Abstract
CD44 is a cell surface receptor for the extracellular matrix glycosaminoglycan hyaluronan and is involved in processes ranging from leukocyte recruitment to wound healing. In the immune system, the binding of hyaluronan to CD44 is tightly regulated, and exposure of human peripheral blood monocytes to inflammatory stimuli increases CD44 expression and induces hyaluronan binding. Here we sought to understand how mouse macrophages regulate hyaluronan binding upon inflammatory and anti-inflammatory stimuli. Mouse bone marrow-derived macrophages stimulated with tumor necrosis factor α or lipopolysaccharide and interferon-γ (LPS/IFNγ) induced hyaluronan binding by up-regulating CD44 and down-regulating chondroitin sulfation on CD44. Hyaluronan binding was induced to a lesser extent in interleukin-4 (IL-4)-activated macrophages despite increased CD44 expression, and this was attributable to increased chondroitin sulfation on CD44, as treatment with β-d-xyloside to prevent chondroitin sulfate addition significantly enhanced hyaluronan binding. These changes in the chondroitin sulfation of CD44 were associated with changes in mRNA expression of two chondroitin sulfotransferases, CHST3 and CHST7, which were decreased in LPS/IFNγ-stimulated macrophages and increased in IL-4-stimulated macrophages. Thus, inflammatory and anti-inflammatory stimuli differentially regulate the chondroitin sulfation of CD44, which is a dynamic physiological regulator of hyaluronan binding by CD44 in mouse macrophages.
Keywords: Cell Adhesion, Cell Surface Protein, Chondroitin Sulfate, Hyaluronate, Macrophage, CD44, Macrophage Activation
Introduction
Macrophages differentiate from peripheral blood monocytes upon recruitment to the tissues. Under homeostatic conditions this process occurs constitutively to maintain a population of resident macrophages that provide a first line of defense against invading microorganisms (for review, see Refs. 1 and 2). This is of particular importance in some tissues, such as the lungs, where phagocytosis of bacteria by resident alveolar macrophages can be sufficient to clear infection (3). During inflammatory conditions, recruitment of a different subpopulation of monocytes occurs, and the number of macrophages in the tissues is greatly increased. Macrophages initially undergo “classical activation” in response to IFNγ and toll-like receptor (TLR)6 ligands such as LPS and display increased anti-microbial abilities due to increased production of reactive nitrogen and oxygen species (for review, see Refs. 4 and 5). The production of proinflammatory and T helper cell 1 (TH1)-polarizing cytokines by these LPS- and IFNγ-polarized (M1) macrophages affects both the local and systemic immune response. Recruited macrophages are also important for resolving inflammation, as they phagocytose inflammation-inducing agents such as bacteria, cellular debris, and degraded extracellular matrix components (6, 7). Furthermore, increased levels of anti-inflammatory or T helper cell 2 (TH2)-polarizing cytokines such as IL-4 result in the “alternative activation” of macrophages. These alternatively activated (M2) macrophages produce anti-inflammatory cytokines such as IL-10 and TGF-β in addition to cytokines and chemokines that promote tissue growth, remodeling, and angiogenesis (for review, see Refs. 8 and 9).
One of the components of the extracellular matrix degraded during an inflammatory response is the glycosaminoglycan hyaluronan. Hyaluronan accumulation at an injury site is important in recruiting macrophages to skin wounds (10). However, the accumulation of fragmented hyaluronan can promote inflammation by activating macrophages and dendritic cells through either TLR2 or 4 (for review, see Ref. 11). Hyaluronan internalization by macrophages requires the cell surface receptor CD44 (12), and hyaluronan uptake by macrophages is important for the local turnover of hyaluronan (13). Consistent with this, hyaluronan accumulated in the inflamed lungs of CD44 null (CD44−/−) mice treated with bleomycin, and this was reduced in mice reconstituted with CD44+/+ bone marrow (14). Together, these studies suggest an important role for macrophage CD44 in the removal of fragmented hyaluronan from inflammatory sites. Although TLR2 and -4 are thought to be the receptors that activate macrophages and dendritic cells in response to fragmented hyaluronan, CD44 can modulate TLR4 responses by negatively regulating TLR signaling (15, 16). Therefore, through the removal of proinflammatory fragmented hyaluronan and down-regulation of TLR signaling, CD44 may dampen the inflammatory response and assist in the resolution of inflammation, as has been observed in the bleomycin model of lung inflammation (14). However, CD44 may also promote the inflammatory response. Reduced macrophage recruitment into inflamed lungs has been observed in CD44−/− mice after Mycobacterium tuberculosis infection (17) or LPS inhalation (18), and CD44−/− macrophages have reduced ability to migrate to atherosclerotic lesions in a mouse model of atherosclerosis (19).
Although CD44 is the primary cell surface receptor for hyaluronan on immune cells, the majority of immune cells do not bind hyaluronan constitutively (for review, see Refs. 20). T lymphocytes are induced to bind hyaluronan after activation with antigen (21, 22), whereas proinflammatory cytokines, such as TNFα, induce hyaluronan binding in human peripheral blood monocytes (23, 24) and endothelial cells (25). M2-inducing cytokines such as IL-4, meanwhile, can inhibit hyaluronan binding in human peripheral blood monocytes (23). Hyaluronan binding is usually associated with increased expression of CD44 but is also affected by various post-translational modifications to CD44 such as glycosylation (26–28), glycosaminoglycan addition (29, 30), sialylation (31), and sulfation (24, 32). Disruption of the actin cytoskeleton, which prevents CD44 clustering, can also affect hyaluronan binding (33). Although artificially altering CD44 post-translational modifications can affect hyaluronan binding, the challenge is to determine which changes occur in response to physiological stimuli. In human monocytic cells, TNFα-induced hyaluronan binding correlated with the increased sulfation of CD44 (24, 32), and further examination revealed that TNFα increased the expression of two carbohydrate sulfotransferases, CHST2 and CHST7 (34), which led to sulfation of CD44 on both O- and N-linked carbohydrates (35) and the expression of a sialylated 6-sulfo-N-acetyl lactosamine epitope on the N-linked carbohydrate of CD44 (34). At the same time the carbohydrate sulfation of CD44 was increased, the percent of sulfation due to chondroitin sulfate on CD44 was decreased (35). Further work identified serine 180 as the site of chondroitin sulfate addition on the standard isoform of human CD44 and showed that chondroitin sulfate addition at this site negatively regulated hyaluronan binding on a CD44-immunoglobulin fusion protein (29). The removal of sialic acid residues also promotes hyaluronan binding, and this is one of the physiological mechanisms that regulates hyaluronan binding in human peripheral blood monocytes after LPS stimulation through the induction of sialidase activity (31). Much less is known about hyaluronan binding in mouse macrophages, although alveolar macrophages are able to bind and degrade hyaluronan without additional stimulation (12, 36). Here we examined the physiological mechanisms responsible for the induction of hyaluronan binding by mouse bone marrow-derived macrophages and peritoneal macrophages exposed to M1 or M2 promoting agents. Both M1- and M2-inducing agents up-regulated CD44 expression and induced hyaluronan binding, albeit to differing extents. The inflammatory mediators TNFα and LPS/IFNγ reduced the chondroitin sulfation of CD44, and the loss of chondroitin sulfate directly contributed to increased hyaluronan binding by CD44. In contrast, the M2-inducing agent IL-4 increased CD44 expression but only induced low levels of hyaluronan binding, which was attributable to the increased chondroitin sulfation of CD44. Thus, both M1 and M2-macrophages regulate hyaluronan binding by differentially regulating the chondroitin sulfation of CD44.
EXPERIMENTAL PROCEDURES
Mice and Primary Cells
Murine bone marrow-derived macrophages were generated by isolating bone marrow from the tibias and femurs of C57BL/6 and CD44−/− mice (37), lysing red blood cells with 0.84% ammonium chloride and then plating 1–2 × 107 cells in a 10-cm2 Petri dish with DMEM (Invitrogen) supplemented with 20% FCS, 1 mm sodium pyruvate (Invitrogen), 2 mm l-glutamine (Sigma), 50 units/ml penicillin/streptomycin (Invitrogen), and 5% L929 cell-conditioned media (LCCM) as a source of macrophage colony-stimulating factor (macrophage differentiation medium). After 4 days cells were re-plated at a 1:4 dilution and stimulated with 20 ng/ml mouse recombinant TNFα (R&D Systems, Minneapolis, MN), 10 ng/ml mouse recombinant IL-4 or 10–50 ng/ml mouse recombinant IFNγ (eBioscience, San Diego, CA) and 100 ng/ml ultrapure LPS (InvivoGen, San Diego, CA) for 2–3 days. In some experiments cells were also grown in the presence of 2 mm p-nitrophenyl β-d-xylopyranoside (β-d-xyloside, Sigma). Resident peritoneal macrophages were isolated by peritoneal lavage on euthanized mice using Ca2+-and Mg2+-free Hanks' balanced salt solution (Invitrogen). Recovered cells were re-suspended in DMEM with 20% FCS and allowed to adhere to the Petri dish for 3 h. Non-adherent cells were removed by washing, and adherent cells were stimulated as above for 48 h but in the absence of L929 cell-conditioned medium. Animal experimentation was conducted in accordance with protocols approved by the University Animal Care Committee and Canadian Council of Animal Care guidelines.
Cell Lines
The human myeloid cell line KG1a from the American Type Culture Collection (ATCC #CRL246.1, Manassas, VA) was cultured in RPMI 1640 (Invitrogen) supplemented with 10% FCS, 1 mm sodium pyruvate, 2 mm l-glutamine, and 50 units/ml penicillin/streptomycin. High and low hyaluronan binding KG1a cells were previously established by cell sorting (33).
