Abstract
Satellite cells are well known as a postnatal skeletal muscle stem cell reservoir that under injury conditions participate in repair. However, mechanisms controlling satellite cell quiescence and activation are the topic of ongoing inquiry by many laboratories. In this study, we investigated whether loss of the cell cycle regulatory factor, pRb, is associated with the re-entry of quiescent satellite cells into replication and subsequent stem cell expansion. By ablation of Rb1 using a Pax7CreER,Rb1 conditional mouse line, satellite cell number was increased 5-fold over 6 months. Furthermore, myoblasts originating from satellite cells lacking Rb1 were also increased 3-fold over 6 months, while terminal differentiation was greatly diminished. Similarly, Pax7CreER,Rb1 mice exhibited muscle fiber hypotrophy in vivo under steady state conditions as well as a delay of muscle regeneration following cardiotoxin-mediated injury. These results suggest that cell cycle re-entry of quiescent satellite cells is accelerated by lack of Rb1, resulting in the expansion of both satellite cells and their progeny in adolescent muscle. Conversely, that sustained Rb1 loss in the satellite cell lineage causes a deficit of muscle fiber formation. However, we also show that pharmacological inhibition of protein phosphatase 1 activity, which will result in pRb inactivation accelerates satellite cell activation and/or expansion in a transient manner. Together, our results raise the possibility that reversible pRb inactivation in satellite cells and inhibition of protein phosphorylation may provide a new therapeutic tool for muscle atrophy by short term expansion of the muscle stem cells and myoblast pool.
Keywords: Gene Knockout, Protein Phosphatase, Protein Phosphorylation, Retinoblastoma (Rb), Stem Cell, Tautomycetin, Muscle, myoblast, Satellite Cell
Introduction
Skeletal muscle is a highly regenerative tissue because of the presence of muscle stem cells intertwined with individual muscle fibers. Muscle stem cells are also referred to as satellite cells because of their location of the cells under the basement membrane of muscle fibers (1). Satellite cells are maintained in quiescence in the adult steady state, while stimuli such as injury activate these myogenic (lineage-restricted) stem cells and induce differentiation into myoblasts then myofibers via the process of muscle regeneration (2, 3). Activated satellite cells divide symmetrically or asymmetrically to either replenish the reservoir of quiescent stem cells and/or to create myogenic cell progeny destined for differentiation (4, 5, 6). Although the regulatory mechanisms modulating the satellite cell quiescence/activation cycle are not yet well understood, it is widely known that the HGF/c-Met signaling axis is one such mechanism regulating the cell cycle re-entry of quiescent satellite cells (3, 7). A related recent study suggested that high concentration of HGF inhibits satellite cell activation by the negative feedback mechanism through enhanced satellite cell expression of Myostatin (GDF8) (8), a potent regulator of quiescence/activation (10). In more broad-reaching studies, Fukada et al. (2007) recently performed a gene expression analysis comparing activated and quiescent satellite cells to shed light on the regulatory mechanism of satellite cell activation and have demonstrated that the calcitonin receptor is associated with satellite cell quiescence (9). Notwithstanding these and other studies, a great deal remains to be learned about satellite cell quiescence and activation.
The mammalian cell cycle is regulated by the E2F transcriptional factors and the retinoblastoma tumor suppressor protein (pRb)2 (11). During myogenic terminal differentiation, pRb is de-phosphorylated, and this hypophosphorylated form leads to withdrawal from the cell cycle by inhibition of E2F transcriptional activity. In contrast, pRb is maintained as hyperphosphorylated form by cycling myoblasts (12). Lack of pRb causes severe deficiency of skeletal muscle in newborn pups of Rb1 knock-out mice, and myoblasts and MyoD-transfected MEFs (mouse embryo fibroblasts) exhibit a defect in terminal differentiation of these myogenic cells when pRb is absent or inactivated (13–16). The Rb family protein, p130, is also highly expressed in the satellite cell-like quiescent reserve cell population of C2C12 cells. Conversely, cyclin-dependent kinase inhibitors (CKI) that modulates pRb family members are up-regulated in quiescent satellite cells compared with activated satellite cells (9, 17). These reports thus indicate the importance of the Rb family for myogenesis. However, the role of the Rb family proteins, especially in postnatal (adolescent and adult) satellite cell kinetics has not been specifically examined.
To study the role of pRb in satellite cells, we employed a conditional mouse model. We and other groups recently generated satellite cell specific Cre-driver mouse lines, which can temporally induce Cre-recombinase expression in Pax7-expressing satellite cells by tamoxifen administration (18, 19). By using these mouse lines, specific gene expression can be ablated or induced in satellite cells of postnatal muscle (18–21). To investigate whether Rb-dependent cell cycle regulation contributes to the satellite cell quiescence/activation circuit and satellite cell expansion in postnatal skeletal muscle, we used Pax7CreERp/WT and Rb1flox/flox mouse lines to ablate Rb1 in satellite cells. We found that lack of Rb1 in satellite cells postnatally caused an increase in the muscle stem cell pool and accelerated quiescent satellite cells to re-enter the cell cycle. We also demonstrated that sustained loss of Rb1 in the satellite cell lineage causes a deficit of muscle fiber accretion despite an increase in myoblast number. Finally, we showed that pharmacological inhibition of protein phosphatase 1 (PP1) can temporally accelerate satellite cell activation and renewal in vitro and in vivo. Our results suggest that reversible (pharmacological) pRb inactivation in satellite cells could be a very useful therapeutic intervention for the muscle atrophy accompanying aging or muscle degenerative disease by short term expansion of the muscle stem cell and myoblast pools in postnatal skeletal muscle.
MATERIALS AND METHODS
Mice
All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Health Science Center at San Antonio or the Oregon Health & Science University. Rb1flox/flox mice were obtained from the Mouse Models of Human Cancer Consortium (MMHCC, NCI-Frederick, National Institutes of Health). Tamoxifen-inducible Pax7CreERp/WT mice and Rosa26LUSEAPm/WT reporter mice were previously described (18, 22) and can be obtained from the MMHCC as strain 01XBS. Pax7CreERp/WT mice were mated with Rb1flox/flox mice to generate the Pax7CreERp/WTRb1flox/WT intermediate mice then, these intermediates were further crossed with Rb1flox/flox to generate Pax7CreERp/WTRb1flox/flox (Pax7CreER,Rb1) mice. For experimental mice, CreER was always inherited from the sire to prevent somatic mosaicism.
