Abstract
We evaluated analgesic use and analgesiometry in aquatic African-clawed frogs (Xenopus laevis). We used the acetic acid test (AAT) to assess the analgesic potential of systemic xylazine hydrochloride, meloxicam, flunixin meglumine, and morphine sulfate after injection into the dorsal lymph sac. Flunixin meglumine provided better analgesia than did the other drugs, most evident at 5 and 9 h after administration. Because the AAT was associated with the development of dermal lesions, we discontinued use of this assay and chose the Hargreaves test as an alternative method of measuring nociception in Xenopus. This assay is commonly performed in rodents, but its efficacy in an aquatic species such as Xenopus was unknown prior to this study. We found that the Hargreaves test was an effective measure of nociception in Xenopus, and we used it to evaluate the effectiveness of the nonopiod agents xylazine hydrochloride, meloxicam, and flunixin meglumine both in the absence of surgery and after surgical oocyte harvest. Similar to findings from the AAT, flunixin meglumine provided better analgesia in the Hargreaves test than did the other agents when analyzed in the absence of surgical intervention. Results were equivocal after oocyte harvest. Although surgical oocyte harvest is a common procedure in Xenopus, and currently there are no published recommendations for analgesia after this invasive surgery. Future studies are needed to clarify the efficacy of nonsteroidal antiinflammatory drugs for that purpose.
Abbreviation: AAT, acetic acid test
Xenopus laevis, the South African-clawed frog, is widely used for developmental, cellular and molecular biology research.30 Additional research uses of amphibians include pain and analgesia, limb regeneration, metamorphosis, hibernation, neurology, developmental biology, and embryology.3,29 During the last 20 y, Xenopus spp. have surpassed Rana spp. as the primary amphibian used in biomedical research.14 Attributes making Xenopus laevis an excellent research animal include resistance to disease and infection, short life cycle, and an aquatic lifestyle, which decreases husbandry efforts when compared with those for terrestrial amphibians.30
Oocytes are commonly collected from Xenopus for use in research. Typically, these oocytes are harvested surgically by making a ceolomic incision and performing a partial ovariectomy. The administration of postoperative analgesics has not been common in Xenopus after oocyte harvest.5 This omission may be in part due to the perception that because amphibians do not possess a limbic or cerebral cortex, they do not perceive pain in the same way as mammals. 4,5,24 However, current information regarding pain in nonmammalian vertebrates suggests that these animals may feel pain similarly to mammals.5,21 The amphibian nervous system contains large, heavily myelinated A fibers; small, thinly myelinated B fibers; and small, unmyelinated C fibers, which share similar characteristics to those in mammals.24 Previous studies in amphibians have demonstrated impulse transmission secondary to a noxious stimulus to these fibers.1,8,15,24 Therefore, the assumption that amphibians feel pain seems reasonable, and therefore they may benefit from analgesics when subjected to potentially painful procedures, in a similar fashion to that in mammals.29 A void currently exists regarding the effectiveness of postoperative analgesia in this species.
Historically, the acetic acid test (AAT) has been used to assess nociception in amphibian species, such as the leopard frog. The AAT is a commonly performed behavioral assay of cutaneous nociception through the measurement of response to noxious chemical stimuli following excitation of afferent fibers.18,22-28,32 Although numerous analgesia studies have been conducted on frog species, most notably the northern leopard frog (Rana pipiens), few reports exist that describe an effective measure of nociception in aquatic species, such as Xenopus laevis. Two previous studies have used the AAT to evaluate anesthesia in Xenopus. One study used the AAT in Xenopus to determine the efficacy of using eugenol for anesthesia,7 and a separate study used the AAT to determine anesthetic properties of propofol.6 Initially we chose the AAT as a measure of nociception in our study.18,22,23,25,26,28,32
We also evaluated the use of the Hargreaves apparatus in Xenopus. The Hargreaves test is an assay of nociception that is commonly used in mice and rats. Previous reports indicate that the time required to move a body part from light source providing radiant heat allows a measure of thermal sensitivity.9 A thermal stimulus can be applied to the skin to measure nociception when testing the analgesic activity of a substance or procedure.2 The Hargreaves test can be performed repeatedly, due to the transient nature and lack of tissue damage associated with this assay.2 In amphibians, 2 studies describe the use of a modified thermal stimulus apparatus to determine nociception in leopard frogs.31,32 The Hargreaves test differs from the AAT in that a thermal stimulus evokes a behavioral response in the Hargreaves test, but it shares similar characteristics to the AAT by relying upon cutaneous stimulation of afferent fibers.2 We attempted to determine whether application of a thermal stimulus could be used to test nociception in Xenopus.