Antibodies and Reagents
Purified rat anti-human/mouse CD44 mAb, IM7.8.1 (ATCC #TIB-235), was conjugated to Alexa 488, Alexa 647, or Pacific blue (Molecular Probes, Eugene, OR) or coupled to CNBr-activated Sepharose 4B (Amersham Biosciences) according to the manufacturer's instructions. The rat anti-mouse CD44 mAb KM201 (ATCC #TIB-240) was from P. Kincade, and the mouse anti-human CD44 mAb 3G12 was from G. Dougherty (38). Rabbit antiserum against the cytoplasmic domain of mouse CD44 (J1WBB) was previously described (39). The anti-chondroitin sulfate stub mAbs 2B6 and 3B3 were from Seikagaku America (East Falmouth, MA). F4/80 conjugated to phycoerythrin-cyanine 7 was from eBioscience. FITC-conjugated goat anti-mouse Ab was from Caltag (Burlingame, CA), and HRP-conjugated goat anti-mouse and HRP-conjugated goat anti-rabbit Abs were from Jackson ImmunoResearch (West Grove, PA). Fluorescein-conjugated hyaluronan (fluorescent-hyaluronan) was made as described (40) using rooster comb hyaluronan from Sigma. Prestained molecular mass standards were purchased from New England Biolabs (Beverly, MA).
Generation of Point Mutations
R43A and S183A mutations in mouse CD44.1H cDNA (41) were created by oligonucleotide site-directed mutagenesis. Six primers were used: primer 1 containing the XhoI site in the pBS vector, 5′-CCCCCCCTCGAGGTCGAC-3′; primer 2 containing the BamHI site in CD44 (5′-TCCAGCTAATTCGGATCCATGAGTCACAGT-3′), complementary primers 3 (5′-CCGGGAGATACTGTAGGCGCCATTTTT-3′) and 4 (5′-AAAAATGGCGCCTACAGTATCTCCCGG-3′) containing the R43A mutation; complementary primers 5 (5′-ATGGTGGAGCCGGCGCTGACATCGTC-3′) and 6 (5′-GACGATGTCAGCGCCGGCTCCACCAT-3′) containing the S183A mutation. The mutated sequences are underlined in each case. Fragments were generated by PCR using primer 1 with primers 3 or 5 and using primer 2 with primers 4 or 6. Each fragment pair was then mixed, and PCR was performed again. The final product was inserted into the CD44 sequence using XhoI and BamHI.
Retroviral Infection
Mouse CD44.1H cDNA was inserted into MIY, a murine stem cell virus-based vector with the internal ribosomal entry site sequence and the gene for yellow fluorescent protein (YFP) (42) from R. K. Humphries. Amphotropic Phoenix packaging cells were transfected with the CD44-MIY vectors using Lipofectamine 2000 (Invitrogen), and the resulting supernatants were used to infect the ecotropic packaging cell line GP+E86, and virus-producing cells were subsequently sorted for high expression of YFP, as previously described (43, 44). For infection of CD44−/− bone marrow, a 10-cm2 tissue culture dish of GP+E86 cells were treated with 25 μg/ml mitomycin C (Calbiochem) for 30 min, washed twice, and then incubated overnight in DMEM supplemented with 10% FCS. The next day, freshly isolated bone marrow cells were added to the GP+E86 monolayer in the presence 5 μg/ml protamine sulfate (Sigma). After 2 days, non-adherent bone marrow cells were removed, resuspended in macrophage differentiation media, and then plated on Petri dishes. On day 4, cells were re-plated and treated as described earlier. Typically, 40–60% of the bone marrow-derived macrophages were positive for YFP and CD44.
Flow Cytometry
Cells (2 × 105) were incubated with 2.4G2 (ATCC #HB-197) tissue culture supernatant for 20 min on ice to block Fc receptors. After removal of supernatant, cells were incubated for 20 min with ∼5 μg/ml IM7-Alexa 488 or fluorescent-hyaluronan in PBS containing 2% FCS and 5 mm EDTA. Alternatively, cells were incubated with both IM7-Alexa 647 or IM7-Pacific blue and fluorescent-hyaluronan. After washing, cells were resuspended in buffer containing 1 μg/ml propidium iodide (PI; Sigma), and a minimum of 5000 live events were collected on a FACScan, FACSVantage, or LSRII and analyzed using CellQuest (BD Biosciences) or FlowJo (Tree Star, Ashland, OR) software. Detection of sulfated epitopes on the cell surface was performed as described (34). Peritoneal macrophages were also stained with F4/80-conjugated phycoerythrin-cyanine 7.
Trypsin Treatment
Bone marrow-derived macrophages (1 × 105) were polarized in the presence of LPS/IFNγ or IL-4 for 24 h and then treated with 0.25% w/v trypsin (Invitrogen) for 5 min on ice, which was confirmed by flow cytometry to remove all surface CD44. The cells were washed once with media, resuspended in their original polarizing media, and further cultured for 3, 18, or 48 h before fluorescent-hyaluronan binding and CD44 expression were analyzed by flow cytometry.
Analysis of Chondroitin Sulfate on CD44
Immunoprecipitation, sulfate labeling, and Western blotting of CD44 was performed as described (29). Briefly, cells were cultured for the last 2 days in Na2[35]SO4 during a 3-day incubation with TNFα and/or β-d-xyloside. Cells were lysed, incubated with IM7-coupled beads, and then immunoprecipitated CD44 was resolved on a 7.5% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Millipore, Mississauga, ON). Membranes were exposed to Kodak BioMax MR film (Interscience, Markham, ON) at −80 °C for 7–10 days. To determine relative CD44 levels, membranes were blotted with antiserum against CD44 (J1WBB) and HRP-goat anti-rabbit Ab. To detect chondroitin sulfate, immunoprecipitated CD44 from ∼2.5 × 107 cells was digested with chondroitinase ABC (Seikagaku America) and blotted with the mAb 2B6 or 3B3, which recognize the 4- or 6-sulfated chondroitin sulfate stub, respectively. After incubation with HRP-goat anti-mouse Ab, membranes were developed with enhanced chemiluminescence (ECL, Amersham Biosciences) according to the manufacturer's instructions.
Semiquantitative PCR
The primers and annealing temperatures for the mouse carbohydrate transferases, chondroitin sulfate N-acetylgalactosamine transferases 1 and 2 (CSGalNAcT1 and CSGalNAcT2) and the mouse carbohydrate sulfotransferases (CHST) N-acetylglucosamine 6-sulfotransferase (CHST2), N-acetylgalactosamine 6-sulfotransferase (CHST3, also known as C6ST1), N-acetylglucosamine/galactosamine 6-sulfotransferase (CHST7, also known as C6ST2), keratan sulfate galactose 6-sulfotransferase (CHST1, also known as KSGal6ST), and GAPDH are as follows: CSGalNAcT1, 56 °C, 5′-AGAAGAAATAAATGAAGTCAAAGGAATAC-3′ and 5′-GAAGTAGATGTCCACATCACAG-3′, which formed a 181-bp fragment; CSGalNAcT2, 40 °C, 5′-CCTAGAATCTGTCACCAGT-3′ and 5′-GTTAAGGAATTCGGCTGAGAAATA-3′, which formed a 172-bp fragment (45); CHST2, 51 °C, 5′-GAGGTGTTCTTCCTCTATGAGCC-3′ and 5′-CCACGAAAGGCTTGGAGGAGG-3′, which formed an 847-bp fragment; CHST3, 60 °C, 5′-GGACCTTGTACACAGCCTAAAGATTCG-3′ and 5′-CTCGGACAGCCACTTCTTCCA-3′, which formed a 928-bp fragment; CHST7, 60 °C, 5′-ACCCAGGAAAAGCAACACATCTATG-3′ and 5′-GGTTAAGAAGAAATCAGCGCGTGG-3′, which formed a 735-bp fragment; CHST1, 60 °C, 5′-AGTACACAGCCATCCGCACTT-3′ and 5′-TGTGCCACGTGACTGTCCA-3′, which formed a 934-bp fragment; and GAPDH, 60 °C, 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-CACCACCCTGTTGCTGTAGCC-3′, which formed a 450-bp fragment. mRNA expression levels were measured using RT-PCR as described below or in Tjew et al. (34). 250 ng of total RNA from unstimulated bone marrow-derived macrophages or macrophages stimulated with LPS/IFNγ or IL-4 for 24 h using TRIzol Reagent (Invitrogen) was reverse-transcribed with iScript (Bio-Rad) according to manufacturer instructions. An aliquot of the cDNA was subjected to PCR (25–35 cycles) with Platinum Taq polymerase (Invitrogen) in 20 μl. The PCR product was electrophoresed in 1.2% agarose gel, stained with SYBR Safe (Invitrogen), and visualized under ultraviolet light.
Quantitative Real Time PCR
Total RNA was extracted from 48-h stimulated bone marrow-derived macrophages using TRIzol reagent (Invitrogen) and reverse-transcribed using the iScript cDNA Synthesis kit (Bio-Rad). Quantitative mRNA expression was analyzed by real-time PCR (Bio-Rad CFX384), with SsoFast EvaGreen (Bio-Rad). CD44s and CD44v10 were amplified using the common forward primer 5′-ACCATCGAGAAGAGCACC-3′ and the reverse primers 5′-TCATAGGACCAGAAGTTGTGG-3′ and 5′-GTCTCGATCTCCTGGTAAGG-3′, respectively. GAPDH served as the endogenous reference gene, and normalized gene expression to GAPDH was calculated by CFX384.
