Abstract
The type I and type II bone morphogenetic protein receptors (BMPRI and BMPRII) are present at the plasma membrane as monomers and homomeric and heteromeric complexes, which are modulated by ligand binding. The complexes of their extracellular domains with ligand were shown to form heterotetramers. However, the dynamics of the oligomeric interactions among the full-length receptors in live cell membranes were not explored, and the roles of BMP receptor homodimerization were unknown. Here, we investigated these issues by combining patching/immobilization of an epitope-tagged BMP receptor at the cell surface with measurements of the lateral diffusion of a co-expressed, differently tagged BMP receptor by fluorescence recovery after photobleaching (FRAP). These studies led to several novel conclusions. (a) All homomeric complexes (without or with BMP-2) were stable on the patch/FRAP time scale (minutes), whereas the heterocomplexes were transient, a difference that may affect signaling. (b) Patch/FRAP between HA- and myc-tagged BMPRII combined with competition by untagged BMPRIb showed that the heterocomplexes form at the expense of homodimers. (c) Stabilization of BMPRII·BMPRIb heterocomplexes (but not homomeric complexes) by IgG binding to same-tag receptors elevated phospho-Smad formation both without and with BMP-2. These findings suggest two mechanisms that may suppress the tendency of preformed BMP receptor hetero-oligomers to signal without ligand: (a) competition between homo- and heterocomplex formation, which reduces the steady-state level of the latter, and (b) the transient nature of the heterocomplexes, which limits the time during which BMPRI can be phosphorylated by BMPRII in the heterocomplex.
Keywords: Bone Morphogenetic Protein (BMP), Membrane Biophysics, Receptor Structure-Function, Receptor Serine/Threonine Kinase, Signal Transduction, BMP Receptor Oligomerization, Fluorescence Recovery after Photobleaching (FRAP)
Introduction
Bone morphogenetic proteins (BMPs)2 are members of the transforming growth factor-β (TGF-β) cytokine superfamily (1–3). They play critical roles in numerous biological processes, including development, differentiation, and tissue regeneration in embryonic and mature tissues (4–8), and have been implicated in disorders such as primary pulmonary hypertension (9, 10), brachydactyly-type dysostosis (11–13), juvenile polyposis syndrome (14–16), and cancer (17). BMPs signal via two receptor Ser/Thr kinases, type I (BMPRIa and -Ib) and type II (BMPRII; as well as the type II activin receptors ActRII and ActRIIb) (5, 18–20). Unlike the related TGF-β receptors where type I (TβRI) fails to bind ligand and type II (TβRII) is the high affinity receptor, BMPRII binds BMP-2 only weakly, whereas BMPRI binds the ligand with high affinity (20–24).
Using immunofluorescence co-patching and co-immunoprecipitation studies, we have demonstrated that even prior to ligand binding the BMP receptors exist at the cell surface as a mixed population largely comprising monomers but also containing homodimers and heteromeric complexes (the latter are termed preformed complexes (PFCs)) (18, 20, 25, 26). Following ligand binding to PFCs (25), BMPRII phosphorylates BMPRI, which proceeds to phosphorylate Smad1/5/8; they then bind to Smad4 and translocate to the nucleus where they regulate the expression of specific target genes (2, 27). BMPs can also signal via non-Smad pathways, which appear to be initiated mainly by ligand-induced BMP receptor heterocomplexes, reflecting a different oligomerization mode (5, 19, 25, 26, 28, 29).
The crystal structures of BMP-2 in complex with the ectodomain (ECD) of BMPRIa (23) and of BMP-7 with the ECD of the ActRII (30, 31) show a dimeric ligand in complex with two receptors (homodimers). Recent studies (24, 32) reported the structures of the ternary heteromeric complexes of BMP-2 with the ECDs of BMPRIa and ActRII or ActRIIb. Interestingly, these heterocomplexes differ from the ternary complex of TGF-β3 with the ECDs of TβRI and TβRII (33) because only the TGF-β receptor heterocomplex displays a direct contact between TβRI and TβRII that contributes to the interactions (20).
Although structural studies have shown that the ECDs of the BMP receptors form homodimers and heterotetramers in the presence of ligand, there is no structural information on their interactions in the absence of ligand or on complex formation among the full-length receptors situated at the plasma membrane. Moreover, the dynamics of BMP receptor homomeric and heteromeric interactions (stable versus transient complexes) were hitherto unexplored. Here, we report a detailed investigation of the dynamics of BMP receptor homomeric and heteromeric complexes in live cells based on patch/fluorescence recovery after photobleaching (FRAP) studies applied to co-expressed BMP receptors carrying different extracellular epitope tags. Our studies demonstrate a striking difference between the dynamics of homomeric and heteromeric interactions in BMP receptor complexes: although the homomeric complexes were stable on the time scale of the FRAP studies, the heteromeric complexes were transient. This is distinct from the situation encountered for the TGF-β receptors where the heteromeric (type I-type II) complexes were stable (34) in line with an additional contribution of the direct contact between the two TGF-β receptor types to heterocomplex stability (33). The transient nature of the BMP receptor heterocomplexes limits the heterocomplex lifetime during which BMPRI is susceptible to phosphorylation by BMPRII; this may restrict the signaling capability of BMP receptor PFCs to signal without ligand. This notion is in line with our finding that stabilization of the BMPRII·BMPRIb complexes by bivalent anti-tag IgG binding to same-tag receptors elevated their Smad signaling in response to BMP-2. Notably, we show that the heteromeric BMPRII·BMPRIb complexes formed at the expense of homodimers. This suggests for the first time a potential role for BMP receptor homodimerization in suppressing the signaling of preformed heteromeric complexes in the absence of ligand.
EXPERIMENTAL PROCEDURES
Materials
COS7 cells (CRL 1651, American Type Culture Collection) were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum as described (18). Recombinant human BMP-2 was a gift from W. Sebald (University of Wuerzburg, Wuerzburg, Germany) or purchased from Peprotech, and fatty acid-free bovine serum albumin (BSA; fraction V) was from Sigma. 9E10 mouse IgG against the myc epitope tag (α-myc), HA.11 rabbit antiserum, and 12CA5 mouse IgG against the influenza hemagglutinin epitope tag (α-HA) were from Covance Research Products. IgG and monovalent Fab′ fragments were prepared as described (35). Normal goat γ-globulin was from Jackson ImmunoResearch Laboratories. Alexa Fluor 488-IgG of goat anti-rabbit (GαR) IgG, Alexa Fluor 488-F(ab′)2 of GαR IgG, and Alexa Fluor 546-F(ab′)2 of goat anti-mouse (GαM) IgG were from Invitrogen-Molecular Probes; fluorescent F(ab′)2 was converted to Fab′ as described (36). Rabbit α-phospho-Smad1/5/8 (C-terminal S*XS* motif where S* stands for phospho-Ser) was from Cell Signaling Technology, rabbit α-Smad1/5/8 was from Santa Cruz Biotechnology, mouse α-β-actin (antibody C4) was from MP Biomedicals, and peroxidase-coupled GαM and GαR IgGs were from Dianova.