Tamoxifen Induction
To induce Cre-recombinase expression in Pax7+ satellite cells, 200 μl of 10 mg/ml tamoxifen (Sigma-Aldrich) suspended in corn oil was injected intraperitoneally into 4-week-old Pax7CreERp/WT Rb1flox/flox, Pax7CreERp/WT Rb1flox/flox Rosa26LUSEAPm/WT, Pax7CreERp/WT Rosa26LUSEAPm/WT, or Rosa26LUSEAPm/WT mice once a day for 5 days at a dose of 2 mg/20 g body weight/day. Rosa26LUSEAPm/WT Cre/LoxP reporter mice that express luciferase following Cre expression have been described previously (18, 22, 23). Mice were euthanized by CO2 asphyxiation at 2 weeks after tamoxifen injection (for Pax7 CreERp mice), and skeletal muscle were harvested for immunohistochemistry and single fiber culture.
Single Fiber Culture
Single fiber culture was carried out in accordance with previous studies (24–26). Briefly, single muscle fibers were isolated from gastrocnemius muscles of mice. Muscles were carefully removed at the tendon and treated with 0.2% (w/v) collagenase type I (Sigma-Aldrich) reconstituted in Dulbecco's Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA) at 37 °C for 60 min with gentle shaking. After trituration with pipette, the muscle was further treated in enzyme solution at 37 °C for 10 min. The muscle was transferred into fresh 10% FBS/DMEM, and fine fibers were liberated from the muscle by further gentle tituration with pipette. Isolated-fine fibers were maintained as non-adherent cultures in 10% FBS/DMEM on 24-well non-coated culture plates (BD Biosciences, San Jose, CA) for 0–3 days and then stained for each markers.
Satellite Cell Progeny Culture
Muscle fibers were picked up from an isolated-fiber pool and placed in a cell culture dish coated with MatrigelTM (BD Biosciences) as a single fiber per well. After 2 days, each muscle fiber was picked up from the well, and cells remaining in the dish were cultured in 10% FBS/DMEM for additional 8 days. Triplicate clones from both control and Pax7CreER,Rb1 mice were used for fusion index quantification. TUNEL assay was also performed on single clones. Briefly, satellite cell progeny clones from wild-type control and Pax7CreER,Rb1 mice were cultured for 10 days in 10% FBS/DMEM, then media was replaced with 2% HS/DMEM and cultured for 36 h to examine differentiation ability.
Immunocytochemistry
For immunocytochemistry, cells on isolated-fibers in culture dishes were fixed with 2–4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) for 20 min at room temperature. Following washing with PBS, cells were incubated in 5% normal goat serum (NGS; Invitrogen)/0.1% Triton-X (Sigma)/PBS to block nonspecific binding of antibodies. Primary antibodies were then applied and cells were incubated overnight at 4 °C. After washing cells with PBS, Alexafluor 594 or 488 conjugated anti-mouse IgG (1:400 diluted with 5% NGS in PBS; Invitrogen) was added and incubated for 1 h at RT. For phosphorylated Histone-H3 staining, Tris-buffered saline (TBS) was used as basal buffer for washing, blocking, and antibody dilution buffers. Slides were mounted using the Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA) and visualized on an Olympus IX81 confocal microscope equipped with FluoroviewTM 1.6A software (Olympus America, Center Valley, PA).
Immunohistochemistry
For immunohistochemistry, tibialis anterior muscle was collected from Pax7CreER,Rb1 and age-matched control mice 6 month after tamoxifen injection. Following fixation in 10% formalin, muscles were gradually transitioned in 10, 20, and 30% sucrose/PBS at 4 °C for 12 h prior to embedding in OCT compound. Staining was performed using the M.O.M. Immunodetection Kit Staining Procedure (Vector Laboratories) following the manufacturer's instructions (18).
Antibodies
Primary antibodies used for immunofluorescence as follows: anti-Pax7 monoclonal (1:100, Developmental Studies Hybridoma Bank (DSHB), University of Iowa, Iowa City, IA), anti-MyoD1 monoclonal (1:100, 5.8A, Novus Biologicals, Littleton, CO), anti-Myosin heavy chain monoclonal (1:50, MF20, DSHB), anti-pRb monoclonal (1:500, BD Bioscience), anti-Myf5 polyclonal (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-histone H3 (Ser-10) polyclonal (1:200, Cell Signaling Technology, Beverly, MA), anti-α2a Laminin polyclonal (1:500, a kind gift from Dr. Peter D. Yurchenco, University of Medicine and Dentistry of New Jersey).
Optical Imaging for Luminescence
In vivo bioluminescence imaging of live female mice was performed using Xenogen IVIS-Spectrum system (Caliper LS; Alameda, CA). Animals were maintained under inhaled anesthesia using 2% isoflurane in 100% oxygen at the rate of 2.5 liter/min. For firefly luciferase imaging, the image acquisition parameters were 50-s exposure time, 2 × 2 binning, 12.6-cm field of view, and f/stop of 1/4. Data were acquired and analyzed using the manufacturer's proprietary Living ImageTM 2.5 software.
Tautomycetin Treatment
Tibialis anterior muscle fibers were isolated from 8-week-old wild-type mouse muscle and were cultured in 10% FBS/DMEM with DMSO or Tautomycetin (TC; 10 nm) (Tocris Bioscience, Ellisville, MO). After 24 h of culture, muscle fibers were fixed with 4% PFA then immunocytochemistry for Pax7 and phosphorylated histone-H3 (pH-H3) was performed and the number of activated satellite cell (pH-H3+/Pax7+) on fibers were counted. For in vivo investigations, TC (30 nm) or vehicle in a volume of 50 μl was directly injected into mouse TA muscle concurrently with cardiotoxin (2.5 μm). After 14 days, skeletal muscle was collected and the frequency of Pax7+ satellite cells were counted for 100 randomly regenerating myofibers with central nuclei for each groups (n = three 6-week-old animals per cohort).
