Abstract
Cefovecin sodium is a long-acting, third-generation, cephalosporin antibiotic approved for the treatment of skin infections in dogs and cats. The pharmacokinetic properties of cefovecin were evaluated in cynomolgus macaques (Macaca fascicularis), olive baboons (Papio anubis), and rhesus macaques (Macaca mulatta) by using a single-dose (8 mg/kg SC) dosing regimen. Plasma cefovecin concentrations were determined by using ultra-performance liquid chromatography with tandem mass spectrometry, and a noncompartmental model was used to determine pharmacokinetic parameters. The half-life of cefovecin was 4.95 ± 1.47 h in cynomolgus macaques, 9.17 ± 1.84 h in olive baboons, and 8.40 ± 2.53 h in rhesus macaques. These values are considerably lower than the half-lives previously published for dogs (133 h) and cats (166 h). The extended half-life of cefovecin in dogs and cats is speculated to be due to active reabsorption of drug in the kidney tubules because plasma clearance is well below the normal glomerular filtration rate. In nonhuman primates, renal clearance rates approximated plasma clearance rates, suggesting that active renal reabsorption of cefovecin does not occur in these species. The pharmacokinetic properties of cefovecin in nonhuman primates are vastly different from the pharmacokinetic properties in dogs and cats, precluding its use as a long-acting antibiotic in nonhuman primates. This study highlights the importance of performing pharmacokinetic studies prior to extralabel drug usage.
Abbreviation: AUC, area under the drug concentration–time curve.
Cefovecin sodium (Convenia, Pfizer Animal Health, New York, NY) is a recently developed third-generation cephalosporin antibiotic labeled for the treatment of skin infections in dogs and cats.11 Cephalosporins are bactericidal, β-lactam antibiotics that act by interfering with bacterial cell-wall synthesis, and are active against a wide range of organisms.12 In particular, third-generation cephalosporins have excellent broad-spectrum antimicrobial activity.12 Cefovecin is administered to both dogs and cats as a single, subcutaneous dose of 8 mg/kg.11 After injection, therapeutic drug concentrations are maintained in dogs for 7 d for Staphylococcus intermedius infections and for 14 d for Staphylococcus canis (group G) infections, whereas therapeutic concentrations are maintained in cats for approximately 7 d for Pasteurella multocida infections.11
A long-acting antibiotic such as cefovecin would be advantageous for treating nonhuman primates, among which animals may be housed in group cages or large outdoor corrals and access to individual animals for daily dosing is problematic. Injectable medications often are preferred over oral dosing in nonhuman primates because oral administration is dependent on appetite and individual taste preferences, making this route of administration less reliable.3 A long-acting injectable antibiotic would decrease the stress placed on animals otherwise requiring daily or even more frequent dosing. For these reasons, we investigated the possibility of using cefovecin against pathogenic bacteria in nonhuman primates. We hypothesized that the pharmacokinetics of cefovecin in nonhuman primates might be similar to those in dogs and cats, thereby supporting its use as a long-acting antibiotic in nonhuman primates. We therefore conducted a pharmacokinetics study of cefovecin in 3 species of nonhuman primates commonly used in research.
Materials and Methods
The pharmacokinetics of cefovecin were studied in cynomolgus macaques at Pfizer (Kalamazoo, MI) and in olive baboons and rhesus macaques at the University of Illinois at Chicago. The bioanalytic and pharmacokinetic phases of all studies were conducted at Pfizer. All studies were conducted under approved protocols to ensure appropriate animal care and use. Cynomolgus macaques and olive baboons received both intravenous and subcutaneous administration of cefovecin, whereas rhesus macaques received only subcutaneous cefovecin.
Cefovecin formulation.
The commercial formulation of cefovecin (Convenia, Pfizer) reconstituted lyophile, with each milliliter containing 80.0 mg cefovecin, 1.8 mg methylparaben, 0.2 mg propylparaben, 5.8 mg sodium citrate dehydrate, and 0.1 mg citric acid monohydrate (sodium hydroxide or hydrochloric acid as required to adjust pH), was used for both intravenous and subcutaneous administration.
Pharmacokinetics of cefovecin in cynomolgus macaques.
Cynomolgus macaques (Macaca fascicularis, Mauritius-origin) were maintained at Pfizer Global Research and Development (Groton, CT), an AAALAC-accredited animal care program, and in accordance with the Guide for the Care and Use of Laboratory Animals and Animal Welfare Act Regulations.1,7 All activities were approved by the facility's IACUC. Macaques (n= 12, 6 male and 6 female) were acclimated to the laboratory environment for at least 30 d prior to initiation of dosing. Macaques were older than 2.5 y, and their weights ranged from 5.3 to 7.9 kg for males and 5.1 to 6.7 kg for females. Subjects were housed in same-sex pairs in stainless steel cages and separated in the morning to accommodate study procedures. Animals were returned to same-sex pairs after study procedures had been performed. Environmental conditions were maintained at 21 ± 3 °C, 50% ± 10% humidity, an approximate 12:12-h light:dark cycle, and a minimum of 12 air exchanges hourly. A standard diet of pelleted food (Certified Primate Diet 5K91, PMI Feeds, St Louis, MO) supplemented with vegetables or fruit or both was provided twice daily. Water was provided ad libitum.
