Abstract
The yeast Bem1p SH3b and Nbp2p SH3 domains are unusual because they bind to peptides containing the same consensus sequence, yet they perform different functions and display low sequence similarity. In this work, by analyzing the interactions of these domains with six biologically relevant peptides containing the consensus sequence, they are shown to possess finely tuned and distinct binding specificities. We also identify a residue in the Bem1p SH3b domain that inhibits binding, yet is highly conserved for the purpose of preventing nonspecific interactions. Substitution of this residue results in a marked reduction of in vivo function that is caused by titration of the domain away from its proper targets through nonspecific interactions with other proteins. This work provides a clear illustration of the importance of intrinsic binding specificity for the function of protein-protein interaction modules, and the key role of “negative” interactions in determining the specificity of a domain.
Keywords: Peptide Interactions, Protein-Protein Interactions, SH3 Domains, Signal Transduction, Yeast, Binding Affinity, Nonspecific Interaction, Protein Binding Specificity
Introduction
Signals are transmitted through cellular pathways by relays of protein-protein interactions resulting in specific outputs, such as cell growth, differentiation, or apoptosis. To generate appropriate responses from signaling pathways, the protein-protein interactions involved must be specific and not lead to misactivation of other pathways. This requisite precision can be readily achieved by proteins that possess “high intrinsic specificity,” directly binding their intended targets much more tightly than any other protein. For protein-DNA interactions, this can involve differences of greater than 3 orders of magnitude or more in the Kd value between target and non-target binding (1). However, protein-protein interactions are often mediated by small conserved modular domains that recognize short sequence motifs in their target proteins and may not possess intrinsically high specificity (2–3). Because members of the same domain family often bind to similar peptide sequences and individual domains have been found to bind many different peptides with similar affinities, many protein-protein interaction modules have been described as “promiscuous,” meaning that they are unable on their own to distinguish correct from incorrect binding sites. In these cases, the interactions of proteins containing these modules may still achieve high specificity through alternative mechanisms, such as coordinated temporal and spatial localization within the cell or participation in cooperative multi-protein complexes. Currently, the mechanisms by which protein interaction specificity within signaling pathways is achieved are not well understood (2, 4). In this work, we address three key questions on this topic: How much intrinsic binding specificity is encoded in small protein-protein interaction modules? What are the mechanisms for encoding this specificity? Are there biological consequences to the alteration of the intrinsic specificity of a domain?
Our studies on binding specificity focus on SH3 domains, which are among the most widespread and best characterized protein interaction domains (5, 6). SH3 domains generally recognize peptides with a core PXXP motif that can be either class I (+XXPXXP) or class II (PXXPX+), where X may be any amino acid and + is Arg or Lys. Many SH3 domains bind diverse PXXP-containing peptides with similar low affinities ranging from 10 to 50 μm and thus appear to be promiscuous binders (7–11). It was concluded by some investigators that factors apart from intrinsic binding specificity must contribute significantly to directing SH3 domains to their biologically relevant binding partners (3, 12–14). However, a biologically relevant target peptide of the yeast Sho1p SH3 domain has been shown to possess a high intrinsic binding specificity for this SH3 domain relative to other yeast SH3 domains, and decreasing this level of specificity was detrimental to the fitness of the cell (15). Other studies have also described higher affinity SH3 domain-mediated interactions (0.05–1 μm Kd values) requiring extended peptide sequences that can range from 12 to 30 residues in length (16–20). The recognition of extended peptide sequences by these domains suggests that their level of intrinsic specificity is higher than those recognizing shorter sequences, but the importance of high intrinsic specificity for the in vivo function of these domains has not been investigated.
To address the role of binding specificity in SH3 domain function, we have examined an unusual pair of yeast SH3 domains from the yeast adaptor proteins Nbp2p and Bem1p. In previous studies, these domains appeared to possess identical binding specificity despite their distinct biological roles and relatively low amino acid sequence identity of 36% (randomly chosen pairs of SH3 domains display 27% sequence identity on average (21)). Nbp2p is an adaptor protein involved in down-regulating the high osmolarity glycerol and cell wall integrity MAPK pathways. It binds to components in these pathways via its SH3 domain and recruits Ptc1p phosphatase (22–24). Bem1p contains two SH3 domains, a PX domain and a PB1 domain, and acts as a scaffold for multiple proteins involved in establishing cell polarity, including Cdc42p and its guanine exchange factor Cdc24p (25–29). Although it has been hypothesized that the SH3 domains in yeast have evolved to resist binding cross-reactivity (15), the Nbp2p SH3 domain (NbpSH3)2 and the second SH3 domain of Bem1p (BemSH3b) were both found to interact with a highly conserved 11-residue site in the Ste20p kinase (30). Moreover, several biologically relevant binding sites of NbpSH3 and BemSH3b (supplemental Table S1) display the same consensus sequence (Fig. 1A), one that also matches a consensus derived from a phage display experiment performed on the NbpSH3 (supplemental Fig. S1A and Ref. 31). The structure of a complex of BemSH3b bound to its target peptide from Ste20p shows that all of the residues at conserved positions in the consensus sequence contribute to the binding interface (32).