Plasmids and Cell Transfection
Expression vectors encoding BMPRIa, BMPRIb, and BMPRII (the long form; Ref. 37) with extracellular myc or HA epitope tags in pcDNA1 (Invitrogen) were described by us earlier (18). The tagged receptors retained the ability to bind iodinated ligand and to induce BMP-2-mediated signaling (18, 25). BMPRIb with a cytoplasmic C-terminal HA tag in pCMV5 (38) was a gift from L. Attisano (University of Toronto, Ontario, Canada). COS7 cells growing in 6-well plates were co-transfected using FuGENE 6 (Roche Applied Science) with different combinations of vectors encoding myc- and HA-tagged BMP receptors, complementing to 2 μg of DNA by empty vector. The DNA amounts of the various vectors were adjusted to yield similar cell surface expression levels of the co-expressed differently tagged BMP receptor pairs and were validated as follows. 24 h post-transfection, the cells from each well were split onto several glass coverslips. After an additional 24 h, nonspecific binding was blocked by normal goat γ-globulin (200 μg/ml, 30 min, 4 °C); one coverslip was labeled for HA, and another was labeled for myc (4 °C surface labeling) by saturating amounts of murine IgG α-HA or α-myc (100 μg/ml, 30 min, 4 °C) to eliminate potential differences due to different affinities of the two antibodies. This was followed by labeling with Alexa Fluor 546-Fab′ GαM (100 μg/ml, 30 min, 4 °C). The cell surface fluorescence levels were quantified by the FRAP apparatus under nonbleaching conditions as described by us earlier (39), focusing the laser beam on the plasma membrane and measuring 200 cells per sample.
IgG-mediated Patching-Cross-linking
At 44–48 h post-transfection, COS7 cells transfected with pairs of BMP receptors carrying different extracellular epitope tags (e.g. myc-BMPRII with HA-BMPRIb) were serum-starved (2 h, 37 °C), washed with cold Hank's balanced salt solution (HBSS) supplemented with 20 mm Hepes (pH 7.4) and 2% BSA (HBSS/Hepes/BSA), and blocked with normal goat γ-globulin (200 μg/ml, 30 min, 4 °C). They were then labeled successively at 4 °C (to avoid internalization and enable exclusive cell surface labeling) in HBSS/Hepes/BSA (45 min incubations) with (i) monovalent mouse Fab′ α-myc (40 μg/ml) together with rabbit IgG α-HA (20 μg/ml) and (ii) Alexa Fluor 546-Fab′ GαM (40 μg/ml) together with Alexa Fluor 488-IgG GαR (20 μg/ml). This protocol results in the HA-tagged receptor cross-linked and immobilized by IgGs, whereas the myc-tagged receptor, whose lateral diffusion is then measured by FRAP (see below), is labeled exclusively by monovalent Fab′. In control experiments, the IgGs were replaced by rabbit Fab′ α-HA followed by Alexa Fluor 488-Fab′ GαR to enable identification of co-expressing cells but avoid cross-linking.
FRAP and Patch/FRAP
Co-expressed epitope-tagged BMP receptors labeled fluorescently by anti-tag Fab′ fragments as described above were subjected to FRAP studies. The FRAP measurements were conducted at 15 °C, replacing the sample with a fresh one after 20 min to minimize internalization during the measurement. For FRAP studies at 37 °C, samples were replaced within 5 min. An argon ion laser beam (Innova 70C, Coherent) was focused through a fluorescence microscope (Axio Imager.D1, Carl Zeiss MicroImaging) to a Gaussian spot of 0.77 ± 0.03 μm (plan apochromat 63×/1.4 numerical aperture oil immersion objective). After a brief measurement at monitoring intensity (528.7 nm, 1 microwatt), a 5-milliwatt pulse (10–20 ms) bleached 60–75% of the fluorescence in the spot, and recovery was followed by the monitoring beam. The lateral diffusion coefficient (D) and the mobile fraction (Rf) were extracted from the FRAP curves by nonlinear regression analysis, fitting to a lateral diffusion process (40). Patch/FRAP studies were performed similarly except that antibody-mediated cross-linking-patching of an HA-tagged BMP receptor (described above) preceded the measurement (41, 42). This enables determination of the effects of immobilizing one receptor type on the lateral diffusion of the co-expressed receptor, allowing identification of complex formation between them and distinction between transient and stable interactions (41, 42).
Smad Phosphorylation Assay and Western Blotting
COS7 cells were transfected with different combinations of BMP receptors as described under “Plasmids and Cell Transfection.” After serum starvation (growth medium without serum, 2 h), cells were stimulated in starvation medium with 10 nm BMP-2 for the periods indicated in the figure legends. Following ligand stimulation, cells were lysed and subjected to electrophoresis by 10% SDS-PAGE (loading 100 μg of protein per lane) followed by immunoblotting as described (43). The blots were probed by rabbit α-phospho-Smad1/5/8 (1:1000) or mouse α-β-actin (1:10,000) followed by peroxidase GαR or GαM IgG (1:5000). In some cases, the blots were stripped (44) and reprobed for total Smad1/5/8 using rabbit α-Smad1/5/8 (1:1000) and peroxidase-GαR IgG. The bands were visualized by ECL (Amersham Biosciences) and quantified by densitometry (EZQuant-Gel 2.2 from EZQuant Ltd. or ImageJ).
RESULTS
BMP Receptors Form Stable Homomeric Complexes
We have formerly used immunofluorescence co-patching and co-immunoprecipitation to demonstrate that a fraction of the cell surface BMP receptors form homomeric and heteromeric complexes even in the absence of ligand; some of these (heteromeric type I-type II and homomeric type I complexes) are enhanced by ligand (18, 25). However, both methods can only detect relatively stable complexes, whereas transient oligomers may dissociate during the patching or immunoprecipitation steps (34). The mode of interaction between the BMP receptors in the homomeric and heteromeric complexes (stable versus transient interactions on the time scale of the FRAP experiments) can be assessed by the combination of IgG-mediated patching with FRAP (patch/FRAP) (34, 41, 42). In this approach, one receptor is patched and laterally immobilized by cross-linking with a double layer of IgGs; the effect on the lateral diffusion of a differently tagged co-expressed receptor (labeled by monovalent Fab′ fragments) is measured by FRAP (see “Experimental Procedures”). Complex formation between the receptors may result in reducing either the Rf or D of the Fab′-labeled (uncross-linked) receptor, depending on the FRAP time scale relative to the rates of the dissociation-association kinetics of the complex. If the complex lifetimes are longer than the characteristic FRAP times, the IgG-mediated immobilization of one receptor type is expected to reduce Rf of the uncross-linked receptor because bleached molecules of the latter would not undergo appreciable dissociation from the immobile patches during the FRAP measurement. On the other hand, if the complex lifetime is short relative to the FRAP time, each molecule of the Fab′-labeled receptor would undergo several association-dissociation cycles during the recovery phase of the FRAP experiment, leading to a reduction in D rather than in Rf (41, 42). When the effect is on Rf, the percentage of oligomerization among different receptors can be calculated from the percent reduction in Rf of the uncross-linked receptor; for example, a reduction of Rf from 0.80 to 0.60 upon patching of the co-expressed receptor implies 100 × (0.20/0.80) = 25% oligomerization. A limitation of the method is that it yields information exclusively on the mobile receptor population; thus, no information can be derived on the receptor population that is immobile already prior to the patching step. It should be noted that when the effect is on D the transient nature of the interactions precludes an exact calculation of the percentage of oligomerization because the extent of retardation in the apparent D value depends not only on the concentrations of complexed versus free receptors but also on the binding-unbinding rates of the Fab′-tagged receptor to the co-expressed immobilized receptors relative to the lateral diffusion rate (41, 42).
