Abstract
The proangiogenic members of VEGF family and related receptors play a central role in the modulation of pathological angiogenesis. Recent insights indicate that, due to the strict biochemical and functional relationship between VEGFs and related receptors, the development of a new generation of agents able to target contemporarily more than one member of VEGFs might amplify the antiangiogenic response representing an advantage in term of therapeutic outcome. To identify molecules that are able to prevent the interaction of VEGFs with related receptors, we have screened small molecule collections consisting of >100 plant extracts. Here, we report the isolation and identification from an extract of the Malian plant Chrozophora senegalensis of the biflavonoid amentoflavone as an antiangiogenic bioactive molecule. Amentoflavone can to bind VEGFs preventing the interaction and phosphorylation of VEGF receptor 1 and 2 (VEGFR-1,VEGFR-2) and to inhibit endothelial cell migration and capillary-like tube formation induced by VEGF-A or placental growth factor 1 (PlGF-1) at low μm concentration. In vivo, amentoflavone is able to inhibit VEGF-A-induced chorioallantoic membrane neovascularization as well as tumor growth and associated neovascularization, as assessed in orthotropic melanoma and xenograft colon carcinoma models. In addition structural studies performed on the amentoflavone·PlGF-1 complex have provided evidence that this biflavonoid effectively interacts with the growth factor area crucial for VEGFR-1 receptor recognition. In conclusion, our results demonstrate that amentoflavone represents an interesting new antiangiogenic molecule that is able to prevent the activity of proangiogenic VEGF family members and that the biflavonoid structure is a new chemical scaffold to develop powerful new antiangiogenic molecules.
Keywords: Cancer Therapy, Cell Migration, Drug Action, Growth Factors, Receptor Tyrosine Kinase, Angiogenesis, VEGF Family, Flavonoids, Protein/Small Molecule Interaction, Small Molecule Libraries
Introduction
In the past few years, it has been demonstrated that the proangiogenic members of VEGF family, VEGF-A, VEGF-B, and placental growth factor (PlGF),4 play a central role in the modulation of pathological angiogenesis (1, 2). VEGFs accomplish their action interacting with two tyrosine kinase receptors, VEGFR-1 and VEGFR-2, also known as Flt-1 and KDR. Pathological neovascularization is a condition associated to many complex diseases such as cancer, atherosclerosis, arthritis, diabetic retinopathy, and age-related macular degeneration (2, 3).
VEGF-A is the most potent proangiogenic factor known showing a crucial stimulating activity on endothelial cells. Indeed, it is the target of the first U. S. Food and Drug Administration-approved antiangiogenic drugs for the therapy of cancer and age-related macular degeneration. Bevacizumab (Avastin), a monoclonal antibody anti-VEGF-A, has been approved for the therapy of metastatic colorectal cancer in combination with chemotherapy (4), whereas pegaptanib (Macugen), an anti-VEGF-A PEGylated aptamer (5), and ranibizumab (Lucentis), a Fab fragment of bevacizumab (6), have been approved for treatment of age-related macular degeneration. Although VEGF-A-targeted therapy has improved cancer survival, many patients are refractory to VEGF-A-targeted therapy, and most patients who initially respond develop resistance (7).
More recently, increasing attention has been addressed to other members of VEGF network involved in angiogenesis modulation. Indeed, the block of VEGFR-1 (8), or of its specific ligand PlGF (9, 10), is sufficient to strongly inhibit pathological angiogenesis in experimental models of pathologies such as cancer, atherosclerosis, arthritis, ocular neovascular diseases, and metastasis formation (11–15), indicating how fine-tuning of the availability of VEGFs and related receptors is required for a correct angiogenesis during pathological conditions. This is also confirmed by data indicating that the up-regulation of PlGF expression provides a tumor escape strategy to anti-VEGF-A therapy (10, 16).
It appears evident that due to the strict biochemical and functional relationship between VEGFs and related receptors (17), the development of a new generation of agents able to target contemporarily more than one member of the VEGFs might amplify the antiangiogenic response. This would overcome some of the difficulties associated with current angiogenesis inhibitors, representing an advantage in terms of therapeutic outcome (17, 18).