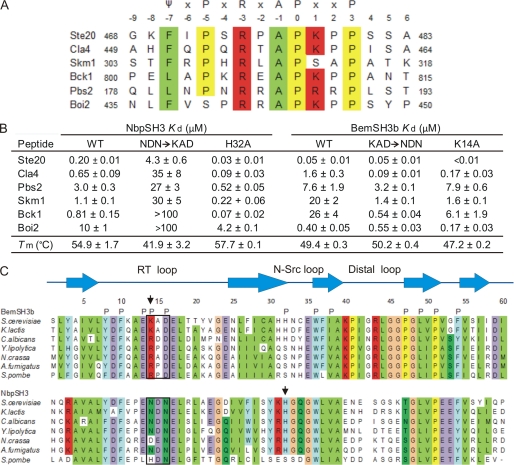
FIGURE 1.
In vitro binding analysis of BemSH3b and NbpSH3 interactions. A, the alignment of yeast peptides used in in vitro binding assays. The consensus sequence for peptides bound by BemSH3b and NbpSH3, which is consistent with the consensus from phage display experiments performed on the NbpSH3, is indicated, where ψ is a hydrophobic residue and X is any amino acid. Peptide numbering is according to Lim et al. (42). B, binding affinities of wild-type and mutant BemSH3b and NbpSH3. The Kd values were determined as described under “Experimental Procedures.” Values are mean ± S.E. The measurements with the wild-type domains were performed at least three times, and the measurements with the mutant domains were performed at least twice. Repeated experiments were performed with independently purified proteins. The thermodynamic stability of each domain was measured by temperature-induced unfolding experiments, and the temperature midpoints (Tm) of these transitions are shown. C, alignments of BemSH3b and NbpSH3 from diverse fungal species. The numbering of SH3 domain residues is according to a standard convention (21). BemSH3b secondary structure and residues involved in peptide binding (P) obtained from the structure of the BemSH3b-Ste20 complex (32) are shown. The RT loop residues exchanged between BemSH3b and NbpSH3 are boxed. The BemSH3b K14 and NbpSH3 H32 residues that interfere with binding are indicated by the arrows. Positions with > 70% conservation are colored: hydrophobic (light green), polar (dark green), positive (red), negative (purple), aromatic and His (light blue), Pro (yellow), and Gly (orange).
To investigate the specificity determinants of NbpSH3 and BemSH3b, we measured the binding affinities of these two domains for six different target sequences. Although both domains were able to bind all six sequences, several sequences were bound with surprisingly different affinities by each of the two domains, demonstrating a finely tuned level of specificity. Further studies on this unique pair of cross-reactive SH3 domains have allowed us to identify residues that are responsible for determining specificity, address the in vivo consequences of altering specificity, and establish a mechanism by which specificity is maintained.
EXPERIMENTAL PROCEDURES
Sample Preparation for in Vitro Binding Studies
NbpSH3 (residues 110–172) and BemSH3b (residues 155–252) were expressed with a C-terminal His6 tag from the pET21d (Novagen) vector. Target peptides were expressed as C-terminal fusions to the bacteriophage λ cI repressor carrying a C-terminal His6 tag, as described previously (33). Proteins were purified using nickel-affinity chromatography and dialyzed against 50 mm sodium phosphate (pH 7.0), 100 mm NaCl buffer. All subsequent assays were carried out in this buffer.
In Vitro Peptide-binding and SH3 Domain Stability Assays
The degree of binding of the SH3 domain to target peptides was assessed by monitoring the increase in SH3 domain tryptophan fluorescence at 326 nm using an Aviv ATF105 spectrofluorometer. To calculate binding affinities, SH3 domains (0.5 μm or 1 μm) were titrated with cI-peptide fusion proteins using a Microlab 500 series automated titrator. After mixing, the samples were equilibrated for 1 min and excited at 295 nm with a 5-s averaging time. The dissociation constant (Kd) values were calculated as described previously (33). The Kd values calculated are not affected by changes to the fluorescence difference between the bound and unbound states that could be caused by some amino acid substitutions. The thermal stability of the wild-type and mutant SH3 domains were measured using an Aviv circular dichroism model 202 spectrometer. Proteins were heated from 20 to 90°C, and protein unfolding was assessed by monitoring the change in ellipticity at 212 nm for the BemSH3b domain and at 230 nm for the NbpSH3 domain, respectively.
SH3 Domain Binding Assays in Yeast Extracts
Yeast BY4741 and BY4741 strains encoding TAP-tagged proteins were grown in yeast peptone dextrose medium to an A600 nm of ∼1.0, and cells from 150 ml of culture were collected by centrifugation. Protein extracts were prepared by resuspending the cell pellet in 1.8 ml of lysis buffer (100 mm Tris-HCl (pH 7.9), 250 mm NaCl, 5 mm EDTA, 50 mm NaF, 0.1% Nonidet P-40, 1 mm DTT, 10% glycerol) and lysing the cells with glass beads. Nickel-nitrilotriacetic acid-agarose resin (50 μl) was preloaded with 100 nmol of purified BemSH3b or NbpSH3 (1 ml of protein at a concentration of 100 μm) and then incubated with 200 μl of the prepared protein extract. The resin was washed three times with 300 μl of lysis buffer, and bound proteins were eluted by resuspending the resin in 40 μl of 2× SDS sample buffer. 20 μl of eluates was loaded onto 15% SDS-polyacrylamide gel and visualized by Coomassie staining or immunoblotting with α-TAP antibody (Open Biosystems, CAB1001). Each experiment was performed at least three times, and one representative gel was chosen for the figures shown.