We first used patch/FRAP to investigate the interaction mode in homomeric BMP receptor complexes at the surface of cells co-expressing differently tagged receptors of the same subtype. Typical curves showing that Fab′-labeled (uncross-linked) BMP receptor (e.g. myc-BMPRII) was laterally mobile whereas IgG patching of e.g. HA-BMPRII resulted in its lateral immobilization are depicted in Fig. 1 (A and B). The average results of many patch/FRAP experiments on all the potential homomeric BMP receptor pairs (including HA-BMPRIa/myc-BMPRIb) are summarized in Fig. 2. IgG-mediated cross-linking and immobilization of one of the co-expressed receptors (e.g. HA-BMPRIa; Fig. 2, A and B) resulted in a significant reduction in Rf of its counterpart (myc-BMPRIa) already in the absence of ligand (Fig. 2B, compare second and third white bars) without affecting the D value (Fig. 2A). Reduction in Rf with no effect on D is typical for stable interactions between receptors on the FRAP time scale (34, 42). Analogous results (reduction in Rf but not in D) were obtained for all the homomeric BMP receptor pairs, including pairs consisting of the two different type I BMP receptors (BMPRIa and BMPRIb). These findings suggest that all the homomeric BMP receptor complexes are stable at the time scale of the FRAP experiments (minutes). Importantly, addition of the ligand BMP-2 resulted in a further significant decrease in Rf of the myc-tagged receptors upon cross-linking of the HA-tagged co-expressed counterparts (Fig. 2, B, D, F, and H, black bars) except for the pair HA-BMPRII/myc-BMPRII (Fig. 2H), whose affinity to BMP-2 in the absence of type I receptors is very low (20). Quantitatively, a statistical correction has to be applied to derive the percentage of receptors in homodimers from the reduction in Rf in the patch/FRAP experiments (45). For homodimers containing myc- and HA-tagged receptors, the probabilities of homodimer formation are 1:2:1 for myc/myc-, myc/HA + HA/myc-, and HA/HA-containing dimers. The myc-tagged receptors would be immobilized along with the patched HA-tagged counterpart only in the differently tagged dimers, whereas myc/myc-containing homodimers would not be affected. In addition, the latter complexes contain two myc tags and would therefore be labeled at twice the intensity of myc/HA-containing homodimers. These statistical considerations (36, 45) imply that for homodimers (but not for heterodimers) the percent reduction in Rf in patch/FRAP should be multiplied by a factor of 2 to obtain the percentage of homodimeric receptors. Thus, the reduction in Rf of the mobile myc-BMPRIa population from 0.77 to 0.64 upon immobilization of HA-BMPRIa (Fig. 2B, white bars) implies a reduction of 100 × (0.17/0.77) = 17%, suggesting that 17 × 2 = 34% of the mobile population of these receptors are in homodimers. The reduction in the Rf values is even stronger (26 × 2 = 52% of the mobile population) in the presence of BMP-2 (Fig. 2B, black bars). Such calculations show analogous increases in the percentage of dimerization of HA-BMPRIb/myc-BMPRIb (16 and 49% without and with BMP-2, respectively; Fig. 2D) and HA-BMPRIa/myc-BMPRIb (28 and 41%, respectively, Fig. 2F; no statistical correction was applied because the Ia and Ib are different receptors). On the other hand, the homodimerization of BMPRII, which binds BMP-2 weakly, did not increase appreciably following BMP-2 binding (30 and 36% without and with BMP-2, respectively; Fig. 2H). These findings are in line with our earlier studies on homomeric BMP receptor complexes by semiquantitative immunofluorescence co-patching measurements (18, 25), although the latter also contain a contribution from the immobile receptor population, which in part may be co-localized in immobile structures, and some loss of complexes may also occur during the longer co-patching procedure.
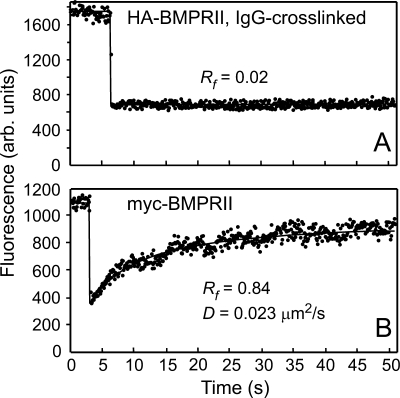
FIGURE 1.
Representative FRAP curves demonstrating lateral diffusion of Fab′-labeled myc-BMPRII and immobilization of HA-BMPRII following IgG cross-linking. COS7 cells co-transfected with myc- and HA-tagged BMPRII were subjected to the antibody-mediated patching-cross-linking protocol detailed under “Experimental Procedures” (A), resulting in HA-BMPRII patched and labeled by Alexa Fluor 488-IgG GαR, whereas myc-BMPRII was labeled exclusively by non-cross-linking Fab′ (secondary Fab′-Alexa Fluor 546 GαM). In B, to avoid cross-linking, the IgGs used to label the HA tag were replaced by Fab′ fragments (see “Experimental Procedures”). FRAP studies were conducted at 15 °C. Solid lines represent the best fit of a nonlinear regression analysis to the lateral diffusion equation (40). A, a representative FRAP curve showing that IgG-cross-linked HA-BMPRII is laterally immobile. Only the Rf value is shown as the recovery was too low to enable determination of D. B, a representative FRAP curve of the lateral diffusion of myc-BMPRII in a cell co-expressing HA-BMPRII (no IgG cross-linking). arb., arbitrary.
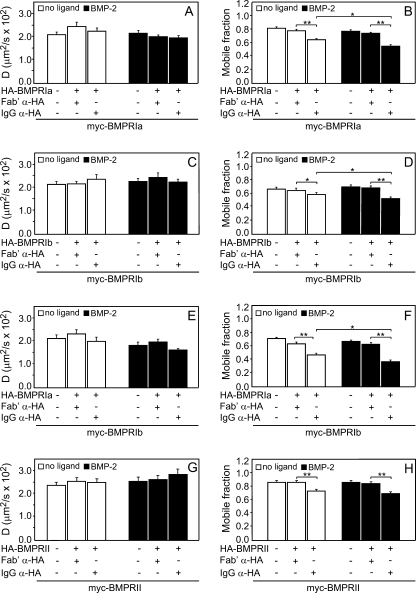
FIGURE 2.
Patch/FRAP studies demonstrate stable interactions in all homomeric BMP receptor complexes. COS7 cells were co-transfected with pairs of expression vectors encoding myc- and HA-tagged BMP receptors of the same subtype (either type I or type II). In experiments with singly expressed myc-tagged receptors, the HA-tagged construct was replaced by empty vector (left-most bars). The cells were subjected to the patching-cross-linking protocol described under “Experimental Procedures.” Control experiments (avoiding cross-linking of co-expressed HA-tagged receptors) were conducted similarly except that exclusive Fab′ labeling was used (“Experimental Procedures”). IgG α-HA indicates experiments where the HA-tagged receptor was cross-linked by a double layer of IgGs; Fab′ α-HA indicates control experiments without HA cross-linking. FRAP experiments were conducted at 15 °C as described under “Experimental Procedures,” measuring the diffusion of the Fab′-labeled myc-tagged receptors. Where indicated, BMP-2 (10 nm) was added at 4 °C 30 min prior to incubation with the antibodies and retained throughout the experiment. A, C, E, and G, average D values derived from 30–40 experiments. B, D, F, and H, average Rf values derived from the patch/FRAP measurements. Bars are mean ± S.E. of 30–40 measurements in each case. Asterisks indicate significant differences between the Rf values of the pairs indicated by brackets (**, p < 0.001; *, p < 0.05; Student's t test). No significant differences were found in the D values following IgG-mediated cross-linking comparing each bar with the co-expressed uncross-linked (Fab′ α-HA) control.
