Abstract
Choosing an appropriate anesthetic protocol that will have minimal effect on experimental design can be difficult. Guinea pigs have highly variable responses to a variety of injectable anesthetics, including ketamine–xylazine (KX). Because of this variability, supplemental doses often are required to obtain an adequate plane of anesthesia. Our group studies the isolated guinea pig heart, and we must anesthetize guinea pigs prior to harvesting this organ. In this study, we sought to determine whether a higher dose of KX protected isolated guinea pig hearts against myocardial ischemia–reperfusion injury. Male Hartley guinea pigs (Crl:HA; 275 to 300 g; n = 14) were anesthetized with either of 2 doses of KX (K: 85 mg/kg, X: 15 mg/kg; or K: 200 mg/kg, X: 60 mg/kg). After thoracotomy, hearts underwent 20 min of ischemia followed by 2 h of reperfusion. The high dose of KX significantly reduced myocardial infarct size as compared with the low dose (36% ± 3% and 51% ± 6%, respectively). Furthermore, the high dose of KX improved hemodynamic function over that associated with the low dose as measured by increases in both left ventricular developed pressure (49 ± 4 and 30 ± 8 mm Hg, respectively) and maximal rate of left ventricular relaxation (−876 ± 70 and −576 ± 120 mm Hg/s, respectively). However, the high dose of KX did not alter the maximal rate of left ventricular contraction or coronary flow. These results suggest that supplementation of KX to ensure an adequate anesthetic plane may introduce unwanted variability in ischemia–reperfusion studies.
Abbreviation: KX, ketamine–xylazine; VF, ventricular fibrillation; VT, ventricular tachycardia
In many studies using animals, measurements on proteins, cells, or organs are collected after anesthesia of the subject. However, use of anesthesia always raises the caveat that the anesthesia itself may alter the behavior of the system in question. Choosing a protocol that provides adequate anesthesia with minimal influence on the outcome measurements is an area of ongoing interest.58
Among laboratory animal models used in biomedical research, guinea pigs are known to have highly variable responses to a variety of anesthetic agents, particularly those injected both intraperitoneally and intramuscularly;2,20,25,27,35,50,57 however, the mechanism for this variation is not known. Because of guinea pigs’ variability in responsiveness from injections and their lack of a tail for intravenous delivery, many researchers prefer halogenated inhalation anesthetics (such as isoflurane, halothane, desflurane, enflurane, and sevoflurane) for anesthesia in guinea pigs. Although the use of volatile gas anesthetics is appropriate in many research disciplines, these agents are contraindicated for studies of ischemia–reperfusion injury due to confounding effects of the anesthetics themselves. For example, previous studies have shown that administration of volatile anesthetics confers protection against ischemia–reperfusion injury across species in tissues including heart,18,21,37,49,56 brain,33,60,68 kidney,31 lung,36 and liver.5 Accordingly, many scientists interested in ischemia–reperfusion injury use various injectable anesthetics. One of the most common injectable anesthetics used for surgical procedures is a ketamine–xylazine (KX) combination. The recommended KX dosage for guinea pigs varies considerably (30 to 120 mg/kg for ketamine and 0.2 to 13 mg/kg for xylazine26). This wide range likely reflects the difficulty in using KX to anesthetize guinea pigs for surgery,13 which often require supplemental injections to achieve adequate anesthetic depth.26
Our group studies cardiac ischemia–reperfusion injury. When compared with other rodents such as rats and mice, guinea pigs are preferred for these studies because the electrophysiologic profile of the guinea pig heart more closely resembles those of larger animals,8,54 yet guinea pigs are easier to manage than larger mammals. Because the effects of KX on cardiac function and infarction are negligible compared with other means of anesthesia,18 KX is often used in cardiac experiments. Like other investigators,2,20,25,27,35,50,57 we have observed that the effectiveness of KX is highly variable among guinea pigs and that supplemental injections of KX are often required to achieve an adequate surgical plane of anesthesia. In previous studies, we anecdotally noticed that guinea pigs that required supplemental KX injections also appeared to have less cardiac damage after ischemia–reperfusion. Given our focus on identifying novel treatments that protect the heart,7-12 variability introduced by the anesthetic regimen could influence the interpretation of our data greatly.
In the current study we sought to determine whether use of high compared with low doses of KX to anesthetize guinea pigs alters the response of isolated hearts to subsequent ischemic injury. We hypothesized that hearts from guinea pigs that received higher doses of KX would be protected against experimental ischemia–reperfusion injury. Here, we show that use of a higher dose of KX significantly reduced myocardial infarct size and preserved hemodynamic function when compared with effects associated with a lower dose of KX.
Materials and Methods
Animals.