Statistical Analysis
All experiments were performed in triplicate and mean ± S.D. was calculated. A Student's two-tailed t test was employed to determine statistical significance. p < 0.05 was considered statistically significant.
RESULTS
Rb1 Inactivation in Satellite Cells and in Their Progeny Accelerates Cell Expansion
We recently generated a tamoxifen-inducible Cre-driver mouse line, Pax7CreERp/WT, which facilitates temporally-specific Cre-recombinase expression in Pax7-expressing cells such as satellite cells following tamoxifen injection (18, 20, 21, 22). To clarify the role of Rb1 in satellite cell homeostasis, we first examined and confirmed that pRb is expressed in Myf5+ satellite cells, the population of satellite cells that are often activated (supplemental Fig. S1). We next generated Pax7CreERp/WTRb1flox/flox (Pax7CreER,Rb1) mice to delete Rb1 in satellite cells of postnatal skeletal muscle. After tamoxifen (TAM) injection, exon 19 of both Rb1 alleles is ablated, resulting in inactivation of Rb1 in satellite cells of postnatal skeletal muscle (Fig. 1A). TAM was injected at postnatal day 30 (P30), and skeletal muscle was harvested from age matched control and Pax7CreER,Rb1 mice 14 days later for a single muscle fiber culture. Isolated fine muscle fibers were cultured for 0 and 72 h, and immunocytochemistry for Pax7 and MyoD was performed on these fibers (Fig. 1B). At 0 h after muscle fiber isolation, Pax7+ satellite cells were increased by 152% in Pax7CreER,Rb1 muscle compared with control (Fig. 1C). Results were similar at 42 h (supplemental Fig. S2). At 0 h, Ki67+ proliferating satellite cells were undetectable (data not shown). This initial quiescence of freshly isolated satellite cells was affirmed by staining with the cell cycle marker, phosphorylated histone-H3 (pH-H3) (supplemental Fig. S3). However, after 3 days in culture, the frequency of MyoD+ myoblast, progeny of satellite cells, was increased by 218% in these satellite cell progeny lacking Rb1 (Fig. 1D). To further examine the role of Rb1 in satellite cell cell-cycle kinetics, isolated single muscle fibers were fixed at 24 h after isolation and presence of pH-H3 was examined by immunocytochemistry. Satellite cells positive for pH-H3 were counted on age-matched control and Pax7CreER,Rb1 mouse muscle fibers. Although activated satellite cells (pH-H3+/Pax7+) were present on muscle fibers of both cohorts, at 24 h the number of activated satellite cells was 210% higher in Pax7CreER,Rb1 mice (Fig. 1E). Together, these data suggest that cell cycle re-entry in satellite cells is increased by Rb1 ablation, resulting in the expansion of activated satellite cells and the progeny of the satellite cell lineage.
FIGURE 1.
Satellite cell-specific Rb1 inactivation results in an increase of satellite cells and their progeny in vitro. A and B, strategy for conditional deletion of Rb1 in satellite cells. Pax7CreERp/WT mice were mated with Rb1flox/flox mice to generate Pax7CreERp/WTRb1flox/flox (Pax7CreER,Rb1) mice. At postnatal day 30 (P30), TAM was intraperitoneally injected to Pax7CreER,Rb1 mice to induce satellite cell-specific conditional ablation of Rb1. To study the role of Rb1 in satellite cells, single muscle fiber isolation was performed on TAM-injected mice. At 14 days after TAM injection, fibers were isolated and cultured for 0 and 72 h then, immunocytochemistry for Pax7 or MyoD was performed. C, Pax7+ cell number on the isolated single muscle fibers was counted at 0 h (0H) after TAM injection (age matched control: n = 3, total 33 fibers; Pax7CreER,Rb1: n = 3, total 57 fibers). Pax7+ satellite cells were increased by inactivation of Rb1 in adolescent mouse muscle (*, p < 0.05). Arrows indicate Pax7+ satellite cells. Control: age-matched control, P7Rb−/−: Pax7CreER,Rb1. D, inactivation of Rb1 in satellite cells caused an increase of their progeny. At 72 h after muscle fiber isolation, MyoD+ myoblast number on fibers was quantified by immunohistochemistry (Control: n = 2, total 27 fibers; Pax7CreER,Rb1: n = 2, total 31 fibers). Myoblast number was significantly increased in Pax7CreER,Rb1 mouse muscle (*, p < 0.05). (E) Rb1 inactivation in satellite cells enhances satellite cell expansion (*, p < 0.05). Immunohistochemistry for phosphorylated histone-H3 (pH-H3) and Pax7 were performed on isolated muscle fibers from Pax7CreER,Rb1 and age-matched control mice at 24 h after plating (age-matched control: n = 2, total 25 fibers, Pax7CreER,Rb1: n = 2, total 21 fibers). Arrowheads indicate expanding satellite cells. Scale bar: 50 μm.