From this perspective, we have performed a screening plan of small molecule collections consisting of >100 extracts from plants used in the traditional medicine collected in various areas of the world. Medicinal plants have become extremely important in drug discovery for the treatment of human diseases, and their secondary metabolites have proven to be the most reliable source of new and effective anticancer agents (19). Moreover, the management of small molecules still offers numerous advantages over biotherapeutics because they can be more easily and cheaply produced. In addition small molecules are more stable and generally free of contaminants of biological origin and may offer more opportunity for delivery.
The screenings have been performed using competitive dose-dependent ELISA assays for PlGF-1 and VEGF-A interaction with high affinity receptor VEGFR-1 (20, 21). The chloroform/methanol extract from leaves of the Malian plant C. senegalensis showed the highest inhibiting activity and its subsequent bioassay-guided fractionation led to the isolation and characterization of amentoflavone (AF) as bioactive constituent able to inhibit the interaction of either PlGF-1 or VEGF-A with VEGFR-1. AF belongs to a unique class of naturally occurring biflavonoids (22).
The binding properties and the antiangiogenic activity of the selected compound have been assessed in in vitro and cell-based assays. The data presented here demonstrate that AF specifically binds to VEGFs, preventing the activation of both VEGFR-1 and VEGFR-2. It is able to significantly inhibit growth and neoangiogenesis in two different tumor models, an orthotropic model of melanoma and a xenograft model of colon carcinoma.
EXPERIMENTAL PROCEDURES
Materials
Analytical grade solvents were obtained from Carlo Erba (Milano, Italy). HPLC grade acetonitrile (CH3N), methanol (MeOH), and formic acid were purchased from J. T. Baker (Baker Mallinckrodt, Phillipsburg, NJ). HPLC grade water (18 mV) was prepared using a Millipore (Bedford, MA) Milli-Q purification system.
Recombinant PlGF-1 protein (amino acids 19–149) (23) produced in Escherichia coli in our laboratory was used in all the experiments. The numeration of PlGF-1 residues reported below is related to this sequence with an additional methionine in position 1. This recombinant form of PlGF-1 shows the same activity in terms of receptor binding of that produced by R&D Systems (amino acids 21–149; Minneapolis, MN). All of the other recombinant growth factors, receptors, and antibodies used were from R&D Systems.
Plant Extract Preparation
The air-dried powdered plant material was extracted by exhaustive maceration with solvents at increasing polarity. Source plants and kinds of extracts are reported in supplemental Table S1.
Purification of Amentoflavone
The air-dried powdered leaves of Chrozophora senegalensis (1 kg) were defatted with n-hexane and extracted successively by exhaustive maceration (3 × 1 liter, for 48 h) with CHCl3, CHCl3-MeOH (9:1) (v/v), and MeOH. The extracts were concentrated under reduced pressure to afford 22.4, 28.4, 23.6, and 104.0 g of dried residues, respectively.
A portion of the CHCl3-MeOH (9:1) (v/v) extract (50.0 g) was partitioned between n-BuOH and H2O to give an n-BuOH soluble portion (19.0 g). 5.0 g of this residue were chromatographed over a Sephadex LH-20 column (100 cm × 5 cm) with MeOH as the eluent. A total of 100 fractions were collected (11-ml each). These were combined according to TLC analysis (silica 60 F254 gel-coated glass sheets with n-BuOH-AcOH-H2O (60:15:25, v/v/v) and CHCl3-MeOH-H2O (40:9:1, v/v/v)) to give ten pooled fractions (A–L).
The bioactive fraction E (220 mg), purified by RP-HPLC using CH3CN-H2O (55:45, v/v), contained a main component (tR = 13.0 min) at 90% w/w (200 mg), which was identified successfully as AF by determination of structural properties (supplemental data). It was obtained at a purity of 98.4%, as assessed by RP-HPLC diode array detector on a C18-Luna column (2 × 50 mm, flow rate of 0.2 ml min−1) (Phenomenex). The chromatogram was acquired at 280 nm. The injection volume was 20 μl (0.1 mg/ml). The solvent gradient changed according to the following conditions (solvent A, H2O + HCOOH 0.1% and solvent B, acetonitrile): 0–1 min, 20% B; 20 min, 70% B; 20–23 min, 70% B, 23–24 min, 9 5% B; 24–30 min 100% B (AF tR = 14.8 min) (supplemental Fig. S1). Purified AF has been used for all in vitro and in vivo assays.