Male Hartley guinea pigs (Cavia porcellus; n = 14; weight, 275 to 300 g; age, approximately 30 d) were obtained from a commercial vendor (Charles River Laboratories, Raleigh, NC). Based on health surveillance programs performed by the vendor and research institution, the guinea pigs were free from: Sendai virus, pneumonia virus of mice, reovirus, lymphocytic choriomeningitis virus, guinea pig adenovirus, Encephalitozoon cuniculi, Bordetella bronchiseptica, Streptococcus pneumoniae, Streptococcus zooepidemicus, Klebsiella spp., Salmonella spp., and ecto- and endoparasites. To minimize the effects of external stimuli, the same environmental conditions were maintained throughout the study. Guinea pigs were group-housed (3 or 4) per cage in a room housing only that species. The cages contained aspen bedding chips (Northeastern Products, Warrensburg, NY). All guinea pigs were allowed a minimum of 5 d to acclimate after shipping prior to their experimental use. Standard guinea pig chow (Lab Diet ProLab 5P18, Purina, St Louis, MO) was fed ad libitum, and all guinea pigs were offered water by automatic watering device ad libitum. Environmental enrichment in the form of PVC tubes and vegetables were given to the guinea pigs. Routine husbandry care was performed by the same husbandry technician, when possible, to help familiarize the animals to routine husbandry procedures.
All research adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals.29 The protocol was approved by the East Carolina University IACUC and was performed in an AAALAC-accredited facility.
Anesthesia and experimental protocol.
On each experimental day, guinea pigs were assigned randomly to 1 of 2 KX anesthesia protocols (Table 1). The low-dose protocol (K, 85 mg/kg; X, 15 mg/kg) used in this study was recommended by the veterinary staff and is the typical dosage of KX used for surgical anesthesia in guinea pigs among investigators at our institution. Although the low-dose protocol is at the higher end of the range of dosages suggested,26 our previous experience indicated that as many as 3 supplemental doses at this level sometimes were needed to achieve a surgical plane of anesthesia suitable for guinea pig thoracotomy, particularly after failure to achieve appropriate anesthetic depth with the initial dose. For this reason and in consultation with the veterinary staff, we chose the concentrations of K and X in the high-dose protocol (Table 1). Each anesthetic cocktail was administered to the guinea pigs by intraperitoneal injection. Fifteen minutes after KX injection, guinea pigs were tested for righting, toe-pinch, and palpebral reflexes. Anesthetized guinea pigs in the low-dose group lacked righting and palpebral reflexes, but several animals retained a toe-pinch reflex (a situation that typically would warrant a supplemental injection). Therefore, because we sought to standardize the anesthetic dose for each animal to ascertain the effects on cardiac function, cervical dislocation was performed in all anesthetized animals immediately prior to thoracotomy to account for varied reflex responsiveness. Cervical dislocation was performed in guinea pigs in a manner similar to the procedure for cervical dislocation in other rodents as described in the 2007 AVMA euthanasia guidelines.1 Fifteen minutes after intraperitoneal injection of KX, cervical dislocation was performed on anesthetized guinea pigs by a highly trained operator under the direct supervision of the attending veterinarian and with prior approval by the IACUC. Although cervical dislocation was performed in 275- to 300-g guinea pigs in this study (that is, larger than the recommendation of less than 200 g for other rodents), the dislocation was performed on anesthetized guinea pigs (that lacked righting and palpebral reflexes), and there were no complications with any of the animals.
Table 1.
Ketamine–xylazine anesthesia doses used in the current study and hemodynamic parameters of guinea pig heart at baseline and after 1 h of reperfusion
| Lower dosea | Higher dosea | P | ||
| Baseline | ||||
| LVDP (mL/min/g wet weight) | 82 ± 8 | 96 ± 10 | 0.28 | |
| +dP/dt (mm Hg/s) | 1947 ± 187 | 2138 ± 97 | 0.41 | |
| -dP/dt (mm Hg/s) | −1512 ± 146 | −1579 ± 85 | 0.71 | |
| Coronary flow (mL/min × g wet weight) | 6.3 ± 0.6 | 7.0 ± 0.6 | 0.43 | |
| Reperfusion | ||||
| LVDP(mL/min/g wet weight) | 30 ± 8 | 49 ± 5 | 0.04b | |
| +dP/dt (mm Hg/s) | 833 ± 186 | 1190 ± 94 | 0.13 | |
| -dP/dt (mm Hg/s) | −576 ± 120 | −876 ± 70 | 0.04b | |
| Coronary flow (mL/min × g wet weight) | 4.5 ± 0.9 | 4.3 ± 0.6 | 0.86 |
+dP/dt, maximal rate of left ventricular contraction; -dP/dt, maximal rate of left ventricular relaxation coronary flow; LVDP, left ventricular developed pressure (mm Hg).
Lower dose: 85 mg/kg ketamine + 15 mg/kg xylazine; higher dose, 200 mg/kg ketamine + 60 mg/kg xylazine.
P < 0.05 compared with corresponding value for low dose.