A parallel study was conducted with 12 cynomolgus macaques (3 per sex per treatment). Macaques were allocated to dosing groups according to body weights by using a computer-assisted randomization procedure, with consideration given to subjects housed in pairs such that 2 subjects in a single cage did not receive the same treatment. Six subjects (3 per sex) received cefovecin (8 mg/kg IV) on day 0; the remaining 6 subjects (3 per sex) received the same dose by the subcutaneous route on day 0. Blood samples were collected before treatment administration (day 0, 0 h) and at 0.25, 0.50, 1, 3, 6, 8, 24, 32, 48, 72, 96, 120, 168, 240, 288, and 336 h after dosing. Blood samples of 2 to 3 mL were collected from each animal at each time point into collection vials containing EDTA. Samples were processed by centrifugation and plasma aliquots frozen at −20 °C until analyzed. Animals were restrained manually during dosing and blood collection procedures.
Pharmacokinetics of cefovecin in olive baboons.
Olive baboons (Papio anubis) were maintained at the University of Illinois at Chicago, an AAALAC-accredited animal care program, and in accordance with the Guideand Animal Welfare Act regulations.1,7 All activities were approved by the facility's IACUC. Baboons used for these studies (n= 12, 6 male and 6 female) had been in the facility's conditioned colony for at least 26 mo prior to the initiation of dosing. Animals were older than 7 y, and their weights ranged from 24 to 33 kg for males and 13 to 19 kg for females. Baboons were housed singly but had visual and auditory contact with other baboons. Environmental conditions were maintained at 22 ± 2 °C, 30% to 70% humidity, a 12:12-h light:dark cycle, and 15 to 17 air exchanges hourly with 100% conditioned air. A standard diet (15% Monkey Diet 8714, Harlan Teklad, Madison, WI) was provided once daily, supplemented with vegetables or fruits or foraging mixture (dried fruits, nuts, seeds) once daily. Water was provided ad libitum.
A parallel study was conducted by using 12 olive baboons. Animals were allocated to dosing groups by using a computer-assisted randomization program to provide 3 per sex per treatment. The study design, treatment groups, sample collection times, and blood sample volumes for the olive baboons were the same as those described for cynomolgus macaques. All animal handling was performed on sedated (ketamine hydrochloride, 10 mg/kg IM; Phoenix Pharmaceuticals, St Joseph, MO) baboons.
Pharmacokinetics of cefovecin in rhesus macaques.
Rhesus macaques (Macaca mulatta, Chinese-origin; n= 6, 4 male and 2 female) were maintained at the University of Illinois at Chicago under the same housing and husbandry conditions as described for olive baboons. All activities were approved by the facility's IACUC. Rhesus macaques had been in the facility's conditioned colony for at least 16 mo prior to initiation of dosing. Animals were older than 7 y, and their weights ranged from 8 to 16 kg (male rhesus) and 5 to 6 kg (female rhesus).
The study design for rhesus macaques was similar to that described for the other 2 species, but due to limitations regarding animal availability, 4 male and 2 female rhesus macaques were included, and subjects received the 8-mg/kg dose subcutaneously only. Therefore, there was no statistical analysis between sex and route of administration for rhesus macaques. Sample collection times and blood sample volumes for rhesus macaques were the same as those described for cynomolgus macaques. All animal handling was performed on ketamine-sedated rhesus macaques.
Determination of cefovecin concentrations in plasma.
Cefovecin concentrations in plasma were determined by using ultra-performance liquid chromatography with tandem mass spectrometry detection. The free acid of cefpodoxime was used as the internal standard. To cover a broader range of concentrations, 2 standard curves (0.500 to 500 µg/mL and 0.001 to 1.00 µg/mL) were used for the analysis. For the bioanalytic phase, quality-control samples were prepared separately and analyzed with processed test samples at intervals based on the total number of samples. Results less than 0.001 µg/mL were reported as being below the limit of quantification. Separate calibration curves were prepared for each species. Sample preparation was simple protein precipitation with acetonitrile containing the internal standard (500 ng/mL), followed by dilution of the supernatant in diluent (0.3% formic acid in water). The supernatant was diluted 50-fold for samples in the high concentration range and 8-fold for samples in the low concentration range. The concentration of the internal standard was reduced 10-fold for samples in the low concentration range.
Gradient separation was achieved by using a ultra-performance liquid chromatography system (Acquity System, Waters, Milford, MA). A 10-μL volume of sample was injected onto a C18 column (Acquity BEH C18 HPLC column, 1.7 μm, 50 mm × 2.1 mm; Waters), and eluted by using a 1-min gradient of 5% to 95% acetonitrile in 0.3% formic acid at a flow rate of 0.5 mL/min. The aqueous phase consisted of 2 mM ammonium acetate in 0.3% formic acid. The initial mobile-phase conditions were held at 5% organic for 1 min prior to injection of the next sample. Detection was performed by using a triple-quadruple mass spectrometer (API 4000, Applied Biosystems, Foster City, CA) with positive electrospray ionization and multiple-reaction monitoring of the transitions 454 to 241 m/z for cefovecin and 428 to 241 m/z for cefpodoxime (internal standard). Peak area ratios were used for quantitation. The tandem mass spectrometry detection system was computer-controlled (Analyst Software, version 1.4.2, Applied Biosystems). Peak chromatograms were integrated (Analyst Software, version 1.4.2, Applied Biosystems), and raw data were imported (Watson LIMS version 7.2.0.03, Thermo Electron, Philadelphia, PA) for standard regression of peak area ratios (quadratic regression, weighting factor 1/χ2).