Heteromeric BMP Receptor Complexes Are Transient
We next explored the dynamics of the interactions between BMPRI and BMPRII residing in heteromeric complexes. To this end, we conducted patch/FRAP studies on differently tagged BMPRI/BMPRII pairs without and with ligand (BMP-2), measuring the effects of patching the HA-tagged receptors on the lateral diffusion of the Fab′-labeled myc-tagged counterparts (Fig. 3). Surprisingly, in sharp contrast to the dynamics of BMP receptor homomeric complexes (Fig. 2), the effects observed in the patch/FRAP studies on heteromeric BMP receptor complexes were exclusively on the D values (no significant effects on Rf), attesting to transient rather than stable interactions in the heteromeric type I·type II BMP receptor complexes. IgG cross-linking of HA-tagged BMPRIa or BMPRIb resulted in a significant reduction in the D values of the co-expressed myc-BMPRII already in the absence of ligand (Fig. 3, A and C, white bars). In both cases, the Rf values were not significantly altered (Fig. 3, B and D). Importantly, the effects of immobilizing HA-BMPRI subtypes by IgG cross-linking on D of myc-BMPRII were significantly enhanced in the presence of BMP-2 (Fig. 3, A and C, black bars). The enhanced reductions in the D values of myc-BMPRII in the presence of ligand were significant not only relative to the D values in uncross-linked co-expressing cells (Fig. 3, A and C, compare the fifth and sixth bars) but also relative to the IgG-cross-linked cells in the absence of ligand (Fig. 3, A and C, compare the third and sixth bars), suggesting that BMP-2 binding enhances the heteromeric interactions. It should be noted that the transient nature of the BMP receptor heterocomplexes precludes a calculation of the percentage of oligomerization from the reduction in D as detailed in the former section. However, an estimate of the level of heterocomplex formation among the different BMP receptors can be obtained from our earlier co-patching studies (18, 25, 46); these provide a lower limit for heterocomplex formation because due to their transient nature some of the complexes may dissociate during the long incubation steps in the co-patching protocol. Calculation of the percentage of oligomerization from these experiments (18, 25, 46) shows significant amounts of heterocomplexes prior to ligand binding (13–19% for BMPRIa·BMPRII and 22–23% for BMPRIb·BMPRII complexes), which increase in the presence of BMP-2 (37–41 and 34–37% for BMPRIa·BMPRII and BMPRIb·BMPRII, respectively). Taken together, the results of the patch/FRAP studies (Figs. 2 and 3) demonstrate a fundamental difference between homomeric and heteromeric BMP receptor complexes: although the homomeric complexes are relatively stable, the heteromeric complexes are highly transient, undergoing several association-dissociation cycles on the time scale of the FRAP experiments (i.e. within several seconds as the half-time of BMP receptor recovery in these experiments is around 7–15 s; see Fig. 1B).
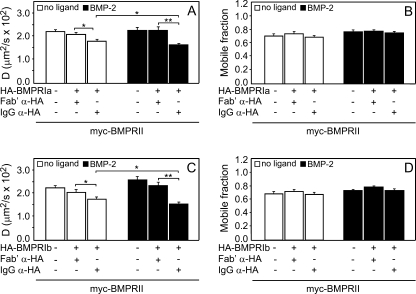
FIGURE 3.
Patch/FRAP studies demonstrate transient interactions between BMPRI and BMPRII in heteromeric complexes. COS7 cells were co-transfected by myc-BMPRII and HA-BMPRIa or -Ib. Patching-labeling of the differently tagged receptors (by IgGs and/or Fab′ fragments) and patch/FRAP experiments were conducted at 15 °C exactly as described in Fig. 2 and under “Experimental Procedures.” In all cases, the FRAP measurements were conducted on the Fab′-labeled myc-BMPRII. IgG α-HA and Fab′ α-HA stand for experiments where the HA-tagged receptors were either IgG-cross-linked or Fab′-labeled (no cross-linking), respectively. A and C, average D values. B and D, average Rf values. In all cases, each bar is the mean ± S.E. of 30–40 measurements. Asterisks indicate significant differences between the D values of the pairs indicated by brackets (**, p < 0.01; *, p < 0.05). No significant differences were found between the Rf values of myc-BMPRII as a result of cross-linking co-expressed HA-BMPRIa or HA-BMPRIb.
To validate that the basic difference between the stability of homomeric and heteromeric BMP receptor complexes holds also at 37 °C, we repeated the core patch/FRAP experiments on homomeric (HA-BMPRII/myc-BMPRII) and heteromeric (HA-BMPRIb/myc-BMPRII) interactions at 37 °C. As shown in Fig. 4, the results were similar to those observed at 15 °C, the only difference being that the D values of myc-BMPRII at 37 °C were 1.5–1.7-fold higher than at 15 °C in accord with the lower viscosity of the membrane at the higher temperature. Thus, the stable versus transient nature of homomeric versus heteromeric BMP receptors holds also at 37 °C.
FIGURE 4.
Patch/FRAP studies at 37 °C. COS7 cells were co-transfected by pairs of vectors encoding HA-BMPRII/myc-BMPRII (A and B) or HA-BMPRIb/myc-BMPRII (C and D). Patching-labeling by IgGs and/or Fab′ fragments and patch/FRAP experiments were as in Figs. 2 and 3 except that the FRAP studies (measuring in all cases the Fab′-labeled myc-BMPRII) were conducted at 37 °C. IgG α-HA and Fab′ α-HA stand for experiments where the HA-tagged receptors were either IgG-cross-linked or Fab′-labeled, respectively. A and C, average D values. B and D, average Rf values. Bars are mean ± S.E. of 30–40 measurements in each case. Asterisks indicate significant differences between values of the pairs indicated by brackets (**, p < 0.01; Student's t test).
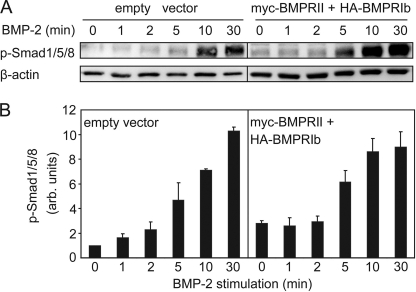
Because BMP signaling is initiated by heterocomplex formation, a prediction that follows from the relatively fast association-dissociation kinetics of BMP receptor heterocomplexes is that BMP-mediated signaling should be detected within a time scale that is only slightly longer than that of heterocomplex dynamics. We therefore investigated the formation of phospho-Smad1/5/8 in response to BMP-2 as a function of time both in mock-transfected COS7 cells (expressing only endogenous receptors) and in COS7 cells co-transfected with myc-BMPRII and HA-BMPRIb. The results (Fig. 5) demonstrate that phospho-Smad1/5/8 formation can be detected in mock-transfected cells already 1 min after addition of BMP-2, increasing further with time. This fits with the above prediction based on BMP receptor heterocomplex dynamics. Notably, a low but detectable level of phospho-Smad1/5/8 is noticed already in the absence of ligand (zero time point) in serum-starved cells transfected with empty vector (Fig. 5A, left lane), suggesting that the endogenous receptors may induce a low level of Smad signaling even without ligand. This notion is supported by the detection of low levels of phospho-Smad1/5/8 in unstimulated C2C12 cells, which were further inhibited by the BMPRI inhibitors dorsomorphin and LDN-193189 (47). As shown in Fig. 5, the level of phospho-Smad1/5/8 at time 0 increased about 2-fold in cells transfected with the BMP receptors, masking the BMP-2-mediated activation at the very short time points and suggesting that increased formation of heterocomplexes in the higher expressing transfected cells may enhance the signaling prior to ligand stimulation. This notion gains further support from data showing that IgG-mediated stabilization of BMPRII·BMPRIb heterocomplexes increases phospho-Smad1/5/8 formation in the absence of BMP-2 (Fig. 7B, compare the seventh bar with the other white bars).
FIGURE 5.
Western blot analysis shows fast Smad1/5/8 phosphorylation in response to BMP-2. COS7 cells were either mock-transfected (empty vector) or co-transfected with HA-BMPRIb together with myc-BMPRII (see “Experimental Procedures”). After serum starvation, cells were stimulated with 10 nm BMP-2 for the indicated periods followed by cell lysis, SDS-PAGE, and Western blotting with α-phospho-Smad1/5/8 (p-Smad1/5/8) as detailed under “Experimental Procedures.” A, representative blots (one of three independent experiments). B, densitometric quantification of the multiple experiments as a function of time, normalizing to β-actin (loading control). Results (mean ± S.E.) were calibrated relative to the value obtained in mock-transfected unstimulated cells at zero time. arb., arbitrary.