Yeast Strains
Yeast strains used in this study are listed in supplemental Table S4. All gene modifications were introduced at chromosomal loci using PCR-based homologous recombination, following standard procedures (34). To construct strains carrying Nbp2/BemSH3b hybrid proteins, the chromosomal region encoding the NbpSH3 domain was first replaced with a URA3 cassette. This cassette was subsequently replaced by homologous recombination with a DNA fragment encoding the BemSH3b flanked by DNA homologous to the NBP2 gene. The desired recombinants lacking URA3 were selected using 5-fluoroorotic acid. The same method was applied to obtain strains encoding single amino acid substitutions in NbpSH3 and BemSH3b. All double mutants were constructed through mating, sporulation of the diploids, and tetrad dissection, where the resulting double mutants were identified by the associated markers. To construct a plasmid expressing wild-type Bem1p, a DNA fragment including the 900-base pair BEM1 promoter region and encoding Bem1p fused to a C-terminal 3HA tag was amplified from genomic DNA of strain MG40 constructed in this study (supplemental Table S4). The amplified DNA was inserted into a centromeric URA3-marked pRS316 plasmid.
Growth Assays
Standard rich medium (yeast peptone dextrose) or minimal medium (synthetic defined) supplemented with the appropriate amino acids were used for growth assays. The cells were grown overnight to saturation, normalized by A600 nm, and spotted in 5-fold serial dilutions. Growth was monitored for 2 days at standard growing temperature (30 °C) or high temperatures (36–39 °C). It should be noted that changes to the incubation temperature as small as 0.5 °C had a significant effect on cell fitness.
RESULTS
NbpSH3 and BemSH3b Display Finely Tuned Specificity
To determine the binding specificities of BemSH3b and Nbp2SH3, we tested the binding of these SH3 domains to six peptides derived from yeast proteins implicated in the function of either Bem1p or Nbp2p (supplemental Table S1). The BemSH3b construct (155–252) used in the binding assays included a C-terminal extension that is required for folding of the domain and contributes to peptide binding by extending the peptide binding surface (32). The interaction of the SH3 domains to target peptides was monitored by measuring the increase in intrinsic Trp fluorescence resulting from peptide binding (supplemental Fig. S1, B and C). Although both SH3 domains bound all of the tested peptides, the affinities of each domain for the set of peptides spanned a broad range of values. NbpSH3 bound with Kd values ranging from 0.2 to 11 μm, whereas BemSH3b bound with an even greater range of affinities varying from 0.05 to 26 μm (Fig. 1B). Whereas some target peptides, including Ste20, Cla4, and Pbs2, were bound with relatively similar affinities by the two domains (< 4-fold difference), the Boi2, Bck1, and Skm1 sites showed a much greater difference in their affinities for the two domains, ranging from 20- to 30-fold. In the case of the Boi2 site, the BemSH3 bound more tightly, whereas the Nbp2SH3 bound the Bck1 and Skm1 sites with a higher affinity. It is also notable that each domain bound to most sites with a relatively high affinity compared with typical SH3 domain target peptide interactions. For example, the Kd value of the BemSH3b-Ste20 interaction (0.05 μm) is among the lowest observed for SH3 domains. In summary, although these domains recognize the same consensus sequence, their specificities are seen to be finely tuned when assayed against a panel of yeast peptides, some of which are proven biological targets of NbpSH3 or BemSH3b domains (supplemental Table S1).
The Conserved Lys-14 Residue of BemSH3b Is a Key Determinant of Specificity
To identify key residues that determine the distinct specificities of BemSH3b and NbpSH3, we exchanged residues 14–16 in the RT loops of these domains (Fig. 1C). We chose these positions because they were occupied by residues with different properties in the two domains (KAD in BemSH3b versus NDN in NbpSH3) and this region of SH3 domains had been implicated in determining specificity in several different systems (35–36). Remarkably, the BemSH3bKAD → NDN mutant bound most sites more tightly than the wild-type domain (Fig. 1B). In particular, it bound the Bck1 and Skm1 sites 50- and 20-fold more tightly, respectively. Overall, the specificity of profile of the BemSH3bKAD → NDN mutant more closely resembles the specificity of the wild-type NbpSH3 except that it is still able to bind the Boi2 site with a much higher affinity than the Nbp2SH3. In contrast to the BemSH3bKAD → INDN mutant, the NbpSH3NDN → KAD mutant displayed significantly reduced binding affinity to all the peptides.
To investigate the mechanism of the changes in specificity induced by the RT loop substitutions of BemSH3b, we tested the effects of substitutions with Ala in this region. Strikingly, the K14A substitution caused large increases in affinity for the Cla4, Skm1, and Bck1 sites, similar to the KAD → NDN substitution, and also bound more tightly to the Ste20 and Boi2 sites (Fig. 1B). A K14A/D16A double mutant showed no increase in affinity for any sites beyond that observed for the K14A mutant (supplemental Table S2). Thus, the altered binding properties of the BemSH3bKAD → NDN mutant can be mostly attributed to the elimination of the Lys-14 side chain, suggesting that this residue interferes with the binding mediated by Bem1SH3b. This result is surprising because the alignment of BemSH3b from widely diverse fungal species (Fig. 1C) shows that a positively charged residue has been conserved at this position over at least 500 million years of evolution. Substitutions at such conserved positions in protein-protein interaction interfaces usually cause large decreases in binding affinity.
