Abstract
Erythropoiesis is a coordinated process by which RBCs are produced. Erythropoietin, a kidney-derived hormone, and iron are critical for the production of oxygen-carrying mature RBCs. To meet the high demands of iron during erythropoiesis, small intestinal iron absorption is increased through an undefined mechanism. In this study, erythropoietic induction of iron absorption was further investigated. Hypoxia-inducible factor-2α (HIF-2α) signaling was activated in the small intestine during erythropoiesis. Genetic disruption of HIF-2α in the intestine abolished the increase in iron absorption genes as assessed by quantitative real-time reverse transcription-PCR and Western blot analyses. Moreover, the increase in serum iron following induction of erythropoiesis was entirely dependent on intestinal HIF-2α expression. Complete blood count analysis demonstrated that disruption of intestinal HIF-2α inhibited efficient erythropoiesis; mice disrupted for HIF-2α demonstrated lower hematocrit, RBCs, and Hb compared with wild-type mice. These data further cement the essential role of HIF-2α in regulating iron absorption and also demonstrate that hypoxia sensing in the intestine, as well as in the kidney, is essential for regulation of erythropoiesis by HIF-2α.
Keywords: Gene Expression, Hypoxia, Intestinal Metabolism, Iron Metabolism, Transport Metals
Introduction
Erythropoiesis is a tightly regulated process involved in maintaining and modulating RBC production under both normal and pathological conditions. Erythropoiesis is controlled by erythropoietin (EPO),2 which is produced by the kidney and secreted into the bloodstream (1). EPO acts on red blood cell progenitors in the bone marrow to stimulate their differentiation (2). When large numbers of erythrocytes are lost due to disease or injury, EPO production increases dramatically, leading to increased erythrocyte production to restore the RBC population (3). Increased erythrocyte production requires an increase in available iron to produce Hb (4). Erythropoiesis stimulates expression of iron absorption genes in the small intestine, although the mechanism by which this occurs is unknown (5–7). Erythropoiesis-stimulating agents are commonly used to increase serum iron levels to combat iron deficiency anemia, particularly in cancer patients and patients with chronic kidney disease (8).
In addition, β-thalassemia is a common genetic disorder caused by mutations in the β-globin gene, which result in ineffective erythropoiesis leading to iron overload (9). In β-thalassemia patients, intestinal iron absorption is three to four times greater than in normal control patients, and the increase in circulating and tissue iron is the major cause of death in β-thalassemia patients (9, 10). Intestinal iron absorption is tightly regulated by the small intestine. Dietary iron is primarily in ferric (Fe3+) form and is reduced to ferrous (Fe2+) iron by the duodenal ferric reductase (DcytB) (11–13). Ferrous iron is then transported into the enterocyte by Dmt1 (divalent metal transporter 1) (12, 14, 15). To enter circulation, ferrous iron passes through the only characterized basolateral iron exporter, Fpn1 (ferroportin-1) (16–19). Hepcidin, a liver-derived peptide that regulates iron homeostasis through Fpn1 degradation (20–22), has been implicated in the increase in iron absorption following erythropoiesis and in thalassemia patients (10, 23–25). However, other mechanisms that regulate intestinal iron absorption are thought to be involved because hepcidin expression does not completely ameliorate the iron overload in β-thalassemic mouse models (23), and the effect of hepcidin on intestinal iron absorption does not appear to be direct and rapid (26–30).
In addition to hepcidin, the transcription factor hypoxia-inducible factor-2α (HIF-2α) is necessary for intestinal iron absorption following iron deficiency (31, 32). HIF-2α is a heterodimeric transcription factor consisting of an iron- and oxygen-regulated α-subunit and a constitutively expressed β-subunit, the aryl hydrocarbon receptor nuclear translocator (also known as HIF-β) (33). Under normal cellular oxygen and iron levels, HIF-2α is hydroxylated at specific proline residues, which are required for Von Hippel-Lindau (VHL) tumor suppressor binding. VHL is the substrate recognition portion of an E3 ubiquitin ligase complex and is necessary for ubiquitination and subsequent proteasomal degradation of HIF-2α. The prolyl hydroxylases responsible for hydroxylation of HIF-2α require oxygen and iron as cofactors (34). During iron deficiency, prolyl hydroxylase activity is inhibited, and HIF-2α accumulates, dimerizes with the aryl hydrocarbon receptor nuclear translocator, and activates transcription of its target genes. HIF-2α binds to consensus hypoxia response elements in the promoters of genes involved in maintaining iron homeostasis, such as Dcytb, Dmt1, and Fpn1 (27, 31, 32). Because HIF-2α is critical in maintaining iron homeostasis following iron deficiency, in this study, we assessed whether similar mechanisms are required for the erythropoietic induction of iron absorption genes in the small intestine. Wild-type and intestinal HIF-2α knock-out mice were administered phenylhydrazine (PhZ), which causes hemolysis and stimulates erythropoiesis (5–7). It is shown that intestinal HIF-2α is critical not only for the erythropoietic induction of iron absorption genes in the small intestine but also for the increase in serum iron, which in turn is necessary for efficient erythropoiesis.