Rb1 Inactivation Results in an Increase of Satellite Cells and Myoblasts in Vivo
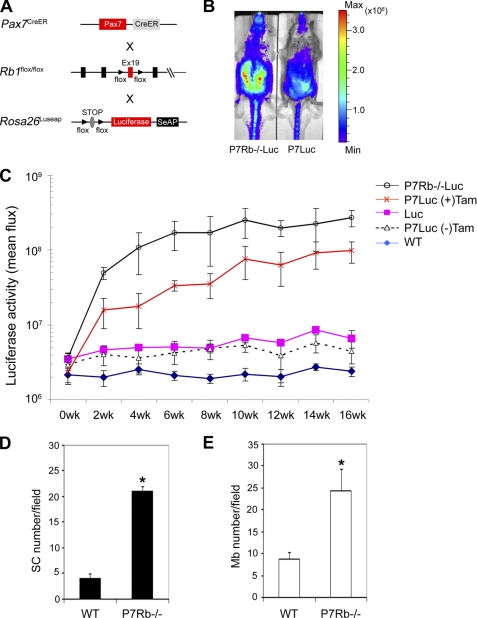
To quantify whole animal satellite cell kinetics when Rb1 is inactivated, Pax7CreER,Rb1 mice were mated with a Rosa26LUSEAPm/WT reporter strain to generate Pax7CreERp/WTRb1flox/floxRosa26LUSEAPm/WT (Pax7CreER,Rb1,Luseap) mice (18) (Fig. 2A). Tamoxifen was intraperitoneally administered to Pax7CreER,Rb1,Luseap mice at P30 then myogenic luciferase signal was measured in vivo every 2 weeks. Pax7CreER,Rb1,Luseap mice had stronger luciferase signal intensity than Pax7CreERp/WTRosa26LUSEAPm/WT mice at 2 weeks after TAM injection (Fig. 2B), and this higher signal for Pax7CreER,Rb1,Luseap mice was sustained for the entire measurement period (16 weeks). As we previously reported that luciferase signal depends on cell differentiation status and cell number (18), higher luciferase signal of Pax7CreER,Rb1,Luseap mice might reflect a higher number of satellite cells and/or myoblasts in the muscle than wild type mice (Fig. 2C). Indeed, Pax7+ satellite cells and MyoD+ myoblasts were increased 5- and 3-fold, respectively, in the muscle of Pax7CreER,Rb1 mice 6 month after TAM injection (Fig. 2, D and E). In related work, Huh et al. (2004) have shown using adenovirus-Cre infection technique that pRb deficiency causes 2.5-fold reduction of doubling time of primary myoblasts (15). Taking together this finding and our in vitro and in vivo results, one can conclude that Rb1 may critically regulate both satellite cell expansion and myoblast proliferation.
FIGURE 2.
Rb1 inactivation increases satellite cell and myoblast number in vivo. A, strategy for in vivo satellite cell tracing in mouse muscle. Pax7CreERp/WTRb1flox/flox (Pax7CreER,Rb1) mice were mated with Rosa26LUSEAPm/WT mice to trace satellite cell kinetics in vivo. Mice were injected with Tamoxifen at P30 to activate the luciferase reporter in satellite cells. B, representative example of qualitative luciferase signal from satellite cells in Pax7CreERp/WTRb1flox/floxRosa26LUSEAPm/WT (P7Rb−/−Luc) mice treated with a tamoxifen pulse at 6 weeks of age versus Pax7CreERp/WTRosa26LUSEAPm/WT (P7Luc) mice. Bioluminescence is seen predominantly from the ventral (abdominal) musculature. Images are displayed at a minimum-maximum scale of 2 × 105 to 5 × 106 photons/s/cm/sr. C, longitudinal, steady state time course of quantitative bioluminescence for Pax7CreERp/WTRb1flox/floxRosa26LUSEAPm/WT mice after tamoxifen injection. Tamoxifen was intraperitoneally injected at P30 after birth and then luciferase signal was analyzed every 2 weeks. Lines represent Pax7CreERp/WTRb1flox/floxRosa26LUSEAPm/WT (black, n = 5), Pax7CreERp/WTRosa26LUSEAPm/WT (with tamoxifen; orange, n = 7), Rosa26LUSEAPm/WT (pink, n = 4), Pax7CreERp/WTRosa26LUSEAPm/WT (without tamoxifen; black dot, n = 5), and wild type (blue, n = 4), respectively. D and E, at 6-month after tamoxifen injection, tibialis anterior muscles were collected and stained with anti-Pax7 (black bars) and anti-MyoD (white bars) antibodies to calculate satellite cell and myoblast number (n = 3). Satellite cell and myoblast number were increased in Pax7CreER,Rb1 mouse muscle (*, p < 0.05).
Sustained Rb1 Inactivation Causes Incomplete Muscle Growth and Delays Muscle Repair
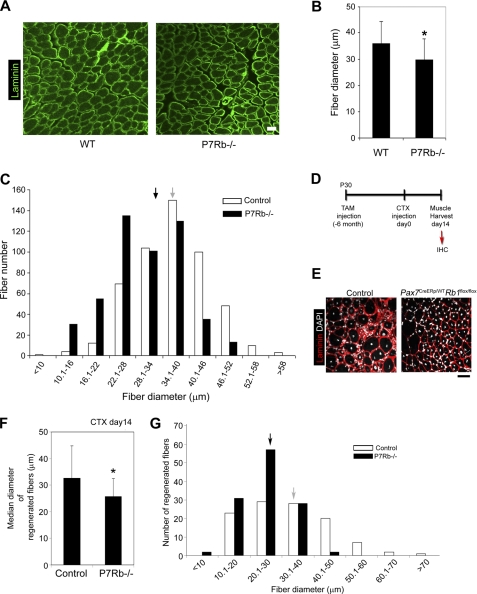
Huh et al. (2004) previously reported that Rb1 inactivation specifically in fetal myoblasts impairs terminal differentiation of the cell by induction of cell apoptosis using in vitro studies (15). Although this study demonstrated the crucial association of Rb1 to terminal differentiation in committed myogenic cells, the homeostatic regulation of Rb1 in satellite cells and myoblasts postnatally (for adolescents and adults) in vivo is still unclear. Herein, we investigated whether Rb1 inactivation for satellite cells and their progeny affects skeletal muscle homeostasis in normally growing muscle as well as experimentally injured muscle in vivo. First, skeletal muscle of Pax7CreER,Rb1 mice was harvested at 6 months after TAM injection and immunohistochemistry for Laminin was performed to compare muscle fiber diameter between age matched control and Pax7CreER,Rb1 mice (Fig. 3A). Muscle fiber diameter of Pax7CreER,Rb1 mice was significantly thinner than controls in normally growing muscle (36 ± 8 versus 30 ± 8 μm, p < 0.001) (Fig. 3B). This result was consistent with altered whole fiber diameter distribution in that Pax7CreER,Rb1 mouse muscle was shifted to thinner distributions (Fig. 3C). Next, we performed muscle injury/regeneration studies to examine whether Rb1 deficiency in satellite cell affect muscle regeneration. CTX was injected into tibialis anterior muscle of 6-month-old Pax7CreER,Rb1 mice, then injured muscle was harvested at day 14 after CTX injection to perform immunohistochemistry for Laminin (Fig. 3D). CTX-injected muscle of Pax7CreER,Rb1 mice appeared to approximate normal regeneration as evidenced by the presence of centrally nucleated muscle fibers in the injury area (Fig. 3E). By close comparison to control mice, however, regenerated fibers of Pax7CreER,Rb1 mice had smaller diameter (Fig. 3, F and G, 33 ± 12 versus 26 ± 7 μm, p < 0.001), indicating a delay of muscle regeneration in Pax7CreER,Rb1 mice.