Pharmacokinetic and statistical analyses.
Pharmacokinetic parameters were calculated by using noncompartmental analysis (Watson LIMS version 7.2.0.03, ThermoElectron). All concentration data lower than 0.1 µg/mL were excluded from these analyses. Because the plasma concentration data indicated a prolonged elimination phase at very low concentrations, a more complicated 2-compartment model would be required to analyze data across the entire plasma concentration–time profile. However, for practical application, concentrations lower than 0.1 µg/mL are minimally relevant for treatment purposes, and these low concentrations contribute to less than 2% of the total area under the drug concentration–time curve (AUC). Therefore, we concluded the noncompartmental analysis was sufficient to describe the apparent pharmacokinetics. The elimination rate constant was estimated by least-squares regression analysis of the log-transformed concentration data. The linear trapezoidal AUC rule for pharmacokinetics analysis was used for calculation of AUC0-tlast from time 0 to the last time point (tlast) with a measurable concentration (Clast) of 0.1 µg/mL or greater; the area from tlast to infinity was estimated as Clast divided by the elimination rate constant and summed with AUC0-t(last) to estimate AUC0-∞. Mean residence time, plasma clearance, plasma terminal half-life, and volume of distribution at steady state were estimated by using standard equations in Watson LIMS.
For studies in cynomolgus macaques, statistical analysis was performed by using SAS software (version 9.1.3, SAS Institute, Cary, NC) with a linear mixed model and 2-way ANOVA to examine the difference between sexes. For data from olive baboons, analysis was performed in GraphPad Prism 5 for Windows (version 5.01, GraphPad Software, La Jolla, CA). To determine the effects of sex and time for analysis of concentration versus time data, the primary model was a repeated-measures 2-way ANOVA (mixed model); for all pharmacokinetic parameters, an unpaired t test (2-tailed) was used. With both analyses, plasma concentration data, AUC0-tlast, AUC0-∞, maximal drug concentration, and the volume of distribution at steady state were log-transformed prior to analysis; half-life was transformed by using a reciprocal transformation. Additional parameters including mean time to maximum plasma concentration, clearance values, elimination rate constants, and mean residence time were analyzed for each model without transformation. The 5% level of significance (α = 0.05) was used throughout for statistical hypothesis tests. There was no statistical analysis of data from rhesus macaques because these animals received cefovecin subcutaneously only, and the treatment group included 4 male and 2 female macaques. For studies in cynomolgus macaques and olive baboons, least-squares mean AUC0-∞ values were used to calculate bioavailability as follows:
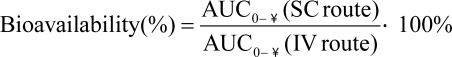 |
Mean values for plasma concentrations and standard deviations were calculated for each sampling time point after administration.
Plasma protein binding.
In vitro estimates of plasma protein binding were determined by equilibrium dialysis. Frozen cynomolgus macaque plasma with sodium EDTA as the anticoagulant (Bioreclamation, Liverpool, NY) was thawed and fortified with stock solutions of cefovecin in water to achieve concentrations of 0.100, 1.00, 10.0, and 100 μg/mL. The total amount of water added to each plasma sample was equal to 1% of the total volume. For in vivo estimates in cynomolgus macaques, selected time points were pooled at 1, 8, 24, and 48 h after dosage (concentrations greater than or equal to 0.1 µg/mL, subcutaneous dose group only). For in vivo estimates in olive baboons and rhesus macaques, additional time points were included at 72 and 96 h after treatment (concentrations greater than or equal to 0.1 µg/mL, subcutaneous dose group only). Plasma samples (in vitro: n = 4 per concentration; in vivo: n = 6 per time point) were dialyzed against PBS (pH 7.4) at 37 °C in a 96-well dialysis chamber, with each well divided by a Spectra/Por cellulose acetate dialysis membrane with a molecular weight cut-off of 12 to 14,000 Da (Spectrum Laboratories, Laguna Hills, CA). Prior to loading them into the 96-well apparatus, dialysis membranes were prepared according to the manufacturer's procedure. Samples were incubated at 37 °C for 6 h at a rotation speed of 100 rotations per minute. After dialysis, both plasma and buffer were recovered and placed in a 96-well plate for analysis to determine concentrations of cefovecin. Plasma and buffer samples were analyzed by liquid chromatography and tandem mass spectrometry similarly as described earlier. The fraction of free cefovecin was estimated by comparing the unbound drug concentration in buffer to the postdialysis plasma concentration and was reported as percentage bound.
Dosing simulation.