EXPERIMENTAL PROCEDURES
Animals and Treatments
VhlF/F (35), VhlΔIE (36), Hif-2αF/F, and Hif-2αΔIE (27) mice were described previously. The mice were housed in a light- and temperature-controlled room and were given water and chow ad libitum. PhZ (Sigma) was dissolved in neutralized saline. Mice were injected at 60 mg/kg of body weight on 2 consecutive days and were killed 2, 3, and 7 days following the second injection. Tissues were harvested and used fresh or flash-frozen in liquid nitrogen and stored at −80 °C for future use. All animal studies were carried out in accordance with Institute of Laboratory Animal Resources guidelines and approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Western Blot Analysis
Tissues were homogenized and lysed in radioimmune precipitation assay buffer for whole cell extracts, and nuclear proteins were isolated using the NE-PER nuclear extraction kit (Pierce). Membrane proteins were isolated as described previously (27). Membrane extracts were incubated for 5 min at 65 °C for detection of DcytB, whereas all other extracts were heated at 95 °C for 5 min. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes using standard methods. Membranes were incubated with antibodies against HIF-1α and HIF-2α (Novus Biologicals, Littleton, CO), Dmt1 and DcytB (Alpha Diagnostic International Inc., San Antonio, TX), and RAN (Santa Cruz Biotechnology, Santa Cruz, CA). For in vivo hypoxia detection, nitroimidazole was dissolved in normal saline with 2.4% ethanol and injected at 200 mg/kg of body weight 90 min before harvesting tissues. The signal was measured by using an antibody to nitroimidazole-protein adducts (HPI Inc., Burlington, MA) in whole cell extracts.
Quantitative Real-time Reverse Transcription-PCR (qPCR)
RNA was isolated from fresh or frozen tissue using Isol-RNA lysis reagent (5 PRIME, Gaithersburg, MD) and reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (Fisher). cDNA was quantified using SYBR Green dye and run on a 7900HT fast real-time PCR system (primers are listed in Table 1). Ct values were normalized to β-actin and expressed as -fold difference from controls.
TABLE 1.
Primers used for qPCR analysis
IRE, iron-responsive element.
| Primer | Sequence |
|---|---|
| β-Actin | |
| Forward | 5′-TATTGGCAACGAGCGGTTCC-3′ |
| Reverse | 5′-GGCATAGAGGTCTTTACGGATGT-3′ |
| Dmt1 IRE | |
| Forward | 5′-TGTTTGATTGCATTGGGTCTG-3′ |
| Reverse | 5′-CGCTCAGCAGGACTTTCGAG-3′ |
| Hepcidin | |
| Forward | 5′-TCTTCTGCATTGGTATCGCA-3′ |
| Reverse | 5′-GAGCAGCACCACCTATCTCC-3′ |
| EPO | |
| Forward | 5′-CATCTGCGACAGTCGAGTTCTG-3′ |
| Reverse | 5′-CACAACCCATCGTGACATTTTC-3′ |
| HIF-2α exon 2 | |
| Forward | 5′-TGAGTTGGCTCATGAGTTGC-3′ |
| Reverse | 5′-TATGTGTCCGAAGGAAGCTG-3′ |
| DcytB | |
| Forward | 5′-CATCCTCGCCATCATCTC-3′ |
| Reverse | 5′-GGCATTGCCTCCATTTAGCTG-3′ |
| Fpn1 | |
| Forward | 5′-ATGGGAACTGTGGCCTTCAC-3′ |
| Reverse | 5′-TCCAGGCATGAATACGGAGA-3′ |
Iron and Hematological Analysis
Serum iron was analyzed using the QuantiChrom iron assay kit (Bioassay Systems, Hayward, CA) following the manufacturer's protocol. Tissue iron in small intestinal epithelium was measured as described previously (37). Briefly, tissues were digested in 3 m HCl and 10% trichloroacetic acid at 65 °C for 20 h and then compared against an iron standard by a colorimetric assay. Complete blood count analysis was performed by the Unit for Laboratory Animal Medicine Pathology Core for Animal Research.
Immunohistochemistry
Duodenums were Swiss-rolled, frozen in cryo-embedding medium, and sectioned at 7 μm. The sections were fixed in 4% paraformaldehyde in PBS and incubated overnight at 4 °C with rabbit anti-mouse Fpn1 antibody (1:100; Alpha Diagnostic International Inc.) diluted in PBS with 2% BSA. Slides were washed twice with PBS and then incubated for 1 h at room temperature with Alexa Fluor® 488-labeled goat anti-rabbit IgG (1:500; Molecular Probes, Inc., Eugene, OR) diluted in PBS with 2% BSA. After incubation with the secondary antibody, all sections were washed three times for 5 min with PBS and mounted with ProLong® gold antifade reagent with DAPI (Molecular Probes, Inc.). Immunofluorescence was visualized using a Nikon Eclipse TE200 microscope with a ×20 objective. Images were acquired using an Olympus DP71 microscope digital camera and processed using an Olympus DP Controller Version 3.2.1.276 software package (Olympus America Inc., Center Valley, PA).