Statistics
Data are shown as the mean ± S.D. Significance was determined by Student's t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
RESULTS
M1- and M2-polarizing Agents Induce CD44-mediated Hyaluronan Binding in Mouse Bone Marrow-derived Macrophages to Differing Extents
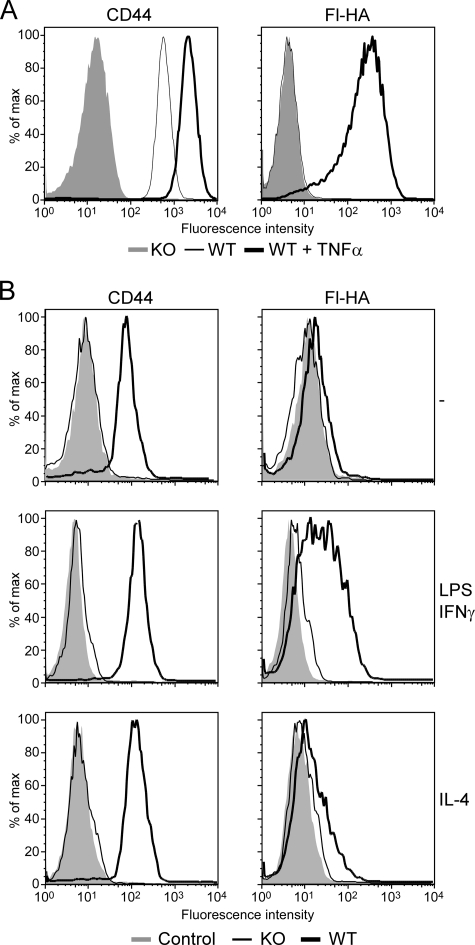
Bone marrow-derived macrophages were generated from the bone marrow of C57Bl/6 and CD44−/− mice and cultured for 2–3 days under either M1-polarizing conditions with 50 ng/ml IFNγ and 100 ng/ml LPS or with 20 ng/ml TNFα or under M2-polarizing conditions with 10 ng/ml IL-4. Fluorescent hyaluronan binding was induced by 24 h and peaked around 48 h (data not shown). Fig. 1 shows CD44 expression levels and fluorescent-hyaluronan binding of both unstimulated and stimulated mouse bone marrow-derived macrophages by flow cytometry. TNFα up-regulated CD44 expression and induced high levels of hyaluronan binding (Fig. 1A), and this was mediated by CD44, as it did not occur in bone marrow-derived macrophages derived from the CD44−/− mice. Stimulation by LPS/IFNγ also induced CD44-mediated hyaluronan binding, as did IL-4, albeit to a lesser extent (Fig. 1B).
FIGURE 1.
Induction of hyaluronan binding and CD44 in bone marrow-derived macrophages. CD44 expression and hyaluronan binding were analyzed by flow cytometry in bone marrow-derived macrophages derived from wild type or CD44−/− mice and stimulated with 20 ng/ml TNFα (A) or 100 ng/ml LPS and 50 ng/ml IFN-γ or with 10 ng/ml IL-4 (B) for 2 days. A, the left panel shows expression levels of CD44, detected using Alexa 647 conjugated IM7, from unstimulated (dashed line) and TNFα-stimulated (thick line) bone marrow-derived macrophages, whereas the right panel shows binding to fluorescent-hyaluronan (Fl-HA). CD44 and Fl-HA binding of CD44−/− bone marrow-derived macrophages is shown by the shaded histograms. B, CD44 expression levels were detected using Pacific blue-conjugated IM7 on unstimulated, LPS/IFNγ stimulated, and IL-4 stimulated bone marrow-derived macrophages derived from wild type (thick line) and CD44−/− mice (thin line). Negative controls (shaded histograms) are unlabeled cells. This is one representative experiment repeated at least three times.
TNFα Reduces CD44 Sulfation in Mouse Bone Marrow-derived Macrophages
We had previously established a correlation between TNFα-induced hyaluronan binding and increased CD44 sulfation in human myeloid cells and peripheral blood monocytes (24, 32). In human monocytes, TNFα increased the carbohydrate sulfation of CD44, up-regulated the expression of two carbohydrate glycosyltransferases, and induced the expression of the sulfate-specific epitope recognized by the mAb, AG107, while simultaneously causing a decrease in the percent of CD44 sulfation due to chondroitin sulfate (34, 35). To determine whether TNFα was inducing similar changes in mouse bone marrow-derived macrophages, we labeled the cells with [35S]sulfate and measured sulfate incorporation by CD44 (Fig. 2A). Surprisingly, unlike human peripheral blood monocytes, TNFα did not increase the sulfate incorporation of CD44, but actually reduced it, suggesting no induction of carbohydrate sulfation in these mouse macrophages in response to TNFα. Consistent with this, TNFα did not up-regulate the expression of two glycosyl sulfotransferases, CHST2 and CHST7 (Fig. 2B), implicated in the induction of the sulfated carbohydrate present on CD44 in human myeloid cells (34). In addition, these TNFα-stimulated bone marrow-derived macrophages did not express any sulfated epitopes detected by the mAbs AG107 or DD2 (46, 47) that recognize 6-sulfo sialyl Lewis x and did not bind the 2F3 mAb (46), which recognizes sialyl Lewis x epitopes (data not shown). Thus, TNFα neither enhances the carbohydrate sulfation of CD44 in mouse bone marrow-derived macrophages nor induces expression of the AG107 epitope (6-sulfo N-acetyllactosamine) on CD44, highlighting significant differences in TNFα-induced post-translational modifications on CD44 between mouse bone marrow-derived macrophages and human peripheral blood monocytes.
FIGURE 2.
TNFα does not increase CD44 sulfation in bone marrow-derived macrophages, and chondroitin sulfate-modified CD44 negatively correlates with hyaluronan binding in human KG1a cells. A, shown is an autoradiograph of CD44 immunoprecipitated from sodium [35S]sulfate-labeled bone marrow-derived macrophages and resolved by SDS-PAGE. CD44 loading (lower panel) was determined by Western blotting with J1WBB. B, CHST2 (GlcNAc6ST-1) and CHST7 (GlcNAc6ST-4) mRNA expression from unstimulated and TNFα-stimulated bone marrow-derived macrophages (BMDM) is shown. Isolated mRNA was subjected to semiquantitative PCR, and β-actin expression was used as a loading control. RNA isolated from mouse brain or kidney was used as a positive control for CHST2 or CHST7, respectively. C, shown is flow cytometry of CD44 expression and fluorescent-hyaluronan (Fl-HA) binding by KG1a cells sorted for either low or high hyaluronan binding. D, detection of chondroitin sulfate on immunoprecipitated CD44 by Western blotting with the anti- 4-sulfated chondroitin sulfate stub mAb, 2B6 is shown. CD44 loading was determined by blotting with the anti-human mAb 3G12 (lower panel). E, densitometry was performed on the Western blots from D, and after controlling for differences in CD44 loading, the amount of 2B6 detected was set to 1 for the high hyaluronan binding cells. Data are the mean ± S.D. of four experiments, and statistical significance (**p < 0.01) is shown compared with low cells.
Chondroitin Sulfate-modified CD44 Inversely Correlates with Hyaluronan Binding in Human Myeloid Cells
This raised the possibility that in human monocytes, it was the reduction in chondroitin sulfate rather than the induction of carbohydrate sulfation on CD44 that may be responsible for induced hyaluronan binding after TNFα stimulation. To evaluate whether hyaluronan binding correlated with the expression of the sulfated carbohydrate epitope AG107 in human myelocytic cells, we selected for AG107 high and low human myelocytic SR91 cells and compared their ability to bind hyaluronan. TNFα-stimulated SR91 cells were neuraminidase-treated (to expose the AG107 epitope) and then sorted for high and low AG107-positive cells. The cells were cultured and then restimulated with TNFα. Although the cells maintained their high and low AG107 reactivity, they showed equivalent fluorescent-hyaluronan binding, indicating no correlation between the expression levels of the AG107 epitope and hyaluronan binding (data not shown). In contrast, the human myeloid progenitor cell line (KG1a) previously sorted for high and low hyaluronan binding populations (Fig. 2C) (33) showed an inverse correlation between hyaluronan binding and chondroitin sulfation of CD44 (Fig. 2, D and E). Western blotting of chondroitinase ABC-treated CD44 immunoprecipitates with the anti-chondroitin sulfate stub mAb, 2B6, which recognizes the 4-sulfated chondroitin sulfate stub, found reduced staining on CD44 in the high hyaluronan binding cells (Fig. 2D). Densitometric analysis of the 2B6 Western blots, which provides an indication of the frequency of chondroitin sulfation or chondroitin sulfate addition as opposed to the length of the chondroitin sulfate chains, revealed that chondroitin sulfate modification of CD44 was ∼3-fold higher in low hyaluronan binding cells compared with high hyaluronan binding cells (Fig. 2E). This indicated an inverse correlation between the chondroitin sulfation of CD44 and hyaluronan binding in these human myeloid cells.