Immediately after cervical dislocation, hearts were excised by means of a midline thoracotomy. The aorta was secured around a cannula of a modified Langendorff apparatus (isolated perfused heart model) and perfused in a retrograde fashion (at 75 mm Hg constant perfusion pressure) with buffer consisting of 118 mM NaCl, 24 mM NaHCO3, 1.2 mM KH2PO4, 4.75 mM KCl, 1.2 mM MgSO4, 2.0 mM CaCl2, and 10 mM glucose (equilibrated with 95% O2/5%CO2), as described previously.8 A latex balloon (size no. 6, Harvard Apparatus, Holliston, MA) was inserted through the mitral valve and into the left ventricle. Cardiac hemodynamic and electrical parameters, including left ventricular developed pressure, perfusion pressure, coronary flow rates, maximal rates of contraction and relaxation, and volume-conducted electrocardiogram, were measured constantly throughout the protocol. These parameters of cardiac function were collected and digitized by using the Powerlab System (AD Instruments, Colorado Springs, CO), and stored on a personal computer for subsequent analysis.
Ischemia–reperfusion protocol.
After a 15-min equilibration period, hearts experienced no-flow ischemia (global ischemia) for 20 min, after which flow was reestablished for 2 h (reperfusion).
Measurement of infarct size.
At the end of the reperfusion period, hearts were disconnected from the cannula, and the right ventricle and atria were removed. To assess the amount of tissue death, infarct size was assessed histologically as previously described.10 Briefly, the left ventricle was sliced into 4 slices from apex to base. Each slice was weighed and incubated in a 1% 2,3,5-triphenyltetrazolium chloride solution for 10 min in a slow-shaking water bath (37 °C). Measurement of infarct size by using triphenyltetrazolium chloride staining is considered the ‘gold standard’ for quantification of cardiac ischemia–reperfusion injury in numerous species, including dogs, rabbits, and rodents.24,30,62 After the brief incubation, both sides of each slice were photographed by using a digital camera associated with a dissecting microscope. Infarct areas were quantified by using computer software (ImageJ Software, NIH, Bethesda, MD). Total area at risk and infarct area were measured for each side of each slice and corrected for the wet weight of each slice. All slices were averaged, and final infarct size was expressed as a percentage of the left ventricle.
Arrhythmia scoring.
Arrhythmias were characterized as previously described,7 by using the guidelines established by the Lambeth Conventions and scored by using a system similar to that previously described.19 Arrhythmias were scored during the reperfusion period as follows: 0, 0 to 49 ventricular premature beats; 1, 50 to 499 ventricular premature beats; 2, more than 499 ventricular premature beats or 1 episode of spontaneously reverting ventricular tachycardia (VT) or ventricular fibrillation (VF) less than 60 s in total duration; 3, multiple episodes of VT or VF that are less than 60 s in total duration; 4, episodes of reverting VT or VF or both that are less than 120 s in total duration; 5, episodes of VT or VF or both that are more than 120 s in combined duration; 6, nonreverting (fatal) VT or VF that began more than 15 min after reperfusion; 7, fatal VT or VF that began 5 to 15 min after reperfusion; and 8, fatal VT or VF that began less than 5 min after reperfusion. In addition to arrhythmia scoring, total time in VT or VF was determined throughout reperfusion.
Statistical analysis.
All data are presented as mean ± SEM. A Student t test was used to determine differences between groups, and statistical significance was established by using an α level of 0.05.
Results
Infarct size.
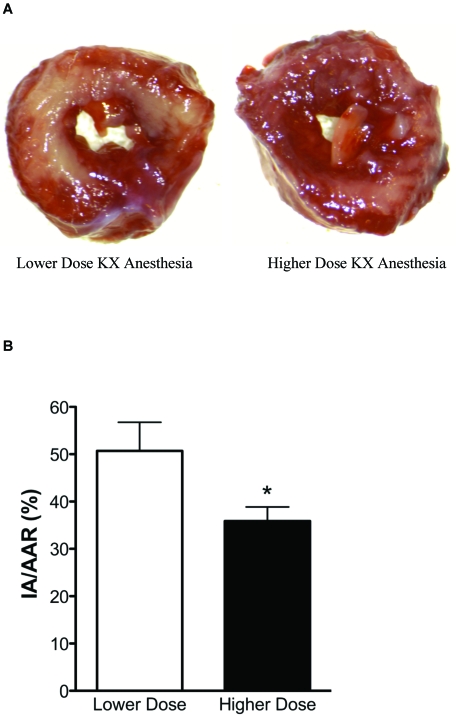
Representative infarct pictures and infarct size data are presented in Figure 1. After 20 min of ischemia and 2 h of reperfusion, infarct size was significantly (P < 0.05) smaller in hearts from guinea pigs that received high-dose KX (n = 7) compared with that from animals that received low-dose KX (n = 7; 36% ± 3% and 51% ± 6%, respectively). This reduction in infarct size suggests that KX dose-dependently influences the development of cell death.
Figure 1.
(A) Representative left ventricular guinea pig heart slices from low-dose (left) and high-dose (right) KX anesthesia groups. Infarcted tissue is represented by the pale tissue, and viable tissue is stained red. (B) Infarct sizes for all hearts in the study, expressed as percentages of the infarct area or area at risk (IA/AAR). Data are shown as mean ± SEM. *, P < 0.05.
Hemodynamics.
