer distance was 26.7 miles, with 96.1% within 100 miles. In 45.8% of cases, patients bypassed a closer hospital to go to farther hospital that had a better 30-day risk standardized mortality rates. However, in 36.8% of cases, another revascularization hospital with lower 30-day risk-standardized mortality was actually closer to the original admitting non-revascularization hospital than the observed transfer destination. Adjusted regression models demonstrated that shorter transfer distances were more common than transfers to the hospitals with lowest 30-day mortality rates. Simulations suggest that an optimized system that prioritized the transfer of AMI patients to a nearby hospital with the lowest 30-day mortality rate might produce clinically meaningful reduction in mortality.
Conclusions
Over 40% of AMI patients admitted to non-revascularization hospitals are transferred to revascularization hospitals. Many patients are not directed to nearby hospitals with the lowest 30-day risk-standardized mortality, and this may represent an opportunity for improvement.
Keywords: patient transfers, revascularization, networks, Medicare, mortality
Most acute-care hospitals in the U.S. are unable to provide coronary revascularization to patients with acute myocardial infarction (AMI).1 Many AMI patients who are admitted to non-revascularization hospitals are therefore transferred to revascularization hospitals during the same admission.2 Yet we know little of the basis for how revascularization hospitals are selected during this process. In particular, it is unclear the extent to which AMI patients are transferred preferentially toward “higher-quality” revascularization hospitals and the role that geographic distances play in such decisions. If transfers between hospitals fail to concentrate AMI patients at the best-performing hospitals within a region, opportunities would exist for improving care in this high-risk group.
As evidence continues to support early cardiac catheterization and revascularization in both STEMI3, 4 and high-risk non-STEMI patients,5, 6 it is increasingly important to understand the ways in which transfers to revascularization hospitals occur in the real world. While who is transferred has been examined in the past, there has been no previous work examining where patients are transferred.7 Because outcomes across all revascularization hospitals are not uniform,8 examining the organizational structure of transfers may provide an empirical basis to assess interventions to optimize the use of transfer in AMI.
Accordingly, we used network analysis to better understand patterns of interhospital transfer among elderly Medicare beneficiaries with AMI who were initially admitted to non-revascularization hospitals in the United States. Our analyses set out to examine: (1) the proportion of AMI patients admitted to hospitals without revascularization capabilities transferred to revascularization hospitals, (2) the frequency with which patients were transferred to a nearby hospital with the lowest 30-day risk-standardized mortality rate for AMI, and (3) the relationship between a hospital’s likelihood as a transfer destination and its 30-day risk-standardized mortality rate for AMI after accounting for geographic distances traveled.
METHODS
Data Sources and Study Population
In this retrospective cohort analysis, we analyzed all fee-for-service Medicare beneficiaries in the 2006 Medicare Provider Analysis and Review (MedPAR) files admitted with a primary diagnosis of AMI, as defined by an International Classification of Diseases, 9th Revision—Clinical Modification (ICD-9-CM) diagnostic code of 410.xx (excluding 410.x2). We excluded cases with a length of stay less than or equal to 1 day – unless that patient died, left against medical advice, or was transferred to another hospital – since such a short length of stay was likely to represent “rule-out” admissions and not true AMI.9
We empirically defined revascularization hospitals, as have others, as those that performed at least 5 coronary bypass grafting (CABG) and percutaneous coronary intervention (PCI) procedures during the year; all others were considered non-revascularization hospitals.10, 11 For this analysis, we only included patients initially admitted to non-revascularization hospitals with at least 10 AMI admissions during the calendar year in order to allow more reliable estimates of our outcomes of interest. We specifically excluded patients from hospitals that performed PCI in Medicare patients but did not perform CABG because such facilities receive very few transfers from non-revascularization hospitals and have distinct rationales for transferring out patients (e.g., emergent CABG after PCI).
We obtained 30-day risk-standardized mortality rates and volume for AMI from each revascularization hospital using publicly-available data from 2006 on the Hospital Compare website.12,13 The approach for calculating these rates and their validation (as compared with clinical chart abstraction) has been described elsewhere.14, 15 Briefly, the rates are calculated from extensive Medicare inpatient and outpatient claims data using hierarchical regression models. Of relevance for this analysis, the approach used by Hospital Compare assigns AMI patients to the first hospital where they received care when calculating these rates, so as not to bias facilities accepting patients in transfer.16 To ensure that our results were not susceptible to year-to-year fluctuations in 30-day risk-standardized mortality rates across hospitals, we also examined the use of rates from a 3-year period between July 2005 and June 2008 during sensitivity analysis.