Surface Plasmon Resonance Analysis
Surface plasmon resonance analyses were performed on a Biacore 3000 (Uppsala, Sweden) optical biosensor equipped with research-grade CM5 sensor chips, as reported elsewhere (24). Briefly, two separate human PlGF-1, VEGF-A, or Fc-VEGFR-1 chimera surfaces, one human serum albumin or human tubulin surfaces and one unmodified reference surface, were prepared for simultaneous analyses.
Immediately after chip docking, the instrument was primed with water, and the sensor chip surfaces were preconditioned by applying two consecutive 20-μl injections each (at a flow rate of 100 μl/min) of 50 mm NaOH, 10 mm HCl, 0.1% SDS (w/v), and 10 mm H3PO4. Data were collected at 2.5 Hz. Proteins (100 μg/ml in 10 mm sodium acetate, pH 5.0) were immobilized on individual sensor chip surfaces at a flow rate of 5 μl/min using standard amine-coupling protocols to obtain densities of 8–12 kilo response units. The AF binding study was performed using a five-point concentration series, typically spanning 0.05–10 μm, and triplicate aliquots of each AF concentration were dispensed into single-use vials. Binding experiments were performed at 25 °C, using a flow rate of 50 μl/min, with a 60 s monitoring of association and a 200 s monitoring of dissociation. Simple interactions were adequately fitted to a single-site bimolecular interaction model (A + B = AB), yielding a single KD. Sensorgram elaborations were performed using the Bioevaluation software provided by Biacore AB.
Competitive ELISA Assays
For competitive ELISA used for the screening of plant extracts and for dose-dependent experiments, see the supplemental data (20, 21, 25). Each point was done in triplicate, and each experiment was repeated twice.
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were grown in standard conditions in endothelial basal medium 2 supplemented with bullet kit (EGM-2) containing hydrocortisone, hFGF-B, VEGF, R3-IGF-1, ascorbic acid, heparin, FBS, hEGF, gentamicin, and amphotericin-B (Cambrex, Charles City, IA).
The cell line overexpressing human VEGFR-1, obtained by stable transfection of human embryonic kidney 293 cells and named 293-hFlt-1, was grown as described elsewhere (25). Human tumor cell lines MeWo (ATCC catalog no. HTB-65, from melanoma) and HCT-116 (ATCC catalog no. CCL-247, from colon carcinoma) were grown in Eagle's minimal essential medium or McCoy's 5a medium, respectively, supplemented with 10% FBS, 2 mm glutamine, and standard concentration of antibiotics.
Cell-based Assays
For receptor phosphorylation assays, HUVECs were starved in absence of growth factors but in the presence of 1% FBS. 293-hFlt-1 cells (25) were starved for 16 h in absence of FBS. To induce VEGFR-1 and VEGFR-2 receptor activation, 20 ng/ml of PlGF-1 or 50 ng/ml of VEGF-A for 10 min were used. AF (2- 25 μm) was added to medium at the same time of inductors. As control, neutralizing anti-PlGF mAb (16D3, Thrombogenics, Leuven, Belgium) or anti-VEGF-A antibody (R&D Systems) were used at ∼3 nm.
To detect phosphorylated forms of receptors in Western blot experiments performed following standard procedures, antibodies anti-p-VEGFR-1 (R&D Systems) diluted 1:500 and anti-p-VEGFR-2 (Cell Signaling, Danvers, MA) diluted 1:1000 were used. Normalization was performed using anti-VEGFR-1, 1/500 (Sigma-Aldrich) or anti-VEGFR-2, 1/500 (Santa Cruz Biotechnology, Santa Cruz, CA). The values of densitometry analyses, performed using ImageQuant software (version 5.2, Molecular Dynamics) are shown. Values (%) were calculated as ratio of degree of receptors phosphorylation with respect to the total receptor amounts. The value of 1.0 has been arbitrarily assigned to PlGF-1- or VEGF-A-induced samples. Data are representative of three independent experiments.
For toxicity and proliferation assays, see supplemental data. The chemotaxis assay (26) and capillary-like tube formation assays (21, 25) were performed as described elsewhere (see also supplemental data).