There were no significant differences in hemodynamic parameters before the 20-min ischemic period (Table 1). During this baseline period, left ventricular developed pressure, maximal rates of contraction and relaxation, and coronary flow rates were similar between the high- and low-dose KX groups. Because normoxic hemodynamic function begins to decline in hearts after approximately 1.5 h in our model,10 we examined hemodynamic differences between groups at 1 h into reperfusion. At this point, there was significant (P < 0.05) improvement in left ventricular developed pressure and maximal rate of relaxation in guinea pigs anesthetized with high-dose KX. There were no significant differences between dose groups in maximal rate of contraction or coronary flow during the reperfusion period (Table 1).
Electrocardiographic changes.
There were no observable differences in the incidence of reperfusion arrhythmias between KX dose groups. Neither arrhythmia scores nor total time in VT or VF were influenced by the anesthetic regimen (data not shown).
Discussion
This study was conducted to determine whether the dose of KX anesthesia influenced cardiac ischemia–reperfusion injury. The variable tolerance of guinea pigs for KX anesthesia often leads to supplemental injections before surgical procedures can be performed. However, this supplementation of anesthetic could alter the physiology of the heart. In this study, we show that a higher dose of KX reduced myocardial infarct size and preserved hemodynamic function in guinea pig hearts exposed to ischemia–reperfusion as compared with a lower dose. To our knowledge, this study is the first demonstration that the use of higher doses of KX to anesthetize guinea pigs protects the heart against ischemic injury.
The appropriate anesthesia protocol for animal studies is something that all laboratory animal veterinarians and investigators must consider. For scientists interested in studying ischemia–reperfusion injury, this decision is complicated. Inhalation agents are easy to administer and effective at providing anesthesia, yet are well known to confer protection against subsequent ischemia–reperfusion injury (that is, to ‘precondition’ the tissue against infarction), as reviewed in reference 21. Anesthetic-induced preconditioning (with inhalant agents) occurs in a variety of tissues5,21,31,33,49,60 and in species ranging from laboratory mice,41 rats,33,34 guinea pigs,51 rabbits,28 dogs,42,61 and human patients undergoing surgery.5,31
In cardiac ischemia–reperfusion studies, anesthesia, rather than euthanasia, is used to obtain a beating heart in order to avoid ischemic preconditioning, a phenomenon that has repeatedly been shown to influence infarct size.4,6,67 In cardiac studies directly comparing the infarct-limiting effect of anesthetic regimens, inhalation anesthetics are more robust than injectable agents in reducing infarct size.18,59 Due to the cardioprotection afforded by halogenated anesthetics, injectable anesthetics such as KX, pentobarbital, and propofol are used most frequently in ischemia–reperfusion studies. Among these injectable regimens, KX anesthesia exerts a smaller influence on infarct size than does propofol or pentobarbital.18,28,59 Recent recommendations against the use of pentobarbital because of a narrow safety window and questions of efficacy14,55,64 provide further support for KX anesthesia. Lending additional support for the use of KX anesthesia, propofol is given intravenously and therefore can be difficult to administer in guinea pigs due to the difficulty associated with gaining intravenous access.
Although KX appears to be an appropriate anesthetic regimen for ischemia–reperfusion studies, the difficulty attaining an adequate plane of anesthesia, especially in guinea pigs, frequently necessitates anesthetic supplementation (often with multiple doses).13 Although supplementation is a common practice that is often necessary to achieve an adequate surgical plane, we are unaware of any reports in which increasing the anesthetic dose was shown to alter ischemia–reperfusion injury. In the current study, we compared high and low doses of KX in guinea pigs and found that the higher KX dose reduced infarct size by approximately 30%, indicating that supplemental anesthetic KX doses may be introducing variability into experimental data by disproportionally preconditioning the tissue.
In addition to examining the effect of varying KX doses on infarct size, we examined the influence of KX dose on cardiac function. The absence of baseline differences in left ventricular function between the KX dose groups (Table 1) is consistent with previous reports showing that KX anesthesia had minimal effects on baseline pressure development.18 This lack of influence on baseline cardiac function also provides support for KX use in cardiac functional studies, given that volatile anesthetics are all potent negative inotropes.16-18,22,23,32,38,39,46,47,52,61,63
Although different types of anesthetic drugs have been shown to influence recovery from and incidence of arrhythmia,3,43,45,65 we are unaware of any studies that have examined the influence on postischemic functional recovery in the context of a specific injectable anesthetic regimen. After ischemia, high-dose KX anesthesia preserved the pumping ability of the left ventricle (Table 1). To our knowledge, the finding that higher doses of KX influence the mechanical recovery of the heart during reperfusion is novel and underscores the need for standardization of anesthetic agents. The dose of KX we used did not influence the propensity for ventricular arrhythmia in our study. These findings are notable, in that investigators who are interested primarily in cardiac electrophysiology can be reassured regarding the negligible influence of KX anesthesia on propensity for arrhythmia.