FIGURE 3.
Loss of Rb1 in satellite cells causes muscle fiber atrophy. A, immunohistochemistry for Laminin on muscle from Pax7CreERp/WTRb1flox/flox (Pax7CreER,Rb1)(P7Rb−/−) and age-matched wild-type control (WT) mice. B, fiber diameter was measured in muscle cross sections, and median diameter was compared between Pax7CreER,Rb1 (P7Rb−/−; n = 500 fibers) and age-matched wild-type control mice (WT; n = 500 fibers) mice. Muscle fibers are hypotrophic in Pax7CreER,Rb1 mice (*, p < 0.05). C, fiber distribution of Pax7CreER,Rb1 (P7Rb−/−) and age-matched wild-type control mice (Control). Arrows represent median (black: P7Rb−/−, gray: control). D, strategy of muscle regeneration induction. CTX was injected to tibialis anterior muscle of age-matched wild-type control and Pax7CreER,Rb1 mice at 6 months after TAM injection. At 14 days after CTX injection, muscles were harvested, and immunohistochemistry (IHC) was performed. E, immunohistochemistry for laminin. Regenerating fibers have central nuclei (DAPI). F and G, at day 14 after CTX injection, a diameter of regenerated muscle fiber which exhibit central nuclei was measured and compared with fiber distribution between age-matched control (n = 110 fibers) and Pax7CreER,Rb1 (n = 120 fibers) mice. Arrows in G indicate median diameter. Loss of Rb1 in satellite cells significantly delayed muscle regeneration (*, p < 0.05). Scale bar: 50 μm.
Satellite Cell Progeny Lacking Rb1 Fail to Differentiate into Myotubes
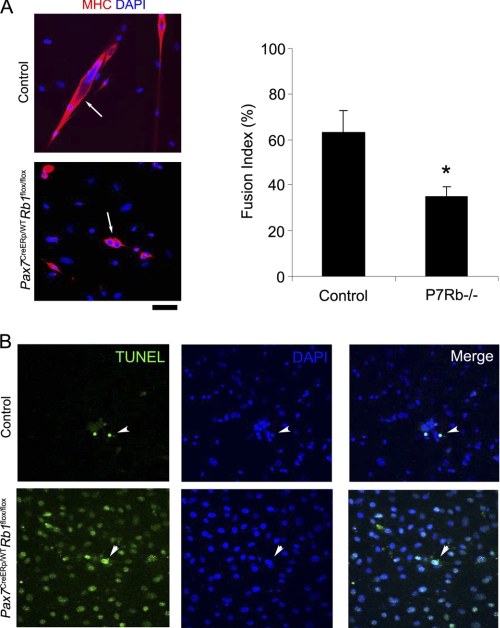
Terminal differentiation of fetal-derived myoblasts and the protection against apoptosis accompanying with differentiation are regulated by cell cycle regulatory components such as p21 and pRb (27, 28, 29). Indeed, when pRb was completely lost in isolated myoblasts using Adenovirus-Cre system, terminal differentiation failed and these cells exhibited accelerated apoptosis under differentiation condition (15). To elucidate the cause of smaller fiber diameter and a delay of muscle regeneration of Pax7CreER,Rb1 mice, we performed TUNEL under conditions of myogenic differentiation for satellite cell progeny. Satellite cell progeny were cloned from individually-isolated single muscle fibers of age-matched control and Pax7CreER,Rb1 mice then cultured on Matrigel-coated cell culture dish for 2 weeks. The fusion index (frequency of multinucleated cells) for satellite cell progeny lacking Rb1 was significantly lower than control myotubes from mice with intact Rb1 (Fig. 4A) and less protection against apoptosis in the differentiation media was afforded to cells lacking Rb1 (Fig. 4B). These results are consistent with a previous report of myoblasts lacking pRb (15) and suggest that muscle hypotrophy and a delay of muscle regeneration observed in Pax7CreER,Rb1 mice may be caused by the decrease of terminal differentiation of satellite cell progeny lacking of Rb1.
FIGURE 4.
Satellite cell progeny derived from Rb1-inactivated satellite cells have less terminal differentiation capacity. A, Rb1 inactivation in satellite cells causes inhibition of terminal differentiation of cell progeny. Satellite cell clones were separated from isolated single muscle fibers and cultured for 7 days in GM (10% FBS/DMEM) and 3 days in DM (2% HS/DMEM) to induce terminal differentiation of myoblasts. Immunohistochemistry for myosin heavy chain (MHC) was performed (left panels), and fusion index (frequency of multinucleated cells) was calculated in three different clones for each groups (n = 3, right graph). Arrows indicate MHC+ myotubes, which were less frequent in Pax7CreERp/WTRb1flox/flox (P7Rb−/−) mice (*, p < 0.05). B, satellite cell progenies lacking Rb1 are predisposed to apoptosis. TUNEL assay was performed on satellite cell progenies derived from isolated single muscle fibers of Pax7CreERp/WTRb1flox/flox and age-matched wild-type control mice. Arrowheads indicate TUNEL+ apoptotic cells. Scale bar: 50 μm.