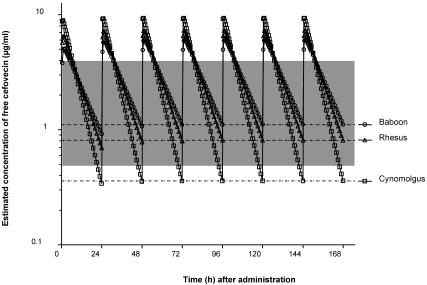
A simulation based on total plasma concentration data was performed to estimate free drug trough concentrations in nonhuman primates for a 7-d period by using a once-daily subcutaneous dose of cefovecin at 8 mg/kg (Figure 1). The simulation was run by using a 2-compartmental model (WinNonLin v5.2, model 12, Pharsight Corporation, Mountain View, CA, weighting factor = 1/Yhat2), which takes into account repeated daily dosing more accurately than does a noncompartmental model.
Figure 1.
Multiple-dose simulation of cefovecin (8 mg/kg SC) for 7 consecutive daily injections in cynomolgus macaques, olive baboons, and rhesus macaques. Dashed lines indicate mean estimated free trough concentrations (geometric means) for each species; shaded areas represent minimal inhibitory concentrations for several bacterial species [Proteus mirabilis (0.5 µg/mL), Streptococcus spp. (0.5 µg/mL), Klebsiella pneumoniae (1 µg/mL), Staphylococcus aureus (2 µg/mL), coagulase-negative Staphylococcus (2 µg/mL), and Corynebacterium spp. (4 µg/mL)].
Results
All animals remained in good health throughout the study. No injection site reactions, anorexia, diarrhea, or other adverse effects were noted in any animal. There were no noteworthy cefovecin-related changes in mean body weight relative to day 0.
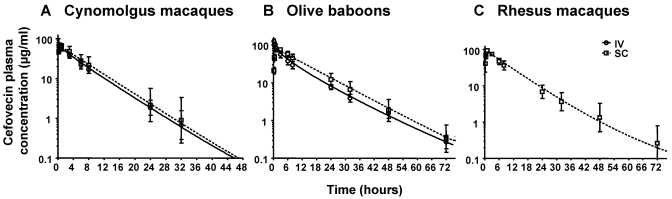
Results for assay standards and quality controls met or exceeded guidelines (15% accuracy and precision for standards and quality controls, 20% at the lower limit of quantification) outlined in the facility's standard operating procedures and industry standards.18 Mean total (bound and free) plasma concentration data are listed in Table 1. The concentration–time profiles in cynomolgus macaques, olive baboons, and rhesus macaques are presented in Figure 2.
Table 1.
Total (bound and free) plasma concentrations (μg/mL; mean ± 1 SD) of cefovecin in nonhuman primates
| Time (h) after | Cynomolgus macaques | Olive baboons | Rhesus macaques |
||
| administration | IV | SC | IV | SC | SC |
| 0.25 | 72.4 ± 23.3 | 48.4 ± 7.73 | 125 ± 17.1 | 21.3 ± 4.46 | 46.1 ± 25.7 |
| 0.5 | 68.6 ± 9.45 | 61.0 ± 11.1 | 103 ± 13.4 | 46.5 ± 7.86 | 69.4 ± 15.0 |
| 1 | 60.4 ± 8.59 | 61.4 ± 14.8 | 87.5 ± 16.8 | 72.1 ± 6.10 | 93.7 ± 6.61 |
| 3 | 37.9 ± 5.54 | 49.6 ± 16.5 | 56.9 ± 13.1 | 74.4 ± 8.61 | 74.8 ± 8.13 |
| 6 | 22.7 ± 5.67 | 29.4 ± 11.2 | 37.7 ± 8.16 | 57.3 ± 10.2 | 47.5 ± 9.83 |
| 8 | 17.1 ± 3.69 | 23.8 ± 12.2 | 29.3 ± 6.02 | 45.9 ± 11.4 | 37.3 ± 10.6 |
| 24 | 1.97 ± 0.809 | 3.49 ± 4.61 | 7.65 ± 1.16 | 12.9 ± 5.68 | 7.33 ± 2.92 |
| 32 | 0.845 ± 0.544 | 1.77 ± 2.47 | 3.97 ± 0.902 | 7.37 ± 4.20 | 4.17 ± 2.29 |
| 48 | 0.0774 ± 0.0660 | 0.292 ± 0.608 | 1.62 ± 0.472 | 2.19 ± 1.39 | 1.81 ± 1.40 |
| 72 | 0.0111 ± 0.00890 | 0.0325 ± 0.0669 | 0.291 ± 0.132 | 0.442 ± 0.362 | 0.411 ± 0.388 |
| 96 | 0.00288 ± 0.00176 | 0.00905 ± 0.0182 | 0.0528 ± 0.0270 | 0.149 ± 0.177 | 0.121 ± 0.112 |
| 120 | not done | not done | 0.0160 ± 0.0101 | 0.0328 ± 0.0373 | 0.0323 ± 0.0309 |
| 168 | not done | not done | 0.00369 ± 0.00175 | 0.00551 ± 0.00462 | 0.00720 ± 0.00647 |
All dose groups contained 6 animals (3 male and 3 female), except for rhesus macaques (4 male and 2 female).