In addition to the evaluation of analgesiometric assays in Xenopus, we wished to explore the efficacy of potential analgesics, particularly of the nonopioid class. The use of nonopioid pharmacologic agents would have multiple benefits to the research community in that these drugs are readily available and specialized licensure is not required to obtain them. Literature exists describing the use of nonsteroidal antiinflammatory drugs in leopard frogs. Stevens and colleagues found that nonsteroidal antiinflammatory agents (indomethacin and ketorolac) produced analgesia after systemic administration.26 A separate study detected long-lasting analgesia with xylazine hydrochloride (10 mg/kg) and found that flunixin meglumine (25 mg/kg) provided good analgesia for 2 and 4 h.29 Therefore, we assessed the effectiveness of nonopioid agents both in the absence of surgical manipulation and after surgical oocyte harvest. Potential painful stimuli secondary to oocyte harvest in Xenopus include incisional pain as well as pain secondary to partial ovariectomy. We did not find any information pertaining to the detection or alleviation of potential visceral pain associated with an oocyte harvest in Xenopus. We hypothesized that the Hargreaves apparatus would support accurate determination of nociception in Xenopus. Another hypothesis was that nonopioid pharmacologic agents would provide discernable analgesia in Xenopus, both in the absence of surgery and perioperatively, as determined by the Hargreaves test. The current study is the first report of the use of the Hargreaves apparatus in Xenopus and concomitant evaluation of nonopioid analgesics in this species.
Materials and Methods
Humane care and use of animals.
The study was conducted in an AAALAC- accredited animal resources facility; all husbandry and experimental procedures were conducted according to the Public Health Service Policy on the Humane Care and Use of Laboratory Animals16 and the Guide for the Care and Use of Laboratory Animals.11 All procedures involving animal use were approved by the Institutional Animal Care and Use Committee at Emory University.
Animals.
A total of 30 sexually mature laboratory-raised female Xenopus laevis frogs (length, 6.25 to 7.5 cm; weight, 48.9 to 78.1 g) were purchased from a commercial source (NASCO, Fort Atkinson, WI). Frogs were free of ranavirus and chytrid fungus and were group-housed in a 50-gal custom-built static tank supplied with multiple aeration devices (Tetra, Blacksburg, VA). Water known to be free of contaminants was used, and water quality reports documenting pH, NH3, NO2, hardness, and Cl were obtained weekly (Freshwater Master Test Kit, That Pet Place, Lancaster, PA). The pH was maintained at 7.2 to 7.6, and daily water temperature measurements were 18 to 23 °C. An estimated 90% to 95% of the water within the tank was replaced 3 times each week. Frogs were maintained on a 12:12-h light:dark cycle and received a commercial frog brittle (NASCO) twice weekly. Commercial floating enrichment devices (Foster and Smith, Rhinelander, WI) were provided in the tank. A 2-wk acclimation period was provided prior to using the frogs in the study.
Drug treatments.