The Difference in Binding Affinities Between BemSH3b and NbpSH3 Is Biologically Significant
To determine whether the differences in binding specificity between Nbp2SH3 and BemSH3b observed in vitro have an effect on in vivo function, we replaced the chromosomally encoded NBP2 gene with one encoding a hybrid protein in which NbpSH3 was replaced with BemSH3b. As shown in Fig. 2, substitution of Nbp2SH3 with BemSH3b resulted in growth defects at 39 °C comparable with those seen with an nbp2Δ mutant (37). To assess the defect in NbpSH3 function conferred by replacing it with BemSH3b, we compared the growth of cells expressing the Nbp2/BemSH3b hybrid protein to cells expressing Nbp2p bearing Y8A and F54A SH3 domain substitutions, which cause large reductions in peptide-binding but are not expected to affect specificity because they interact with the PXXP region (21). The level of growth mediated by the Nbp2/BemSH3b hybrid protein was intermediate between the NbpSH3 Y8A and F54A mutants which display 45- and 280-fold reductions, respectively, in binding affinity to the Ste20 peptide (supplemental Table S2). This suggested that in the Nbp2/BemSH3b hybrid protein, SH3 domain function is significantly affected but not completely lost. In contrast to cells expressing the Nbp2/BemSH3b hybrid protein, those expressing the Nbp2/BemSH3bKAD → NDN mutant grew as well as cells expressing wild-type Nbp2p (Fig. 2). All of these mutant proteins were expressed at levels similar to the wild type (supplemental Fig. S2). These data show that the specificity differences between NbpSH3 and BemSH3b observed in vitro result in an inability of BemSH3b to replace NbpSH3 in vivo. However, the BemSH3bKAD → NDN mutant was able to replace NbpSH3 because its specificity much more closely resembles that of NbpSH3.
FIGURE 2.
Biological activity of Nbp2/BemSH3b hybrid proteins. An nbp2Δ strain and strains expressing Nbp2p bearing the indicated SH3 domain amino acid substitutions, which impair peptide binding (supplemental Table S2), were included for comparison. Strains were grown overnight, normalized to an A600 nm of 1.0, and spotted onto yeast peptone dextrose plates in 5-fold serial dilutions.
Altering the Binding Specificity of the BemSH3b Domain Impairs Bem1p Function
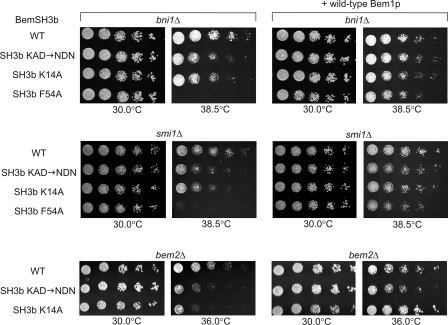
To investigate the effects of altered binding specificity on the in vivo function of the Bem1p protein, we constructed yeast strains in which the BEM1 gene was replaced by genes encoding the BemSH3b KAD → NDN or K14A mutants. Although strains bearing these mutations displayed no reduction in viability in a wild-type strain background (supplemental Fig. S3A), they displayed marked reductions in cell growth at high temperature (Fig. 3) when combined with smi1Δ, bni1Δ, or bem2Δ gene deletions, which are synthetically lethal with bem1Δ (supplemental Fig. S3B and Ref. 38). The in vivo effects of the altered specificity mutants were not the result of altered Bem1p expression levels or BemSH3b instability (Fig. 1B and supplemental Fig. 3E). To gauge the importance of BemSH3b function in these backgrounds, we also tested the effect of the BemSH3b F54A substitution, which causes a large reduction in binding affinity (supplemental Table S2). Introduction of this substitution resulted in complete lack of growth at high temperature (Fig. 3) or synthetic lethality in the case of bem2Δ (supplemental Fig. S3C), emphasizing a critical role of SH3 domain activity in these backgrounds.
FIGURE 3.
The effect of BemSH3b altered specificity mutants on cell growth. Chromosomally expressed mutants of Bem1p bearing the indicated amino acid substitutions in the SH3b domain were assayed in smi1Δ, bni1Δ, and bem2Δ genetic backgrounds. A BemSH3bF54A mutant that significantly impairs peptide binding (supplemental Table S2) was included for comparison. All strains also carried a centromeric plasmid. The plasmid in strains shown in the right panels expressed wild-type Bem1p under the control of its own promoter, whereas the plasmid in strains shown in the left panels contained no insert. Strains were grown overnight, normalized to A600 nm of 0.2, and spotted in 5-fold serial dilutions on synthetic defined plates lacking uracil.
The phenotypes caused by the BemSH3b KAD → NDN and K14A mutations could have been the result of a gain of function whereby the altered binding specificity led to deleterious misactivation of a pathway. To explore this possibility, we tested the effect of plasmid-mediated expression of wild-type Bem1p within these mutant cells. As shown in Fig. 3, expression of wild-type Bem1p at endogenous levels (supplemental Fig. S3D) led to normal levels of growth for the BemSH3b mutants in all three backgrounds. This recessive-like behavior indicated that altering the binding specificity of BemSH3b caused a loss of Bem1p function that could be overcome by expressing wild-type Bem1p. If these mutations had caused a gain of function, we would have expected their effect to be dominant.