We defined interhospital transfers as temporally adjacent hospitalizations in the same patient at 2 different facilities; the discharge day for the non-revascularization hospital had to be the same or one day less than the admitting date of the revascularization hospital.17, 18 For each transfer, straight-line distances between the hospitals involved were calculated.19 Additional data on geographic location and academic affiliation were obtained from the 2005 American Hospital Association (AHA) Annual Survey.20 For subgroup analyses, we defined hospitals as being an urban or rural facility using metropolitan statistical areas.
We limited our analyses to AMI patients at hospitals in the 50 states and the District of Columbia. We also excluded those patients treated at non-revascularization hospitals with incomplete data on facility characteristics (n=18), and at revascularization hospitals with insufficient geographical information (n=8).
Statistical Analysis
We graphed the nationwide interhospital network of transfers for AMI patients between non-revascularization and revascularization hospitals in the United States during 2006 using ArcGIS software. In the network representation, hospitals are nodes, and the transfer of a patient from a non-revascularization hospital to a revascularization hospital forms an edge.
To determine the relative importance of 30-day risk-standardized mortality rates and geographical proximity for a hospital, we used McFadden’s discrete choice framework with a logistic regression implementation.21 (Chapter 7),22 This model quantifies tradeoffs that are made between several competing alternatives, each of which has several measurable characteristics of value. In a general sense, it quantitatively asks the question: what characteristics of hospital A made it more likely to be chosen as a transfer destination than other nearby hospitals? For each patient, we compared the 30-day risk-standardized mortality of and distance to the revascularization hospital that was the final transfer destination with the outcomes of and distance to other revascularization hospitals that were competing alternatives for that transfer. Both distance and RSMR were continuous variables. This approach only compares characteristics of competing alternatives – as such, it takes into account the number of alternatives available from any given non-revascularization hospital. We separately defined this set of competing alternatives to include all other revascularization hospitals within 25- and 100-miles of the non-revascularization hospitals, as both are plausible distances over which transfers might be considered.
During sensitivity analyses, we replicated this last analysis in several important subgroups. First, we considered only patients with a primary diagnosis of AMI at both the non-revascularization and revascularization hospitals. Second, we replicated the analysis separately for non-revascularization hospitals located in urban and rural areas. Third, we evaluated several different radii within 25 miles and 100 miles of the non-revascularization hospital to define our set of alternative revascularization hospitals. Further, we also controlled for the designation as better or worse than the national average in Hospital Compare, as well as hospital AMI volume and teaching status. Finally, we also evaluated the impact of using distance traveled from the patient’s home (based on the centroid of his or her ZIP code) to the transfer destination to define the set of alternative revascularization hospitals.
We assessed the network’s overall tendency to favor transfers to revascularization hospitals with lower 30-day risk-standardized mortality for AMI. To do so, we tested for a bivariate association between outcomes at a revascularization hospital and the number of non-revascularization hospitals sending patients to that facility, after dividing hospitals into quintiles based on the number of sending hospitals and using Cuzick’s Wilcoxon-type non-parametric test for trend. 23 Using negative binomial regression, we asked whether revascularization hospitals with lower RSMR tended to receive more transfers, after adjusting for academic affiliation, urban/rural location, and region of the country defined by U.S. Census division.24 These analyses were conducted at the hospital level for all revascularization hospitals. For the negative binomial regression, we conducted and report parallel analyses with 2 dependent variables: the number of hospitals from which patients are transferred, and the number of patients transferred in. This form of regression was used because the dependent variables were counts, as in Poisson regression; however, negative binomial regression allows for over-dispersion in the counts.
To estimate the potential population impact of an optimized transfer system, we performed a Monte Carlo simulation in which each patient was transferred to the hospital with the lowest published RSMR within a given radius. The aggregate potential reduction in mortality was calculated as the sum, across all patients, of the difference between the RSMRs for the observed transfer destination and that of an optimized transfer destination. To incorporate the uncertainty in the actual RSMRs, we repeated each simulation after sampling the RSMR for both the observed and optimized destination from a probability distribution based on its 95% confidence intervals. The differences in sampled RSMRs were then summed across all patients, and compared with the expected mortality (based on a mean 30-day risk-standardized mortality rate among all revascularization hospitals in the sample of 0.162, applied to the same population). The simulations were repeated 1000 times and the resultant numerical 95% confidence intervals were reported. (A worked example with greater detail and sensitivity analyses is available from the authors on request.) This analysis assumes that the average mortality risk of transferred patients was the same as patients who were directly admitted; that no transfers were refused or provided lower-quality care due to capacity constraints25; that transfers were accomplished with the high level of safety currently observed26, 27; and that revascularization hospitals provided the same outcomes of care to transferred patients as to directly admitted patients.
All analyses were conducted in Stata 10. 28 All regression standard errors were adjusted for potential clustering of AMI patients within a non-revascularization hospital using Huber and White robust standard errors.