The agents we evaluated were xylazine hydrochloride (10 mg/kg; α2 adrenergic agonist), meloxicam (0.2 mg/kg) and flunixin meglumine (25 mg/kg; both nonsteroidal antiinflammatory drugs), and morphine sulfate (40 mg/kg; opioid). The dosages of xylazine hydrochloride (Lloyd Laboratories, Shenandoah, IA), flunixin meglumine (Banamine, Schering-Plough Animal Health, Union, NJ), and morphine sulfate (Baxter Healthcare, Deerfield, IL) were based on previously published doses that were administered to amphibians including Rana spp., because of the lack of published data from Xenopus.20,29 The dosage for meloxicam (Boehringer Ingelheim, St Joseph, MO) was based on recommended doses used for companion animals, because of the lack of published data in Xenopus.19 Sterile 0.9% sodium chloride (Braun Medical, Irvine, CA) served as the control agent, in addition to being the substance used to dilute doses of the test agents to a final volume of 0.25 mL. Needles (22-gauge, 1-in.; Tyco Healthcare Group LP, Mansfield, MA) were used to inject the selected agents into the dorsal lymph sac.
Acetic acid test.
The acetic acid test was performed according to previously published guidelines.17,25,26,28,29,31,32 Frogs were separated into pairs 24 h prior to performing the AAT. During AAT, individual frogs were placed into polycarbonate cages that contained 0.5 cm water in the bottom. Glacial acetic acid (EK Industries, Joliet, IL) was serially diluted to 10 strengths equally spaced on a logarithmic scale and numbered 1 to 10 according to increasing concentration. Acetic acid test solutions were delivered as a single drop to the dorsal thigh of the frog, and distilled water was used to rinse the region after 5 s. If the animal did not show a wiping response, the test was repeated with the next highest concentration of acetic acid on the opposite thigh. The nociceptive threshold was determined to be the concentration of acetic acid that elicited a wiping response.
We used the AAT to determine the effectiveness of selected pharmacologic agents (xylazine hydrochloride, meloxicam, flunixin meglumine, and morphine sulfate) in Xenopus. Frogs were assigned randomly to 5 groups (4 drug treatments and 1 control), with each group containing 6 frogs. Personnel donned personal protective equipment, including a laboratory coat and moistened nitrile gloves (Kimberly-Clark Worldwide, Roswell, GA), prior to frog manipulation. Frogs were tested 1 h prior to and at 1, 5, 9, and 24 h after administration of the test agent. The staff person who performed the AAT was blinded to the treatments. Frogs were monitored continuously for 1 h after completion of the AAT to ensure they maintained the ability to swim and move normally around the tank.
Hargreaves test.
The Hargreaves apparatus (Ugo Basile model 7370 Plantar Test, Collegeville, PA) was used to measure thermal nociceptive threshold. Frogs were acclimated to the glass test chamber for 5 min before a focus of high-intensity light was directed through the chamber onto the medial aspect of the thigh (Figure 1). The latency of inner thigh movement in response to the radiant heat source was measured by the apparatus. The infrared intensity was maintained at 90 mW/cm2. The time from activation of the thermal stimulus to withdrawal response was recorded as the thermal nociceptive threshold. The cutoff time of the apparatus was set at 29.1 s.
Figure 1.
Photograph of the testing mechanism, including the Hargreaves apparatus, glass chamber, and Xenopus laevis.
We sought to determine which, if any, of the selected agents (xylazine hydrochloride at 10 mg/kg, meloxicam at 0.2 mg/kg, and flunixin meglumine at 25 mg/kg) provided analgesia in Xenopus as defined by an increased latency of inner thigh movement in response to the radiant heat source. Frogs were assigned randomly to 4 groups (3 drug treatments and 1 control), with each group containing 6 frogs. Frogs were removed from the primary tank and placed in pairs within separate polycarbonate cages 24 h prior to the onset of testing and remained in the cage for observation an additional 24 h after the completion of testing. Each polycarbonate cage contained water from the primary tank. Individual body weights and identifying phenotypic characteristics were determined at this time. A single, blinded observer performed nociceptive testing by using the Hargreaves apparatus.