In Vivo Analyses
For chorioallantoic membrane assay, see supplemental data.
For tumor models, MeWo human melanoma cells (5 × 105) were inoculated intradermally in the right flank of 24 8-week-old CD-1 male nude athymic mice (Charles River, Chatillon-sur-Chalaronne, France) by using a 100-μl Hamilton syringe. Then, animals were subdivided into two groups (n = 9). Starting 3 days after injection, every group was treated as follows: vehicle, 1% gum acacia in sterile water (i.p.) and AF, 50 mg/10 ml/kg (i.p.) as described previously (27). Mice were sacrificed by CO2 inhalation 14 days after cell injection and tumor nodules were surgically removed, measured, and weighed.
For xenograft tumor experiments, 5 × 106 HCT-116 colon carcinoma cells were injected subcutaneously in 20 8-week-old CD1 nude athymic mice. After 7 days, when tumors reach a volume between 50 and 100 mm3, animals were divided into two groups (n = 8) and treated with AF or vehicle as described before. Tumor growth was followed by three weekly measurements of tumor diameters with a caliper. Tumor volume (TV) was calculated according to the formula: TV (mm3) = d2 × D/2, where d and D are the shortest and the longest diameters, respectively. For ethical reasons, mice were sacrificed when control tumors reached in average a volume of 1500 mm3, after 14 days of drug treatment.
For animal experiments, the care and husbandry of mice and tumor experimental procedures were in accordance with European Directive 86/609 and with Italian Decreto Legge (D.L.) 116. All experiments were approved by the Institute of Genetics and Biophysics and the sigma-tau veterinarians.
Immunohistochemical analyses were performed on 4-μm-thick deparaffined tumor sections incubated overnight at 4 °C with the following primary antibodies: rat anti-mouse PECAM-1 (anti-CD31; 1:1000; BD Pharmingen, San Jose, CA) and rat anti-mouse F4/80 (1:50; Serotec, Oxford, UK). The staining procedure was continued using specific secondary biotinylated antibody (all from DAKO, Glostrup, Denmark). Slides were counterstained with hematoxylin. Images were recorded with a digital camera Leica DC480 (Milano, Italy). Vessel density was manually measured. Densitometric analysis for F4/80 staining was performed with QwinPro software (Leica). Analyses were performed on five optical fields for each tumor.
Structural Characterization of PlGF-1·AF Complex and Molecular Docking
For UV cross-linking, limited proteolysis, mass spectrometry, and docking analyses (28), see supplemental data.
Statistical Analysis
Data are expressed as mean ± S.E., with p < 0.05 considered statistically significant. Differences among groups were tested by one-way analysis of variance; Tukey HD test was used as post hoc test to identify which group differences account for the significant overall analysis of variance. All calculations were carried out using the SPSS statistical package (version 12.1, Chicago, IL). The IC50 and LD50 have been calculated using linear regression fitted using the least squares method.
RESULTS
Identification of Amentoflavone as Bioactive Compound
Polar and apolar plant extracts (supplemental Table S1) were screened for their ability to inhibit the in vitro interaction between PlGF-1 and VEGF-A with VEGFR-1. About 5% of extracts tested by competitive ELISA-based assays met the criteria for activity showing a dose-dependent response on both the ELISAs with an inhibition over 50% at a concentration of 100 μg/ml.
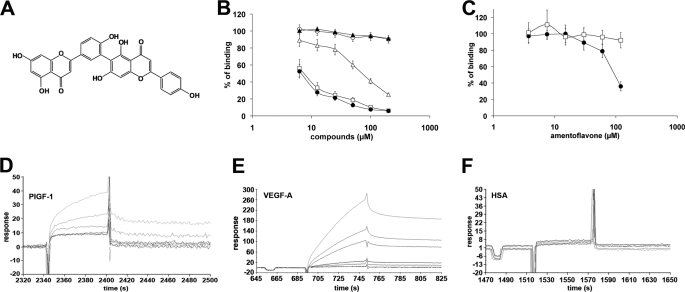
The deconvolution of active extracts was thus performed by bioassay-guided fractionation. Among the fractions obtained from active extracts, only four of ten fractions obtained from the C. senegalensis CHCl3-MeOH (9:1, v/v) extract showed a dose-dependent inhibition (supplemental Fig. S2). Chromatographic separation of the most active fraction (fraction E, supplemental Fig. S2) by RP-HPLC allowed identification of the main component as the AF at 98.4% purity (supplemental Fig. S1). The AF structure was elucidated by one- and two-dimensional NMR spectroscopy as well as ESI mass spectrometry and comparison with published data (Fig. 1A and supplemental data).