Cell Culture
Caco-2 cells were maintained at 37 °C in 5% CO2 and 21% O2. Cells were cultured in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic. For EPO experiments, cells were switched to serum-free medium 24 h before EPO incubation. Recombinant human erythropoietin (Prospec, East Brunswick, NJ) was used at a concentration of 10 or 100 IU/ml, and cells were incubated for 24 h. Cells were lysed in radioimmune precipitation assay buffer, and Western blot analysis was performed. For hypoxia experiments, cells were incubated in 1% O2 and 5% CO2 with balance N2 at 37 °C for 24 h.
RESULTS
PhZ Treatment Induces Expression of Epo in the Kidney and Iron Absorption Genes in the Small Intestine
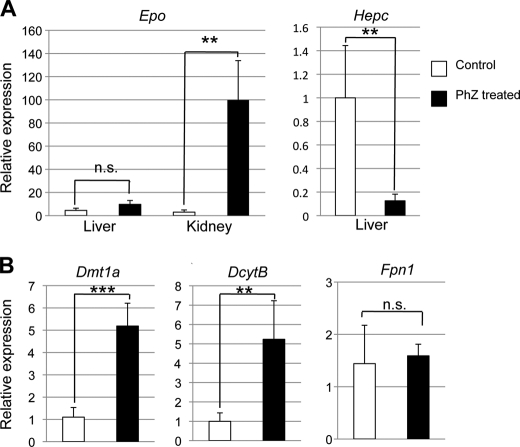
PhZ is commonly used to study hemolytic anemia-induced erythropoiesis (5–7). A dramatic decrease in RBCs following PhZ treatment leads to hypoxia in the kidney (1). This stabilizes HIF-2, activating EPO expression (1), and stimulates RBC differentiation and maturation into erythrocytes (2). Wild-type mice were treated with PhZ for 2 consecutive days and killed 48 h following final treatment. Epo mRNA expression was highly induced in the kidney, but not in the liver. Also, liver hepcidin mRNA expression was strongly suppressed (Fig. 1A), consistent with a previous report (25). Expression of Dmt1a and Dcytb was significantly increased in the duodenums of treated mice compared with untreated controls as assessed by qPCR. Fpn1 expression was not affected, however (Fig. 1B). Because erythropoiesis increases the demand for iron, these integrative changes provide an efficient system to increase the available iron stores. Moreover, PhZ treatment provides an ideal model to assess the mechanism by which iron absorption is induced following erythropoiesis.
FIGURE 1.
PhZ-induced hemolysis activates expression of EPO in the kidney and iron absorption genes in the small intestine. Wild-type mice were injected twice 24 h apart with 60 mg of PhZ/kg of body weight or with normal saline (Control) and killed 2 days later. qPCR was performed in the kidney or liver for Epo and hepcidin (Hepc) (A) or in the duodenum for Dmt1, Dcytb, and Fpn1 (B). Expression was normalized to β-actin. Values are expressed as -fold change compared with untreated controls. Four to five animals for each treatment group were assessed. Statistical analyses were performed using Student's t test. Each bar represents the mean ± S.D. **, p < 0.01; ***, p < 0.005; n.s., not significant.
Erythropoietic Induction of Iron Absorption Genes in the Small Intestine Is HIF-2α-dependent
Chronic low iron treatment induces the expression of iron absorption genes in the small intestine through a HIF-2α-dependent mechanism (27, 31, 32). However, the precise molecular mechanisms by which iron absorption is increased to satisfy systemic iron requirements during erythropoiesis are unknown. To investigate whether HIF-2α is also critical in erythropoietic induction of iron absorption, mice with an intestinal disruption of HIF-2α (Hif-2αΔIE) and wild-type littermate controls (Hif-2αF/F) were assessed following PhZ treatment. Hif-2αΔIE and Hif-2αF/F mice were treated with PhZ for 2 days and then killed 2 or 3 days post-treatment. In Hif-2αF/F mice, PhZ increased iron absorption genes in the duodenum as assessed by qPCR. However, PhZ induction of intestinal Dmt1a and Dcytb was completely abolished in the Hif-2αΔIE mice (Fig. 2A). Kidney Epo expression was highly induced to the same extent in Hif-2αΔIE mice and wild-type littermates, demonstrating that these changes are not due to dysregulation of kidney Epo expression (Fig. 2B). Western blotting was performed on protein extracts from the duodenum. In Hif-2αF/F mice, DcytB and Dmt1 expression was strongly induced in membrane duodenal extracts 2 and 3 days post-PhZ treatment. Consistent with the mRNA data, in Hif-2αΔIE mice, there was no increase in either Dmt1 or DcytB protein expression following PhZ treatment (Fig. 2C). Because Fpn1 expression was not detected by Western blotting consistently after PhZ treatment (data not shown), a highly sensitive immunohistochemistry assay was performed using intestines from PhZ-treated wild-type and Hif-2αΔIE mice. Fpn1 expression was similar following PhZ administration in Hif-2αΔIE mice compared with Hif-2αF/F mice, consistent with qPCR data (Fig. 2D). These data demonstrate that the induction of the apical iron absorption genes in the small intestine is dependent on HIF-2α expression.