Because the BemSH3bKAD → NDN mutant displayed specificity that was similar to that of NbpSH3, we postulated that Bem1p carrying this substitution might interfere with Nbp2p function by competing for NbpSH3 binding sites within the cell. However, in strains carrying this mutation, we failed to observe any phenotypes indicative of a defect in the function of Nbp2p. These might have included hyperactivation of the cell wall integrity pathway (22) and growth defects in strains sensitized to Nbp2p SH3 function by deletion of additional genes (supplemental Fig. S4).
The K14A Substitution Causes an Increase in Nonspecific Binding
The recessive behavior of BemSH3b altered specificity mutants led us to hypothesize that the impairment of Bem1p function caused by these mutations might be due to increased nonspecific binding, which might divert Bem1p from its proper target proteins. To investigate this idea, we compared the abilities of the wild-type domain and mutant BemSH3b coupled to nickel affinity resin to bind proteins from yeast extracts. These assays were performed with high concentrations of SH3 domains so that nonspecific binding could be detected. Although both the wild-type and mutant domains bound to a number of different proteins, the K14A mutant clearly bound more tightly to these proteins, as indicated by the greater intensity of many bands in the lane of the gel showing the eluate from the K14A column (Fig. 4A). The large number of proteins bound in these assays and their high concentration (i.e. they were easily visualized in a Coomassie-stained SDS-PAGE gel) suggested that these interactions were nonspecific. Most of the bound proteins were absent in the eluate of BemSH3b containing the F54A substitution, which abrogates PXXP binding interface. These indicated that these nonspecific interactions still required Pro-rich sequences.
FIGURE 4.
BemSH3b K14A and Nbp2 H32A mutants display increased nonspecific binding. A, binding of SH3 domains to proteins in yeast extracts. Purified SH3 domains bound to nickel affinity resin were incubated with yeast extracts. Bound proteins were eluted and visualized by Coomassie staining of SDS-PAGE gels. Protein bands with higher intensity in the NbpSH3 H32A and BemSH3b K14A lanes are indicated by arrows. The bands corresponding to the eluted SH3 domains (intense bands) are indicated. B, binding of the BemSH3K14A mutant to TAP-tagged proteins. The same procedure was used as in A, except proteins bound by the SH3 domain were detected by immunoblotting with and antibody directed against the TAP tag. The tested TAP-tagged proteins included Tif32p (130,343 Da), Cdc48p (111,995 Da), and Las17p (87,571 Da). C and D, binding of BemSH3b and NbpSH3 to a nonspecific RXXPXXP-containing target. Representative Trp fluorescence spectra of SH3 domains at a concentration of 1 μm alone or in the presence of 100 μm target peptide are shown. Kd values were not obtained for nonspecific binding reactions because the low affinity of these reactions made it impossible to reach binding saturation with the quantities of peptide-fusion protein available.
With this in mind, we used the same assay to test binding of BemSH3b to six different TAP-tagged proteins containing RXXPXXP motifs, which have no functional connection to Bem1p (supplemental Table S3). Among these proteins, Tif32p, Cdc48p, and Las17p showed increased binding to the K14A mutant when compared with the wild-type domain but absence of binding to the F54A mutant (Fig. 4B). These results mirrored those seen in the binding assay to total yeast lysates.
To confirm increased nonspecific peptide binding activity of the K14A mutant, we assessed its binding to an RXXPXXP-containing peptide that possessed none of the other conserved features of peptides bound tightly by BemSH3b (Fig. 4C). Wild-type BemSH3b displayed little detectable binding to this peptide, as detected by examination of Trp fluorescence spectra, even when the peptide was present in 100-fold excess. By contrast, addition of this peptide to the K14A mutant caused an approximately 5-fold increase in Trp fluorescence intensity at 330 nm compared with the wild-type domain and a blue shift in the peak wavelength of emission, indicating that the mutant bound significantly more strongly to the nonspecific target. In summary, these results demonstrate an increased level of nonspecific binding by the BemSH3bK14A mutant, which could reduce its ability to bind sufficiently to its required partners in vivo.
An NbpSH3 Mutant Also Displays Increased Nonspecific Binding
Our findings regarding the Lys-14 position of BemSH3b led us to seek similar specificity-regulating residues within NbpSH3. Through investigation of several conserved positions within the putative peptide interaction interface of this domain, we discovered that the H32A substitution led to an approximately 10-fold increase in affinity for most of the tested target peptides (Fig. 2B). Interestingly, BemSH3b also possesses a His residue at this position, but substitution of this residue had little effect on binding affinity (supplemental Table S2). Like the BemSH3bK14A mutant, the NbpSH3H32A mutant showed an increased level of nonspecific binding to proteins in a yeast cell extract as compared with the wild-type domain (Fig. 4A). This increased nonspecific binding was still observable even though the wild-type NbpSH3 displayed an overall higher level of nonspecific binding as compared with BemSH3b. Also similar to the BemSH3bK14A mutant, the NbpSH3H32A mutant displayed increased nonspecific binding in vitro (Fig. 4D). However, we were not able to detect an in vivo phenotype for the H32A substitution despite testing its effect in a number of different genetics backgrounds (data not shown).