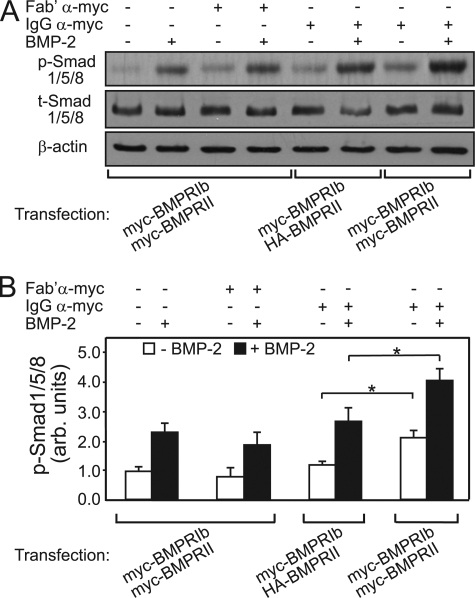
FIGURE 7.
Stabilization of myc-BMPRIb·myc-BMPRII complexes by IgG α-myc enhances phosphorylation of BMP-responsive Smads. COS7 cells were co-transfected with myc-BMPRIb together with either myc-BMPRII or HA-BMPRII as described under “Experimental Procedures.” After serum starvation (2 h), the cells were incubated (4 °C, 45 min) in buffer alone (HBSS/Hepes/BSA; control) or in buffer containing either monovalent Fab′ α-myc (40 μg/ml; uncross-linked control) or IgG α-myc (20 μg/ml). Where indicated, BMP-2 (10 nm) was added with the antibodies. This was followed by incubation in starvation medium prewarmed to 37 °C (with or without BMP-2) for 20 min. The cells were lysed and subjected to SDS-PAGE and Western blotting with α-phospho-Smad1/5/8 (p-Smad1/5/8) and with antibodies to β-actin as described under “Experimental Procedures.” The blot membranes were then stripped and reprobed for total Smad1/5/8 (t-Smad1/5/8). A, representative immunoblots (one of five experiments). B, densitometric analysis of data derived from multiple experiments (mean ± S.E.; n = 5), normalizing to β-actin (loading control). The results were calibrated relative to the value obtained in control cells (incubated without Fab′ or IgG α-myc) in the absence of BMP-2 stimulation. Asterisks indicate significant differences between the phospho-Smad1/5/8 levels of the pairs indicated by the brackets either in the absence (white bars) or presence (black bars) of BMP-2 (*, p < 0.05; Student's t test). These differences were similarly significant also relative to the other controls (myc-BMPRIb·myc-BMPRII-expressing cells incubated in buffer or incubated with monovalent Fab′ α-myc; first and second bar pairs). arb., arbitrary.
Heteromeric BMP Receptor Complexes Are Formed at Expense of Homomeric Complexes
The existence of both homomeric and heteromeric BMP receptor complexes at the cell surface raises interesting questions pertaining to the potential roles of homomeric complexes in the regulation of BMP signaling. Because BMP signaling requires both type I and type II receptors and is initiated via the heteromeric complexes (2, 19, 20, 26, 27), an attractive possibility is that the formation of homomeric complexes may compete with heterocomplex formation, reducing the steady-state levels of heteromeric PFCs and suppressing unwanted spontaneous signaling in the cell.
A prediction of this hypothesis is that the formation of heteromeric complexes would proceed at the expense of homomeric complexes. To experimentally test this prediction, we used patch/FRAP to determine whether expression of a type I BMP receptor (BMPRIb-HA; HA-tagged at the intracellular C terminus and thus unable to bind the extracellularly applied IgG α-HA) reduces the level of myc-BMPRII·HA-BMPRII homomeric complexes (Fig. 6). If heteromeric complexes between BMPRIb and BMPRII are formed at the expense of homomeric myc-BMPRII·HA-BMPRII complexes, one would expect a weaker effect of immobilizing HA-BMPRII on the Rf of myc-BMPRII. As shown in Fig. 6A, co-expression of BMPRIb-HA significantly decreased the reduction in Rf of myc-BMPRII following immobilization of HA-BMPRII; the D values were not affected (Fig. 6B) in keeping with the relatively stable nature of the homomeric complexes. This effect of BMPRIb on the Rf of myc-BMPRII in the patch/FRAP experiments was augmented following addition of BMP-2 in line with the ability of BMP-2 to increase heteromeric BMP receptor complexes (in this experiment, BMPRIb·BMPRII) but not homomeric BMPRII complexes (compare Figs. 2H and 3C), supporting our earlier immunofluorescence co-patching studies (18). We conclude that the heteromeric complexes are formed at the expense of the homomeric BMPRII complexes in keeping with the notion that homomeric BMP receptor complexes may limit excessive formation of heteromeric PFCs in the absence of ligand.
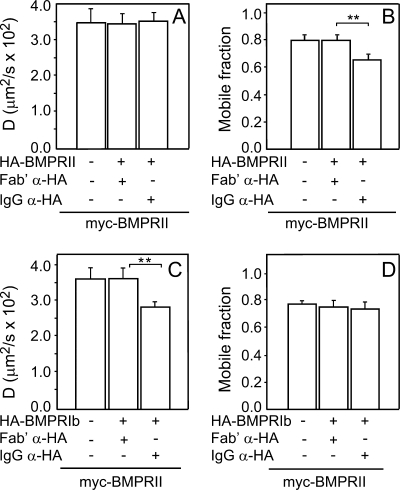
FIGURE 6.
Co-expression with BMPRIb reduces homomeric complexes between HA-BMRII and myc-BMPRII. COS7 cells were co-transfected by extracellularly tagged myc-BMPRII and HA-BMPRII along with intracellularly tagged BMPRIb-HA or empty vector. Patching-labeling of the differently tagged receptors (by IgGs and/or Fab′ fragments) and patch/FRAP experiments were as in Fig. 2 (see “Experimental Procedures”). All FRAP measurements were conducted at 15 °C on the Fab′-labeled myc-BMPRII. IgG α-HA and Fab′ α-HA stand for experiments where the extracellularly HA-tagged receptors were either IgG-cross-linked or Fab′-labeled (no cross-linking), respectively. A, average Rf values derived from 30–40 measurements. B, average D values of the same experiments. Each bar is the mean ± S.E. of 30–40 measurements. Asterisks indicate significant differences between the Rf values of the pairs indicated by brackets (**, p < 0.01; *, p < 0.05). The D values of myc-BMPRII were not significantly affected by overexpression of BMPRIb or by cross-linking of HA-BMPRII.