FIGURE 2.
Erythropoietic induction of iron absorption genes in the small intestine is HIF-2α-dependent. Wild-type (Hif-2αF/F) and intestinal HIF-2 knock-out (Hif-2αΔIE) mice were treated with PhZ or saline (control) and killed 48 h post-treatment. qPCR was performed in the duodenum for Dmt1, Dcytb, and Fpn1 (A) or in the kidney for Epo (B). Expression was normalized to β-actin. Values are expressed as -fold change compared with untreated controls. C, Hif-2αF/F and Hif-2αΔIE mice were treated with PhZ or saline (control (Con)) and killed 48 and 72 h post-treatment. Western blot analysis measuring Dmt1a and DcytB expression in membrane extracts and Coomassie Blue staining of total membrane proteins were assessed for loading controls. D, immunofluorescence staining of Fpn1 in the duodenums of Hif-2αF/F and Hif-2αΔIE mice following PhZ treatment. Four to five animals for each treatment group were assessed. Statistical analyses were performed using Student's t test. Each bar represents the mean ± S.D. *, p < 0.05; **, p < 0.01; n.s., not significant. For Western blot analysis and immunofluorescence, a representative image from an individual mouse for each treatment group or time point is shown.
Erythropoiesis Stabilizes HIF-2α, but Not HIF-1α
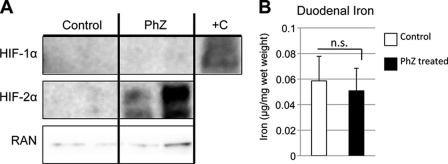
The gene expression data strongly suggest that HIF-2α is necessary for PhZ-induced expression of intestinal iron absorption genes. Consistent with these data, Western blotting of nuclear extracts prepared from the duodenums of PhZ-treated and untreated wild-type mice demonstrated a robust induction of HIF-2α, whereas expression of HIF-1α was not detected in duodenal nuclear extracts from PhZ-treated mice (Fig. 3A). Intestinal HIF-2α is rapidly stabilized by iron deficiency (27, 31, 32); therefore, the possibility that a decrease in duodenal iron stabilizes HIF-2α was assessed. Non-heme iron was extracted from PhZ-treated and untreated duodenal epithelia and quantified (Fig. 3B). No significant difference was detected, demonstrating that a decrease in tissue iron is not responsible for HIF-2α stabilization following PhZ treatment.
FIGURE 3.
PhZ treatment induces HIF-2α (but not HIF-1α) expression in the small intestine. A, Western blot analysis of duodenal nuclear extracts for HIF-1α and HIF-2α following PhZ or saline (Control) treatment. Hypoxia-treated Caco-2 cells were used as a positive control (+C) for HIF-1α. Expression was normalized to RAN protein expression. B, tissue non-heme iron quantification of the duodenums of PhZ- and saline-treated mice. Four to five animals for each treatment group were assessed. For Western blot analysis, representative images from two individual mice for each treatment group are shown. n.s., not significant.
Intestinal Hypoxia Stabilizes HIF-2α following PhZ-induced Erythropoiesis
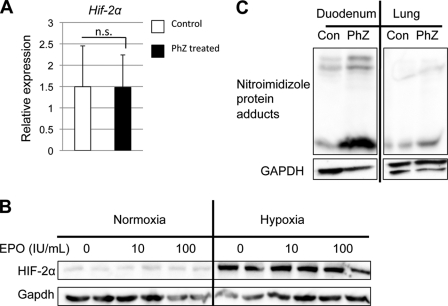
Hif-2α has recently been identified as a direct STAT5 target gene in hematopoietic stem cells (38). EPO is a known activator of the JAK2/STAT5 signaling cascade (39), and therefore, Hif-2α mRNA was assessed. No significant increase was detected in duodenal epithelial cells from PhZ-treated and untreated wild-type mice (Fig. 4A). Although Hif-2α mRNA was unchanged, EPO stabilization of HIF-2α protein was assessed. Caco-2 cells, a human colon cancer cell line, were incubated for 24 h in the presence or absence of recombinant human EPO. No change in HIF-2α expression was observed following EPO incubation (Fig. 4B). In addition, we assessed whether EPO and hypoxia could synergistically stabilize HIF-2α. Hypoxia strongly stabilized HIF-2α in Caco-2 cells. However, EPO incubation did not potentiate the increase in HIF-2α protein expression (Fig. 3B). Moreover, acute treatment of EPO (2, 4, and 8 h) in Caco-2 cells was performed, and EPO did not alter HIF-2α expression at any time points that were assessed (data not shown). These data clearly establish that circulating EPO is not involved in intestinal HIF-2α expression. An initiating event in erythropoiesis is kidney hypoxia stimulating Epo expression in a HIF-2α-dependent manner (40, 41). Therefore, the possibility that oxygen sensing in the small intestine is responsible for HIF-2α stabilization was evaluated. The hypoxia-detecting reagent nitroimidazole was used to investigate tissue hypoxia. Nitroimidazole forms protein adducts only in tissues with low oxygen tension but will not bind to proteins under normoxic conditions (42, 43). It was shown recently that Western blotting performed on extracts from cells treated with nitroimidazole is a sensitive and quantitative method for measuring hypoxic adduct formation (44). Wild-type mice were treated with PhZ for 2 days and injected with nitroimidazole 90 min prior to tissue collection. Nitroimidazole-protein adducts were detected by Western blotting in PhZ-treated and control duodenums, whereas no signal was detected in the oxygen-replete lung. In the duodenum, nitroimidazole staining was significantly stronger in the PhZ-treated mice compared with the control mice (Fig. 4C). These data suggest that following PhZ treatment, HIF-2, but not HIF-1, is stabilized in the intestine by hypoxia, a distinct mechanism from HIF-2α stabilization during iron deficiency.