To determine whether differences in coronary perfusion could account for the cardioprotection we noted with high KX, we measured coronary flow rates from hearts in the study. Given that xylazine is agonistic to α2 adrenergic receptors, we postulated that the high-dose KX regimen might constrict the coronary arteries and lead to hypoxic–ischemic preconditioning of the myocardium. A higher dose of KX did not alter the coronary flow rate during the baseline period or reperfusion, indicating that the cardioprotection that occurs after higher doses of KX is not related to altered cardiac perfusion.
In terminal surgeries in which supplemental KX could confound the outcome variables, we propose an alternative means for tissue harvest. The combination of KX (K, 85 mg/kg; X, 15 mg/kg) followed by a physical method of euthanasia in anesthetized animals by appropriately trained operators successfully standardizes the dose of anesthetic yet ensures that animals are rendered insensate prior to thoracotomy and heart removal. This alternative is only appropriate for acute terminal procedures. In survival or in vivo studies in which procedures are performed on anesthetized animals, dose supplementation to maintain a surgical plane of anesthesia may be unavoidable. In that case, the need to document the amount of anesthetic administered to each animal is crucial, and potential variability introduced by anesthetic supplementation must be acknowledged as a limitation to the experimental design.
Although our study examined the effects of KX dose on cardiac ischemia–reperfusion injury, we should note that the dose of KX anesthesia also may alter infarct size in other tissues exposed to ischemia–reperfusion, such as brain, liver, and kidney. We suspect that use of supplemental KX doses during anesthesia also may reduce infarct size after ischemia in these tissues, as we show occurs in cardiac tissue, but future studies are necessary to test this idea.
Our study altered the dosages of both K and X and determined that in combination, KX acts as a cardioprotectant after ischemia–reperfusion injury. Although we cannot differentiate the individual effects of the higher doses of K and X on myocardial ischemia–reperfusion injury, we can speculate on mechanisms of action for each compound. Ketamine is a dissociative anesthetic that exerts its inhibitory actions by deactivating the N-methyl D-aspartate receptor, a nonselective cation channel. We are unaware of any studies that examined the cardioprotective effects of K directly, but ethanol, another N-methyl D-aspartate receptor antagonist, has been shown to have cardioprotective properties.34,48,69 Increased glutamate release after inhibition of the N-methyl D-aspartate receptor, which preconditions other tissues against cell death,53 could be responsible for the reduction in ischemia–reperfusion injury we observed in the high-dose KX group. K equilibrates into tissues rapidly, with a serum half-life of 13 min in rodents after intraperitoneal injection.40 We cannot rule out the possibility that the rats that received the high dose of K had greater heart uptake of the drug and that the protection observed may have involved increased tissue levels of K. However, in a previous study,44 administration of K to isolated hearts immediately before ischemia had no effect on infarct size, arguing against a correlation between tissue ketamine levels and protection from infarction.
Xylazine is an α2-adrenergic agonist with both analgesic and sedative properties.15 Stimulation of α2-adrenergic receptors in heart leads to vasoconstriction, which might result in ischemic preconditioning of the tissue. However, we saw no differences in baseline coronary flow rates between KX doses, suggesting that any vasoconstricting properties of X must be minimal or short-lived. Based on previous reports that increasing the X content dose-dependently influenced cardiac function66 and other studies in which K alone immediately prior to ischemia did not alter infarct size,44 we speculate that higher doses of X may be responsible for the cardioprotection we observed. Future studies are needed to determine whether K or X alone contributes to cardioprotection or whether higher doses of both agents is necessary for cardioprotection.
One limitation to our study is lack of a true control group, as all animals received KX prior to harvest of the heart. Although we cannot determine the extent of protection induced by the low-dose KX group compared with a ‘no-drug’ group, we chose not to perform cervical dislocation on unanesthetized animals, in accordance with AVMA guidelines.1 Studies in the literature that compare different anesthetics also lack a true control group.18,28,44 Among the studies comparing different anesthetics, the magnitude of infarct-size reduction after KX anesthesia is modest in comparison with volatile anesthetics, which exert a profound influence on infarct size.18,28
Furthermore, our study tested 2 distinct concentrations of KX. Anesthetic supplementation with small incremental doses may not precondition the heart as much as did the dose we used, but our data show clearly that large differences in KX doses can influence the susceptibility of the heart to injury substantially. Awareness of the potentially confounding effects of anesthetic supplementation, especially in species in which an adequate plane of anesthesia is difficult to achieve, likely will help investigators to interpret their data more accurately. Our purpose is not to suggest the use of the high-dose protocol for anesthetizing guinea pigs; however, we want to emphasize the fact that the use of supplemental doses of KX may confound the interpretation of data obtained in cardiac ischemia–reperfusion studies.
In conclusion, we found that higher doses of KX used to anesthetize guinea pigs led to reduction in myocardial infarct size and improved hemodynamic function after experimental ischemia–reperfusion. In studies examining ischemic injury, supplementation of KX to ensure adequate anesthesia in guinea pigs may be introducing unwanted variability.
References
- 1.American Veterinary Medical Association. [Internet]. 2007. AVMA guidelines on euthanasia, 2007 update. [Cited 16 August 2010]. Available at: http://www.avma.org/issues/animal_welfare/euthanasia.pdf.