This research was reviewed and approved by the University of Michigan Institutional Review Board.
RESULTS
We identified 71,336 Medicare beneficiaries admitted with AMI at 1,684 non-revascularization hospitals in 2006. Of these, 31,607 (44.3%) were transferred to another hospital, with 30,875 (97.7% of transfers) directed to revascularization hospitals. The mean age of AMI Medicare beneficiaries who were transferred was 74.2 years; 87.6% were white and 52.8% were men. The mean length of stay at the non-revascularization hospital prior to transfer was 2.5 days (standard deviation: 3.0) with a median of 2 days. Non-revascularization hospitals transferred AMI patients to a median of 3 different revascularization hospitals (25th to 75th percentile: 2 to 4), although 72.2% of patients were transferred to its most common destination facility. After arrival at the revascularization hospital, 19,513 (61.7%) patients underwent coronary revascularization with 14,452 (45.7%) receiving PCI.
We constructed the 2006 nationwide interhospital network of transfers for AMI patients between non-revascularization and the 1,104 revascularization hospitals (Figure 1). The median distance patients traveled to a revascularization hospital was 26.7 miles (IQR, 11.6 to 46.7 miles). Overall, 48.2% of transfers were within 25 miles and 96.1% of transfers were within 100 miles. Transfers originating in rural areas traveled a median of 47.3 miles (IQR 32.8 to 69.2 miles), whereas those originating in urban settings traveled a median of 13.9 miles (IQR, 6.6 to 25.0 miles). The median number of revascularization hospitals within 25 and 100 miles of each non-revascularization hospital was 3 and 18, respectively.
FIGURE 1. Transfers of AMI Patients from Non-Revascularization Hospitals.
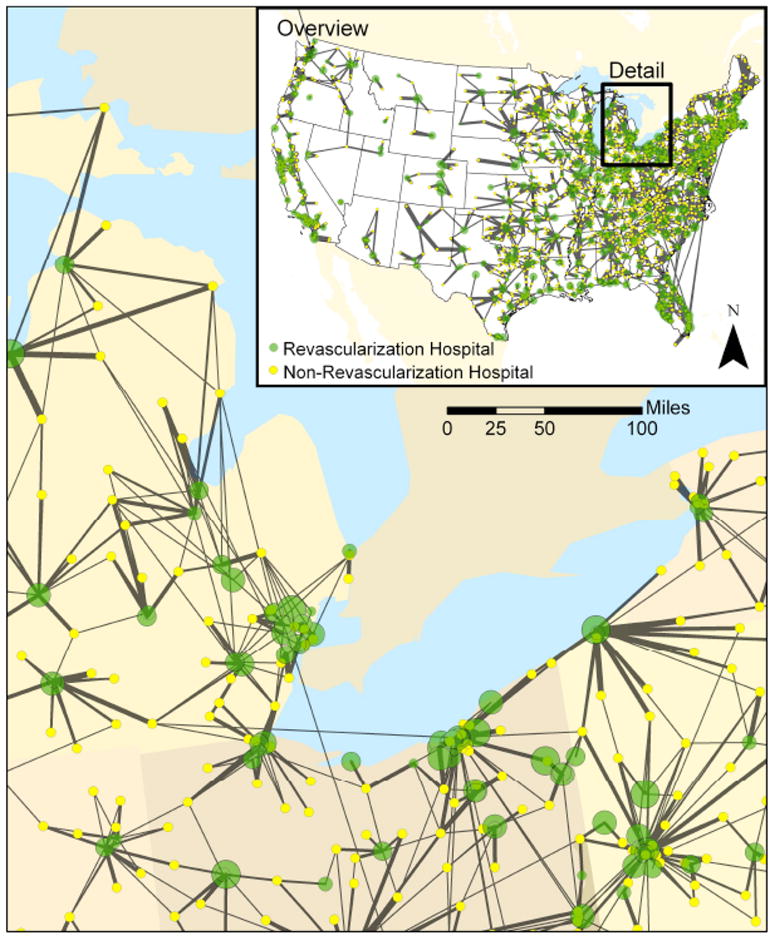
This map shows transfers of patients from non-revascularization hospitals (yellow) to revascularization hospitals (green). The thickness of the lines is proportional to the number of transfers between the hospitals. The diameter of the markers for revascularization hospitals is inversely proportional to their 30-day risk-standardized mortality, with hospitals with better outcomes having larger diameter.