A baseline thermal nociceptive threshold was obtained by using the Hargreaves test 30 min prior to administration of the drug. Each agent was injected into the dorsal lymph sac, and the Hargreaves test was repeated at 1, 5, 9, and 24 h after injection. Frogs were monitored continuously for 1 h after completion of all testing to ensure they maintained the ability the swim and move normally around the tank.
Evaluation of perioperative analgesics.
This aim was designed to determine the nociceptive effect of nonopiod agents after surgical oocyte harvest in Xenopus. Tricaine methanesulfonate (Argent Chemical Laboratories, Redmond, WA) was used as the anesthetic agent for all frogs. Sodium bicarbonate (Hospira, Lake Forest, IL) was used to buffer the solution to a pH of 7.0, and pH strips (EMD Chemicals, Gibbstown, NJ) were used to ensure that the appropriate pH was achieved. Individual frogs were immersed into the buffered anesthetic solution until anesthetized, as determined by loss of righting and withdrawal reflexes. Total induction time was between 10 and 15 min for each frog. The selected pharmacologic agent was injected systemically into the dorsal lymph sac after a surgical plane of anesthesia was detected. Frogs were placed in ventrodorsal recumbency on a saline-moistened paper towel; moistened gauze was placed on all exposed dermal regions excluding the surgical site. Nonsterile, 12-ply gauze (Henry Schein, Melville, NY) was moistened with saline and used to gently prep the surgical site. The surgeon donned white latex powdered surgical gloves (Ansell Healthcare Products LLC, Dothan, AL) and used sterile saline to remove powder from the exterior of the gloves.
A 1- to 2-cm paramedian ceolomic incision was created by using a no.15 scalpel blade (Henry Schein). Autoclaved surgical instruments were used to harvest oocytes. A section of ovary containing a dozen oocytes was maneuvered gently through the incision with sterile forceps and excised with scissors (sharp/sharp). An identifying microchip was implanted into the ceolom of each frog. Gas-sterilized microchips (Destron Fearing, South St Paul, MN) were used for individual frog identification by using a reader (Pocket Reader version 0218-S63, Destron Fearing). A 2-layer abdominal closure was performed. PDS II (4-0; Ethicon, Somerville, NJ) clear monofilament synthetic absorbable suture was used to appose the muscular layer of the abdomen, and Prolene suture (4-0; Ethicon) was used to appose the skin edges in a simple interrupted pattern. Frogs were replaced into polycarbonate cages for postoperative recovery. The Hargreaves test was repeated at 2, 5, 9, and 24 h after injection. The initial postoperative nociceptive assay was performed 2 h after agent administration rather than at the previously selected 1-h time point because some of the frogs had not recovered from anesthesia at the 1-h time point. Body weights and individual observations were obtained daily for 7 d after oocyte harvest.
Data analysis.
Data were analyzed by random-effects modeling within the JMP statistical package (SAS Institute, Cary, NC). In this model, drug, time of administration, and the drug×time interaction were considered as fixed effects, and animal ID nested within drug was considered as a random effect. A P value of less than 0.05 was considered statistically significant. To identify specific differences, we used the Tukey HSD test as a correction for multiple comparisons.
Results
Analgesiometric testing using the acetic acid test.
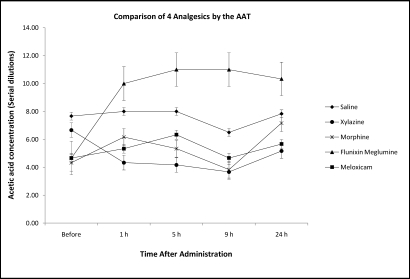
The nociceptive threshold of Xenopuswas increased most markedly after injection of flunixin meglumine (Figure 2) and was significantly (P < 0.05) greater than those associated with morphine and xylazine at 5 and 9 h after administration. Compared with responses after injection of saline, the nociceptive threshold of flunixin meglumine increased significantly (P < 0.05) at all time points after the pretreatment AAT, whereas neither morphine nor xylazine showed any difference. In addition, dermal lesions including ulceration and inflammation, particularly of the hindlimb region to which acetic acid had been applied, appeared in 12 of 30 frogs (40%) within 1 wk of performing the AAT. Furthermore, 2 frogs were found dead in the tank before treatment or euthanasia could be initiated. Because of these adverse reactions, we discontinued using the AAT in our frogs.