- 2.Asch RH, Greenblatt RB. 1978. Primary and membranous dysmenorrhea. South Med J 71:1247–1249, 1252 [DOI] [PubMed] [Google Scholar]
- 3.Aya AG, Robert E, Bruelle P, Lefrant JY, Juan JM, Peray P, Eledjam JJ, de La Coussaye JE. 1997. Effects of ketamine on ventricular conduction, refractoriness, and wavelength: potential antiarrhythmic effects. A high-resolution epicardial mapping in rabbit hearts. Anesthesiology 87:1417–1427 [DOI] [PubMed] [Google Scholar]
- 4.Baxter GF, Ferdinandy P. 2001. Delayed preconditioning of myocardium: current perspectives. Basic Res Cardiol 96:329–344 [DOI] [PubMed] [Google Scholar]
- 5.Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, Jochum W, Spahn DR, Graf R, Clavien PA. 2008. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg 248:909–918 [DOI] [PubMed] [Google Scholar]
- 6.Bolli R. 2007. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292:H19–H27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O'Rourke B. 2008. Effects of 4’-chlorodiazepam on cellular excitation–contraction coupling and ischaemia–reperfusion injury in rabbit heart. Cardiovasc Res 79:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O'Rourke B. 2010. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol 48:673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. 2005. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol 569:913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. 2003. Exercise training preserves coronary flow and reduces infarct size after ischemia–reperfusion in rat heart. J Appl Physiol 95:2510–2518 [DOI] [PubMed] [Google Scholar]
- 11.Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, Moore RL. 2005. Susceptibility of the heart to ischaemia–reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol 564:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DA, O'Rourke B. 2010. Cardiac mitochondria and arrhythmias. Cardiovasc Res 88:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchanan KC, Burge RR, Ruble GR. 1998. Evaluation of injectable anesthetics for major surgical procedures in guinea pigs. Contemp Top Lab Anim Sci 37:58–63 [PubMed] [Google Scholar]
- 14.Buelke-Sam J, Holson JF, Bazare JJ, Young JF. 1978. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci 28:157–162 [PubMed] [Google Scholar]
- 15.Cabral AD, Kapusta DR, Kenigs VA, Varner KJ. 1998. Central α2-receptor mechanisms contribute to enhanced renal responses during ketamine–xylazine anesthesia. Am J Physiol 275:R1867–R1874 [DOI] [PubMed] [Google Scholar]
- 16.Coetzee A, Brits W, Genade S, Lochner A. 1991. Halothane does have protective properties in the isolated ischemic rat heart. Anesth Analg 73:711–719 [DOI] [PubMed] [Google Scholar]
- 17.Coetzee A, Moolman J. 1993. Halothane and the reperfusion injury in the intact animal model. Anesth Analg 76:734–744 [DOI] [PubMed] [Google Scholar]
- 18.Cope DK, Impastato WK, Cohen MV, Downey JM. 1997. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology 86:699–709 [DOI] [PubMed] [Google Scholar]
- 19.Curtis MJ, Walker MJ. 1988. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res 22:656–665 [DOI] [PubMed] [Google Scholar]
- 20.D'Alleinne CP, Mann DD. 1982. Evaluation of ketamine–xylazine anesthesia in the guinea pig: toxicological parameters. Vet Hum Toxicol 24:410–412 [PubMed] [Google Scholar]
- 21.De Hert SG, Turani F, Mathur S, Stowe DF. 2005. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg 100:1584–1593 [DOI] [PubMed] [Google Scholar]
- 22.Drenger B, Ginosar Y, Chandra M, Reches A, Gozal Y. 1994. Halothane modifies ischemia-associated injury to the voltage-sensitive calcium channels in canine heart sarcolemma. Anesthesiology 81:221–228 [DOI] [PubMed] [Google Scholar]
- 23.Drenger B, Ginosar Y, Gozal Y. 1994. Effect of halothane on sarcolemmal calcium channels during myocardial ischemia and reperfusion. Adv Pharmacol 31:89–97 [DOI] [PubMed] [Google Scholar]
- 24.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W. 1981. Early-phase acute myocardial infarct-size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J 101:593–600 [DOI] [PubMed] [Google Scholar]
- 25.Flecknell PA. 1987. Anaesthesia of guinea pigs. Vet Rec 120:167. [DOI] [PubMed] [Google Scholar]
- 26.Gaertner DJ, Batchelder M, Hankenson FC, Hallman TM. 2008. Anesthesia and analgesia for laboratory rodents, p 239–297 : Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals, 2nd ed New York (NY): Elsevier [Google Scholar]
- 27.Green CJ, Knight J, Precious S, Simpkin S. 1981. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10-year experience. Lab Anim 15:163–170 [DOI] [PubMed] [Google Scholar]