When a patient is to be transferred, a potential destination hospital’s proximity and 30-day risk-standardized mortality rates might both be taken into consideration. In 6.0% of cases, patients went to the nearest revascularization hospital, and that hospital also had the best 30-day risk-standardized mortality of all revascularization hospitals within 100 miles. In 45.8% of cases, patients bypassed a closer hospital to go to farther hospital that had a better 30-day risk standardized mortality rates. However, patients also frequently bypassed hospitals with better mortality outcomes to go to hospitals farther away. In 36.8% of cases, another revascularization hospital with lower 30-day risk-standardized mortality was actually closer to the original admitting non-revascularization hospital than the observed transfer destination. In 27.2% of cases, another revascularization hospital was closer and had a 30-day risk-standardized mortality rate more than 1% better than the observed transfer destination. As Table 1 shows, patients were infrequently transferred to the hospital with the lowest 30-day risk-standardized mortality rate within any given radius.
Table 1. Fraction of Transfers that are to the Revascularization Hospital with Best Outcomes within Different Radii Around Non-PCI Admitting Hospital.
Unadjusted counts and fractions are presented.
| Radius (miles) | Observed transfers | % of all transfers that were within radius | % to best | % to within 0.5 percentage points of best * | % to within 1.0 percentage points of best * |
|---|---|---|---|---|---|
| 100 | 29,654 | 93.8% | 13.1% | 15.5% | 20.0% |
| 75 | 28,264 | 89.4% | 17.7% | 21.1% | 28.3% |
| 50 | 24,029 | 76.0% | 24.9% | 30.3% | 38.9% |
| 25 | 14,883 | 47.1% | 41.1% | 44.1% | 51.4% |
0.5 refers to a one-half percentage point of the best 30-day risk-adjusted mortality rate of any revascularization hospital within the given radius, and 1.0 refers to within one percentage point of the best. These columns are not exclusive – all patients transferred to the best were, by definition, also transferred to a hospital within 1.0 of best.
Patients were transferred farther to go to a revascularization hospital with improved 30-day risk-standardized mortality when such an option was available. However, there appeared to be a trade-off between these two factors, and the additional distances traveled were small on average. In rural settings, each 1 percentage point improvement in 30-day risk-standardized mortality rate increased a revascularization hospital’s odds of being the destination by 19% (95% CI: 13%, 26%), whereas being 10 miles closer doubled the odds of being chosen (OR: 2.05, 95% CI: 1.94, 2.18). In urban settings, proximity was even more important: while each 1 percentage point improvement in its 30-day risk-standardized mortality rate increased its odds of being the destination by 16% (95% CI: 10%, 21%), being 10 miles closer nearly quadrupled the odds of being chosen (OR: 3.87, 95% CI: 3.41, 4.38).
The high value of proximity relative to observed 30-day risk-standardized mortality persisted when controlling for other characteristics of the revascularization hospital that have been associated with improved outcomes, such as total volume of AMI patients and teaching status (Table 2). Still, high volume hospitals and teaching hospitals (particularly in rural areas) were more likely to be destinations than other equally close hospitals, showing that these factors were not ignored. In sensitivity analyses, very similar results were obtained when considering distance traveled from the patient’s home ZIP code rather than from the non-revascularization hospital initiating the transfer. Likewise, substantively identical results were obtained when using the 30-day risk standardized mortality rates for 2005–2008, or when controlling for a hospital’s designation as better or worse than the national average in Hospital Compare.
Table 2. Association Between Destination Hospital Characteristics and Their Odds of Being Transferred a Patient.
These odds ratios are from a McFadden’s discrete choice model, implemented with conditional logistic regression. Characteristics of the revascularization hospital that received each transfer are compared to those of all other revascularization hospitals within 100 miles of the sending nonrevascularization hospital.
| Urban Hospitals | OR | 95% CI | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| Proximity (per 10 miles closer to nonrevascularization hospital) | 3.87 | 3.41 | 4.38 | 3.94 | 3.48 | 4.46 |
| 30-Day Risk-Standardized Mortality (per 1% absolute improvement) | 1.16 | 1.10 | 1.21 | 1.07 | 1.02 | 1.12 |
| AMI Volume (per 100 patients) | 1.67 | 1.55 | 1.80 | |||
| Hospital Residency Program (versus not) | 1.10 | 0.88 | 1.37 | |||
| Affiliated with Medical School | 1.18 | 0.93 | 1.51 | |||
| Member of the Council of Teaching Hospitals | 1.20 | 1.00 | 1.43 | |||
| Rural Hospitals | OR | 95% CI | OR | 95% CI | ||
| Proximity (per 10 miles closer to nonrevascularization hospital) | 2.05 | 1.94 | 2.18 | 2.22 | 2.09 | 2.36 |
| 30-Day Risk-Standardized Mortality (per 1% absolute improvement) | 1.19 | 1.13 | 1.26 | 1.05 | 0.99 | 1.11 |
| AMI Volume (per 100 patients) | 2.11 | 1.91 | 2.32 | |||
| Hospital Residency Program (versus not) | 1.60 | 1.23 | 2.09 | |||
| Affiliated with Medical School | 0.96 | 0.73 | 1.25 | |||
| Member of the Council of Teaching Hospitals | 1.85 | 1.51 | 2.25 | |||
Despite the apparent high value placed on proximity, revascularization hospitals with lower 30-day mortality measures received more transfers. Revascularization hospitals varied substantially in the number of non-revascularization hospitals that sent them transfers (Table 3): 147 (13.3% of 1,104) received no transfers while 49 (4.4%) hospitals received transfers from 15 or more other hospitals. Revascularization hospitals with lower 30-day risk-standardized mortality rates, in general, received transfers from more hospitals. After adjusting for academic affiliation, urban/rural location and region of the country, a 1% lower 30-day risk-standardized mortality rate was associated with a 6.5% increase in the number of hospitals from which transfers were received (p<0.001). Similarly, revascularization hospitals with better outcomes also received more total AMI patients in transfer.