The Glycosaminoglycan Inhibitor, β-d-Xyloside, Increases Hyaluronan Binding by Bone Marrow-derived Macrophages
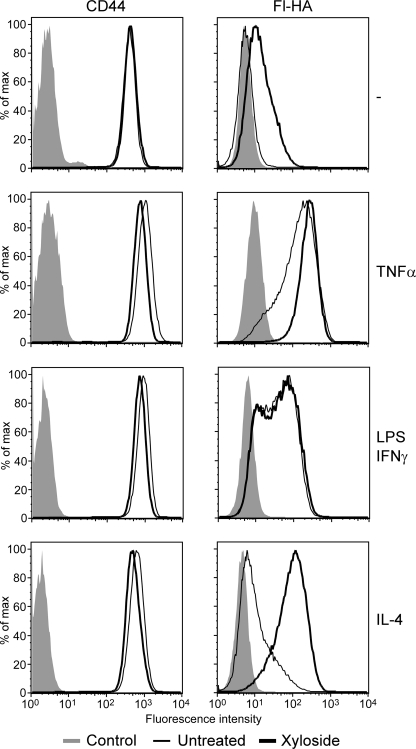
The reduction in CD44 sulfation in mouse bone marrow-derived macrophages as well as the inverse correlation between hyaluronan binding and chondroitin sulfate addition on CD44 in human myeloid cells suggested that chondroitin sulfate may negatively regulate hyaluronan binding in mouse bone marrow-derived macrophages. In addition, we had previously shown that chondroitin sulfate addition to a purified human CD44-Ig fusion protein directly reduces its affinity for hyaluronan (29). As a first step to establish whether CD44 was modified with chondroitin sulfate in mouse bone marrow-derived macrophages, the cells were treated with chondroitinase ABC, but this had little effect on hyaluronan binding (data not shown). Because this lack of effect could be due to the inability of the enzyme to access the chondroitin sulfate chain on CD44 or due to a continued negative effect of the residual chondroitin sulfate stub left after digestion (29), we treated cells with 2 mm β-d-xyloside, a competitive inhibitor of glycosaminoglycan addition, and analyzed hyaluronan binding (Fig. 3). Although a few unstimulated cells bound to hyaluronan, treatment with β-d-xyloside caused ∼50% of the cells to bind low levels of hyaluronan. This binding by β-d-xyloside-treated cells remained CD44-specific, as it could be blocked with the anti-CD44 mAb, KM201 (data not shown). In TNFα-stimulated cells, the majority of cells already bound high levels of hyaluronan, and this was slightly increased by β-d-xyloside. Interestingly, β-d-xyloside had no discernable effect on the level or percent of cells binding hyaluronan after LPS/IFNγ stimulation. In contrast, β-d-xyloside had a dramatic effect on IL-4-stimulated cells, increasing both the level and percent of hyaluronan binding. This indicated that prevention of glycosaminoglycan addition increased hyaluronan binding in bone marrow-derived macrophages, most noticeably in unstimulated and IL-4-treated cells, and suggested that chondroitin sulfate addition to CD44 negatively affected hyaluronan binding in these cells. The reduced ability of β-d-xyloside to increase binding in M1-polarized bone marrow-derived macrophages also raised the possibility that TNFα and LPS/IFNγ may reduce chondroitin sulfate addition to CD44.
FIGURE 3.
Inhibition of glycosaminoglycan addition by β-d-xyloside increases hyaluronan binding by bone marrow-derived macrophages. CD44 expression and hyaluronan binding were analyzed by flow cytometry in unstimulated bone marrow-derived macrophages or bone marrow-derived macrophages stimulated with 20 ng/ml TNFα, 100 ng/ml LPS and 10 ng/ml IFNγ, or 10 ng/ml IL-4 for 2 days in the presence (thick line) or absence (thin line) of 2 mm β-d-xyloside. The left panel shows expression levels of CD44, detected using Alexa 647-conjugated IM7, whereas the right panel shows binding to fluorescent-hyaluronan (Fl-HA). Unlabeled cells (shaded histograms) were used as negative controls. This is one representative experiment repeated three times.
CD44 Is Modified by Chondroitin Sulfate in Mouse Bone Marrow-derived Macrophages, and Chondroitin Sulfation Is Reduced by Inflammatory Agents
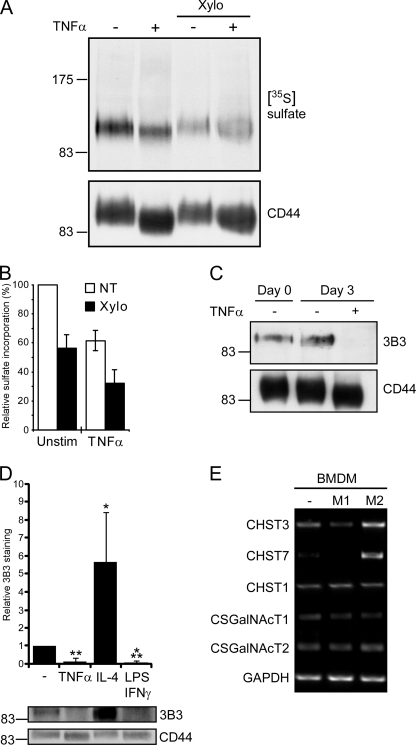
To directly test whether glycosaminoglycan addition to CD44 was reduced upon TNFα stimulation, [35S]sulfate was added to bone marrow-derived macrophages during the second and third day of TNFα stimulation in the presence or absence of 2 mm β-d-xyloside (Fig. 4A). Analysis of sulfate incorporation by autoradiography revealed that sulfated glycosaminoglycans (removed by β-d-xyloside) were responsible for about half of CD44 sulfation in both unstimulated (44 ± 9%) and TNFα-stimulated (49 ± 12%) cells (Fig. 4B). However, as TNFα stimulation reduced overall CD44 sulfation by 38 ± 7%, the total amount of glycosaminoglycan-dependent sulfation on CD44 was reduced by about 40%.
FIGURE 4.
Chondroitin sulfate addition to CD44 is reduced by inflammatory cytokines. A, shown is an autoradiograph of CD44 immunoprecipitated from sodium [35S]sulfate-labeled bone marrow-derived macrophages grown in the presence or absence of 2 mm β-d-xyloside (Xylo) and resolved by SDS-PAGE. CD44 loading was determined by Western blotting with J1WBB. B, relative sulfate incorporation by CD44 as measured by densitometry is shown. Sulfate incorporation per unit CD44 was assessed and set to 100% in unstimulated, non-treated (NT) cells. Values represent the mean ± S.D. of six independent experiments. C, detection of chondroitin sulfate on immunoprecipitated CD44 from unstimulated and TNFα-stimulated bone marrow-derived macrophages by Western blotting with the anti-6-sulfated chondroitin sulfate stub mAb, 3B3, is shown. CD44 loading levels were determined by Western blotting with the CD44 antiserum, J1WBB. D, analysis of chondroitin sulfate addition to CD44 from bone marrow-derived macrophages stimulated with TNFα, IL-4, or with LPS and IFNγ. Western blots (a representative is shown in the bottom panel) were analyzed by densitometry, and after taking into account variations in CD44 loading, the intensity of 3B3 staining for CD44 from unstimulated bone marrow-derived macrophages was set to 1. Data are shown as the mean ± S.D. of 3 experiments with significance indicated as: *, p < 0.05; **, p < 0.01; ***, p < 0.001. E, CHST3 (C6ST1), CHST7 (C6ST2), CHST1 (KSGal6ST), CSGalNAcT1, and CSGalNAcT2 mRNA expression from unstimulated (−), 100 ng/ml LPS and 50 ng/ml IFNγ-stimulated (M1), and 10 ng/ml IL-4-stimulated (M2) bone marrow-derived macrophages is shown. Isolated mRNA was subjected to semiquantitative PCR, and GAPDH expression was used as a loading control. These are representative data that was reproduced with macrophages from four mice.
To determine whether chondroitin sulfate was the glycosaminoglycan being decreased on CD44 upon TNFα stimulation, CD44 was immunoprecipitated from unstimulated and TNFα-stimulated cells and analyzed for chondroitin sulfate addition by Western blotting with the anti-chondroitin sulfate stub mAb 3B3 (Fig. 4C). The 3B3 mAb is similar to the 2B6 mAb, except that it recognizes the 6-sulfated rather than the 4-sulfated chondroitin sulfate stub. A 3B3 signal was detected on CD44 from both day 0 and day 3 unstimulated bone marrow-derived macrophages, but this signal was absent on CD44 immunoprecipitated from macrophages stimulated with TNFα for 3 days, indicating that TNFα stimulation had reduced either chondroitin sulfation or chondroitin sulfate addition to CD44. Consistent with this, a loss of 3B3 staining was also observed in LPS/IFNγ-stimulated macrophages (Fig. 4D). In contrast, IL-4 treatment increased the intensity of 3B3 mAb staining by ∼5-fold, indicating that IL-4 enhances chondroitin sulfation or chondroitin sulfate addition to CD44. This may help explain why these cells bound much lower levels of hyaluronan than either TNFα- or LPS/IFNγ-stimulated macrophages despite a similar or increased expression of CD44. These results indicate the differential regulation of chondroitin sulfation or chondroitin sulfate addition to CD44 after M1 or M2 polarization.
LPS/IFNγ and IL-4 Differentially Effect the Chondroitin Sulfation of CD44 by Modulating the Expression of Chondroitin 6-Sulfotransferases
To investigate how LPS/IFNγ and IL-4 induce changes to the chondroitin sulfation of CD44, we sought to evaluate the effect of these agents on the expression of enzymes involved in chondroitin sulfation and chain elongation. Because the 3B3 mAb detects a terminal 6-sulfated N-acetylgalactosamine (GalNAc) on the chondroitin sulfate stub, we examined the mRNA levels of both sulfotransferases capable of this sulfation, CHST3 and -7 (48) by PCR. We also examined the levels of GalNAcT1 and GalNacT2, two enzymes implicated in the synthesis and elongation of chondroitin sulfate (48). Finally, we examined a galactosamine 6-sulfotransferase, CHST1. CHST3 was expressed in unstimulated macrophages, and this was down-regulated in M1-macrophages and up-regulated in M2-macrophages (Fig. 4E). A similar pattern of expression was observed with CHST7, although expression was low in the unstimulated macrophages. In contrast, no changes were observed in either GalNAc transferase or in CHST1, the galactosamine 6-sulfotransferase. These data suggest that regulation of 6-sulfation of GalNAc residues in the chondroitin sulfate chain, rather than regulation of the GalNAc transferases that would promote chain elongation, is responsible for decreased chondroitin 6-sulfation after LPS/IFNγ stimulation and increased chondroitin 6-sulfation after IL-4 stimulation.