- 28.Haessler R, Kuzume K, Chien GL, Wolff RA, Davis RF, Van Winkle DM. 1994. Anaesthetics alter the magnitude of infarct limitation by ischaemic preconditioning. Cardiovasc Res 28:1574–1580 [DOI] [PubMed] [Google Scholar]
- 29.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 30.Johnson MS, Moore RL, Brown DA. 2006. Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol Heart Circ Physiol 290:H2644–H2647 [DOI] [PubMed] [Google Scholar]
- 31.Julier K, da Silva R, Garcia C, Bestmann L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von Segesser LK, Pasch T, Spahn DR, Zaugg M. 2003. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: a double-blinded, placebo-controlled, multicenter study. Anesthesiology 98:1315–1327 [DOI] [PubMed] [Google Scholar]
- 32.Kanaya N, Kobayashi I, Nakayama M, Fujita S, Namiki A. 1995. ATP sparing effect of isoflurane during ischaemia and reperfusion of the canine heart. Br J Anaesth 74:563–568 [DOI] [PubMed] [Google Scholar]
- 33.Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. 2002. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO-synthase–dependent. Stroke 33:1889–1898 [DOI] [PubMed] [Google Scholar]
- 34.Kehl F, Krolikowski JG, LaDisa JF, Jr, Kersten JR, Warltier DC, Pagel PS. 2003. Adenosine type 1 (A1) receptors mediate protection against myocardial infarction produced by chronic, intermittent ingestion of ethanol in dogs. Int J Cardiol 88:175–182 [DOI] [PubMed] [Google Scholar]
- 35.Kellner S. 1987. Anaesthesia of guinea pigs. Vet Rec 121:360. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Ishibe Y, Ueda M. 2000. Isoflurane–sevoflurane adminstration before ischemia attenuates ischemia–reperfusion-induced injury in isolated rat lungs. Anesthesiology 92:833–840 [DOI] [PubMed] [Google Scholar]
- 37.Lucchinetti E, Jamnicki M, Fischer G, Zaugg M. 2008. Preconditioning by isoflurane retains its protection against ischemia–reperfusion injury in postinfarct remodeled rat hearts. Anesth Analg 106:17–23 [DOI] [PubMed] [Google Scholar]
- 38.Mangano DT. 1990. Perioperative cardiac morbidity. Anesthesiology 72:153–184 [DOI] [PubMed] [Google Scholar]
- 39.Mattheussen M, Rusy BF, Van Aken H, Flameng W. 1993. Recovery of function and adenosine triphosphate metabolism following myocardial ischemia induced in the presence of volatile anesthetics. Anesth Analg 76:69–75 [DOI] [PubMed] [Google Scholar]
- 40.Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. 2006. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther 316:315–324 [DOI] [PubMed] [Google Scholar]
- 41.McAuliffe JJ, Joseph B, Vorhees CV. 2007. Isoflurane-delayed preconditioning reduces immediate mortality and improves striatal function in adult mice after neonatal hypoxia–ischemia. Anesth Analg 104:1066–1077 [DOI] [PubMed] [Google Scholar]
- 42.Meissner A, Weber TP, Van Aken H, Zbieranek K, Rolf N. 2000. Recovery from myocardial stunning is faster with desflurane compared with propofol in chronically instrumented dogs. Anesth Analg 91:1333–1338 [DOI] [PubMed] [Google Scholar]
- 43.Morey TE, Martynyuk AE, Napolitano CA, Raatikainen MJ, Guyton TS, Dennis DM. 1997. Ionic basis of the differential effects of intravenous anesthetics on erythromycin-induced prolongation of ventricular repolarization in the guinea pig heart. Anesthesiology 87:1172–1181 [DOI] [PubMed] [Google Scholar]
- 44.Mullenheim J, Frassdorf J, Preckel B, Thamer V, Schlack W. 2001. Ketamine, but not S(+)-ketamine, blocks ischemic preconditioning in rabbit hearts in vivo. Anesthesiology 94:630–636 [DOI] [PubMed] [Google Scholar]
- 45.Napolitano CA, Raatikainen MJ, Martens JR, Dennis DM. 1996. Effects of intravenous anesthetics on atrial wavelength and atrioventricular nodal conduction in guinea pig heart. Potential antidysrhythmic properties and clinical implications. Anesthesiology 85:393–402 [DOI] [PubMed] [Google Scholar]
- 46.Oguchi T, Kashimoto S, Yamaguchi T, Nakamura T, Kumazawa T. 1993. Is pentobarbital appropriate for basal anesthesia in the working rat heart model? J Pharmacol Toxicol Methods 29:37–43 [DOI] [PubMed] [Google Scholar]
- 47.Oguchi T, Kashimoto S, Yamaguchi T, Nakamura T, Kumazawa T. 1995. Comparative effects of halothane, enflurane, isoflurane and sevoflurane on function and metabolism in the ischaemic rat heart. Br J Anaesth 74:569–575 [DOI] [PubMed] [Google Scholar]
- 48.Pagel PS, Krolikowski JG, Kehl F, Mraovic B, Kersten JR, Warltier DC. 2002. The role of mitochondrial and sarcolemmal K(ATP) channels in canine ethanol-induced preconditioning in vivo. Anesth Analg 94:841–848 [DOI] [PubMed] [Google Scholar]