All animals received a single dose of cefovecin (8 mg/kg SC or IV).
Figure 2.
Total (bound and free) plasma concentrations (µg/mL; mean ± 1 SD) of 8 mg/kg cefovecin after intravenous and subcutaneous administration to (A) cynomolgus macaques (n = 6 per dose group, 3 male and 3 female), (B) olive baboons (n = 6 per dose group, 3 male and 3 female), and (C) rhesus macaques (subcutaneous only; n = 6, 4 male and 2 female).
Pharmacokinetics.
Mean pharmacokinetics parameters for cynomolgus macaques, olive baboons, and rhesus macaques are presented in Table 2. After intravenous administration of cefovecin at a dose of 8 mg/kg to cynomolgus macaques, cefovecin had a half-life of 4.79 ± 0.730 h, mean clearance of 0.293 ± 0.046 mL/kg/min, mean residence time of 6.12 ± 0.887 h, and mean volume of distribution at steady state of 0.105 ± 0.018 L/kg. The relatively small distribution volume most likely reflects that cefovecin is relatively highly bound to plasma proteins. Statistical analysis showed no significant effects (P ≥ 0.05) of administration route or sex in any pharmacokinetic variables, with the exception of distribution volume, which was significantly (P = 0.0162) larger in males (0.146 L/kg; 95% confidence interval, 0.128 to 0.167 L/kg) as compared with females (0.122 L/kg; 95% confidence interval, 0.098 to 0.128 L/kg). Mean AUC0-∞ was 461 ± 75.3 μg·h/mL for the intravenous treatment group and 546 ± 298 μg·h/mL for those that received cefovecin subcutaneously. This difference resulted in a bioavailability estimate of 119%. The estimate of greater than 100% bioavailability is likely because subject 012 (female cynomolgus macaque; subcutaneous dose) had much greater plasma concentrations of cefovecin than did other subjects (intravenous or subcutaneous); plasma concentrations were measurable through 336 h after dosage in subject 012 (data not shown). Despite the variability in the subcutaneous dose group, the bioavailability of cefovecin after subcutaneous dosing is quite high in cynomolgus macaques. The mean maximal cefovecin concentration for the subcutaneous group was 62.8 ± 13.8 µg/mL, time to maximal plasma concentration was 0.750 ± 0.274 h, and the half-life was 4.95 ± 1.47 h.
Table 2.
Pharmacokinetic parameters [mean ± 1 SD (95% confidence interval)] of cefovecin in plasma of nonhuman primates
| Cynomolgus macaques | Olive baboons | Rhesus macaques |
|||
| IV | SC | IV | SC | SC | |
| AUC0-t(last)a (μg· h/mL) | 457 ± 75.6 (338 to 617) | 542 ± 296 (401 to 732) | 860 ± 141 (731 to 1010) | 1130 ± 289 (884 to 1440) | 961 ± 205 (767 to 1200) |
| AUC0-∞a (μg· h/mL) | 461 ± 75.3 (342 to 623) | 546 ± 298 (405 to 738) | 863 ± 139 (735 to 1010) | 1130 ± 288 (888 to 1440) | 965 ± 204 (771 to 1210) |
| Elimination rate constantb (h−1) | 0.145 ± 0.024 (0.115 to 0.174) | 0.140 ± 0.036 (0.111 to 0.170) | 0.080 ± 0.007 (0.073 to 0.087) | 0.076 ± 0.014 (0.061 to 0.091) | 0.083 ± 0.024 (0.057 to 0.108) |
| Half-lifeb, (h) | 4.79 ± 0.730 (3.98 to 6.02) | 4.95 ± 1.47 (4.09 to 6.26) | 8.70 ± 0.78 (7.93 to 9.56) | 9.17 ± 1.84 (7.49 to 11.4) | 8.40 ± 2.53 (6.37 to 11.7) |
| Clearancec (mL/kg/min) | 0.293 ± 0.046 (0.236 to 0.349) | not done | 0.156 ± 0.023 (0.132 to 0.182) | not done | not done |
| Mean residence timec (h) | 6.12 ± 0.887 (5.34 to 6.90) | not done | 10.5 ± 1.3 (9.2 to 11.9) | not done | not done |
| Volume of distribution at steady statea (L/kg) | 0.105 ± 0.018d (0.095 to 0.117) | not done | 0.097 ± 0.024 (0.076 to 0.123) | not done | not done |
| Maximal plasma concentrationa (μg/mL) | not done | 62.8 ± 13.8 (60.0 to 77.5) | not done | 75.9 ± 8.4 (68.0 to 84.7) | 93.5 ± 6.61 (86.8 to 101) |
| Time at maximal plasma concentrationc (h) | not done | 0.750 ± 0.274 (0.423 to 1.08) | not done | 2.83 ± 1.83 (0.91 to 4.76) | 0.875 ± 0.306 (0.554 to 1.20) |
| Bioavailability (%) | not done | 119 | not done | 131 | not done |
All dose groups contained 6 animals (3 male and 3 female), except for rhesus macaques (4 male and 2 female).
All animals received a single dose of cefovecin (8 mg/kg SC or IV).
Back-transformed (log-transformed) least-squares mean.