FIGURE 4.
HIF-2α is stabilized by hypoxia in the small intestine following PhZ treatment. A, qPCR analysis of duodenal Hif-2α mRNA expression following PhZ or saline (Control) treatment in wild-type mice. B, Western blot analysis of Caco-2 cells treated with recombinant human EPO under normoxia and hypoxia for 24 h. Expression was normalized to GAPDH protein expression. C, Western blot analysis of nitroimidazole-protein adducts in extracts from the duodenums and lungs of PhZ-treated and control (Con) wild-type mice. Expression was normalized to GAPDH. Four to five animals for each treatment group were assessed. For Western blot analysis, a representative image from an individual mouse for each treatment group is shown. n.s., not significant.
Intestinal HIF-2α Is Necessary for the Erythropoietic Increase in Serum Iron
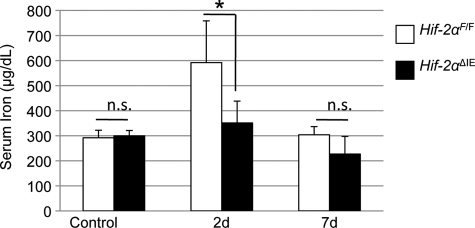
To determine whether HIF-2α-dependent regulation of iron absorption genes is required for the increase in systemic iron levels during erythropoiesis, serum iron was assessed in control and PhZ-treated wild-type and Hif-2αΔIE mice. No significant difference in basal serum iron levels was observed in Hif-2αΔIE mice compared with wild-type mice. At 2 days post-PhZ treatment, serum iron levels were significantly increased in wild-type mice, and the adaptive increase in serum iron following PhZ treatment was completely abolished in Hif-2αΔIE mice. At 7 days post-PhZ treatment, serum iron levels were back to normal in wild-type mice (Fig. 5). These data demonstrate that erythropoietic induction of iron absorption is dependent on HIF-2α.
FIGURE 5.
Intestinal HIF-2 expression is critical for the erythropoietic increase in serum iron. Wild-type (Hif-2αF/F) and intestinal HIF-2 knock-out (Hif-2αΔIE) mice were treated with PhZ or saline (Control), and sera were collected 2 (2d) and 7 (7d) days after the last injection. Serum was assayed for iron content. Four to five animals for each treatment group were assessed. Statistical analyses were performed using Student's t test. Error bars indicate S.D. *, p < 0.05; n.s., not significant.
Intestinal HIF-2α Expression Is Required for Efficient Erythropoiesis
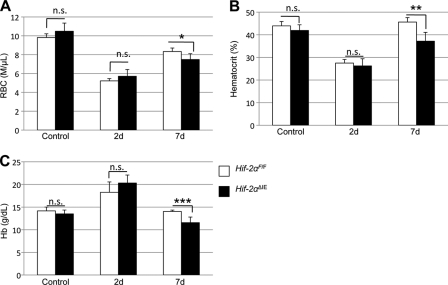
The erythropoietic induction of iron absorption is completely abolished in Hif-2αΔIE mice; therefore, these mice provide an ideal model to assess the significance of intestinal HIF-2α and iron absorption in erythropoiesis. To observe how inhibition of iron absorption in Hif-2αΔIE mice could affect erythropoiesis, complete blood count analysis was performed on Hif-2αF/F and Hif-2αΔIE mice treated with PhZ. Whole blood was taken 2 and 7 days following PhZ treatment. Control Hif-2αF/F and Hif-2αΔIE mice had normal RBC, hematocrit (HCT), and Hb levels (Fig. 6, A–C). Two days following PhZ treatment, RBC and HCT levels decreased significantly in both Hif-2αF/F and Hif-2αΔIE mice, and Hb levels were increased. At 7 days post-PhZ treatment, HCT and Hb levels were completely recovered in wild-type mice, and RBCs were increased compared with 2 days post-treatment. However, Hif-2αΔIE mice demonstrated significantly reduced RBC, HCT, and Hb levels compared with wild-type littermates. The changes observed in RBCs, HCT, and Hb in the Hif-2αΔIE mice reflect a critical role of intestinal HIF-2α in erythropoiesis.