Stabilization of BMPRI·BMPRII Heteromeric Complexes by Limited IgG Cross-linking Enhances Smad Phosphorylation
The transient nature of the BMP receptor heterocomplexes may provide yet another mechanism to restrict signaling from PFCs by limiting the lifetime of the heterocomplexes where BMPRI is phosphorylated and activated by BMPRII. A prediction of this hypothesis is that stabilization of the transient heterocomplexes would enhance signaling through the Smad pathway already in the absence of ligand and increase also BMP-2-mediated Smad phosphorylation. To test this prediction, we used a single layer of bivalent IgG α-myc to induce limited cross-linking and stabilization of myc-tagged receptor complexes in cells co-expressing myc-BMPRIb and myc-BMPRII. The probability that two myc epitopes will be bridged by the IgG is highest for tags located at close proximity, i.e. on BMP receptor complexes. Because BMP signaling is mediated by the heterocomplexes, stabilization of type I/type II receptor interactions within them would be the most relevant for signaling. Indeed, in the crystal structure of BMP-2·type I·type II BMP receptor heterocomplexes (24, 32), the N termini (where the single myc tag is located in our constructs) of the type I and type II receptors are located at the closest proximity, 38 Å (see Research Collaboratory for Structural Bioinformatics Protein Data Bank information in Ref. 32). Moreover, the N-terminal regions are unstructured, and the N-terminal amino acids missing in the structure (34 in BMPRIa and six in ActRIIb; Ref. 32) are also predicted (Predator) to be unstructured. Because even in a β-sheet conformation an amino acid occupies 3.32 Å, this addition alone can easily cover the entire distance between the N termini of the different BMP receptors in the heterocomplex, enabling their bridging by the IgG. This notion is validated by the experiments depicted in Fig. 7 clearly demonstrating that limited cross-linking by IgG α-myc significantly increased phospho-Smad1/5/8 formation already in the absence of ligand (therefore mediated via PFCs) with a further significant enhancement upon stimulation with BMP-2 (Fig. 7, rightmost bar pair relative to all other pairs). The effect of α-myc cross-linking is unlikely to overlap with that of ligand stimulation because the myc tags bridged by the IgG are in the unstructured N termini of the BMP receptors remote from the ligand-binding regions. Thus, although the ligand induces the formation of a high level of BMP receptor heterocomplexes (18, 25), these complexes remain highly transient (Fig. 3). On the other hand, bridging by IgG α-myc stabilizes the heterocomplexes, thereby enhancing signaling both in the absence and presence of ligand.
Several controls demonstrate that these effects are due to cross-linking/stabilization of the heteromeric myc-BMPRIb·myc-BMPRII complexes. First, monovalent Fab′ α-myc, which does not induce cross-linking, had no effect on Smad phosphorylation (Fig. 7, first and second bar pairs). Second, to rule out that some of the effects of IgG α-myc are due to cross-linking of myc-tagged receptors of the same type (within the heteromeric complexes or in homodimers), we tested the effect of IgG α-myc cross-linking on cells co-expressing myc-BMPRIb and HA-BMPRII; under these conditions, only myc-BMPRIb receptors can be cross-linked among themselves. The results (Fig. 7, third bar pair) show no significant increase relative to the other controls (first and second bar pairs). Taken together, the experiments depicted in Fig. 7 demonstrate that stabilization of heteromeric BMP receptor complexes increases Smad phosphorylation both in the absence and presence of ligand, supporting the notion that the transient nature of the heteromeric complexes can contribute to the attenuation of their spontaneous signaling.
DISCUSSION
Regulation of BMP signaling occurs on many levels, including receptor oligomerization and endocytosis, interactions of modulatory proteins with either the ligands or the receptors (co-receptors), interactions with scaffold proteins, and modulation of transcriptional activation (for reviews, see Refs. 2–4, 19, 20, and 26). These regulatory mechanisms operate on different time scales from slow (e.g. effects on development and transcriptional regulation) to fast responses. An important level of regulation on a near-immediate time scale is interactions among the receptors and their modulation in response to ligand binding. The current work presents biophysical studies aimed to explore the interactions among BMP receptors situated at the plasma membrane of live cells, focusing on the hitherto unknown interaction dynamics among the different types of BMP receptor complexes. We show that the interaction dynamics in homomeric and heteromeric BMP receptor complexes are distinctly different independent of ligand binding; although the homomeric complexes were stable for at least several minutes, heteromeric complexes between type I and type II BMP receptors were transient. Accordingly, stabilization of the heteromeric complexes by limited cross-linking (bivalent anti-tag IgG) facilitated signaling to the Smad pathway, suggesting a role for the dynamic interactions in the heterocomplexes in restricting signaling from unstimulated PFCs. Notably, based on the finding that the level of BMP receptor homodimers was reduced upon elevation of heterocomplex formation, we propose that BMP receptor homodimerization may limit the formation of BMP receptor PFCs, suppressing their signaling in the absence of ligand.
Recent crystallographic studies on the structure of ternary heterocomplexes comprising the ECDs of BMPRIa and ActRII (or ActRIIb) and BMP-2 (24, 32) have shown these complexes to be heterotetrameric with respect to the receptors where the receptor ECDs interact only via the dimeric ligand (for a review, see Ref. 20). This suggests that the ECDs of BMP receptors are sufficient for heterocomplex formation in the presence of ligand as was also shown for the BMP-2-bound homodimer of the BMPRIa ECD (23) and for the ActRII ECD bound to BMP-7 (30, 31). However, the crystallographic studies on the ECDs of the receptors do not address the oligomeric structure of the full-length receptors in the cell membrane or the potential contribution of their transmembrane and cytoplasmic regions to the formation of homomeric and/or heteromeric complexes. Moreover, although crystallographic studies yield static structure information, they cannot probe the dynamics of the complexes (i.e. stable versus transient interactions). Here, we addressed these issues by biophysical studies (patch/FRAP) that quantitatively measure the interaction dynamics between full-length BMP receptors at the surface of live cells.
In the patch/FRAP studies (Figs. 1–4), we co-expressed differently tagged pairs of BMPRI and BMPRII, immobilized the HA-tagged receptor by IgG-mediated patching, and measured the effects on the lateral diffusion of the co-expressed Fab′-labeled (uncross-linked) myc-tagged counterpart. For all co-expressed receptors of the same type (e.g. HA-BMPRIa and myc-BMPRIa to measure homomeric interactions), a partial reduction in Rf was observed already without ligand (Figs. 2 and 4A). The fact that Rf was only partially reduced suggests the existence of a mixed population of BMP receptors at the cell surface, a fraction of which resides in homomeric complexes. Except for BMPRII, which has low affinity to BMP-2, the reduction in Rf became stronger following BMP-2 binding for all other homomeric receptor pairs (Fig. 2). These results are in agreement with our earlier immunofluorescence co-patching studies (18, 25, 46). The fact that the immobilization of the HA-tagged BMP receptor reduced the Rf of its myc-tagged counterpart without affecting D demonstrates that there is no significant exchange between the monomeric and homodimeric receptor populations on the time scale of the FRAP measurements (minutes), suggesting that the BMP receptor homomeric complexes are stable for at least several minutes (Figs. 1 and 2). These findings resemble our observations with the related TGF-β receptors that detected a fraction of the receptors in stable homodimers with a significant increase in homodimerization of the ligand-binding TβRII in the presence of TGF-β1 (34, 36). Recent single molecule imaging studies on GFP-tagged TβRII supported TGF-β1-enhanced homodimerization of TβRII, although the homodimerization level measured without ligand was lower most likely due to the lower TβRII expression levels (48). Importantly, analogous patch/FRAP studies on co-expressed different types of BMP receptors (HA-BMPRIa or HA-BMPRIb with myc-BMPRII to measure heteromeric interactions) demonstrated a reduction in D of the Fab′-labeled receptor rather than in its Rf; the reduction was augmented in the presence of BMP-2 (Fig. 3). This is typical of transient interactions because Fab′-labeled receptors can dissociate and reassociate with their immobilized counterparts several times during the FRAP measurement (41, 42). The formation of heteromeric BMP receptor complexes, which appear here to be in dynamic exchange with other cell surface receptor populations, is in line with earlier immunofluorescence co-patching and co-immunoprecipitation studies (18, 25), which showed the existence of PFCs and ligand-mediated heteromeric complexes among type I and type II BMP receptors. Notably, the dynamic nature of the BMP receptor heteromeric complexes is strikingly different not only from the stable BMP receptor homodimers but also from the stable TβRI/TβRII heterodimers as shown both in patch/FRAP (34) and by the stability of the heterocomplexes during hours-long size determination by ultracentrifugation (49). The higher stability of TGF-β receptor heterocomplexes, at least in the presence of ligand, is in accord with the crystallographic studies on ligand-bound ECD heteromeric complexes of BMP and TGF-β receptors. These studies demonstrated that although the BMP receptor ECDs interact only via the ligand the TGF-β receptor ECDs display a direct contact between the TβRII-TGF-β3 interface and TβRI (20, 33) that may add a further element of stability to the TGF-β receptor complexes.