Table 3.
Association Between Number of Hospitals From Which Transfers are Received and 30-day Risk-Standardized Mortality Rate Across Quintiles of Revascularization Hospitals
| Number of Hospitals From Which Transfers Are Received | Number of Revascularization Hospitals | 30-Day Mortality Rate | Standard Deviation of Mortality Rate |
|---|---|---|---|
| 0 – 1 | 309 | 16.39 | 1.57 |
| 2 | 155 | 16.51 | 1.65 |
| 3 – 4 | 257 | 16.22 | 1.64 |
| 5 – 7 | 188 | 16.01 | 1.55 |
| 8 – 39 | 195 | 15.67 | 1.84 |
For ease of display, hospitals were divided into quintiles based on the number of hospitals from which transfers were received. Groups were divided as evenly as possible given the clumping of hospitals receiving few transfers. Cuzick’s Wilcoxon-type non-parametric test for trend was p< 0.001 across groups.
In order to contextualize these results, we simulated the potential impact of a system that optimized patient transfers exclusively on the basis of 30-day risk-standardized mortality rates (Table 4). In the baseline case, an optimized system that always transferred patients to the lowest 30-day mortality hospital within 100 miles might reduce absolute mortality 2.7 percentage points (95% CI: 2.6, 2.7 percentage points), a relative reduction of 16.5% at 30 days after AMI (95% CI: 16.3%, 16.8%). An optimized system that never transferred patients farther than they were currently transferred and never more than 100 miles might reduce absolute mortality by 0.78 percentage points (95% CI: 0.77, 0.80 percentage points), a relative reduction of 4.8% (95% CI: 4.7%, 5.0%) while also reducing travel time. Table 4 shows the extent to which an optimized transfer system might offer meaningful reductions in 30-day AMI mortality as the maximum transfer distance is varied.
TABLE 4. Potential Impact of Optimized Transfer on 30-day Mortality.
The potential reductions in mortality are the summed differences between risk-standardized mortality rate of the observed transfer destination and a system that always transferred a patient to the revascularization hospital with the lowest 30-day risk-standardized mortality. Please see text for a discussion of the important simplifying assumptions used to generate these estimates. 95% confidence intervals take into account the uncertainty about actual RSMRs of observed and optimized transfer hospitals, using 1000 Monte Carlo simulations.
| Radius (miles) | Observed Transfers | % of all transfers that were within radius | Optimized Transfer Potential Reduction in Mortality (95% CI) | |
|---|---|---|---|---|
| Relative | Absolute | |||
| 100 | 29,654 | 93.8% | 16.5% (16.3, 16.7) | 2.7% (2.6, 2.7) |
| 75 | 28,264 | 89.4% | 14.4% (14.2, 14.6) | 2.3% (2.3, 2.4) |
| 50 | 24,029 | 76.0% | 11.9% (11.7, 12.1) | 1.9% (1.9, 2.0) |
| 25 | 14,883 | 47.1% | 9.4% (9.2, 9.6) | 1.5% (1.5, 1.6) |
| No Further Than Observed & within 100 Miles * | 29,654 | 93.8% | 4.8% (4.7, 5.0) | 0.78% (0.77, 0.80) |
The restriction to within 100 miles is based on the assumption that longer range transfers may occur for idiosyncratic reasons; the inclusion of such transfers might inappropriately bias the potential benefits upwards.
DISCUSSION
Over 40% of elderly Medicare beneficiaries with AMI who are admitted at hospitals without revascularization are ultimately transferred to revascularization hospitals during their hospitalization. We find evidence that these patients are preferentially transferred towards hospitals with lower 30-day risk-standardized mortality rates, but that this pattern of movement between facilities with different levels of services provided may be suboptimal. For example, only a minority of patients are transferred and of those transferred, many are transferred to revascularization hospitals with higher mortality rates than other revascularization hospitals within similar distances. Indeed, more than one-third of patients had a revascularization hospital that was both closer and with better outcomes than the observed destination. This suggests that, in general, optimizing the transfer process might result in clinically meaningful improvements in patient outcomes.