FIGURE 1.
Inhibitory and binding properties of amentoflavone. A, AF structure. B, dose-dependent inhibition of the PlGF-1/VEGFR-1 (filled circles), VEGF-A/VEGFR-1 (open squares), and VEGF-A/VEGFR-2 (open triangles) interaction exerted by AF at concentration ranging between 3.75 and 200 μm in ELISA-based assays. As a negative control of inhibition fraction G (supplemental Fig. S2) was used at same concentration for PlGF-1/VEGFR-1 (filled triangles) and VEGF-A/VEGFR-2 (open circles) interactions. Each point was done in triplicate, and each experiment was repeated twice. Data are represented as the mean ± S.E. C, dose-dependent inhibition of VEGF-B/VEGFR-1 (filled circles) and PDGF-B/PDGFR-β (open squares) interactions exerted by AF at concentration ranging between 3.75 and 200 μm in ELISA-based assays. Each point was done in triplicate, and each experiment was repeated twice. Data are represented as the mean ± S.E. D–F, sensograms obtained injecting increasing concentrations (from 0.05 to 10 μm) of AF on PlGF-1-, VEGF-A-, and human serum albumin-coated Biacore chips.
Amentoflavone Specifically Inhibits VEGFs Binding to Related Receptors
As shown in Fig. 1B, AF inhibited in a similar dose-dependent manner PlGF-1/VEGFR-1 and VEGF-A/VEGFR-1 interactions with an IC50 of 4.8 ± 0.3 and 6.8 ± 0.5 μm, respectively. Additionally, it also was able to inhibit the VEGF-A/VEGFR2 interaction but with reduced efficacy (IC50 = 104.4 ± 8.4 μm). As negative control, a nonactive fraction from the same extracts (fraction G, supplemental Fig. S2) was used. AF showed similar inhibitory activity also for VEGF-B/VEGFR-1 interaction (IC50 = 100.7 ± 7.6 μm) (Fig. 1C).
To evaluate the specificity of AF activity, we investigated its inhibitory properties of the interaction between a member of the more structural VEGF-related growth factor family, the PDGF B with related PDGFR-β receptor (PDGFR), and as shown in Fig. 1C, no activity was observed.
Amentoflavone Binds VEGFs
To define the molecular target of AF, surface plasmon resonance experiments by Biacore technology were performed. Recombinant PlGF-1, VEGF-A Fc-VEGFR-1 chimera, and as controls, tubulin, and human serum albumin were coated to a Biacore chip and incubated with increasing concentration of AF starting from 0.05 to 10 μm, measuring the association and dissociation to the coated proteins. AF was able to interact with PlGF-1 and VEGF-A, as demonstrated by the concentration-dependent responses and the clearly discernible exponential curves during both the association and dissociation phases (Fig. 1, D and E). A thermodynamic dissociation constant (KD) of 16.45 ± 0.59 nm and 8.15 ± 0.28 nm was measured for VEGF-A·AF and the PlGF-1·AF complexes, respectively. No significant interaction was detected with human serum albumin (Fig. 1F) or other controls (data not shown). Because AF is a dimer of apigenin (29), we evaluated whether apigenin and structurally related compounds such as quercetin, naringenin, and catechin were able to bind VEGFs, but none of them interacted with PlGF-1 or VEGF-A in surface plasmon resonance experiments (data not shown).