FIGURE 6.
Intestinal HIF-2 expression is essential for erythropoiesis. Wild-type (Hif-2αF/F) and intestinal HIF-2 knock-out (Hif-2αΔIE) mice were treated with PhZ or saline (Control), and blood was collected 2 (2d) and 7 (7d) days after the last injection. Complete blood count analysis was performed measuring RBCs (A), HCT (B), and Hb (C). Four to five animals for each treatment group were assessed. Statistical analyses were performed using Student's t test. Error bars indicate S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.005; n.s., not significant.
DISCUSSION
The induction of iron absorption genes in the intestine by erythropoiesis has been described previously, but a mechanism had not been provided prior to this study (5–7). Similar to iron absorption induced by iron deficiency, HIF-2α is a critical regulator of iron absorption during erythropoiesis (31, 32). Intestinal HIF-2α signaling is essential for the increase in serum iron following induction of erythropoiesis by PhZ treatment. Furthermore, the increase in serum iron mediated by HIF-2α is essential for erythropoiesis, as it is severely inhibited in Hif-2αΔIE mice compared with Hif-2αF/F mice. The results of this study and of previous work demonstrate a central role of HIF-2α in erythropoiesis. Together, these data provide a working model for the induction of erythropoiesis (Fig. 7). A decrease in RBCs results in hypoxic kidneys due to decreased delivery of oxygen, which activates HIF-2α expression and subsequently Epo mRNA (40). The decrease in RBCs stabilizes intestinal HIF-2α, which activates iron absorption genes, leading to an increase in serum iron. Activation of HIF-2α signaling in the kidney and intestine is required for efficient RBC synthesis. Moreover, EPO acts on the bone marrow to stimulate erythrocyte differentiation and maturation, and the renewal of bone marrow-derived hematopoietic stem cells is also activated in a HIF-2α-dependent pathway (38).
FIGURE 7.
HIF-2 is a central regulator of erythropoiesis. Following hemolysis, the kidney and intestine experience hypoxia due to poor oxygen delivery. In the kidney, HIF-2α is stabilized, allowing it to bind to and activate the Epo promoter. In the small intestine, HIF-2α activates the Dcytb and Dmt1 genes, increasing iron absorption. Increased levels of circulating EPO and iron stimulate erythropoiesis and supply new erythrocytes with iron for hemoglobin production. ARNT, aryl hydrocarbon receptor nuclear translocator.
One finding of note is that basal erythropoiesis appears to be largely unaffected in Hif-2αΔIE mice compared with Hif-2αF/F mice under control conditions. Following erythropoietic stress, hematologic parameters are significantly different between Hif-2αΔIE and Hif-2αF/F mice, albeit the changes are moderate. Given the essential role of HIF-2α in intestinal iron absorption, one might expect a dramatic difference in RBCs, Hb, and HCT in basal as well as stress-induced erythropoiesis. One explanation is that a grain-based laboratory diet contains 350 ppm of ferrous iron, which is 10-fold higher than the normal dietary intake of mice. Moreover, ferrous iron does not require ferric reductase activity to be absorbed in the duodenum. It is possible that a diet consisting of ferric iron, which makes up the majority of normal dietary iron intake, would lead to more striking changes in hematologic parameters in Hif-2αΔIE mice.
Fpn1 is up-regulated in a HIF-2α-dependent manner following low iron treatment (27). However, HIF-2α activation following PhZ treatment did not increase Fpn1 expression. Also, an increase in Fpn1 does not appear to be required for erythropoiesis in a PhZ-induced erythropoiesis mouse model. It is possible that the acute HIF-2α activation following PhZ treatment is not sufficient to increase Fpn1 expression in comparison with the chronic activation seen with a low iron diet (27). In addition, basal Fpn1 expression appears to be sufficient to transport iron, despite increased iron uptake due to increased DcytB and Dmt1 expression. Together, these data suggest that regulation of Fpn1 expression is more important in low iron treatment than erythropoiesis, despite the increase in iron absorption in both cases.
Interestingly, in PhZ-treated mice, liver hepcidin expression was strongly repressed despite the mice having high serum iron levels. A similar phenomenon is observed in mouse models and patients with β-thalassemia. Hypoxia and EPO have been shown to repress hepcidin levels, and the increase in hypoxia and or EPO due to ineffective erythropoiesis may override the increase in hepcidin due to high serum iron levels (45, 46). Further experiments are needed to elucidate the role of hepcidin in erythropoiesis. Although the role of hepcidin in erythropoiesis is unclear, this study has clearly demonstrated an essential role of intestinal hypoxia sensing and HIF-2α activity in the regulation of erythropoiesis. HIF-2α-dependent increases in DcytB and Dmt1 at the mRNA and protein levels are required for the increased systemic iron demand during erythropoiesis. These findings present new therapeutic opportunities for treatment of injuries and diseases involving erythropoiesis. Thalassemia major leads to severe iron overload and is treated with chelation therapy (9, 10). It would be beneficial to explore targeted abrogation of intestinal HIF-2α activity, which would reduce iron absorption and could alleviate iron overload. Conversely, intestinal HIF-2α agonists could be used to increase intestinal iron absorption, which could help control iron deficiency anemia without requiring oral iron supplementation. HIF-2α is a central regulator of intestinal iron absorption, and more studies are needed to determine how modulation of intestinal HIF-2α activity can be used to treat diseases of iron overload and iron deficiency anemia.