Least squares mean (no transformation).
Back-transformed (reciprocal transformation) least-squares mean.
Significant (P = 0.0261) difference between male and female animals.
After intravenous administration of cefovecin at a dose of 8 mg/kg to olive baboons, cefovecin had a half-life of 8.70 ± 0.78 h, clearance rate of 0.156 ± 0.023 mL/kg/min, mean residence time of 10.5 ± 1.3 h, and mean volume of distribution at steady state of 0.097 ± 0.024 L/kg. Statistical analysis showed no significant effects (P ≥ 0.05) of administration route or sex in any pharmacokinetic variable. The mean AUC0-∞ was 863 ± 139 µg·h/mL L for the intravenous treatment group and 1130 ± 288 µg·h/m for olive baboons treated subcutaneously. This difference resulted in a bioavailability estimate of 131%. The estimate of greater than 100% bioavailability likely is due to the increased exposure in male baboons, with AUC 30% higher than that in female baboons in the subcutaneous treatment group (data not shown), although this effect was not significant (P = 0.2215). As with the cynomolgus macaques, the volume of distribution in olive baboons (0.097 L/kg) was small, most likely because cefovecin is relatively highly bound to plasma proteins. The mean maximal drug concentration for the subcutaneous group was 75.9 ± 8.4 μg/mL and the mean time at which maximal drug concentration occurred was 2.83 ± 1.83 h after dosage. After subcutaneous administration at a dose of 8 mg/kg to olive baboons, cefovecin had a mean half-life of 9.17 ± 1.84 h.
After subcutaneous administration at a dose of 8 mg/kg to rhesus macaques, cefovecin had a mean half-life of 8.40 ± 2.53 h. The mean maximal drug concentration after subcutaneous dosage was 93.5 ± 6.61 µg/mL, which occurred at the mean time of 0.875 ± 0.306 h.
Plasma protein binding.
In vitro estimates of plasma protein binding in cynomolgus macaques ranged from 82.5% to 88.5% bound across the concentration range (that is, a free fraction of 0.115 to 0.176). Mean recovery ranged from 87.1% to 114% across the concentration range. Total solublized protein in the control plasma was determined to be 62.5 mg/mL (data not shown). The extent of in vivo plasma protein binding in cynomolgus macaques ranged from 78.9% to 87.6% across all time points. Mean recovery ranged from 93.5% to 122% across all samples. Recovery was highest (122%) when the fraction bound was lowest (0.789; that is, a free fraction of 0.211), and variability was higher for equilibrium dialysis results of in vivo samples. To obtain a true estimate of the fraction of bound cefovecin, we compared the in vitro and in vivo results for concentrations greater than or equal to 0.1 µg/mL, thus focusing on relevant concentrations. The mean fraction bound was not significantly different for in vitro and in vivo samples greater than or equal to 0.1 µg/mL (unpaired t test, P = 0.6738). Combining in vitro and in vivo estimates resulted in a mean fraction bound of 85.3% ± 4.13% (95% confidence interval of 84.0% to 86.6%, or a free fraction of 13.4% to 16.0%).
Plasma protein binding of cefovecin in olive baboons and rhesus macaques was evaluated by using in vivo samples only at 1, 4, 24, 48, 72, and 96 h after dosage (concentrations greater than or equal to 0.1 µg/mL). The extent of in vivo plasma protein binding ranged from 89.2% to 96.2% in baboons and from 88.5% to 94.3% in rhesus macaques. The mean fraction bound in olive baboons was 93.1% ± 2.65% (95% confidence interval, 92.0% to 94.2%) and in rhesus macaques was 92.0% ± 2.42% (95% confidence interval, 91.0% to 93.0%). Mean recovery ranged from 76.2% to 96.4% across all samples (data not shown).
Dosing simulation.
As determined by the once-daily dosing simulation, in the cynomolgus macaque, olive baboon, and rhesus macaque, the 24-h plasma free drug trough concentrations on day 7 had estimated means of 0.359 ± 0.770 µg/mL (95% confidence interval, 0.127 to 1.02), 1.11 ± 0.612 µg/mL (95% confidence interval, 0.683 to 1.80 µg/mL), and 0.809 ± 0.458 µg/mL (95% confidence interval, 0.477 to 1.37 µg/mL), respectively (Figure 1).