- 49.Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. 2009. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein kinase Cϵ-mediated pathway. Anesthesiology 111:267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radde GR, Hinson A, Crenshaw D, Toth LA. 1996. Evaluation of anaesthetic regimens in guinea pigs. Lab Anim 30:220–227 [DOI] [PubMed] [Google Scholar]
- 51.Riess ML, Kevin LG, McCormick J, Jiang MT, Rhodes SS, Stowe DF. 2005. Anesthetic preconditioning: the role of free radicals in sevoflurane-induced attenuation of mitochondrial electron transport in guinea pig isolated hearts. Anesth Analg 100:46–53 [DOI] [PubMed] [Google Scholar]
- 52.Sahlman L, Waagstein L, Haljamae H, Ricksten SE. 1995. Protective effects of halothane but not isoflurane against global ischaemic injury in the isolated working rat heart. Acta Anaesthesiol Scand 39:312–316 [DOI] [PubMed] [Google Scholar]
- 53.Schurr A, Payne RS, Tseng MT, Gozal E, Gozal D. 2001. Excitotoxic preconditioning elicited by both glutamate and hypoxia and abolished by lactate transport inhibition in rat hippocampal slices. Neurosci Lett 307:151–154 [DOI] [PubMed] [Google Scholar]
- 54.Shuba LM, Kasamaki Y, Jones SE, Ogura T, McCullough JR, McDonald TF. 1999. Action potentials, contraction, and membrane currents in guinea pig ventricular preparations treated with the antispasmodic agent terodiline. J Pharmacol Exp Ther 290:1417–1426 [PubMed] [Google Scholar]
- 55.Skolleborg KC, Gronbech JE, Grong K, Abyholm FE, Lekven J. 1990. Distribution of cardiac output during pentobarbital versus midazolam–fentanyl–fluanisone anaesthesia in the rat. Lab Anim 24:221–227 [DOI] [PubMed] [Google Scholar]
- 56.Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. 2007. Volatile anesthetic-induced cardiac preconditioning. J Anesth 21:212–219 [DOI] [PubMed] [Google Scholar]
- 57.Suckling AJ. 1987. Anaesthesia of guinea pigs. Vet Rec 120:192. [DOI] [PubMed] [Google Scholar]
- 58.Vogler GA. 2006. Anesthesia and analgesia, p 627–664 : Suckow MA, Weisbroth SH, Franklin CL. The laboratory rat, 2nd ed Burlington (MA): Academic Press [Google Scholar]
- 59.Walsh RS, Tsuchida A, Daly JJ, Thornton JD, Cohen MV, Downey JM. 1994. Ketamine–xylazine anaesthesia permits a KATP channel antagonist to attenuate preconditioning in rabbit myocardium. Cardiovasc Res 28:1337–1341 [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Jin Lee J, Jung HH, Zuo Z. 2007. Pretreatment with volatile anesthetics, but not with the nonimmobilizer 1,2-dichlorohexafluorocyclobutane, reduced cell injury in rat cerebellar slices after an in vitro simulated ischemia. Brain Res 1152:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warltier DC, al-Wathiqui MH, Kampine JP, Schmeling WT. 1988. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology 69:552–565 [DOI] [PubMed] [Google Scholar]
- 62.Weinbrenner C, Liu GS, Downey JM, Cohen MV. 1998. Cyclosporine A limits myocardial infarct size even when administered after onset of ischemia. Cardiovasc Res 38:678–684 [DOI] [PubMed] [Google Scholar]
- 63.White JL, Myers AK, Analouei A, Kim YD. 1994. Functional recovery of stunned myocardium is greater with halothane than fentanyl anaesthesia in dogs. Br J Anaesth 73:214–219 [DOI] [PubMed] [Google Scholar]
- 64.Wixson SK, White WJ, Hughes HC, Jr, Lang CM, Marshall WK. 1987. The effects of pentobarbital, fentanyl–droperidol, ketamine–xylazine and ketamine–diazepam on arterial blood pH, blood gases, mean arterial blood pressure, and heart rate in adult male rats. Lab Anim Sci 37:736–742 [PubMed] [Google Scholar]
- 65.Wright M, Heath RB, Wingfield WE. 1987. Effects of xylazine and ketamine on epinephrine-induced arrhythmia in the dog. Vet Surg 16:398–403 [DOI] [PubMed] [Google Scholar]
- 66.Xu Q, Ming Z, Dart AM, Du XJ. 2007. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin Exp Pharmacol Physiol 34:499–507 [DOI] [PubMed] [Google Scholar]
- 67.Yellon DM, Downey JM. 2003. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151 [DOI] [PubMed] [Google Scholar]
- 68.Zhao P, Peng L, Li L, Xu X, Zuo Z. 2007. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic–ischemic brain injury in neonatal rats. Anesthesiology 107:963–970 [DOI] [PubMed] [Google Scholar]
- 69.Zhu P, Zhou HZ, Gray MO. 2000. Chronic ethanol-induced myocardial protection requires activation of mitochondrial K(ATP) channels. J Mol Cell Cardiol 32:2091–2095 [DOI] [PubMed] [Google Scholar]