Transient Inhibition of Protein Phosphatase 1 Activity Enhances Satellite Cell Activation and/or Expansion
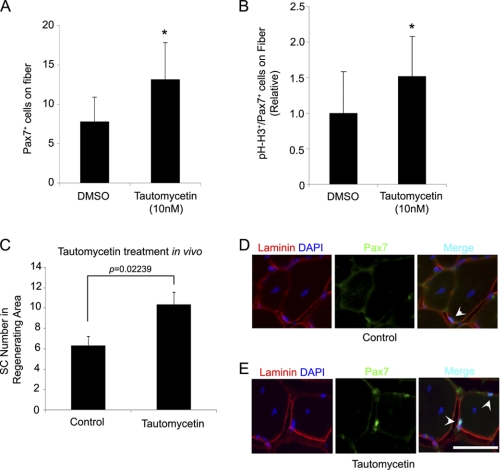
Activity of pRb is strictly regulated by the protein phosphorylation at the serine residues Ser-249, Ser-612, Ser-780, Ser-795, and Ser-807/811, and hyperphosphorylation of pRb is needed to inhibit pRb activity and to release of E2F protein, resulting in entry to the cell cycle (12). The serine/threonine protein phosphatase, PP1, is a dephosphorylation enzyme for pRb, and the enzyme activity of PP1 can be effectively inhibited by phosphatase inhibitors (30). To identify reagents which can exogenously increase satellite cell number in skeletal muscle, we examined whether inhibition of protein phosphatase by the phosphatase inhibitor, Tautomycetin (TC), a specific PP1 inhibitor (31), could cause cell cycle re-entry and pool expansion of satellite cells. Single muscle fibers were isolated from 8 week old wild-type mice and cultured in 10% FBS/DMEM with DMSO or TC (10 nm). At 24 h, Pax7+ satellite cell number was significantly increased on isolated muscle fibers by TC (Fig. 5A). We also calculated activated satellite cell number on DMSO- and TC-treated isolated muscle fibers by immunocytochemistry for both Pax7 and pH-H3 because previous reports have implicated PP1 in cell cycle progression (32). Interestingly, activated satellite cells were increased 1.5-fold in TC-treated muscle fibers compare with DMSO-treated (p = 0.0007) (Fig. 5B). We further investigated whether pharmacological inhibition of pRb activity could increase satellite cell number in vivo during an injury and regeneration cycle. TC was directly injected into mouse skeletal muscle with cardiotoxin. After 14 days, Pax7+ satellite cell number increased 1.6-fold in the regenerated area (p = 0.02239) (Fig. 5C-E). Taken together, our results suggest that pharmacological inhibition of PP1 in satellite cells results in accentuated satellite cell activation similar to genetic loss of Rb1.
FIGURE 5.
Protein phosphatase inhibitor accelerates satellite cells expansion in vitro. A, muscle fibers were isolated from wild-type mice muscle and cultured with TC (10 nm) or DMSO. Satellite cell (Pax7+) number on fibers was measured at 24 h after plating (DMSO-treated: n = 54 fibers, TC-treated: n = 51 fibers). Protein phosphatase inhibitor, TC, accelerates satellite cell expansion (p < 0.05). B, At 24 h for TC treatment (10 nm), activated satellite cell (pH-H3+/Pax7+) number was calculated on isolated muscle fibers (DMSO-treated: n = 30 fibers, TC-treated: n = 32 fibers). Inhibition of protein phosphorylation enhanced activation and/or expansion of satellite cells on muscle fibers (p < 0.05). C, tautomycetin (30 nm) was injected into mouse TA muscle concurrently with CTX (2.5 μm). Fourteen days after injection, satellite cell numbers in the regenerating area were compared between CTX-treated (Control, n = 3) and CTX/TC-treated (tautomycetin, n = 3) TA muscles (p < 0.05). Randomly chosen regenerating fibers (n = 100) were counted for each muscle. D and E, satellite cells were increased in vivo by TC treatment. Arrows indicate Pax7+ satellite cells. Scale bar: 100 μm.
DISCUSSION
Although satellite cells express many factors such as Syndecan-4 and M-cadherin, most of these genes are not specific for satellite cells in postnatal skeletal muscle (33, 34). Among candidate biomarkers, however, Pax7 is one of the most reliable specific markers of quiescent and activated satellite cells in postnatal skeletal muscle (35). Previously, we generated tamoxifen-inducible Pax7-CreER mouse line, in which Cre recombinase expression is driven by Pax7 and Cre-targeted gene expression can be temporally and specifically ablated or induced satellite cells (18, 20, 21). In this study, we examined using Pax7-CreER mouse line whether cell cycle regulatory factor, Rb1, critically regulates satellite cell quiescence/activation and expansion. Our in vivo results in the setting of homeostatic adolescent and young adult muscle growth, as well as in acute injury, are unprecedented for the postnatal context and are consistent with Rb1 playing a critical role in both processes.
Satellite cells symmetrically and asymmetrically divide to generate daughter cells for self-renewal and/or differentiation to myogenic lineage (4, 36), and these symmetric and asymmetric divisions are regulated by cell polarity (5, 6). At first, we expected the deficit of Rb1 in satellite cells might have an effect on this symmetry decision. However, the rate of myogenic differentiation from satellite cells (the percentage of MyoD+/Pax7+ cells in total Pax7+ cells on isolated muscle fibers at 42 h culture) were not significantly different between control and Pax7CreER,Rb1 mice (67.4 ± 20.6% versus 75.7 ± 17.3%, p = 0.13, data not shown), suggesting that Rb1 is not responsible for regulating satellite cell symmetric versus asymmetric division. Nevertheless, pRb does very positively regulate satellite cell and myoblast kinetics and the fate of daughter myoblasts in the differentiation process once activation has occurred.
In that regard, loss of Rb1 in satellite cells caused an increase in activated satellite cell number on isolated muscle fibers at 24 h culture, although baseline (zero hour) levels of Ki67 and pH-H3 proliferation markers were similar to control, suggesting that Rb1 loss/phosphorylation is a necessary but not sufficient cue for satellite cell activation. A general assumption is that satellite cells are kept stringently in a quiescent state but can be activated when these cells received activating signals such as HGF (3). However, in this specific example, the intracellular regulatory event for satellite cell activation following stimulation of HGF/c-Met axis is still unclear. A possible lead comes from work of Leshiem and Halevy (2002) who demonstrated that overexpression of HGF in chicken primary myoblasts enhanced proliferation through hyper-phosphorylation of pRb (37). Taken together, we speculate that pRb might be one of 2 or more necessary targets of the HGF/c-Met axis in quiescent satellite cells that allow cells to enter the cell cycle. Rather than being a sole mediator of activation, loss of Rb1 in satellite cells might be thought of as an accelerator of activation for previously quiescent satellite cells. Whether Rb1 is indeed a target of HGF signaling pathway as a critical factor for the satellite cell activation will be a topic of ongoing investigation.