Past work has examined the characteristics of patients most likely to be transferred; ours is the first study to examine the system-level patterning of those transfers.17, 18 For example, an analysis of the CRUSADE initiative showed that 46.1% of patients with non-ST elevation acute coronary syndromes were transferred to revascularization centers from community hospitals.29 Further, lower-risk patients appear to be preferentially transferred early in their hospital course. These findings reinforce the pattern noted in 1994–1995 data on Medicare beneficiaries from the Cooperative Cardiovascular Project within Michigan.7 Claims data lack the clinical granularity to reexamine the appropriateness of any given decision to transfer or not. Instead, our data raise the possibility that patients often bypass nearby revascularization hospitals with better outcomes, once the decision to transfer has been made. Future studies will need to evaluate how the decision to transfer is initiated, how patients are selected, and what processes are in place for determining the destination.
There are several potential reasons why patients may not be transferred to nearby revascularization hospitals with the best outcomes. First, logistical barriers due to bed occupancy or physician availability may prevent a revascularization hospital from accepting patients at the moment a transfer is needed. Timeliness of revascularization is an essential part of the care of AMI patients, and theoretically, better hospitals must be available when transfers are required. Second, the choice of transfer destination may be a routine, rather than conscious decision for any particular patient. Over 70% of transfers from non-revascularization hospitals went to a single revascularization hospital, suggesting well-established relationships between facilities. These relationships may be driven by the presence of supplemental insurance or by corporate relationships between hospitals that send and receive patients; however, our evidence emphasizes that such non-clinical issues may have clinical consequences for patients, who may not be sent to the best available facility.
Third, publicly-available information on hospital performance in AMI patients may not have been available for sufficiently long time to patients and providers. Data from Hospital Compare used in this analysis have only recently become available and may not have become incorporated into these decisions by 2006. However, the impact of public information about the quality of healthcare providers has not been substantial to date.30–32 Fourth, it is possible that providers and patients considered the individual “rankings” of hospital performance in Hospital Compare unreliable even when they were familiar with the information. Finer grain information that allows individuals to make reliable decisions is urgently needed to change these population-level outcomes.
Finally, another important possibility is that patients might highly value proximity of care relative to outcomes even if these other challenges were resolved.33 In one recent survey of AMI patients who had been transferred for primary PCI in the setting of ST-elevation, over 15% of respondents responded that they would have preferred receiving care locally despite the potential mortality benefits associated with transfer.34 Efforts to optimize the care of patients need to consider strategies to minimize the psychological distress associated with transfer while achieving the best outcomes possible. 35
Despite these multiple barriers, there is evidence that current patterns of transfer at least partially consider hospital performance. We observe that on average hospitals with better outcomes received more patients from more hospitals. More reliable and accessible information might take advantage of these decentralized mechanisms, potentially if coupled to incentives to optimize outcomes. Linking accountability for transfers back to the non-revascularization hospital could also be helpful, a practice that is already in place through the publicly-reported data from Hospital Compare where 30-day AMI mortality rates for non-revascularization hospitals includes patients who were ultimately transferred to a revascularization hospital. There may be a role for individual physicians and hospitals to view their own referral practices through a quality-improvement lens, independent of any system-level reform to improve transfers.
This empirical work has several limitations that should be kept in mind. First, we studied transfer patterns within Medicare and after hospital admission. Other insurers may actively shape transfers to meet different clinical or economic objectives. Similarly, our analyses do not address transfers that occur between emergency departments prior to hospital admission. Second, we lacked detailed clinical information to evaluate whether the decision to transfer was “right” or “optimal” for particular patients. Medicare claims did not allow for us to reliably determine why more than 50% of AMI patients were not transferred, nor to distinguish STEMI and NSTEMI. For some patients, this may be due to patient and family preference to undergo non-invasive stress testing or advanced co-morbidities or other relative contraindications to cardiac catheterization or revascularization. Some have even suggested that sicker patients may be less likely to be transferred from community hospitals to academic centers.2, 29 However, these issues are beyond the scope of our work. Our primary goal was to evaluate where patients were directed after the decision for transfer had been made. Third, the 30-day risk-standardized mortality for a specific hospital is a point estimate with a 95% confidence interval (95% CI). Comparing the decision to transfer a given patient between 2 specific hospitals is not possible from this analysis. Instead, we focused on the patterns of transfer from a population-level perspectiv—which is more critical for policy-makers. We also accounted for uncertainty in estimates of a hospital’s 30-day risk-standardized mortality in our Monte Carlo simulations. Finally, we assumed that revascularization hospitals perform as well for transferred patients as they do for patients directly admitted with AMI; in theory, some hospitals might perform disproportionately better in one or the other population. Indeed, some decision-makers may prefer to focus on outcomes other than mortality, such as a risk-stratified readmission rate or other process measures; it would be valuable if our work were replicated with other measures. Future work explicitly measuring the care provided to transfer patients may be of substantial benefit in guiding policy and clinical decision-making on this important issue.