This work was supported, in whole or in part, by National Institutes of Health Grant CA148828 (to Y. M. S.). This work was also supported by a grant from the University of Michigan Gastrointestinal Peptide Center (to Y. M. S.).
- EPO
- erythropoietin
- HIF-2α
- hypoxia-inducible factor-2α
- VHL
- Von Hippel-Lindau
- PhZ
- phenylhydrazine
- qPCR
- quantitative real-time reverse transcription-PCR
- HCT
- hematocrit.
REFERENCES
- 1. Jelkmann W. E. (2011) J. Physiol. 589, 1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dev A., Fang J., Sathyanarayana P., Pradeep A., Emerson C., Wojchowski D. M. (2010) Blood 116, 5334–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corwin H. L. (2006) Transfus. Med. Rev. 20, 27–33 [DOI] [PubMed] [Google Scholar]
- 4. Tanno T., Miller J. L. (2010) Adv. Hematol. 2010, 358283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson G. J., Powell L. W., Halliday J. W. (1990) Gastroenterology 98, 576–585 [DOI] [PubMed] [Google Scholar]
- 6. Conrad M. E., Weintraub L. R., Crosby W. H. (1965) Blood 25, 990–998 [PubMed] [Google Scholar]
- 7. Latunde-Dada G. O., Vulpe C. D., Anderson G. J., Simpson R. J., McKie A. T. (2004) Biochim. Biophys. Acta 1690, 169–176 [DOI] [PubMed] [Google Scholar]
- 8. Schmid H., Schiffl H. (2010) Cardiovasc. Hematol. Agents Med. Chem. 8, 164–172 [DOI] [PubMed] [Google Scholar]
- 9. Weatherall D. J. (1998) Baillieres Clin. Haematol. 11, 127–146 [DOI] [PubMed] [Google Scholar]
- 10. Gardenghi S., Marongiu M. F., Ramos P., Guy E., Breda L., Chadburn A., Liu Y., Amariglio N., Rechavi G., Rachmilewitz E. A., Breuer W., Cabantchik Z. I., Wrighting D. M., Andrews N. C., de Sousa M., Giardina P. J., Grady R. W., Rivella S. (2007) Blood 109, 5027–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latunde-Dada G. O., Simpson R. J., McKie A. T. (2008) J. Nutr. 138, 991–995 [DOI] [PubMed] [Google Scholar]
- 12. Mackenzie B., Garrick M. D. (2005) Am. J. Physiol. Gastrointest. Liver Physiol. 289, G981–G986 [DOI] [PubMed] [Google Scholar]
- 13. McKie A. T., Barrow D., Latunde-Dada G. O., Rolfs A., Sager G., Mudaly E., Mudaly M., Richardson C., Barlow D., Bomford A., Peters T. J., Raja K. B., Shirali S., Hediger M. A., Farzaneh F., Simpson R. J. (2001) Science 291, 1755–1759 [DOI] [PubMed] [Google Scholar]
- 14. Fleming M. D., Trenor C. C., 3rd, Su M. A., Foernzler D., Beier D. R., Dietrich W. F., Andrews N. C. (1997) Nat. Genet. 16, 383–386 [DOI] [PubMed] [Google Scholar]
- 15. Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. (1997) Nature 388, 482–488 [DOI] [PubMed] [Google Scholar]
- 16. Abboud S., Haile D. J. (2000) J. Biol. Chem. 275, 19906–19912 [DOI] [PubMed] [Google Scholar]
- 17. Donovan A., Brownlie A., Zhou Y., Shepard J., Pratt S. J., Moynihan J., Paw B. H., Drejer A., Barut B., Zapata A., Law T. C., Brugnara C., Lux S. E., Pinkus G. S., Pinkus J. L., Kingsley P. D., Palis J., Fleming M. D., Andrews N. C., Zon L. I. (2000) Nature 403, 776–781 [DOI] [PubMed] [Google Scholar]
- 18. Donovan A., Lima C. A., Pinkus J. L., Pinkus G. S., Zon L. I., Robine S., Andrews N. C. (2005) Cell Metab. 1, 191–200 [DOI] [PubMed] [Google Scholar]
- 19. McKie A. T., Marciani P., Rolfs A., Brennan K., Wehr K., Barrow D., Miret S., Bomford A., Peters T. J., Farzaneh F., Hediger M. A., Hentze M. W., Simpson R. J. (2000) Mol. Cell 5, 299–309 [DOI] [PubMed] [Google Scholar]
- 20. Park C. H., Valore E. V., Waring A. J., Ganz T. (2001) J. Biol. Chem. 276, 7806–7810 [DOI] [PubMed] [Google Scholar]
- 21. Krause A., Neitz S., Mägert H. J., Schulz A., Forssmann W. G., Schulz-Knappe P., Adermann K. (2000) FEBS Lett. 480, 147–150 [DOI] [PubMed] [Google Scholar]
- 22. Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. (2004) Science 306, 2090–2093 [DOI] [PubMed] [Google Scholar]