The importance of the Rb family proteins in terminal differentiation of fetal myoblasts has been well described. Lack of pRb in myoblasts leads to a severe defect of myogenesis and terminal differentiation as a result of apoptosis, while proliferation is transiently enhanced (15, 16). In our study, sustained loss of Rb1 in the lineage of postnatal satellite cells causes apoptosis of myoblast progeny whereas proliferation is accelerated, consistent with former studies. In this study, we also demonstrated that irreversible loss of Rb1 in satellite cells and their progeny results in muscle hypotrophy in adolescent muscle and a delay of muscle regeneration in experimentally injured muscle. These muscle fiber abnormalities in Pax7CreER,Rb1 mice are likely due to impaired but not absent terminal differentiation. In adult muscle injury experiments with Pax7CreER,Rb1 mice, interestingly, regeneration was surprisingly near-sufficient as evidenced by muscle fibers having central nuclei (albeit these fiber diameters were smaller). Considering together the bimodal distribution of the homeostatic muscle fiber diameters in Pax7CreER,Rb1 mice and the finding that acute injury can result in small, non-multinucleated fibers with central nuclei strongly suggest that some if not many myoblasts can become new myofibers and sometimes escape the apoptotic cell fate observed in vitro. Furthermore, we have not ruled out the contribution of non-myogenic cells to either the growth or repair processes: future studies will interrogate whether compensation can be accounted for by intramuscular stem-like cells such as MDSC (muscle-derived stem cell) and muscle SP (side population) cells that participate in muscle regeneration with or without passing through the Pax7 lineage (38, 39, 40).
Perhaps the most exciting finding from these studies is the expansion of satellite cells during the pharmacological inhibition of a protein phosphatase. If inactivation of pRb and Rb-associating factors can be induced reversibly (temporarily) in satellite cells, the expansion of satellite cells and myoblasts in skeletal muscle may be possible without myoblast apoptosis or muscle fiber hypotrophy because pRb function would be restored when the pharmacological agent is withdrawn (washed out). Protein phosphorylation is a well known biological regulatory mechanism and is controlled by the balance between protein phosphatases and protein kinases. Protein phosphatases are numerous and include PP1, P2A, and PP2B, and now a growing number of specific protein phosphatase inhibitors have been identified (41, 42). Among these inhibitors, TC is a selective PP1 inhibitor and induces growth arrest of some types of tumor cell lines by increasing net protein phosphorylation (31, 43, 44). Conversly, for non-tumor tissue, genetic studies suggest that pRb activity is regulated by PP1 (45). For this reason, we explored whether TC would enhance satellite cell number in normal skeletal muscle through hyper-phosphorylation of pRb by PP1 inhibition. Indeed, by TC treatment we found that PP1-specific inhibition induces an increase of satellite cell number on isolated muscle fibers. Interestingly, PP1-specific inhibition also increased phosphorylated histone-H3+ activated satellite cells on the fibers. These results indicate that PP1-specific inhibition in satellite cells accelerates expansion of the muscle stem cell pool similar to the genetic loss of Rb1. Our results strongly suggest that pharmacological inhibition of PP1 or pRb activity in satellite cells could be novel therapeutic approach for aging-associated muscle atrophy, sarcopenia, muscular dystrophy, and other neuromuscular disorders. Exciting future studies will investigate the safety and efficacy of tautomycetin and its derivatives in preclinical models of these diseases.
Supplementary Material
Acknowledgments
We thank Jennifer Alabran for thoughtful comments on this study. The anti-Pax7 monoclonal antibody was obtained from the Developmental Studies Hybridoma Bank developed under the NICHD and maintained by the University of Iowa.
This study was supported by start-up funds (to C. K.) from the Doernbecher Children's Hospital Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- pRb
- retinoblastoma tumor suppressor protein
- CKI
- cyclin-dependent kinase inhibitor
- PP1
- protein phosphatase 1
- TC
- tautomycetin
- TAM
- tamoxifen
- CTX
- cardiotoxin.