LPS/IFNγ and IL-4 Differentially Regulate CD44v10 Expression
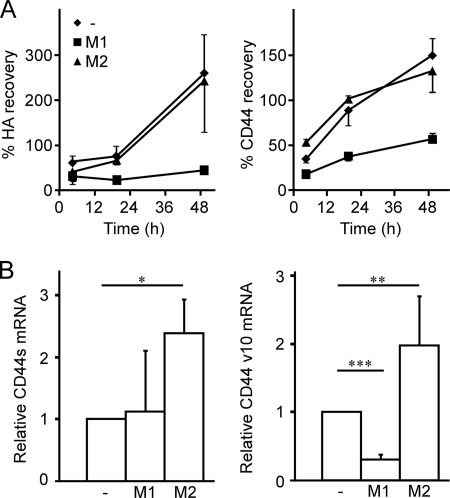
To better understand how IL-4 stimulated bone marrow-derived macrophages bind hyaluronan despite increased levels of chondroitin 6-sulfation, we sought to examine whether stimulation altered patterns of CD44 expression. New CD44 protein expression was necessary to enhance hyaluronan binding after stimulation, as evidenced by the ability of cycloheximide to largely prevent stimulation-induced hyaluronan binding (data not shown). To determine whether CD44 turnover was different in M1- and M2- macrophages, the cells were treated with trypsin to remove surface CD44, then the recovery of surface CD44 was monitored by flow cytometry. Interestingly, both unstimulated and IL-4-stimulated macrophages quickly recovered their CD44 surface expression, whereas LPS/IFNγ-stimulated macrophages only recovered to 50% that of their original value, even after 48 h (Fig. 5A). This suggests that CD44 synthesis or turnover is different in the M1 and M2-macrophages.
FIGURE 5.
Macrophage polarization affects CD44 turnover and expression of variable exon 10. A, percent recovery of fluorescent-hyaluronan (HA) binding and CD44 expression after trypsin treatment of unstimulated, M1, and M2 bone marrow-derived macrophages is shown. Bone marrow-derived macrophages were unstimulated (−) or stimulated with either 100 ng/ml LPS and 50 ng/ml IFNγ (M1) or 10 ng/ml IL-4 (M2) for 1 day. Cells were then treated with trypsin for 5 min or untreated, then further cultured for 3, 18, and 48 h. The percent recovery was calculated from flow cytometry, by dividing the mean fluorescence value of fluorescent-hyaluronan or CD44 from the trypsin-treated samples by the value of the non-trypsin treated cells at each time point. Data shown are the mean ± S.D. of three biological replicates. B, relative mRNA expression of CD44s and CD44v10 in bone marrow-derived macrophages is shown. Bone marrow-derived macrophages were cultured in media (−) or stimulated for 48 h with 50 ng/ml IFNγ and 100 ng/ml LPS (M1) or 10 ng/ml IL-4 (M2). Total mRNA was extracted and subjected to reverse transcription. CD44s (left panel) and CD44v10 (right panel) transcripts were measured by quantitative-PCR and normalized to GAPDH expression. Expression is shown relative to the unstimulated control. Graphs show the average relative expression from five mice over three experiments ± S.D. with significance indicated as p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
Immunoprecipitation of CD44 from unstimulated and M1- and M2-stimulated macrophages did not indicate any higher molecular mass proteins other than the 90-kDa protein that is typical of the standard CD44 isoform, CD44s. Quantitative PCR using primers spanning the variable exon insertion site also indicated the predominance of the CD44s isoform under both unstimulated and stimulated conditions (data not shown). Notably, CD44s mRNA expression was significantly up-regulated in M2-macrophages (Fig. 5B). However, using a PCR primer specific for variable exon 10, the CD44v10 isoform was detected in unstimulated macrophages and was significantly down-regulated in M1-macrophages and markedly up-regulated in M2-macrophages (Fig. 5B). Thus M1 and M2 stimulation differentially regulate CD44v10 expression at the mRNA level, although it remains to be determined whether this is also reflected at the protein level.
LPS Substantially Enhances Hyaluronan Binding by M2-macrophages, but IL-4 Has Little Effect on M1-macrophages
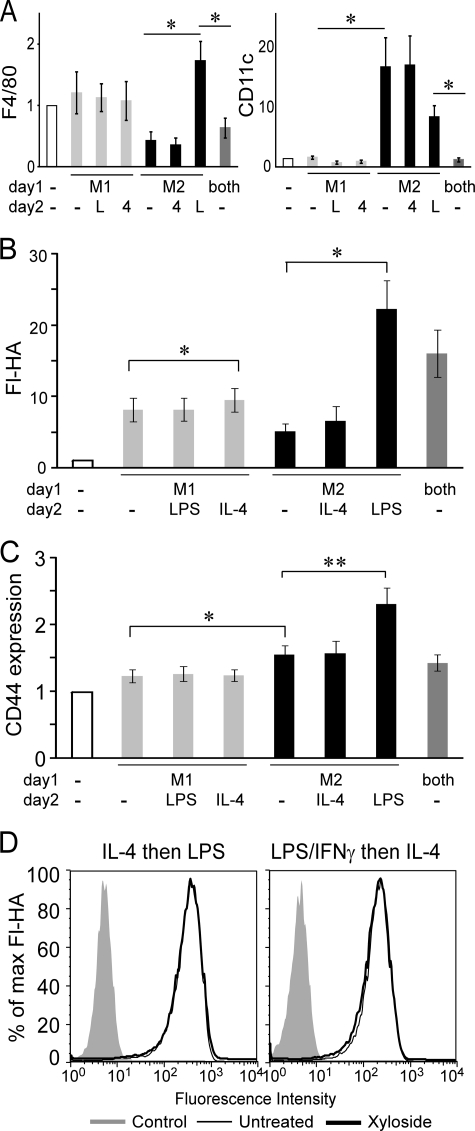
M1-macrophages are characterized by TNFα and nitric oxide production, whereas M2-macrophages, induced by IL-4, show increased arginase activity and no TNFα or nitric oxide (for review, see Ref. 49). However, the subsequent exposure of M2-macrophages to LPS induces the secretion of TNFα (50) suggestive of their conversion to an M1-macrophage and indicative of the plasticity of this M1/M2 designation. We observed that M1- and M2-bone marrow-derived macrophages can also be distinguished phenotypically by surface marker expression, as LPS-stimulated macrophages displayed increased levels of the F4/80 macrophage marker and lower levels of CD11c compared with IL-4-stimulated macrophages (Fig. 6A). Treatment of M1-macrophages with IL-4 had little effect on F4/80 or CD11c expression levels, whereas LPS increased F4/80 expression and reduced CD11c expression on M2-macrophages, consistent with the ability of LPS to both functionally and phenotypically convert M2-macrophages to M1-macrophages.
FIGURE 6.
Distinct phenotypes of classically activated (M1) and alternatively activated (M2) bone marrow-derived macrophages and the relative expression levels of CD44 and fluorescent-hyaluronan binding. A–C, bone marrow-derived macrophages were stimulated with either 100 ng/ml LPS and 50 ng/ml IFN-γ (M1), 10 ng/ml IL-4 (M2), 100 ng/ml LPS, 50 ng/ml IFN-γ, and 10 ng/ml IL-4 (both) or media control (−) on day 1, then re-stimulated with LPS (L), IL-4 (4) or media alone (−) on day 2. Graphs show the mean fluorescent intensity ± S.E. of at least three experiments with significance indicated as: *, p < 0.05; **, p < 0.01. A, shown is the relative increase in expression levels of F4/80 (left panel) and CD11c (right panel) of activated versus unstimulated bone marrow-derived macrophages. B and C, shown is the relative increase in fluorescent-hyaluronan (Fl-HA) binding (B) and CD44 expression levels (C) compared with unstimulated bone marrow-derived macrophages. D, bone marrow-derived macrophages were stimulated in the presence (black line) or absence (thin line) of 2 mm β-d-xyloside, and the negative control (shaded histogram) was unlabeled cells. Cells were stimulated with IL-4 followed by LPS on the second day (IL-4 then LPS) or stimulated with LPS and IFN-γ followed by IL-4 on the second day (LPS/IFN-γ then IL-4). One of four experiments is shown.
LPS treatment of M2-macrophages substantially enhanced fluorescent-hyaluronan binding and CD44 expression, whereas IL-4 had minimal effect on hyaluronan binding by M1-macrophages (Fig. 6, B and C), suggesting that LPS was able to overcome the inhibitory effect of IL-4. To evaluate whether LPS could stimulate the removal of IL-4-induced chondroitin sulfate, LPS-treated M2-macrophages as well as IL-4 treated M1-macrophages were treated with β-d-xyloside to evaluate the presence of chondroitin sulfate. Unlike IL-4-activated macrophages where hyaluronan binding was greatly enhanced by β-d-xyloside treatment, hyaluronan binding by LPS-treated M2-macrophages or IL-4-treated M1-macrophages were not affected by β-d-xyloside (Fig. 6D). This implies that LPS stimulation can counter the induction of chondroitin sulfation on CD44 that normally occurs after IL-4 stimulation alone and illustrates that hyaluronan binding can be dynamically regulated by inflammatory and anti-inflammatory signals via the regulation of chondroitin sulfation on CD44.