DISCUSSION
To understand how cells are able to elicit specific responses to changes in environmental conditions, it is essential to delineate the mechanisms by which protein-protein interaction specificity is generated. A key question is how much of the information governing pathway specificity is encoded within individual protein-protein interaction modules. In this work, we have addressed this question using BemSH3b and NbpSH3, and demonstrated that these domains possess finely tuned intrinsic binding specificities, despite recognizing a common consensus sequence. Furthermore, we have shown that altering this intrinsic binding specificity through amino acid substitutions markedly impairs function in vivo, and that a highly conserved residue in BemSH3b plays a key functional role in reducing nonspecific interactions. This work provides a clear illustration of the importance of intrinsic binding specificity for the function of protein-protein interaction modules, and the key role of “negative” interactions in determining the specificity of a domain.
Although BemSH3b and NbpSH3 both bound the same six biologically relevant peptide sequences, they did so with distinct specificities. They bound some sites with very similar affinities, whereas affinity differences of up to 30-fold were seen for other sites (Fig. 1B). Remarkably, the observed binding affinities of these domains for the six sites varied over a 500-fold range, even though these sites all conform to the same consensus sequence. These results illustrate that the intrinsic binding specificity of SH3 domains can be highly nuanced, and that residues outside of the conserved positions of the consensus play important roles. The distinct binding specificities of NbpSH3 and BemSH3b are likely a result of different binding mechanisms. This is illustrated by the drastic decrease in binding affinity of NpbSH3 caused by replacement of three of its RT loop residues with the corresponding residues from the BemSH3b (NbpSH3NDN → KAD mutant). BemSH3b must make stronger interactions with peptides in a region distinct from the RT loop to compensate for the inhibitory effect on binding of the residues in its RT loop. Supporting this idea, the recently solved structure of BemSH3b in complex with the target peptide from Ste20p showed extensive interactions with Phe-7 made by a unique 40 residue extension at the C terminus of the domain not found in NbpSH3 (32). Another piece of evidence supporting the existence of different binding mechanisms utilized by these domains was provided by our observation that substitution of position 32, which is occupied by His in both domains, significantly increased the binding affinity of NbpSH3 (Fig. 1B) but decreased the affinity of BemSH3b (supplemental Table S2). Overall, these results indicate that different SH3 domains are able to recognize the same target peptides utilizing distinctive binding modes that contributes to their unique intrinsic binding specificities. At the same time, our observation that BemSH3b and NbpSH3 can interact with the same site with similar high affinity (e.g. Cla4) suggests that these domains may at times function by competing for binding to the same protein. There is a biological rationale for such a competition because these proteins appear to generate opposite responses: Nbp2p negatively regulates MAP kinase pathways (22–24), whereas Bem1p activates them (30).
The changes in binding specificity resulting from amino acid substitutions in BemSH3b caused significant in vivo effects. Although the function of NbpSH3 could not be assumed by the wild-type version of BemSH3b, a variant of Nbp2p bearing the mutant BemSH3bKAD → NDN domain was able to function normally (Fig. 2). The ability of this mutant BemSH3b domain to replace NbpSH3 is likely due to its increased affinity for sites that are bound strongly by NbpSH3. For example, the BemSH3bKAD → NDN mutant bound 50-fold more tightly to the site from the Bck1p MAP kinase, which is a required component of the cell wall integrity pathway. This pathway has been implicated in many of the phenotypes resulting from the deletion of NBP2 (22, 37). We also found that replacement of the genomic copy of BEM1 with a gene encoding either of the mutations affecting specificity in vitro (i.e. KAD → NDN or K14A) caused a marked decrease in viability in several different genetic backgrounds (Fig. 3). It is remarkable that despite the increased binding affinity of these mutants for all of the sites tested, they were still debilitated in their function. This implies that the altered binding specificity of these mutants is the cause of their in vivo deficiency. Although a number of studies have investigated amino acid substitutions in protein-protein interaction modules that alter binding specificity (35–36, 39), this study provides the first easily demonstrable viability phenotype for such a mutant. One comparable result was obtained in a study of the yeast Sho1p SH3 domain, in which amino acid substitutions that caused reduced specificity were generated in the target site for this domain. The presence of these promiscuous sites within a yeast strain decreased viability, but this phenotype only became evident after many generations of growth under stressful conditions in competition with a wild-type strain (15). In another study, similar promiscuous binding sites for the Gads SH3 domain were isolated, but these sites showed no alteration in in vivo activity (40).
Another important observation in this study is that the function of Lys-14 within BemSH3b is to prevent interactions with non-target proteins. Our experiments showed that the K14A mutant bound more tightly than the wild-type to at least 10 different proteins (Fig. 4A). Because the specific targets of NbpSH3 and BemSH3b investigated in this study are expressed at a level that would not be detected on a Coomassie-stained gel (< 2000 molecules per cell, (41)), the proteins detected in our experiments are likely to be abundant proteins that bound nonspecifically and with low affinity. Although both the wild-type and K14A mutant domains bind to these proteins, the K14A mutant clearly displays increased nonspecific binding. This conclusion is further supported by our observation that the K14A mutant bound more tightly in pull-down experiments to Las17p, Cdc48p, and Tif32p, which possess only sites conforming to the RXXPXXP consensus (Fig. 4B), and the K14A mutant bound more tightly in vitro to a nonspecific site (Fig. 4C). We conclude that despite the increased affinity of the K14A mutant for biologically relevant target proteins such as Ste20p and Cla4p (27, 30), its increased affinity for numerous abundant non-target proteins causes it to be titrated away from its correct biological targets, resulting in a relative deficiency of Bem1p activity. This suggestion is supported by our finding that the loss in viability resulting from chromosomally introduced KAD → NDN and K14A mutations could be rescued by wild-type Bem1p expressed from a plasmid. This recessive behavior would not have been observed if loss of viability were caused by activation of an inappropriate pathway. Furthermore, we also observed that in the bni1Δ diploid background the BEM1/bem1Δ heterozygote displayed some loss in viability at high temperature (supplemental Fig. S3F), which implies that cells are sensitive to decreased levels of Bem1p as would result from the nonspecific binding activity of the K14A mutant. In summary, our data indicate that the primary function of Lys-14 is not to form stabilizing interactions with biologically relevant target peptides but to prevent nonspecific interactions with other cellular proteins.