REFERENCES
- 1. Mauro A. (1961) J. Biophys. Biochem. Cytol. 9, 493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chargé S. B., Rudnicki M. A. (2004) Physiol. Rev. 84, 209–238 [DOI] [PubMed] [Google Scholar]
- 3. Tatsumi R., Anderson J. E., Nevoret C. J., Halevy O., Allen R. E. (1998) Dev. Biol. 194, 114–128 [DOI] [PubMed] [Google Scholar]
- 4. Shinin V., Gayraud-Morel B., Gomès D., Tajbakhsh S. (2006) Nat. Cell Biol. 8, 677–687 [DOI] [PubMed] [Google Scholar]
- 5. Kuang S., Kuroda K., Le Grand F., Rudnicki M. A. (2007) Cell 129, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Grand F., Jones A. E., Seale V., Scimè A., Rudnicki M. A. (2009) Cell Stem. Cell. 4, 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tatsumi R., Hattori A., Ikeuchi Y., Anderson J. E., Allen R. E. (2002) Mol. Biol. Cell. 13, 2909–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamada M., Tatsumi R., Yamanouchi K., Hosoyama T., Shiratsuchi S., Sato A., Mizunoya W., Ikeuchi Y., Furuse M., Allen R. E. (2010) Am. J. Physiol. Cell Physiol. 298, C465–C476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukada S., Uezumi A., Ikemoto M., Masuda S., Segawa M., Tanimura N., Yamamoto H., Miyagoe-Suzuki Y., Takeda S. (2007) Stem Cells 25, 2448–2459 [DOI] [PubMed] [Google Scholar]
- 10. McCroskery S., Thomas M., Maxwell L., Sharma M., Kambadur R. (2003) J. Cell Biol. 162, 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lussac J. (1980) Rev. Odontostomatol. Midi. Fr. 38, 113–115 [PubMed] [Google Scholar]
- 12. Corbeil H. B., Whyte P., Branton P. E. (1995) Oncogene. 11, 909–920 [PubMed] [Google Scholar]
- 13. Novitch B. G., Mulligan G. J., Jacks T., Lassar A. B. (1996) J. Cell Biol. 135, 441–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Bruin A., Wu L., Saavedra H. I., Wilson P., Yang Y., Rosol T. J., Weinstein M., Robinson M. L., Leone G. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 6546–6551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huh M. S., Parker M. H., Scimè A., Parks R., Rudnicki M. A. (2004) J. Cell Biol. 166, 865–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zacksenhaus E., Jiang Z., Chung D., Marth J. D., Phillips R. A., Gallie B. L. (1996) Genes. Dev. 10, 3051–3064 [DOI] [PubMed] [Google Scholar]
- 17. Cao Y., Zhao Z., Gruszczynska-Biegala J., Zolkiewska A. (2003) Mol. Cell. Biol. 23, 6725–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishijo K., Hosoyama T., Bjornson C. R., Schaffer B. S., Prajapati S. I., Bahadur A. N., Hansen M. S., Blandford M. C., McCleish A. T., Rubin B. P., Epstein J. A., Rando T. A., Capecchi M. R., Keller C. (2009) FASEB. J. 23, 2681–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lepper C., Conway S. J., Fan C. M. (2009) Nature 460, 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brack A. S., Conboy M. J., Roy S., Lee M., Kuo C. J., Keller C., Rando T. A. (2007) Science 317, 807–810 [DOI] [PubMed] [Google Scholar]
- 21. Shea K. L., Xiang W., LaPorta V. S., Licht J. D., Keller C., Basson M. A., Brack A. S. Cell Stem. Cell. 6, 117–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hosoyama T., Nishijo K., Garcia M. M., Schaffer S. B., Ohshima-Hosoyama S., Prajapati I. S., Davis D. M., Grant F. W., Scheithauer W. B., Marks L. D., Rubin P. B., Keller C. (2010) Genes Cancer 1, 388–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soriano P. (1999) Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- 24. Bischoff R. (1986) Dev. Biol. 115, 129–139 [DOI] [PubMed] [Google Scholar]
- 25. Shefer G., Yablonka-Reuveni Z. (2005) Methods Mol. Biol. 290, 281–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hosoyama T., Ishiguro N., Yamanouchi K., Nishihara M. (2009) Differentiation 77, 350–359 [DOI] [PubMed] [Google Scholar]
- 27. Jiang Z., Liang P., Leng R., Guo Z., Liu Y., Liu X., Bubnic S., Keating A., Murray D., Goss P., Zacksenhaus E. (2000) Dev. Biol. 227, 8–41 [DOI] [PubMed] [Google Scholar]
- 28. Peschiaroli A., Figliola R., Coltella L., Strom A., Valentini A., D'Agnano I., Maione R. (2002) Oncogene. 21, 8114–8127 [DOI] [PubMed] [Google Scholar]
- 29. Ho A. T., Li Q. H., Hakem R., Mak T. W., Zacksenhaus E. (2004) EMBO. J. 23, 460–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ludlow J. W., Glendening C. L., Livingston D. M., DeCarprio J. A. (1993) Mol. Cell. Biol. 13, 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitsuhashi S., Matsuura N., Ubukata M., Oikawa H., Shima H., Kikuchi K. (2001) Biochem. Biophys. Res. Commun. 287, 328–331 [DOI] [PubMed] [Google Scholar]
- 32. Ceulemans H., Bollen M. (2004) Physiol. Rev. 84, 1–39 [DOI] [PubMed] [Google Scholar]
- 33. Cornelison D. D., Filla M. S., Stanley H. M., Rapraeger A. C., Olwin B. B. (2001) Dev. Biol. 239, 79–94 [DOI] [PubMed] [Google Scholar]
- 34. Irintchev A., Zeschnigk M., Starzinski-Powitz A., Wernig A. (1994) Dev. Dyn. 199, 326–337 [DOI] [PubMed] [Google Scholar]
- 35. Seale P., Sabourin L. A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M. A. (2000) Cell 102, 777–786 [DOI] [PubMed] [Google Scholar]
- 36. Conboy M. J., Karasov A. O., Rando T. A. (2007) PLoS. Biol. 5, e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leshem Y., Halevy O. (2002) J. Cell. Physiol. 191, 173–182 [DOI] [PubMed] [Google Scholar]
- 38. Gussoni E., Soneoka Y., Strickland C. D., Buzney E. A., Khan M. K., Flint A. F., Kunkel L. M., Mulligan R. C. (1999) Nature 401, 390–394 [DOI] [PubMed] [Google Scholar]
- 39. Qu-Petersen Z., Deasy B., Jankowski R., Ikezawa M., Cummins J., Pruchnic R., Mytinger J., Cao B., Gates C., Wernig A., Huard J. (2002) J. Cell Biol. 157, 851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asakura A., Seale P., Girgis-Gabardo A., Rudnicki M. A. (2002) J. Cell Biol. 159, 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishida Y., Furukawa Y., Decaprio J. A., Saito M., Griffin J. D. (1992) J. Cell. Physiol. 150, 484–492 [DOI] [PubMed] [Google Scholar]
- 42. MacKintosh C., Klumpp S. (1990) FEBS. Lett. 277, 137–140 [DOI] [PubMed] [Google Scholar]
- 43. Mitsuhashi S., Shima H., Tanuma N., Matsuura N., Takekawa M., Urano T., Kataoka T., Ubukata M., Kikuchi K. (2003) J. Biol. Chem. 278, 82–88 [DOI] [PubMed] [Google Scholar]
- 44. Lee J. H., Lee J. S., Kim S. E., Moon B. S., Kim Y. C., Lee S. K., Choi K. Y. (2006) Mol. Cancer Ther. 5, 3222–3231 [DOI] [PubMed] [Google Scholar]
- 45. Kim T. H., Goodman J., Anderson K. V., Niswander L. (2007) Dev. Cell. 13, 87–102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.