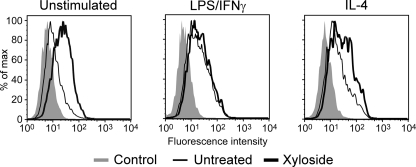
Inhibiting Glycosaminoglycan Addition Induces Hyaluronan Binding in Peritoneal Macrophages
To extend our findings to ex vivo macrophages, cells were isolated from the peritoneum of untreated mice, and adherent cells were stimulated with IL-4 or LPS/IFNγ in the presence or absence of β-d-xyloside. After 48 h, F4/80-positive peritoneal macrophages were analyzed for binding to fluorescent-hyaluronan (Fig. 7). As with bone marrow-derived macrophages, unstimulated cells were predominately non-hyaluronan binding but were able to bind an increased amount of hyaluronan when treated with β-d-xyloside. Hyaluronan binding was also increased by β-d-xyloside treatment of IL-4-stimulated cells, although to a lesser extent than observed in bone marrow-derived macrophages. Similar to bone marrow-derived macrophages, LPS/IFNγ treatment increased hyaluronan binding, and hyaluronan binding was not affected by β-d-xyloside treatment. This suggests that CD44 is modified by chondroitin sulfate in peritoneal macrophages, and this is removed when peritoneal macrophages are exposed to inflammatory stimuli.
FIGURE 7.
Effect of inhibition of glycosaminoglycan addition on hyaluronan binding by peritoneal macrophages. Fluorescent-hyaluronan (Fl-HA) binding was analyzed by flow cytometry after gating on F4/80-positive macrophages. These peritoneal macrophages were stimulated ex vivo with 20 ng/ml IL-4 or 100 ng/ml LPS and 10 ng/ml IFNγ for 2 days in the presence (thick line) or absence (thin line) of 2 mm β-d-xyloside. Unlabeled cells (shaded histograms) were used as negative controls. One of four experiments is shown.
Chondroitin Sulfate Addition to CD44 Regulates Hyaluronan Binding during TNFα Stimulation of Bone Marrow-derived Macrophages
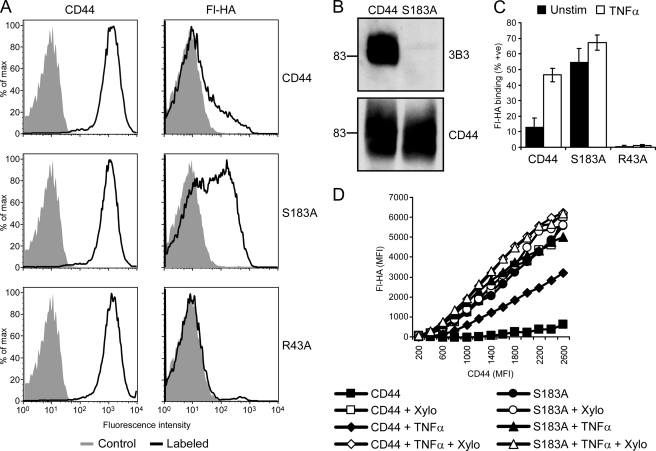
Although β-d-xyloside is an extremely useful tool for studying the effects of glycosaminoglycan addition, it is not specific to CD44. In addition, β-d-xyloside treatment results in the formation of a large amount of free chondroitin sulfate chains in the cell (51), potentially leading to other glycosylation changes. Therefore, we chose to express a mutated form of CD44s that cannot be modified by chondroitin sulfate (S183A-CD44) to directly assess its contribution to hyaluronan binding in bone marrow-derived macrophages. We used retroviral infection to express YFP and wild type CD44, S183A-CD44, or R43A-CD44 in CD44−/− bone marrow-derived macrophages. The R43A mutation is equivalent to the R41A mutation in human CD44, which prevents hyaluronan binding (52), and Ser-183 in mouse is equivalent to Ser-180 in human CD44, which was previously established to be the site of chondroitin sulfate addition (29). Fig. 8A shows the CD44 expression and fluorescent-hyaluronan binding in the YFP positive cells. As expected, cells expressing R43A-CD44 did not bind fluorescent-hyaluronan under any condition tested. Before stimulation, only a small percent of wild type CD44-expressing cells bound hyaluronan, whereas about 50% of S183A-CD44-expressing cells bound hyaluronan constitutively. S183A was confirmed as the site of chondroitin sulfate addition on murine CD44 by the absence of reactivity with the anti-6-sulfated chondroitin sulfate stub mAb (3B3) compared with wild type CD44 (Fig. 8B). Similar to bone marrow-derived macrophages, TNFα induced hyaluronan binding in CD44−/− bone marrow-derived macrophages reconstituted with wild type CD44 (Fig. 8C). In contrast to wild type CD44-expressing macrophages, TNFα stimulation of S183A-CD44-expressing cells resulted in only a minor increase in the percent of fluorescent-hyaluronan binding cells. The constitutive hyaluronan binding of S183A-CD44 and the lack of a major increase in hyaluronan binding upon TNFα stimulation further supports our conclusion that TNFα induces hyaluronan binding by reducing the chondroitin sulfate on CD44 and indicates that the loss of chondroitin sulfate on CD44 is one mechanism induced by TNFα to regulate hyaluronan binding by CD44.
FIGURE 8.
Chondroitin sulfate addition to CD44 regulates hyaluronan binding in bone marrow-derived macrophages. During the generation of bone marrow-derived macrophages, bone marrow from CD44−/− mice was infected with a retrovirus expressing the YFP marker and wild type CD44, S183A mutant CD44, or R43A mutant CD44. A, shown is CD44 expression and fluorescent-hyaluronan (Fl-HA) binding by YFP-positive cells, with unlabeled cells as negative controls (shaded histogram). B, shown is detection of chondroitin sulfate on immunoprecipitated CD44 from CD44 and S183A-CD44 infected bone marrow-derived macrophages by Western blotting with the anti-chondroitin sulfate mAb 3B3. CD44 levels were determined using J1WBB antiserum. C, shown is the percent of fluorescent-hyaluronan binding bone marrow-derived macrophages expressing wild type, S183A mutant CD44, or R43A mutant CD44 after culture in the presence or absence of TNFα for 3 days. Cells were analyzed by flow cytometry after labeling with Alexa 647-conjugated IM7 and fluorescent-hyaluronan. Data are shown as the mean ± S.D. of 5 experiments. D, shown is fluorescent-hyaluronan binding versus CD44 expression in bone marrow-derived macrophages cultured with or without TNFα in the presence or absence of β-d-xyloside (Xylo) for 3 days. Cells were analyzed by flow cytometry as above, and numerous gates were drawn based on CD44 expression levels to determine the mean fluorescent intensity (MFI) for fluorescent-hyaluronan binding in each of these gates. To control for leakage of the YFP signal, the MFI of cells infected with CD44 containing the R43A mutation was subtracted from the original values. The final MFI value for fluorescent-hyaluronan was then plotted against the MFI used for each CD44 gate.
As binding to hyaluronan is highly dependent upon CD44 expression levels and expression varied between conditions, a comparison of fluorescent-hyaluronan binding by cells expressing equivalent amounts of CD44 was performed. As shown in Fig. 8D, unstimulated bone marrow-derived macrophages expressing wild type CD44 bound very low levels of fluorescent-hyaluronan over a wide range of CD44 expression levels, whereas fluorescent-hyaluronan binding per unit CD44 was significantly increased upon TNFα stimulation, with binding increasing with CD44 expression. Notably, treatment of both unstimulated and TNFα-stimulated cells with β-d-xyloside increased fluorescent-hyaluronan binding per unit CD44 to levels comparable with cells expressing S183A-CD44. This demonstrates that the chondroitin sulfate added to Ser-183 on CD44 restricts the binding of hyaluronan. It also indicates that TNFα stimulation does not remove all the chondroitin sulfate on CD44, consistent with our sulfate labeling experiments (Fig. 4B). As expected, the treatment of S183A-CD44-expressing cells with β-d-xyloside had no significant effect on fluorescent-hyaluronan binding due to the inability of S183A-CD44 to be modified by chondroitin sulfate. TNFα stimulation also had little effect on fluorescent-hyaluronan binding in S183A-CD44-expressing cells, again consistent with reduced chondroitin sulfate being one of the primary mechanisms regulating hyaluronan binding in these cells.
DISCUSSION
Here we find an inverse correlation between chondroitin sulfate-modified CD44 and hyaluronan binding in mouse macrophages and show that the regulation of chondroitin sulfate is a physiological mechanism used to regulate hyaluronan binding in these cells. Inflammatory agents such as LPS/IFNγ and TNFα induce hyaluronan binding at least in part by reducing chondroitin sulfation on CD44, whereas the M2-inducing cytokine, IL-4, limits hyaluronan binding by enhancing chondroitin sulfation on CD44. Inflammatory and anti-inflammatory agents differentially regulate the chondroitin sulfation on CD44 by their opposing effects on the expression of the chondroitin 6-sulfotransferases, CHST3 and -7, at the mRNA level. We, therefore, conclude that the regulation of chondroitin sulfation on CD44 is one mechanism to dynamically regulate hyaluronan binding in myeloid cells and macrophages.