Figure 2.
Nociceptive threshold (mean ± SEM) as determined by using the AAT in Xenopus laevis after systemic injection of xylazine hydrochloride (10 mg/kg), meloxicam (0.2 mg/kg), morphine sulfate (40 mg/kg), and flunixin meglumine (25 mg/kg).
Analgesiometric testing using the Hargreaves test.
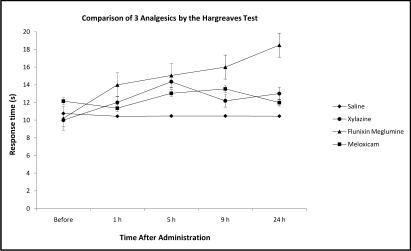
At 24 h after administration, flunixin meglumine provided significantly (P < 0.05) greater thermal stimulus response latency compared with saline and baseline values (Figure 3). There was no statistical difference in the thermal nociceptive thresholds between saline, xylazine hydrochloride (10 mg/kg), meloxicam (0.2 mg/kg), and flunixin meglumine (25 mg/kg) at the 1, 5, or 9 h postinjection time points. Similar to the findings from the AAT, flunixin meglumine appeared to hold some promise as an analgesic in the Hargreaves test. None of the treatments caused changes in normal behavior, such as swimming, righting reflex, and response to tactile stimulation.
Figure 3.
Nociceptive threshold (mean ± SEM) as determined by using the Hargreaves test in Xenopus laevis after systemic injection of xylazine hydrochloride (10 mg/kg), meloxicam (0.2 mg/kg), and flunixin meglumine (25 mg/kg).
Evaluation of perioperative analgesics.
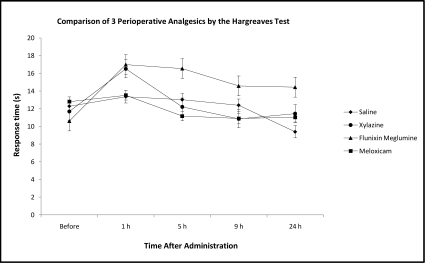
Comparison of the nonopiod agents after oocyte harvest revealed no statistical difference in thermal nociceptive threshold between the drugs (Figure 4). One frog in the flunixin meglumine group was found dead in the tank prior to the 5-h time point, after an uneventful recovery from anesthesia. Surgical complications were not apparent during the gross necropsy, and diagnostic tests including bacterial cultures and Mycobacterium PCR were negative. Histopathologic findings included congested kidneys with mild inflammatory cell infiltrates within the interstitium. In addition, one frog in the xylazine group was slightly sedated with a delayed righting reflex although swimming at the 2-h time point; sedation had resolved prior to the 5-h time point.
Figure 4.
Perioperative nociceptive threshold (mean ± SEM) as determined by using the Hargreaves test in Xenopus laevis after systemic injection of xylazine hydrochloride (10 mg/kg), meloxicam (0.2 mg/kg), and flunixin meglumine (25 mg/kg).
Discussion
In this study, we used the AAT and Hargreaves test to assess the efficacy of analgesic medications in Xenopus laevis. We initially chose the AAT to evaluate cutaneous nociception in Xenopus given previous literature in northern leopard frogs, and we added the Hargreaves test because of complications we noted after the AAT. Flunixin meglumine seemed to be an auspicious drug for the provision of analgesia in Xenopus, as evidenced by increased analgesia in both the AAT and Hargreaves test. However, this trend did not hold true after surgical oocyte harvest. This finding underscores the challenges of evaluating response to analgesics, particularly in a less-well-studied species, such as Xenopus.