Although the patch/FRAP experiments were necessarily conducted on transfected cells expressing epitope-tagged receptors, there are several indications that the receptor interactions measured are relevant also for low expression levels and for the endogenous receptors. First, in patch/FRAP studies, single cells are selected under the microscope for each measurement based on their expression level. The high sensitivity of our FRAP microscope setup (high numerical aperture (63×/1.4 numerical aperture) oil immersion objective) and a specially selected high sensitivity photomultiplier tube) allows FRAP studies on cells expressing as low as 4000–5000 receptors (evaluated as described by us earlier; Refs. 34, 35, and 49), which is only about 2-fold higher than endogenous expression levels on many cell types. Cells with these expression levels yielded results similar to those obtained on cells with up to 10-fold higher expression levels. Moreover, using co-immunoprecipitation by specific antibodies raised against the native receptors, we have previously demonstrated the existence of heteromeric BMP receptor complexes among the endogenous receptors both in the absence of ligand (PFCs) and after ligand binding (18, 25). The formation of PFCs among the endogenous BMP receptors indicates that their formation is not due to the epitope tags (18, 25). Importantly, although BMP-2 binding is clearly required for optimal signaling, we found that the heteromeric PFCs are endowed with a low but detectable signaling capability to the Smad pathway prior to ligand binding (Figs. 5 and 7). This is demonstrated by Fig. 5, which shows some Smad1/5/8 phosphorylation prior to BMP-2 stimulation, increasing ∼2-fold in cells co-transfected with myc-BMPRII and HA-BMPRIb (left and right panels, compare the “0 min” bars). It is further supported by Fig. 7 where limited cross-linking and stabilization of type I and type II BMP receptors carrying the same extracellular tag (myc-BMPRIb·myc-BMPRII) by IgG α-myc enhanced phospho-Smad1/5/8 formation by a factor of 2 already in the absence of ligand. These results are in line with our earlier suggestion that the PFCs activate the Smad pathway (25) based on studies with a BMPRII truncation mutant lacking most of the cytoplasmic region (including the kinase domain) that failed to form PFCs with BMPRI but retained BMP-induced heterocomplex formation. This mutant was dominant-negative for p38 phosphorylation and alkaline phosphatase induction (i.e. this non-Smad signaling pathway was disrupted in line with the ability of the mutant to form BMP-induced heterocomplexes) but did not interfere with Smad signaling presumably due to its failure to interfere with PFC formation (25). The current results (Figs. 5 and 7), along with analogous findings on Smad1/5/8 signaling in unstimulated C2C12 cells (47), support this notion by demonstrating phospho-Smad1/5/8 formation without ligand; as there were no BMP-induced complexes under these conditions, this signaling can only arise from PFCs.
The current results suggest two mechanisms that can act to restrict signaling from ligand-free heteromeric BMP receptor PFCs. The first is based on the instability of the BMP receptor heterocomplexes. Because BMPRI activation depends on its phosphorylation by BMPRII, short heterocomplex lifetimes would limit the duration of the association cycle during which BMPRI can be phosphorylated by BMPRII, thereby attenuating its activation. In accord with this mechanism, stabilization of myc-BMPRIb·myc-BMPRII heteromeric complexes by limited cross-linking with bivalent IgG α-myc enhanced phospho-Smad1/5/8 formation already prior to ligand binding (Fig. 7). On the other hand, limited IgG α-myc cross-linking/stabilization of homomeric interactions (in cells co-expressing myc-BMPRIb and HA-BMPRII where the α-myc can bridge only one myc-BMPRIb molecule with another) had no effect (Fig. 7, third bar pair) most likely because of the already stable nature of the interactions within the homodimers (Fig. 2). The second mechanism is based on competition between homomeric and heteromeric complex formation among the BMP receptors. An important but still unknown aspect of BMP receptor oligomeric structure-function relationship is the potential role of preassembled BMP receptor homodimers at the cell surface. This role is unclear because at least the ECD regions should dissociate from each other before reassociation into a heterotetrameric receptor complex bridged by the dimeric ligand unless many of the heteromeric complexes are arranged in a multimeric higher ordered structure, a much less likely possibility (20). We therefore propose that BMP receptor homodimerization may attenuate the formation of and signaling from heteromeric PFCs prior to ligand binding. This notion is supported by the demonstration (Fig. 6) that the heteromeric BMP receptor complexes formed at the expense of the homodimers.
In conclusion, the heterogeneous population of BMP receptors at the cell surface (a mixture of monomers, homodimers, heteromeric PFCs, and ligand-bound heteromeric complexes, which in turn may interact with a variety of co-receptors and scaffold proteins) endows BMP signaling with high degrees of flexibility and diversity. At the same time, however, some restraints are required to prevent excessive signaling by the heteromeric complexes in the absence of ligand. We propose that such an additional level of regulation is provided by the interplay between stable BMP receptor homodimers and the transient signaling heteromeric complexes.
Acknowledgments
We are grateful to O. Gutman for excellent technical assistance. We thank L. Attisano for the C-terminally HA-tagged BMPRIb plasmid and W. Sebald for recombinant BMP-2.
This work was supported in part by Grant 932-244.13/2006 from the German-Israeli Foundation for Scientific Research and Development (to Y. I. H. and P. K.).
- BMP
- bone morphogenetic protein
- ActRII and ActRIIb
- types II and IIb activin receptors
- BMPRIa
- BMPRIb, and BMPRII, types Ia, Ib, and II BMP receptors
- D
- lateral diffusion coefficient
- ECD
- extracellular domain
- FRAP
- fluorescence recovery after photobleaching
- GαM
- goat anti-mouse
- GαR
- goat anti-rabbit
- HBSS
- Hank's balanced salt solution
- PFC
- preformed complex
- Rf
- mobile fraction
- TβRI and TβRII
- types I and II TGF-β receptors.