There are at least three possible mechanisms to explain how chondroitin sulfate addition to serine 180 in the standard form of human CD44, or serine 183 in mice, can negatively regulate hyaluronan binding. The first is blocking hyaluronan binding by the direct binding of the chondroitin sulfate side chain in the hyaluronan binding pocket of CD44. The second is the binding of the negatively charged chondroitin sulfate to a positively charged region on CD44 resulting in a non-favorable hyaluronan binding conformation. The third possibility is that the rigid chondroitin sulfate modified side chain of CD44 prevents CD44 clustering and creates an unfavorable spacing of CD44 for hyaluronan-binding. The standard form of CD44 only binds chondroitin sulfate with a low affinity compared with hyaluronan (53), and although the proximity of chondroitin sulfate to CD44 may enhance binding kinetics, this seems unlikely to overcome the large differences in affinity. Our demonstration that short chondroitin sulfate chains are able to restrict hyaluronan binding, including the six-sugar chondroitin sulfate stub remaining after chondroitinase ABC digestion, and that this effect can be observed in vitro with purified CD44 fusion proteins when spacing and clustering is less likely to be an issue (29) indicates that this is also unlikely to explain the effect of chondroitin sulfate. We thus favor the second possibility, as the crystal structure of the amino-terminal region of CD44 identified two conformational forms of CD44, a hyaluronan-binding conformation and another in the absence of hyaluronan (54), supporting the possibility that chondroitin sulfate could bind to the hyaluronan binding domain and stabilize a non-binding conformation. Because a basic residue within a Bx7B motif present in variable exon 10 of CD44 is required for the chondroitin sulfate- and CD44-dependent cell-cell adhesion (55), it is possible that chondroitin sulfate-modified CD44 may also interact with a basic residue within a Bx7B motif located at the back of the hyaluronan binding domain to stabilize a non-hyaluronan binding conformation. Although we do not know to what extent CD44v10 is expressed in M2-macrophages or whether it plays a role in regulating hyaluronan binding, it is tempting to speculate that the Bx7B motif in v10 may compete with the Bx7B motif in CD44s for the binding of chondroitin sulfate and thus relieve some of its inhibitory effect on hyaluronan binding.
It should be noted that the modification of CD44 by chondroitin sulfate in bone marrow-derived macrophages does not significantly alter the molecular mass of CD44, implying that the chondroitin sulfate chains of CD44 are small. In addition, chondroitinase ABC did not affect hyaluronan binding in unstimulated macrophages, suggesting that either the enzyme could not access the site on CD44 on the macrophage surface or that the six-sugar stub left after chondroitinase digestion is sufficient to mediate its negative effect. It is also possible that regulation by chondroitin sulfate may operate in other immune cells as the expression of the human CD44s mutant, S180A, which lacks the site of chondroitin sulfate addition, led to constitutive hyaluronan binding in Jurkat T cells (56), raising the possibility that T cells, which up-regulate hyaluronan binding upon antigen recognition (21, 22), may also regulate hyaluronan binding by regulating chondroitin sulfate addition to CD44.
In human peripheral blood monocytes, β-d-xyloside prevents IL-4 from down-regulating TNFα-induced hyaluronan binding (30), consistent with IL-4 increasing the amount of chondroitin sulfate-modified CD44 and inhibiting hyaluronan binding. Here, LPS treatment of M2-macrophages enhanced hyaluronan binding by further increasing CD44 expression and reversing the IL-4-induced increase in chondroitin sulfate, whereas IL-4 treatment of M1-macrophages had little effect on CD44 expression or hyaluronan binding. Similarly, whereas it has been shown that removal of sialic acid residues by LPS-induced sialidase increases hyaluronan binding in human monocytes (31), the removal of sialic acid residues by neuraminidase treatment in mouse bone marrow-derived macrophage caused only a slight increase in hyaluronan binding in both unstimulated and TNFα-stimulated cells (data not shown). However, it is unlikely that hyaluronan binding in macrophages is regulated by CD44 expression and chondroitin sulfate addition alone, given the numerous modifications to CD44 that can affect hyaluronan binding. We did observe increased mRNA expression of variable exon 10 in macrophages stimulated with IL-4, and as this region contains a putative chondroitin sulfate addition site and a Bx7B motif (55), it is foreseeable that these may also modulate hyaluronan binding.
Here we have shown that inflammatory stimuli and IL-4 down- or up-regulate mRNA expression of two chondroitin GalNAc 6-sulfotransferases in mouse bone marrow-derived macrophages, which would be predicted to alter the sulfation of all chondroitin sulfated proteins, not just CD44. There are examples of chondroitin sulfate synthesis being responsive to cytokine treatment, including the anti-inflammatory and M2 cytokine TGF-β, which modifies the expression of various chondroitin sulfate proteoglycans and increases the length of chondroitin sulfate chains on secreted biglycan (57), decorin (57, 58), and versican (59). Chondroitin sulfate chain length is also increased on CD44 in human lung fibroblasts (60) and mouse melanoma cells (61) upon TGF-β treatment, raising the possibility that this cytokine may reduce hyaluronan binding in some conditions. Platelet-derived growth factor also increases chondroitin sulfate addition to CD44 in human dermal fibroblasts (62) and can increase the ratio of 6-to 4-sulfation (57, 59), although this has not been shown for CD44.
In addition to regulating hyaluronan binding, it is possible that the regulation of chondroitin sulfation to CD44 may have additional consequences. Several studies have suggested that chondroitin sulfate addition to CD44 is necessary for CD44-mediated binding to other extracellular matrix components, including fibronectin (63) and collagen (64, 65). The induced changes in chondroitin sulfate seen after cytokine treatment are important in promoting CD44-mediated motility on these substrates. Platelet-derived growth factor induces invasive migration of human dermal fibroblasts into a fibronectin/fibrin gel that can be blocked by both β-d-xyloside and anti-CD44 mAbs (62), whereas β-d-xyloside also inhibits invasion of rabbit wound microvascular endothelial cells into a fibrin matrix (66). Similarly, the invasion and migration of mouse melanoma on type I collagen is increased after TGF-β treatment and blocked by β-d-xyloside (61). Human melanoma cell migration on type IV collagen also involves chondroitin sulfate-modified CD44 in a possible cooperative role with integrins (65). Thus the presence of chondroitin sulfate can promote the migration of adherent cells on the extracellular matrix. It will be interesting to see if the regulation of chondroitin sulfate influences the migration of immune cells in inflamed tissues, particularly as we have shown that modulation of chondroitin sulfate on CD44 regulates its adhesion to the extracellular matrix component, hyaluronan.
Acknowledgments
We thank Andy Johnson at the University of British Columbia Flow Cytometry Facility for cell sorting. We also thank R. Keith Humphries and Bob Argiropoulos for providing and helping us set up the MIY retroviral system.
This work was supported in part by Canadian Institutes of Health Research Grant 81314 (to P. J.) and a grant from the Heart and Stroke Foundation of British Columbia and Yukon, Canada.
- TLR
- toll-like receptor
- β-d-xyloside
- p-nitrophenyl β-d-xylopyranoside
- CHST1
- KSGal6ST, galactose 6-sulfotransferase
- CHST2
- GlcNAcST1, N-acetylglucosamine 6-sulfotransferase
- CHST3
- C6ST-1, GlcNAcST4, N-acetylglucosamine and galactosamine 6-sulfotransferase
- CHST7
- C6ST-2, N-acetylgalactosamine 6-sulfotransferase
- fluorescent-hyaluronan
- fluorescein-conjugated hyaluronan
- GalNAc
- N-acetylgalactosamine
- M0-
- M1-, and M2-macrophages, unstimulated, LPS/IFNγ-stimulated, and IL-4-stimulated bone marrow-derived macrophages
- MFI
- mean fluorescent intensity
- PI
- propidium iodide
- CSGalNAcT
- chondroitin sulfate N-acetylgalactosamine transferase.