Consistent with our findings, a recent study of SH2 domains showed that residues preventing binding to certain sequences play an important role in determining binding specificity in vitro and that both positive and negative interactions allow for a high degree of nuance in the specificities of these domains. Similarly, the promiscuous target of the Sho1p SH3 domain, mentioned above, contained alanine substitutions that did not reduce its affinity for the Sho1p SH3 domain, yet caused it to bind other SH3 domains with a higher affinity (15). Similar to these previous studies, our work emphasizes the importance of high intrinsic specificity for the function of protein-protein modules and the key role of negative selection in determining specificity. However, we have broken new ground by demonstrating a pronounced in vivo phenotype resulting from loss of binding specificity and identifying a single residue within a binding domain that appears to been highly conserved for the sole purpose of preventing nonspecific interactions.
Although Lys-14 of the BemSH3b domain is the first residue to be identified in a protein-protein module that is likely conserved for the purpose of preventing nonspecific interactions, we expect that many other such residues exist. A hallmark of such residues may be similar to that seen for Lys-14, where elimination of the side chain increases affinity to biologically relevant sites. Interestingly, the Ala substitution of the conserved His-32 position of NbpSH3 also increased binding affinity (Fig. 1B) and nonspecific binding (Fig. 4, A and D). Despite our inability to identify an in vivo phenotype for this substitution, the possible specificity-enhancing activity of His-32 may play an important functional role under some, as yet untested, conditions. Our ability to identify residues in two different domains that may be conserved for specificity determination whereas other studies have failed to identify such residues could be a result of a dearth of systematic mutagenesis studies of residues comprising the binding interface of protein-protein interaction modules. Most studies involving mutagenesis of such domains have focused on positions that are conserved across the whole domain family and thus are not expected to be specificity determinants. Future studies, aimed at locating such determinants, should focus on positions that are only conserved among orthologous domains, such as Lys-14 of BemSH3b and His-32 of NbpSH3. In this way, further insight into the importance of intrinsic binding specificity for the function of these domains will be obtained.
Supplementary Material
Acknowledgments
We thank Brenda Andrews and Charlie Boone for supplying the yeast strains used in this study. We also thank Paul Sadowski and Karen Maxwell for critical reading of the manuscript.
This work was supported by Canadian Institutes of Health Research Operating Grant MOP-13609 and by a Natural Sciences and Engineering Research Council PGS-D scholarship (to M. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4, Tables S1–S4, and additional references.
- NbpSH3
- Nbp2p SH3 domain
- BemSH3b
- Bem1p SH3b domain
- PX
- phox homology
- TAP
- tandem affinity purification.
REFERENCES
- 1. Jen-Jacobson L. (1997) Biopolymers 44, 153–180 [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharyya R. P., Remenyi A., Yeh B. J., Lim W. A. (2006) Annu. Rev. Biochem. [DOI] [PubMed] [Google Scholar]
- 3. Castagnoli L., Costantini A., Dall'Armi C., Gonfloni S., Montecchi-Palazzi L., Panni S., Paoluzi S., Santonico E., Cesareni G. (2004) FEBS Lett. 567, 74–79 [DOI] [PubMed] [Google Scholar]
- 4. Pawson T. (2007) Curr. Opin Cell Biol. 19, 112–116 [DOI] [PubMed] [Google Scholar]
- 5. Dalgarno D. C., Botfield M. C., Rickles R. J. (1997) Biopolymers 43, 383–400 [DOI] [PubMed] [Google Scholar]
- 6. Zarrinpar A., Bhattacharyya R. P., Lim W. A. (2003) Sci. STKE 2003, RE8. [DOI] [PubMed] [Google Scholar]
- 7. Viguera A. R., Arrondo J. L., Musacchio A., Saraste M., Serrano L. (1994) Biochemistry 33, 10925–10933 [DOI] [PubMed] [Google Scholar]
- 8. Cussac D., Frech M., Chardin P. (1994) EMBO J. 13, 4011–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feller S. M., Knudsen B., Hanafusa H. (1995) Oncogene 10, 1465–1473 [PubMed] [Google Scholar]
- 10. Kaneko T., Kumasaka T., Ganbe T., Sato T., Miyazawa K., Kitamura N., Tanaka N. (2003) J. Biol. Chem. 278, 48162–48168 [DOI] [PubMed] [Google Scholar]