We noted a high incidence of dermal lesions in our frogs after the AAT. The application of acetic acid potentially disrupted both portions of the skin, leading to secondary bacterial infections with ulceration or sepsis. Review of the literature showed that another study encountered dermal lesions in leopard frogs after the AAT, resulting in euthanasia of severely affected frogs,29 and others have accounted for frog lethality secondary to drug dose or route of administration during experiments.26,28 Our initial goal was to use the AAT in Xenopus in the hope of using the same subjects for the surgical portion of the study. However, this goal was not feasible, given the unexpected morbidity associated with the AAT. The unexpected morbidity was noted subsequent to completion of this aim and did not have a direct effect on statistical significance. The study began with a total of 30 subjects, a number that accommodated an additional experimental group given morphine sulfate. The focus of the project changed due to the unexpected adverse events, and the morphine sulfate group was removed from the study so that group size could be maintained as 6 frogs during evaluation of the Hargreaves test. Because of the potential for adverse events associated with the use of the AAT, we discourage the use of this test in aquatic species such as Xenopus during nonacute studies.
Because we could no longer use the AAT, we elected to evaluate the suitability of the Hargreaves test in Xenopus. This test has been described in rodents,9 and we hypothesized that it would serve the same function in Xenopus. Furthermore, 2 additional studies31,32 described the use of a modified thermal stimulus apparatus in leopard frogs. Our study showed that the Hargreaves test can be used to determine cutaneous thermal nociceptive threshold in aquatic species such as Xenopus, as determined by the thermal stimulus response latencies of the frogs.
Our data suggest that flunixin meglumine is a potential analgesic in Xenopus, although more studies are needed to optimize administration. The Hargreaves test showed that the dose we chose (25 mg/kg), administered by way of the dorsal lymph sac, provided longer and more effective analgesia compared with that in control frogs and relative to the analgesia provided by xylazine hydrochloride and meloxicam at the 24-h time point. The lack of statistically significant results at the 5- and 9-h time points may be indicative of a confounding effect of tricaine methanesulfonate and a small sample size. In future studies, Xenopus should be monitored for longer than 24 h, because the effectiveness of flunixin meglumine was still increasing at the final time point in the current study. Results from the AAT were similar to those from the Hargreaves test, with flunixin meglumine surpassing the other treatments at 5 and 9 h after administration. Our findings are similar to those of another study, in which flunixin meglumine (25 mg/kg) was effective for 2 to 4 h in leopard frogs.29 Therefore, flunixin meglumine appears to warrant further evaluation as a potentially useful analgesic in Xenopus.
After assessing the effectiveness of analgesics in the absence of surgical manipulation, we evaluated their efficacies after surgical oocyte harvest. None of the tested analgesics increased the nociceptive threshold in this portion of the current study. Graphs of the data suggested that the thermal stimulus response latencies of flunixin meglumine might be higher than those of the other agents. The lack of significance could be due to the nociceptive assays used, sample size, or the anesthetic agent. The AAT and Hargreaves test measure behavioral responses to different (that is, chemical or thermal) cutaneous stimuli. Potential visceral pain resulting from oocyte harvest may require measurement of variables such as appetite and activity levels. A larger sample size, resulting in increased power, potentially could elicit statistically significant results in future studies. Previous reports indicate that tricaine methanesulfonate provides analgesia by unknown mechanisms,10,12 and further studies are needed to determine the analgesic potential of this drug. Tricaine methanesulfonate may have a confounding effect on nociceptive studies if the agent provides analgesia to the subjects. In addition, frogs in the saline group had higher nociceptive thresholds before and after oocyte collection than did other groups. The increased baseline values of saline, potentially due to tricaine methanesulfonate, increase the difficulty of obtaining statistical significance in the other groups.