REFERENCES
- 1. Zhang X., Mosser D. M. (2008) J. Pathol. 214, 161–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. (2010) Science 327, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marriott H. M., Dockrell D. H. (2007) Exp. Lung Res. 33, 493–505 [DOI] [PubMed] [Google Scholar]
- 4. Van Ginderachter J. A., Movahedi K., Hassanzadeh Ghassabeh G., Meerschaut S., Beschin A., Raes G., De Baetselier P. (2006) Immunobiology 211, 487–501 [DOI] [PubMed] [Google Scholar]
- 5. Gordon S. (2007) Eur. J. Immunol. 37, S9–S17 [DOI] [PubMed] [Google Scholar]
- 6. Leibovich S. J., Ross R. (1975) Am J. Pathol. 78, 71–100 [PMC free article] [PubMed] [Google Scholar]
- 7. Duffield J. S., Forbes S. J., Constandinou C. M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J. P. (2005) J. Clin. Invest. 115, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crowther M., Brown N. J., Bishop E. T., Lewis C. E. (2001) J. Leukoc. Biol. 70, 478–490 [PubMed] [Google Scholar]
- 9. Gordon S., Martinez F. O. (2010) Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 10. Jameson J. M., Cauvi G., Sharp L. L., Witherden D. A., Havran W. L. (2005) J. Exp. Med. 201, 1269–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang D., Liang J., Noble P. W. (2007) Annu. Rev. Cell Dev. Biol. 23, 435–461 [DOI] [PubMed] [Google Scholar]
- 12. Culty M., Nguyen H. A., Underhill C. B. (1992) J. Cell Biol. 116, 1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Underhill C. B., Nguyen H. A., Shizari M., Culty M. (1993) Dev. Biol. 155, 324–336 [DOI] [PubMed] [Google Scholar]
- 14. Teder P., Vandivier R. W., Jiang D., Liang J., Cohn L., Puré E., Henson P. M., Noble P. W. (2002) Science 296, 155–158 [DOI] [PubMed] [Google Scholar]
- 15. Kawana H., Karaki H., Higashi M., Miyazaki M., Hilberg F., Kitagawa M., Harigaya K. (2008) J. Immunol. 180, 4235–4245 [DOI] [PubMed] [Google Scholar]
- 16. Liang J., Jiang D., Griffith J., Yu S., Fan J., Zhao X., Bucala R., Noble P. W. (2007) J. Immunol. 178, 2469–2475 [DOI] [PubMed] [Google Scholar]
- 17. Leemans J. C., Florquin S., Heikens M., Pals S. T., van der Neut R., Van Der Poll T. (2003) J. Clin. Invest. 111, 681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hollingsworth J. W., Li Z., Brass D. M., Garantziotis S., Timberlake S. H., Kim A., Hossain I., Savani R. C., Schwartz D. A. (2007) Am. J. Respir. Cell Mol. Biol. 37, 248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuff C. A., Kothapalli D., Azonobi I., Chun S., Zhang Y., Belkin R., Yeh C., Secreto A., Assoian R. K., Rader D. J., Puré E. (2001) J. Clin. Invest. 108, 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruffell B., Johnson P. (2009) in Glycoforum: Science of Hyaluronan Review Series (Hascall V. C., Toole B. P. eds) Vol. 33, pp. 1–20 [Google Scholar]
- 21. Lesley J., Howes N., Perschl A., Hyman R. (1994) J. Exp. Med. 180, 383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maeshima N., Poon G. F. T., Dosanjh M., Felberg J., Lee S. S. M., Cross J. L., Birkenhead D., Johnson P. (2011) Eur. J. Immunol. 41, 1108–1119 [DOI] [PubMed] [Google Scholar]
- 23. Levesque M. C., Haynes B. F. (1997) J. Immunol. 159, 6184–6194 [PubMed] [Google Scholar]
- 24. Brown K. L., Maiti A., Johnson P. (2001) J. Immunol. 167, 5367–5374 [DOI] [PubMed] [Google Scholar]
- 25. Mohamadzadeh M., DeGrendele H., Arizpe H., Estess P., Siegelman M. (1998) J. Clin. Invest. 101, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. English N. M., Lesley J. F., Hyman R. (1998) Cancer Research 58, 3736–3742 [PubMed] [Google Scholar]
- 27. Skelton T. P., Zeng C., Nocks A., Stamenkovic I. (1998) J. Cell Biol. 140, 431–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katoh S., Zheng Z., Oritani K., Shimozato T., Kincade P. W. (1995) J. Exp. Med. 182, 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruffell B., Johnson P. (2005) Biochem. Biophys. Res. Commun. 334, 306–312 [DOI] [PubMed] [Google Scholar]
- 30. Levesque M. C., Haynes B. F. (1999) Cell. Immunol. 193, 209–218 [DOI] [PubMed] [Google Scholar]
- 31. Katoh S., Miyagi T., Taniguchi H., Matsubara Y., Kadota J., Tominaga A., Kincade P. W., Matsukura S., Kohno S. (1999) J. Immunol. 162, 5058–5061 [PubMed] [Google Scholar]
- 32. Maiti A., Maki G., Johnson P. (1998) Science 282, 941–943 [DOI] [PubMed] [Google Scholar]
- 33. Brown K. L., Birkenhead D., Lai J. C., Li L., Li R., Johnson P. (2005) Exp. Cell Res. 303, 400–414 [DOI] [PubMed] [Google Scholar]
- 34. Tjew S. L., Brown K. L., Kannagi R., Johnson P. (2005) Glycobiology 15, 7C–13C [DOI] [PubMed] [Google Scholar]
- 35. Delcommenne M., Kannagi R., Johnson P. (2002) Glycobiology 12, 613–622 [DOI] [PubMed] [Google Scholar]
- 36. Culty M., O'Mara T. E., Underhill C. B., Yeager H., Jr., Swartz R. P. (1994) J. Leukoc. Biol. 56, 605–611 [DOI] [PubMed] [Google Scholar]
- 37. Schmits R., Filmus J., Gerwin N., Senaldi G., Kiefer F., Kundig T., Wakeham A., Shahinian A., Catzavelos C., Rak J., Furlonger C., Zakarian A., Simard J. J., Ohashi P. S., Paige C. J., Gutierrez-Ramos J. C., Mak T. W. (1997) Blood 90, 2217–2233 [PubMed] [Google Scholar]
- 38. Dougherty G. J., Cooper D. L., Memory J. F., Chiu R. K. (1994) J. Biol. Chem. 269, 9074–9078 [PubMed] [Google Scholar]
- 39. Li R., Wong N., Jabali M. D., Johnson P. (2001) J. Biol. Chem. 276, 28767–28773 [DOI] [PubMed] [Google Scholar]
- 40. de Belder A. N., Wik K. O. (1975) Carbohydr. Res. 44, 251–257 [DOI] [PubMed] [Google Scholar]
- 41. Zhou D. F., Ding J. F., Picker L. J., Bargatze R. F., Butcher E. C., Goeddel D. V. (1989) J. Immunol. 143, 3390–3395 [PubMed] [Google Scholar]
- 42. Antonchuk J., Sauvageau G., Humphries R. K. (2001) Exp. Hematol. 29, 1125–1134 [DOI] [PubMed] [Google Scholar]
- 43. Kinsella T. M., Nolan G. P. (1996) Hum. Gene Ther. 7, 1405–1413 [DOI] [PubMed] [Google Scholar]
- 44. Markowitz D., Goff S., Bank A. (1988) J. Virol. 62, 1120–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Izumikawa T., Okuura Y., Koike T., Sakoda N., Kitagawa H. (2011) Biochem. J. 434, 321–331 [DOI] [PubMed] [Google Scholar]
- 46. Ohmori K., Takada A., Ohwaki I., Takahashi N., Furukawa Y., Maeda M., Kiso M., Hasegawa A., Kannagi M., Kannagi R. (1993) Blood 82, 2797–2805 [PubMed] [Google Scholar]
- 47. Schäkel K., Kannagi R., Kniep B., Goto Y., Mitsuoka C., Zwirner J., Soruri A., von Kietzell M., Rieber E. (2002) Immunity 17, 289–301 [DOI] [PubMed] [Google Scholar]
- 48. Kusche-Gullberg M., Kjellén L. (2003) Curr. Opin Struct. Biol. 13, 605–611 [DOI] [PubMed] [Google Scholar]
- 49. Mosser D. M., Edwards J. P. (2008) Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brown K. L., Poon G. F. T., Birkenhead D., Pena O. M., Falsafi R., Dahlgren C., Karlsson A., Bylund J., Hancock R. E. W., Johnson P. (2011) J. Immunol. 186, 5497–5505 [DOI] [PubMed] [Google Scholar]
- 51. Schwartz N. B., Galligani L., Ho P. L., Dorfman A. (1974) Proc. Natl. Acad. Sci. U.S.A. 71, 4047–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peach R. J., Hollenbaugh D., Stamenkovic I., Aruffo A. (1993) J. Cell Biol. 122, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. (1990) Cell 61, 1303–1313 [DOI] [PubMed] [Google Scholar]
- 54. Banerji S., Wright A. J., Noble M., Mahoney D. J., Campbell I. D., Day A. J., Jackson D. G. (2007) Nat. Struct. Mol. Biol. 14, 234–239 [DOI] [PubMed] [Google Scholar]
- 55. Hayes G. M., Chiu R., Carpenito C., Dougherty S. T., Dougherty G. J. (2002) J. Biol. Chem. 277, 50529–50534 [DOI] [PubMed] [Google Scholar]
- 56. Ruffell B., Johnson P. (2008) J. Immunol. 181, 7044–7054 [DOI] [PubMed] [Google Scholar]
- 57. Schönherr E., Järveläinen H. T., Kinsella M. G., Sandell L. J., Wight T. N. (1993) Arterioscler. Thromb. 13, 1026–1036 [DOI] [PubMed] [Google Scholar]
- 58. Kähäri V. M., Larjava H., Uitto J. (1991) J. Biol. Chem. 266, 10608–10615 [PubMed] [Google Scholar]
- 59. Schönherr E., Järveläinen H. T., Sandell L. J., Wight T. N. (1991) J. Biol. Chem. 266, 17640–17647 [PubMed] [Google Scholar]
- 60. Romarís M., Bassols A., David G. (1995) Biochem. J. 310, 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Faassen A. E., Mooradian D. L., Tranquillo R. T., Dickinson R. B., Letourneau P. C., Oegema T. R., McCarthy J. B. (1993) J. Cell Sci. 105, 501–511 [DOI] [PubMed] [Google Scholar]
- 62. Clark R. A., Lin F., Greiling D., An J., Couchman J. R. (2004) J. Invest. Dermatol. 122, 266–277 [DOI] [PubMed] [Google Scholar]
- 63. Jalkanen S., Jalkanen M. (1992) J. Cell Biol. 116, 817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ehnis T., Dieterich W., Bauer M., Lampe B., Schuppan D. (1996) Exp. Cell Res. 229, 388–397 [DOI] [PubMed] [Google Scholar]
- 65. Knutson J. R., Iida J., Fields G. B., McCarthy J. B. (1996) Mol. Biol. Cell 7, 383–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Henke C. A., Roongta U., Mickelson D. J., Knutson J. R., McCarthy J. B. (1996) J. Clin. Invest. 97, 2541–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]