- 23. Gardenghi S., Ramos P., Marongiu M. F., Melchiori L., Breda L., Guy E., Muirhead K., Rao N., Roy C. N., Andrews N. C., Nemeth E., Follenzi A., An X., Mohandas N., Ginzburg Y., Rachmilewitz E. A., Giardina P. J., Grady R. W., Rivella S. (2010) J. Clin. Invest. 120, 4466–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Papanikolaou G., Tzilianos M., Christakis J. I., Bogdanos D., Tsimirika K., MacFarlane J., Goldberg Y. P., Sakellaropoulos N., Ganz T., Nemeth E. (2005) Blood 105, 4103–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kong W. N., Chang Y. Z., Wang S. M., Zhai X. L., Shang J. X., Li L. X., Duan X. L. (2008) J. Gastroenterol. 43, 136–143 [DOI] [PubMed] [Google Scholar]
- 26. Brasse-Lagnel C., Karim Z., Letteron P., Bekri S., Bado A., Beaumont C. (2011) Gastroenterology 140, 1261–1271 [DOI] [PubMed] [Google Scholar]
- 27. Taylor M., Qu A., Anderson E., Matsubara T., Martin A., Gonzalez F. J., Shah Y. M. (March 18, 2011) Gastroenterology 10.1053/j.gastro.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaston T., Chung B., Mascarenhas M., Marks J., Patel B., Srai S. K., Sharp P. (2008) Gut 57, 374–382 [DOI] [PubMed] [Google Scholar]
- 29. Mena N. P., Esparza A., Tapia V., Valdés P., Núñez M. T. (2008) Am. J. Physiol. Gastrointest. Liver Physiol. 294, G192–G198 [DOI] [PubMed] [Google Scholar]
- 30. Laftah A. H., Ramesh B., Simpson R. J., Solanky N., Bahram S., Schümann K., Debnam E. S., Srai S. K. (2004) Blood 103, 3940–3944 [DOI] [PubMed] [Google Scholar]
- 31. Mastrogiannaki M., Matak P., Keith B., Simon M. C., Vaulont S., Peyssonnaux C. (2009) J. Clin. Invest. 119, 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah Y. M., Matsubara T., Ito S., Yim S. H., Gonzalez F. J. (2009) Cell Metab. 9, 152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tian H., McKnight S. L., Russell D. W. (1997) Genes Dev. 11, 72–82 [DOI] [PubMed] [Google Scholar]
- 34. Ivan M., Haberberger T., Gervasi D. C., Michelson K. S., Günzler V., Kondo K., Yang H., Sorokina I., Conaway R. C., Conaway J. W., Kaelin W. G., Jr. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 13459–13464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haase V. H., Glickman J. N., Socolovsky M., Jaenisch R. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah Y. M., Ito S., Morimura K., Chen C., Yim S. H., Haase V. H., Gonzalez F. J. (2008) Gastroenterology 134, 2036–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torrance J. D., Bothwell T. H. (1968) S. Afr. J. Med. Sci. 33, 9–11 [PubMed] [Google Scholar]
- 38. Fatrai S., Wierenga A. T., Daenen S. M., Vellenga E., Schuringa J. J. (2011) Blood 117, 3320–3330 [DOI] [PubMed] [Google Scholar]
- 39. Bittorf T., Jaster R., Lüdtke B., Kamper B., Brock J. (1997) Cell. Signal. 9, 85–89 [DOI] [PubMed] [Google Scholar]
- 40. Kapitsinou P. P., Liu Q., Unger T. L., Rha J., Davidoff O., Keith B., Epstein J. A., Moores S. L., Erickson-Miller C. L., Haase V. H. (2010) Blood 116, 3039–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rankin E. B., Biju M. P., Liu Q., Unger T. L., Rha J., Johnson R. S., Simon M. C., Keith B., Haase V. H. (2007) J. Clin. Invest. 117, 1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hodgkiss R. J. (1998) Anticancer Drug Des. 13, 687–702 [PubMed] [Google Scholar]
- 43. Koch C. J. (2002) Methods Enzymol. 352, 3–31 [DOI] [PubMed] [Google Scholar]
- 44. Sato Y., Endo H., Okuyama H., Takeda T., Iwahashi H., Imagawa A., Yamagata K., Shimomura I., Inoue M. (2011) J. Biol. Chem. 286, 12524–12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pinto J. P., Ribeiro S., Pontes H., Thowfeequ S., Tosh D., Carvalho F., Porto G. (2008) Blood 111, 5727–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peyssonnaux C., Zinkernagel A. S., Schuepbach R. A., Rankin E., Vaulont S., Haase V. H., Nizet V., Johnson R. S. (2007) J. Clin. Invest. 117, 1926–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]