- 11. Landgraf C., Panni S., Montecchi-Palazzi L., Castagnoli L., Schneider-Mergener J., Volkmer-Engert R., Cesareni G. (2004) PLoS Biol. 2, E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ladbury J. E., Arold S. (2000) Chem. Biol. 7, R3–8 [DOI] [PubMed] [Google Scholar]
- 13. Mayer B. J. (2001) J. Cell Sci. 114, 1253–1263 [DOI] [PubMed] [Google Scholar]
- 14. Li S. S. (2005) Biochem. J. 390, 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zarrinpar A., Park S. H., Lim W. A. (2003) Nature 426, 676–680 [DOI] [PubMed] [Google Scholar]
- 16. Ghose R., Shekhtman A., Goger M. J., Ji H., Cowburn D. (2001) Nat. Struct. Biol. 8, 998–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kami K., Takeya R., Sumimoto H., Kohda D. (2002) EMBO J. 21, 4268–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewitzky M., Harkiolaki M., Domart M. C., Jones E. Y., Feller S. M. (2004) J. Biol. Chem. 279, 28724–28732 [DOI] [PubMed] [Google Scholar]
- 19. Bauer F., Schweimer K., Meiselbach H., Hoffmann S., Rösch P., Sticht H. (2005) Protein Sci. 14, 2487–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoelz A., Janz J. M., Lawrie S. D., Corwin B., Lee A., Sakmar T. P. (2006) J. Mol. Biol. 358, 509–522 [DOI] [PubMed] [Google Scholar]
- 21. Larson S. M., Davidson A. R. (2000) Protein Sci. 9, 2170–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du Y., Walker L., Novick P., Ferro-Novick S. (2006) EMBO J. 25, 4413–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mapes J., Ota I. M. (2004) EMBO J. 23, 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hruby A., Zapatka M., Heucke S., Rieger L., Wu Y., Nussbaumer U., Timmermann S., Dünkler A., Johnsson N. (2011) J. Cell Sci. 124, 35–46 [DOI] [PubMed] [Google Scholar]
- 25. Bender L., Lo H. S., Lee H., Kokojan V., Peterson V., Bender A. (1996) J. Cell Biol. 133, 879–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irazoqui J. E., Gladfelter A. S., Lew D. J. (2003) Nat. Cell Biol. 5, 1062–1070 [DOI] [PubMed] [Google Scholar]
- 27. Bose I., Irazoqui J. E., Moskow J. J., Bardes E. S., Zyla T. R., Lew D. J. (2001) J. Biol. Chem. 276, 7176–7186 [DOI] [PubMed] [Google Scholar]
- 28. Gulli M. P., Jaquenoud M., Shimada Y., Niederhäuser G., Wiget P., Peter M. (2000) Mol. Cell 6, 1155–1167 [DOI] [PubMed] [Google Scholar]
- 29. Kozubowski L., Saito K., Johnson J. M., Howell A. S., Zyla T. R., Lew D. J. (2008) Curr. Biol. 18, 1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winters M. J., Pryciak P. M. (2005) Mol. Cell. Biol. 25, 2177–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tong A. H., Drees B., Nardelli G., Bader G. D., Brannetti B., Castagnoli L., Evangelista M., Ferracuti S., Nelson B., Paoluzi S., Quondam M., Zucconi A., Hogue C. W., Fields S., Boone C., Cesareni G. (2002) Science 295, 321–324 [DOI] [PubMed] [Google Scholar]
- 32. Takaku T., Ogura K., Kumeta H., Yoshida N., Inagaki F. (2010) J. Biol. Chem. 285, 19346–19353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maxwell K. L., Davidson A. R. (1998) Biochemistry 37, 16172–16182 [DOI] [PubMed] [Google Scholar]
- 34. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 35. Hiipakka M., Saksela K. (2007) FEBS Lett. 581, 1735–1741 [DOI] [PubMed] [Google Scholar]
- 36. Panni S., Dente L., Cesareni G. (2002) J. Biol. Chem. 277, 21666–21674 [DOI] [PubMed] [Google Scholar]
- 37. Ohkuni K., Okuda A., Kikuchi A. (2003) Genetics 165, 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tong A. H., Lesage G., Bader G. D., Ding H., Xu H., Xin X., Young J., Berriz G. F., Brost R. L., Chang M., Chen Y., Cheng X., Chua G., Friesen H., Goldberg D. S., Haynes J., Humphries C., He G., Hussein S., Ke L., Krogan N., Li Z., Levinson J. N., Lu H., Ménard P., Munyana C., Parsons A. B., Ryan O., Tonikian R., Roberts T., Sdicu A. M., Shapiro J., Sheikh B., Suter B., Wong S. L., Zhang L. V., Zhu H., Burd C. G., Munro S., Sander C., Rine J., Greenblatt J., Peter M., Bretscher A., Bell G., Roth F. P., Brown G. W., Andrews B., Bussey H., Boone C. (2004) Science 303, 808–813 [DOI] [PubMed] [Google Scholar]
- 39. Fazi B., Cope M. J., Douangamath A., Ferracuti S., Schirwitz K., Zucconi A., Drubin D. G., Wilmanns M., Cesareni G., Castagnoli L. (2002) J. Biol. Chem. 277, 5290–5298 [DOI] [PubMed] [Google Scholar]
- 40. Seet B. T., Berry D. M., Maltzman J. S., Shabason J., Raina M., Koretzky G. A., McGlade C. J., Pawson T. (2007) EMBO J. 26, 678–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., Dephoure N., O'Shea E. K., Weissman J. S. (2003) Nature 425, 737–741 [DOI] [PubMed] [Google Scholar]
- 42. Lim W. A., Richards F. M., Fox R. O. (1994) Nature 372, 375–379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.