Conclusion
The transfer of patients to revascularization hospitals is a frequent part of the care of AMI patients at non-revascularization hospitals. Although revascularization hospitals with lower 30-day risk-standardized mortality rates receive more of these transfers, many patients are not directed toward local hospitals with the best outcome rates. Consequently, efforts to systematically improve these transfer decisions, both at the level of the health systems and individual referring physicians, may represent an important opportunity to positively impact outcomes for patients with AMI.
Acknowledgments
The authors thank Maxim Herlim, Lili Deng and particularly Tish Shapiro for programming assistance; Danielle Gwinn of the University of Michigan Center for Statistical Consultation and Research for assistance with data visualization; and David Asch, John Birkmeyer, Jill Horwitz, and Carla Keirns and the 2009 International Network for Social Network Analysis Conference for helpful comments.
Funding Sources: This work was supported by 1K08HL091249-01 from the NIH/NHLBI, and utilized the Measurement Core of the Michigan Diabetes Research & Training Center (NIH/NIDDK, P60DK-20572). This project was also funded, in part, under a grant from the Pennsylvania Department of Health, which specifically disclaims responsibility for any analyses, interpretations, or conclusions. The funders were not involved in study design, interpretation or the decision to publish.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Keeley EC, Grines CL. Primary percutaneous coronary intervention for every patient with ST-segment elevation myocardial infarction: what stands in the way? Ann Intern Med. 2004;141:298–304. doi: 10.7326/0003-4819-141-4-200408170-00010. [DOI] [PubMed] [Google Scholar]
- 2.Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138:688–695. doi: 10.1016/s0002-8703(99)70184-5. [DOI] [PubMed] [Google Scholar]
- 3.Grines CL, Westerhausen DR, Jr, Grines LL, Hanlon JT, Logemann TL, Niemela M, Weaver WD, Graham M, Boura J, O’Neill WW, Balestrini C. A randomized trial of transfer for primary angioplasty versus on-site thrombolysis in patients with high-risk myocardial infarction: the Air Primary Angioplasty in Myocardial Infarction study. J Am Coll Cardiol. 2002;39:1713–1719. doi: 10.1016/s0735-1097(02)01870-3. [DOI] [PubMed] [Google Scholar]
- 4.Cantor WJ, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen EA, Morrison LJ, Langer A, Dzavik V, Mehta SR, Lazzam C, Schwartz B, Casanova A, Goodman SG. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705–2718. doi: 10.1056/NEJMoa0808276. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001:3441879–1887. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly Patients at Highest Risk with Acute Myocardial Infarction are more Frequently Transferred from Community Hospitals to Tertiary Centers: Reality or myth? American Heart Journal. 1999;138:688–695. doi: 10.1016/s0002-8703(99)70184-5. [DOI] [PubMed] [Google Scholar]
- 8.Krumholz HM, Normand S-LT, Spertus JA, Shahian DM, Bradley EH. Measuring Performance for Treating Heart Attacks and Heart Failure: The Case for Outcomes Measurement. Health Affairs. 2007;26:75–85. doi: 10.1377/hlthaff.26.1.75. [DOI] [PubMed] [Google Scholar]
- 9.Epstein AJ, Rathore SS, Krumholz HM, Volpp KG. Volume-Based Referral for Cardiovascular procedures in the United States: a Cross-Sectional Regression Analysis. BMC Health Services Research. 2005;5:42. doi: 10.1186/1472-6963-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magid DJ, Calonge BN, Rumsfeld JS, Canto JG, Frederick PD, Every NR, Barron HV for the National Registry of Myocardial Infarction 2 and 3 Investigators. Relation Between Hospital Primary Angioplasty Volume and Mortality for Patients With Acute MI Treated With Primary Angioplasty vs Thrombolytic Therapy. JAMA. 2000;284:3131–3138. doi: 10.1001/jama.284.24.3131. [DOI] [PubMed] [Google Scholar]
- 11.Nallamothu BK, Young J, Gurm HS, Pickens G, Safavi K. Recent trends in hospital utilization for acute myocardial infarction and coronary revascularization in the United States. Am J Cardiol. 2007;99:749–753. doi: 10.1016/j.amjcard.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services. Hospital Compare. Centers for Medicare and Medicaid Services; Available at: www.hospitalcompare.hhs.gov. [Google Scholar]