Discussion
Pharmacokinetics is a division of pharmacology that uses mathematical models to describe what happens to a chemical substance within the body.17 Pharmacokinetics models focus on how the concentration of drug in the body changes with time, which depends on the relative rates of drug absorption, distribution, metabolism, and excretion processes taking place.17 Pharmacodynamics describes the relationship between drug concentrations in the body (plasma) and the resulting pharmacologic effect.5,17 For β-lactam antibiotics, the length of time that the free drug plasma concentration exceeds the minimum inhibitory concentration is the only consistent pharmacokinetics–pharmacodynamics parameter that correlates with therapeutic efficacy,4,8,9,19 because bactericidal activity is concentration-independent (that is, there is no linear relationship between concentration of the antibiotic and bactericidal rate).5 In addition, β-lactam antibiotics have no postantibiotic effect, meaning there is no residual bactericidal activity once the drug concentration falls below the minimal inhibitory concentration.5
Our results demonstrate that the pharmacokinetics of cefovecin in nonhuman primates is vastly different from the pharmacokinetics in dogs and cats. The estimated effective half-lives (based on a last-measurable concentration of at least 0.1 µg/mL) in cynomolgus macaques (4.95 ± 1.47 h), olive baboons (9.17 ± 1.84 h), and rhesus macaques (8.40 ± 2.53 h) are much shorter than the those reported for dogs (133 h or 5.5 d)15 and cats (166 h or 6.9 d).16 In addition, total drug exposure (estimated by the AUC) is substantially lower in nonhuman primates than are values reported for dogs and cats. Mean AUC values after subcutaneous dosing for cynomolgus macaques, olive baboons, and rhesus macaques were 546 ± 298, 1130 ± 288, and 965 ± 204 µg·h/mL, respectively, whereas mean AUC after subcutaneous dosing for dogs and cats were 10,400 ± 296 and 22,700 ± 3450 µg·h/mL, respectively.15,16 Our findings correlate well with those reported previously for cynomolgus macaques (Mauritius-origin) and rhesus macaques (Indian-origin), in which the half-life of cefovecin was 6.3 ± 1.8 and 8.0 ± 0.6 h, respectively, and mean AUC was 468 ± 97 and 520 ± 70 µg·h/mL, respectively.10
Elimination of cefovecin occurs primarily through urinary excretion in the dog and cat,15,16 and we assume that this route of elimination is the same for nonhuman primates. Our assumption is supported by previous findings showing that significant liver metabolism of cefovecin does not occur in nonhuman primates and that most of a cefovecin dose is recovered in the urine unchanged after intravenous dosing.10
Because protein-bound drug is not filtered by the glomerulus, filtration by the kidneys is restricted to the free fraction of drug in plasma.17 Plasma protein binding of cefovecin in nonhuman primates, although relatively high, is considerably less than values reported in dogs (96.0% to 98.7%) and cats (99.5% to 99.8%) across the concentration range of 10 to 100 µg/mL.15,16 This difference in protein binding could explain, at least in part, the shorter terminal half-life in nonhuman primates, in that more of the drug is unbound and available for renal clearance.
Protein binding disparity alone cannot explain the considerably large differences reported for total drug exposure and half-lives between nonhuman primates and dogs and cats. Previous studies done in dogs and cats show that plasma clearance of cefovecin is well below the glomerular filtration rates in these species.15,16 This decreased rate of clearance suggests that active reabsorption of cefovecin occurs in the kidney tubules which is a major factor accounting for the long half-life of cefovecin in dogs and cats.14,15 To determine whether active reabsorption of cefovecin also occurs in nonhuman primates, renal clearance was compared with plasma clearance. Given the fraction of free drug and glomerular filtration rate in nonhuman primates (2.2 mL/min/kg),2,13 we estimate that renal clearance (the glomerular filtration rate multiplied by the fraction of free drug) would be 0.323 mL/min/kg for cynomolgus macaques and 0.152 mL/min/kg for olive baboons. In the noncompartmental model, mean clearance was estimated as 0.293 mL/min/kg for cynomolgus macaques and 0.156 mL/min/kg for olive baboons; therefore, renal clearance is equivalent to plasma clearance in these species, a finding that is consistent with a drug eliminated primarily by glomerular filtration with no significant reabsorption of drug. Given the similar glomerular filtration rates and total drug exposures (after a subcutaneous dose) in rhesus macaques as for the other 2 species we studied, we assume that plasma clearance, mean residence time, and distribution volume in rhesus macaques also depend highly on protein binding and glomerular filtration rate and approximate values obtained for cynomolgus macaques and olive baboons. The active renal reabsorption of cefovecin that appears to occur in dogs and cats but not in nonhuman primates most likely accounts for the vastly different pharmacokinetics values seen between these species.
The plasma free drug concentration of cefovecin can be correlated to its potential efficacy by quantifying the time the plasma free drug concentration is above the minimum inhibitory concentration for a given bacterium. According to the simulation of once-daily dosing, the plasma free drug concentration of cefovecin in rhesus macaques and olive baboons would remain above the minimal inhibitory concentration for several potential pathogens, including Pasteurella multocida (minimum inhibitory concentration is 0.06 µg/mL or less), Proteus mirabilis (0.5 µg/mL), and Streptococcus spp. (0.5 µg/mL).14 In cynomolgus macaques, once-daily dosing would keep the plasma free-drug concentration of cefovecin above the minimal inhibitory concentration for P. multocidaonly. Based on this simulation, cefovecin likely would have to be administered to nonhuman primates several times daily for the plasma free drug concentration to remain above the minimal inhibitory concentration for other bacteria including Klebsiella pneumoniae (minimal inhibitory concentration of 1 µg/mL), Staphylococcus aureus (2 µg/mL), coagulase-negative Staphylococcus (2 µg/mL), and Corynebacterium spp. (4 µg/mL).14 This hypothesis assumes that the target minimum inhibitory concentration of these pathogens in nonhuman primates is similar to that determined from canine isolates, because specific data for cefovecin in nonhuman primate isolates have not yet been determined.