A frog in the flunixin meglumine group was found dead prior to the 5-h time point. The frog had recovered uneventfully from surgery, and subsequent histopathology indicated bilateral kidney congestion with inflammatory infiltrates within the interstitium. The metabolism of flunixin meglumine in Xenopus is unknown, and drug toxicity could be a contributing factor to the histopathology findings and ultimate death of this frog. Despite the lack of significant findings in the perioperative portion of the current study, we suggest that flunixin meglumine is an encouraging analgesic in Xenopus and that further studies should be conducted to refine the analysis of and techniques for administration. Also needed are studies to elucidate safe and toxic drug doses in Xenopus. The current study also provides data indicating that Xenopus can and do perceive pain, as evidenced by the responses observed after administering various analgesics.
Literature on the experience of pain in amphibians is conflicting. Some publications suggest that amphibians may not feel pain or have less appreciation of pain,4,5,24 whereas others describe pain pathways for amphibians,12,13,24,27 suggesting that amphibians can and do perceive pain. Although Xenopus laevis is one of the most widely used nonmammalian laboratory animals,14 most nociceptive testing in frogs is currently conducted in northern leopard frogs. Because frogs may experience pain, emphasis must be placed on determining the effectiveness of analgesics in Xenopus. The present study did not indicate definitively the best analgesic for perioperative use in Xenopus but suggested that flunixin meglumine offers promise as a potential analgesic medication in Xenopus and provided insight with respect to the use of nociceptive assays, such as the AAT and Hargreaves test in Xenopus. Further studies evaluating flunixin meglumine, potentially using larger sample sizes or different dosages, are needed. In addition, studies evaluating the analgesic potential of tricaine methanesulfonate and the analgesic effect, if any, on oocyte production are needed. Current nociceptive assays and new technologies will continue to drive the understanding of nociception in amphibians, particularly aquatic species such as Xenopus.
Acknowledgments
We thank Dr James G Herndon, Jr, and Allison Martin for providing statistical assistance; Marsha Howard and Andre Worthy for providing daily husbandry; Karen Lieber for technical assistance; and other members of the husbandry and veterinary staff for assistance during this study.
References
- 1.Adrian ED, Cattell M, Hoagland H. 1931. Sensory discharges in single cutaneous nerve fibres. J Physiol 72:377–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JW, Yaksh TL. 2004. Assessment of acute thermal nociception in laboratory animals. Methods Mol Med 99:11–23 [DOI] [PubMed] [Google Scholar]
- 3.Anver MR, Pond CL. 1984. Laboratory animal medicine. New York (NY): Academic Press [Google Scholar]
- 4.Duellman WE, Trueb L. 1994. Biology of amphibians. Baltimore (MD): Johns Hopkins University Press [Google Scholar]
- 5.Green SL. 2003. Postoperative analgesics in South African clawed frogs (Xenopus laevis) after surgical harvest of oocytes. Comp Med 53:244–247 [PubMed] [Google Scholar]
- 6.Guenette SA, Beaudry F, Vachon P. 2008. Anesthetic properties of propofol in African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 47:35–38 [PMC free article] [PubMed] [Google Scholar]
- 7.Guenette SA, Helie P, Beaudry F, Vachon P. 2007. Eugenol for anesthesia of African clawed frogs (Xenopus laevis). Vet Anaesth Analg 34:164–170 [DOI] [PubMed] [Google Scholar]
- 8.Hamamoto DT, Simone DA. 2003. Characterization of cutaneous primary afferent fibers excited by acetic acid in a model of nociception in frogs. J Neurophysiol 90:566–577 [DOI] [PubMed] [Google Scholar]