REFERENCES
- 1. Hogan B. L. (1996) Genes Dev. 10, 1580–1594 [DOI] [PubMed] [Google Scholar]
- 2. Shi Y., Massagué J. (2003) Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 3. Derynck R., Miyazono K. (2008) in The TGF-β Family (Derynck R., Miyazono K. eds) pp. 29–43, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 4. Reddi A. H. (2005) Cytokine Growth Factor Rev. 16, 249–250 [DOI] [PubMed] [Google Scholar]
- 5. Canalis E., Economides A. N., Gazzerro E. (2003) Endocr. Rev. 24, 218–235 [DOI] [PubMed] [Google Scholar]
- 6. Varga A. C., Wrana J. L. (2005) Oncogene 24, 5713–5721 [DOI] [PubMed] [Google Scholar]
- 7. Lories R. J., Luyten F. P. (2005) Cytokine Growth Factor Rev. 16, 287–298 [DOI] [PubMed] [Google Scholar]
- 8. Schier A. F., Talbot W. S. (2005) Annu. Rev. Genet. 39, 561–613 [DOI] [PubMed] [Google Scholar]
- 9. Lane K. B., Machado R. D., Pauciulo M. W., Thomson J. R., Phillips J. A., 3rd, Loyd J. E., Nichols W. C., Trembath R. C. (2000) Nat. Genet. 26, 81–84 [DOI] [PubMed] [Google Scholar]
- 10. Thomson J. R., Machado R. D., Pauciulo M. W., Morgan N. V., Humbert M., Elliott G. C., Ward K., Yacoub M., Mikhail G., Rogers P., Newman J., Wheeler L., Higenbottam T., Gibbs J. S., Egan J., Crozier A., Peacock A., Allcock R., Corris P., Loyd J. E., Trembath R. C., Nichols W. C. (2000) J. Med. Genet. 37, 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas J. T., Kilpatrick M. W., Lin K., Erlacher L., Lembessis P., Costa T., Tsipouras P., Luyten F. P. (1997) Nat. Genet. 17, 58–64 [DOI] [PubMed] [Google Scholar]
- 12. Oldridge M., Fortuna A. M., Maringa M., Propping P., Mansour S., Pollitt C., DeChiara T. M., Kimble R. B., Valenzuela D. M., Yancopoulos G. D., Wilkie A. O. (2000) Nat. Genet. 24, 275–278 [DOI] [PubMed] [Google Scholar]
- 13. Lehmann K., Seemann P., Stricker S., Sammar M., Meyer B., Süring K., Majewski F., Tinschert S., Grzeschik K. H., Müller D., Knaus P., Nürnberg P., Mundlos S. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12277–12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howe J. R., Bair J. L., Sayed M. G., Anderson M. E., Mitros F. A., Petersen G. M., Velculescu V. E., Traverso G., Vogelstein B. (2001) Nat. Genet. 28, 184–187 [DOI] [PubMed] [Google Scholar]
- 15. He X. C., Zhang J., Tong W. G., Tawfik O., Ross J., Scoville D. H., Tian Q., Zeng X., He X., Wiedemann L. M., Mishina Y., Li L. (2004) Nat. Genet. 36, 1117–1121 [DOI] [PubMed] [Google Scholar]
- 16. Haramis A. P., Begthel H., van den Born M., van Es J., Jonkheer S., Offerhaus G. J., Clevers H. (2004) Science 303, 1684–1686 [DOI] [PubMed] [Google Scholar]
- 17. Hardwick J. C., Kodach L. L., Offerhaus G. J., van den Brink G. R. (2008) Nat. Rev. Cancer 8, 806–812 [DOI] [PubMed] [Google Scholar]
- 18. Gilboa L., Nohe A., Geissendörfer T., Sebald W., Henis Y. I., Knaus P. (2000) Mol. Biol. Cell 11, 1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyazono K., Kamiya Y., Morikawa M. (2010) J. Biochem. 147, 35–51 [DOI] [PubMed] [Google Scholar]
- 20. Nickel J., Sebald W., Groppe J. C., Mueller T. D. (2009) Cytokine Growth Factor Rev. 20, 367–377 [DOI] [PubMed] [Google Scholar]
- 21. Massagué J. (1998) Annu. Rev. Biochem. 67, 753–791 [DOI] [PubMed] [Google Scholar]
- 22. Kirsch T., Nickel J., Sebald W. (2000) EMBO J. 19, 3314–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirsch T., Sebald W., Dreyer M. K. (2000) Nat. Struct. Biol. 7, 492–496 [DOI] [PubMed] [Google Scholar]
- 24. Allendorph G. P., Vale W. W., Choe S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7643–7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nohe A., Hassel S., Ehrlich M., Neubauer F., Sebald W., Henis Y. I., Knaus P. (2002) J. Biol. Chem. 277, 5330–5338 [DOI] [PubMed] [Google Scholar]
- 26. Sieber C., Kopf J., Hiepen C., Knaus P. (2009) Cytokine Growth Factor Rev. 20, 343–355 [DOI] [PubMed] [Google Scholar]
- 27. Feng X. H., Derynck R. (2005) Annu. Rev. Cell Dev. Biol. 21, 659–693 [DOI] [PubMed] [Google Scholar]
- 28. Derynck R., Zhang Y. E. (2003) Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 29. Moustakas A., Heldin C. H. (2005) J. Cell Sci. 118, 3573–3584 [DOI] [PubMed] [Google Scholar]
- 30. Greenwald J., Groppe J., Gray P., Wiater E., Kwiatkowski W., Vale W., Choe S. (2003) Mol. Cell 11, 605–617 [DOI] [PubMed] [Google Scholar]
- 31. Sebald W., Mueller T. D. (2003) Trends Biochem. Sci. 28, 518–521 [DOI] [PubMed] [Google Scholar]
- 32. Weber D., Kotzsch A., Nickel J., Harth S., Seher A., Mueller U., Sebald W., Mueller T. D. (2007) BMC Struct. Biol. 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Groppe J., Hinck C. S., Samavarchi-Tehrani P., Zubieta C., Schuermann J. P., Taylor A. B., Schwarz P. M., Wrana J. L., Hinck A. P. (2008) Mol. Cell 29, 157–168 [DOI] [PubMed] [Google Scholar]
- 34. Rechtman M. M., Nakaryakov A., Shapira K. E., Ehrlich M., Henis Y. I. (2009) J. Biol. Chem. 284, 7843–7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henis Y. I., Moustakas A., Lin H. Y., Lodish H. F. (1994) J. Cell Biol. 126, 139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gilboa L., Wells R. G., Lodish H. F., Henis Y. I. (1998) J. Cell Biol. 140, 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenzweig B. L., Imamura T., Okadome T., Cox G. N., Yamashita H., ten Dijke P., Heldin C. H., Miyazono K. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7632–7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoodless P. A., Haerry T., Abdollah S., Stapleton M., O'Connor M. B., Attisano L., Wrana J. L. (1996) Cell 85, 489–500 [DOI] [PubMed] [Google Scholar]
- 39. Ehrlich M., Shmuely A., Henis Y. I. (2001) J. Cell Sci. 114, 1777–1786 [DOI] [PubMed] [Google Scholar]
- 40. Petersen N. O., Felder S., Elson E. L. (1986) in Handbook of Experimental Immunology (Weir D. M., Herzenberg L. A., Blackwell C. C., Herzenberg L. A. eds) pp. 24.21–24.23, Blackwell Scientific Publications, Edinburgh, UK [Google Scholar]
- 41. Eisenberg S., Shvartsman D. E., Ehrlich M., Henis Y. I. (2006) Mol. Cell. Biol. 26, 7190–7200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Henis Y. I., Katzir Z., Shia M. A., Lodish H. F. (1990) J. Cell Biol. 111, 1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hartung A., Bitton-Worms K., Rechtman M. M., Wenzel V., Boergermann J. H., Hassel S., Henis Y. I., Knaus P. (2006) Mol. Cell. Biol. 26, 7791–7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kfir S., Ehrlich M., Goldshmid A., Liu X., Kloog Y., Henis Y. I. (2005) Mol. Cell. Biol. 25, 8239–8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dorsch S., Klotz K. N., Engelhardt S., Lohse M. J., Bünemann M. (2009) Nat. Methods 6, 225–230 [DOI] [PubMed] [Google Scholar]
- 46. Sieber C., Plöger F., Schwappacher R., Bechtold R., Hanke M., Kawai S., Muraki Y., Katsuura M., Kimura M., Rechtman M. M., Henis Y. I., Pohl J., Knaus P. (2006) Biol. Chem. 387, 451–460 [DOI] [PubMed] [Google Scholar]
- 47. Boergermann J. H., Kopf J., Yu P. B., Knaus P. (2010) Int. J. Biochem. Cell Biol. 42, 1802–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang W., Jiang Y., Wang Q., Ma X., Xiao Z., Zuo W., Fang X., Chen Y. G. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 15679–15683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wells R. G., Gilboa L., Sun Y., Liu X., Henis Y. I., Lodish H. F. (1999) J. Biol. Chem. 274, 5716–5722 [DOI] [PubMed] [Google Scholar]