With any administered medication, plasma concentrations must be maintained within a therapeutic window in which the drug is both efficacious and safe. In the current study, a single 8-mg/kg dose was well-tolerated by all animals. In general, third-generation cephalosporins are considered remarkably safe.6 However, until safety data for cefovecin in nonhuman primates are available for daily or multiple-daily dosing, these regimens cannot be recommended until further testing is conducted.
Veterinarians in a research environment often work with nondomestic species, including nonhuman primates. Most drugs available for treatment of these nondomestic animals have not been studied in these species, and off-label drug usage is common. The current study shows that the pharmacokinetic properties of cefovecin in nonhuman primates differ markedly from those in dogs and cats, precluding its use as a long-acting antibiotic in nonhuman primates. In addition, the current study highlights that dosing regimens may differ vastly between species, and one should not assume that a drug will act the same way in all species. The only way to be certain how long adequate plasma drug levels are maintained in a certain species is to analyze the pharmacokinetics of the drug in that species.
References
- 1.Animal Welfare Act Regulations. 2008. 9 CFR § 3.75–3.85.
- 2.Birrell AM., Heffernan SJ., Kirwan P., McLennan S., Gillin AG., Yue DK. 2002. The effects of aminoguanidine on renal changes in a baboon model of Type 1 diabetes. J Diabetes Complications 16:301–309 [DOI] [PubMed] [Google Scholar]
- 3.Butler TM., Brown BG., Dysko RC., Ford EW., Hoskins DE., Klein HJ., Levin JL., Murray KA., Rosenberg DP., Southers JL., Swenson RB. 1995. Medical management, p 255–334 In: Bennett BT., Abee CR., Henrickson R. Nonhuman primates in biomedical research: biology and management. San Diego (CA): Academic Press [Google Scholar]
- 4.Craig WA. 1998. Pharmacokinetic–pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12 [DOI] [PubMed] [Google Scholar]
- 5.Ebert SC. 2004. Application of pharmacokinetics and pharmcodynamics to antibiotic selection. P T 29:244–250 [Google Scholar]
- 6.Fekety RF. 1990. Safety of parenteral third-generation cephalosporins. Am J Med 88:38S–44S [DOI] [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 8.Leggett JE., Fantin B., Ebert S., Totsuka K., Vogelman B., Calame W., Mattie H., Craig WA. 1989. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis 159:281–292 [DOI] [PubMed] [Google Scholar]
- 9.McKellar QA., Sanchez Bruni SF., Jones DG. 2004. Pharmacokinetic–pharmacodynamic relationship of antimicrobial drugs used in veterinary medicine. J Vet Pharmacol Ther 27:503–514 [DOI] [PubMed] [Google Scholar]
- 10.Papp R., Popovic A., Kelly N., Tschirret-Guth R. 2010. Pharmacokinetics of cefovecin in squirrel monkey (Saimiri sciureus), rhesus macaques (Macaca mulatta), and cynomolgus macaques (Macaca fascicularis). J Am Assoc Lab Anim Sci 49:805–808 [PMC free article] [PubMed] [Google Scholar]
- 11.Pfizer Animal Health. 2008. Convenia package insert. New York (NY)
- 12.Plumb DC. 2002. Veterinary drug handbook, 4th edition Ames (IA): Blackwell Publishing [Google Scholar]
- 13.Schaer GL., Fink MP., Chernow B., Ahmed S., Parrillo JE. 1990. Renal hemodynamics and prostaglandin E2 excretion in a nonhuman primate model of septic shock. Crit Care Med 18:52–59 [DOI] [PubMed] [Google Scholar]
- 14.Stegemann MR., Passmore CA., Sherington J., Lindeman CJ., Papp G., Weigel DJ., Skogerboe TL. 2006. Antimicrobial activity and spectrum of cefovecin, a new extended-spectrum cephalosporin, against pathogens collected from dogs and cats in Europe and North America. Antimicrob Agents Chemother 50:2286–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegemann MR., Sherington J., Blanchflower S. 2006. Pharmacokinetics and pharmacodynamics of cefovecin in dogs. J Vet Pharmacol Ther 29:501–511 [DOI] [PubMed] [Google Scholar]
- 16.Stegemann MR., Sherington J., Coati N., Brown SA., Blanchflower S. 2006. Pharmacokinetics of cefovecin in cats. J Vet Pharmacol Ther 29:513–524 [DOI] [PubMed] [Google Scholar]
- 17.Taft DR. 2009. Drug excretion, p 175–199 In: Hacker M., Bachmann K., Messer W. Pharmacology in principles and practice. San Deigo (CA): Academic Press [Google Scholar]
- 18.United States Department of Health and Human Services, Food and Drug Administration 2001. Guidance for industry: bioanalytical method validation. Washington (DC): United States Department of Health and Human Services [Google Scholar]
- 19.Vogelman B., Gudmundsson S., Leggett J., Turnidge J., Ebert S., Craig WA. 1988. Correlation of antimicrobial pharmacokinetics parameters with therapeutic efficacy in an animal model. J Infect Dis 158:831–847 [DOI] [PubMed] [Google Scholar]