- 13.Ross JS, Normand SL, Wang Y, Ko DT, Chen J, Drye EE, Keenan PS, Lichtman JH, Bueno H, Schreiner GC, Krumholz HM. Hospital volume and 30-day mortality for three common medical conditions. N Engl J Med. 2010;362:1110–1118. doi: 10.1056/NEJMsa0907130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krumholz HM, Normand S-LT. Public Reporting of 30-Day Mortality for Patients Hospitalized With Acute Myocardial Infarction and Heart Failure. Circulation. 2008;118:1394–1397. doi: 10.1161/CIRCULATIONAHA.108.804880. [DOI] [PubMed] [Google Scholar]
- 15.Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand S-LT. An Administrative Claims Model Suitable for Profiling Hospital Performance Based on 30-Day Mortality Rates Among Patients With an Acute Myocardial Infarction. Circulation. 2006;113:1683–1692. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting Critically Ill Transfer Patients: Adverse Effect on a Referral Center’s Outcome and Benchmark Measures. Annals of Internal Medicine. 2003;138:882–890. doi: 10.7326/0003-4819-138-11-200306030-00009. [DOI] [PubMed] [Google Scholar]
- 17.Iwashyna TJ, Christie JD, Kahn JM, Asch DA. Uncharted paths: hospital networks in critical care. Chest. 2009;135:827–833. doi: 10.1378/chest.08-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The Structure of Critical Care Transfer Networks. Medical Care. 2009;47:787–793. doi: 10.1097/MLR.0b013e318197b1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinnott RW. Virtues of the Haversine. Sky and Telescope. 1984;68:159. [Google Scholar]
- 20.American Hospital Association. American Hospital Association Annual Survey Database for Fiscal Year 2005. Chicago: American Hospital Association; 2005. [Google Scholar]
- 21.Allison PD. Logistic Regression Using the SAS Sytem: Theory and Applications. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 22.Hoffman SD, Duncan GJ. Multinomial and Conditional Logit Discrete-Choice Models in Demography. Demography. 1988;25:415–427. [PubMed] [Google Scholar]
- 23.Cuzick J. A Wilcoxon-type test for trend. Statistics In Medicine. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 24.Gelman A, Hill J. Data Analysis Using Regression and Multi-level/Hierarchical Models. New York: Cambridge University Press; 2007. [Google Scholar]
- 25.Iwashyna TJ, Kramer AA, Kahn JM. Intensive care unit occupancy and patient outcomes. Crit Care Med. 2009;37:1545–1557. doi: 10.1097/CCM.0b013e31819fe8f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh JM, MacDonald RD, Bronskill SE, Schull MJ. Incidence and predictors of critical events during urgent air–medical transport. CMAJ. 2009;181:579–584. doi: 10.1503/cmaj.080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan E, McDonald RD, Adhikari NKJ, Scales DC, Wax RS, Stewart TE, Ferguson ND. Outcomes of interfacility critical care adult patient transport: a systematic review. Critical Care. 2006;10:R6. doi: 10.1186/cc3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.StataCorp. Stata Statistical Software: Release 10.1. College Station, TX: Stata Corporation; 2009. [Google Scholar]
- 29.Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE, Jr, Cairns CB, Smith SC, Jr, Pollack CV, Jr, Brindis RG, Califf RM, Gibler WB, Ohman EM, Peterson ED. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156:185–192. doi: 10.1016/j.ahj.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Schneider EC, Epstein AM. Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA. 1998;279:1638–1642. doi: 10.1001/jama.279.20.1638. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz LM, Woloshin S, Birkmeyer JD. How do elderly patients decide where to go for major surgery? Telephone interview survey. BMJ. 2005;331:821. doi: 10.1136/bmj.38614.449016.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenberg NE, Amey CH, Stoller EP, Muldoon SB. Lay referral patterns involved in cardiac treatment decision making among middle-aged and older adults. Gerontologist. 2003;43:493–502. doi: 10.1093/geront/43.4.493. [DOI] [PubMed] [Google Scholar]
- 33.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF., Jr Patient preferences for location of care: implications for regionalization. Medical Care. 1999;37:204–209. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Henry J, Lindsay B, Menssen K, Christiansen E, Unger B, Larson D, Henry T. Impact of emergent transfer for percutaneous coronary intervention on ST-elevation myocardial infarction patients and their families. J Am Coll Cardiol. 2009;53:A374. [Google Scholar]
- 35.Tel H, Tel H. The effect of individualized education on the transfer anxiety of patients with myocardial infarction and their families. Heart & Lung. 2006;35:101–107. doi: 10.1016/j.hrtlng.2005.09.001. [DOI] [PubMed] [Google Scholar]