- 9.Hargreaves K, Dubner R, Brown F, Flore C, Joris J. 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88 [DOI] [PubMed] [Google Scholar]
- 10.Hawkins MG. 2006. The use of analgesics in birds, reptiles, and small exotic mammals. J Exotic Pet Medicine 15:177–192 [Google Scholar]
- 11.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 12.Machin KL. 1999. Amphibian pain and analgesia. J Zoo Wildl Med 30:2–10 [PubMed] [Google Scholar]
- 13.Machin KL. 2001. Fish, amphibian, and reptile analgesia. Vet Clin North Am Exot Anim Pract 4:19–33 [DOI] [PubMed] [Google Scholar]
- 14.Major N, Wassersug RJ. 1998. Survey of current techniques in the care and maintenance of the African clawed frog (Xenopus laevis). Contemp Top Lab Anim Sci 37:57–60 [PubMed] [Google Scholar]
- 15.Maruhashi J, Mizuguchi K, Tasaki I. 1952. Action currents in single afferent nerve fibres elicited by stimulation of the skin of the toad and the cat. J Physiol 117:129–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Office of Laboratory Animal Welfare [Internet]. 2002. Public health policy on humane care and use of laboratory animals. [Cited June 2010]. Available at: http://grants.nih.gov/grants/olaw/references/phspol.htm
- 17.Pezalla PD. 1983. Morphine-induced analgesia and explosive motor behavior in an amphibian. Brain Res 273:297–305 [DOI] [PubMed] [Google Scholar]
- 18.Pezalla PD, Stevens CW. 1984. Behavioral effects of morphine, levorphanol, dextrorphan and naloxone in the frog Rana pipiens. Pharmacol Biochem Behav 21:213–217 [DOI] [PubMed] [Google Scholar]
- 19.Plumb DC. 2008. Plumb's veterinary drug handbook. Hoboken (NJ): Wiley-Blackwell [Google Scholar]
- 20.Smith SA. 2007. Appendix: compendium of drugs and compounds used in amphibians. ILAR J 48:297–300 [DOI] [PubMed] [Google Scholar]
- 21.Spray DC. 1976. Pain and temperature receptors of anurans. In: Llinas R, Precht W. Frog neurobiology: a handbook. Berlin (Germany): Springer-Verlag [Google Scholar]
- 22.Stevens CW. 1988. Opioid antinociception in amphibians. Brain Res Bull 21:959–962 [DOI] [PubMed] [Google Scholar]
- 23.Stevens CW. 1996. Relative analgesic potency of µ, δ, and κ opioids after spinal administration in amphibians. J Pharmacol Exp Ther 276:440–448 [PubMed] [Google Scholar]
- 24.Stevens CW. 2004. Opioid research in amphibians: an alternative pain model yielding insights on the evolution of opioid receptors. Brain Res Brain Res Rev 46:204–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens CW, Brenner GM. 1996. Spinal administration of adrenergic agents produces analgesia in amphibians. Eur J Pharmacol 316:205–210 [DOI] [PubMed] [Google Scholar]
- 26.Stevens CW, MacIver DN, Newman LC. 2001. Testing and comparison of nonopioid analgesics in amphibians. Contemp Top Lab Anim Sci 40:23–27 [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens CW, Martin KK, Stahlheber BW. 2009. Nociceptin produces antinociception after spinal administration in amphibians. Pharmacol Biochem Behav 91:436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens CW, Rothe KS. 1997. Supraspinal administration of opioids with selectivity for µ-, δ-, and κ-opioid receptors produces analgesia in amphibians. Eur J Pharmacol 331:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terril-Robb LA, Suckow MA. 1996. Evaluation of the analgesic effects of butorphanol tartrate, xylazine hydrochloride, and flunixin meglumine in leopard frogs (Rana pipiens). Contemp Top Lab Anim Sci 35:54–56 [Google Scholar]
- 30.Tinsley RC, Kobel HR. 1996. The biology of Xenopus. Oxford (UK): Clarendon Press [Google Scholar]
- 31.Willenbring S, Stevens CW. 1996. Thermal, mechanical, and chemical peripheral sensation in amphibians: opioid and adrenergic effects. Life Sci 58:125–133 [DOI] [PubMed] [Google Scholar]
- 32.Willenbring S, Stevens CW. 1997. Spinal µ, δ, and κ opioids alter chemical, mechanical, and thermal sensitivities in amphibians. Life Sci 61:2167–2176 [DOI] [PubMed] [Google Scholar]