Amentoflavone Prevents VEGF-induced Receptor Activation, Cell Migration, and Capillary-like Tube Formation
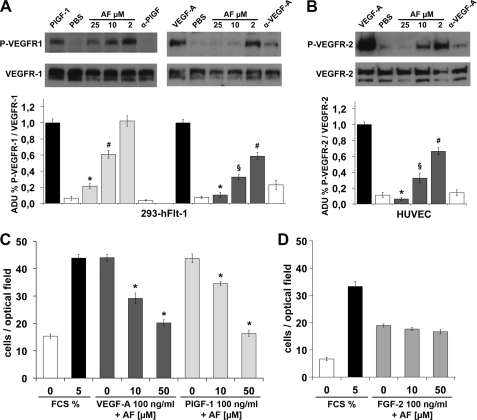
The neutralizing activity of AF observed in vitro was therefore assessed in receptor phosphorylation assays. AF was able to reduce, in a dose-dependent manner, PlGF-1- and VEGF-A-induced phosphorylation of human VEGFR-1 in the cell line stably overexpressing the receptor (293-hFlt-1) (Fig. 2A). Notably, the inhibition activity was higher for VEGF-A-induced VEGFR-1 activation, with an inhibition of ∼40% using 2 μm AF (p < 0.005), whereas at 10 μm, the observed inhibition was similar to that obtained with a neutralizing anti-VEGF-A antibody (R&D Systems) used at ∼3 nm (∼70%, p < 0.001). At 25 μm AF, ∼90% of inhibition was obtained (p < 0.0005). In the VEGFR-1 phosphorylation induced by PlGF-1, AF at 10 μm was able to induce ∼50% of inhibition (p < 0.005), whereas at 25 μm the extent of inhibition increased up to 75% (p < 0.0005).
FIGURE 2.
Amentoflavone inhibits VEGFR phosphorylation and HUVEC migration. A, representative pictures of Western blot analysis of VEGFR-1 phosphorylation (P-VEGFR-1) induced by 20 ng/ml of PlGF-1 or 50 ng/ml VEGF-A on cells overexpressing VEGFR-1 (293-Flt-1). AF was used at a concentration ranging between 2 and 25 μm. As control of inhibition neutralizing antibody anti-PlGF (α-PlGF) or anti-VEGF-A (α-VEGF-A) were used at 3.3 nm. Anti-VEGFR-1 antibody was used for normalization. B, Western blot analysis of VEGFR-2 phosphorylation (P-VEGFR-2) performed in the same condition as reported in A but on HUVECs. Anti-VEGFR-2 antibody was used for normalization. Histograms represent densitometry analysis of three independent experiments, and data are represented as the mean ± S.E. ADU, arbitrary densitometric unit. *, p < 0.0005 versus PlGF-1 or VEGF-A; §, p < 0.001 versus VEGF-A; #, p < 0.005 versus PlGF-1 or VEGF-A. C, cell migration induced by VEGF-A or PlGF-1 was significantly inhibited by AF used at 10 and 50 μm. Conversely, AF failed to inhibit FGF-2-stimulated cell migration D, 0% or 5% FCS were used as negative and positive control of migratory stimulus. *, p < 0.0001 versus VEGF-A, PlGF-1, and FCS (5%). Each point was done in quadruplicate, and the experiments were performed twice. Data are represented as the mean ± S.E.
To evaluate whether AF was able to inhibit also VEGF-A-induced VEGFR-2 phosphorylation, we used primary HUVECs. As shown in Fig. 2B, AF also gave a strong dose-dependent inhibition of VEGFR-2 phosphorylation (p < 0.0005, p < 0.001, and p < 0.005 for AF at 25, 10, and 2 μm, respectively).
Before evaluating further inhibitory activities of AF on endothelial cells, we investigated the toxicity in vitro of the molecule evaluating its concentration able to kill 50% of proliferating HUVECs (LD50). The analysis reported in supplemental Fig. S3A allowed the calculation of an LD50 of 290.0 ± 17.8 μm.
Because cell migration represents one of the crucial events for neovessel formation, we evaluated the effects of AF on HUVEC migration in response to VEGF-A and PlGF-1. Exposure to 10 μm AF for 4 h led to a significant decrease of the migratory movement toward both stimulus (p < 0.0001). Moreover, at 50 μm, the number of migrating cells was reduced to that of basal levels (p < 0.0001) (Fig. 2C). At the same concentrations AF resulted ineffective in inhibiting the HUVEC migratory movement elicited by FGF2 (Fig. 2D), further indicating the specificity of AF versus VEGFs.
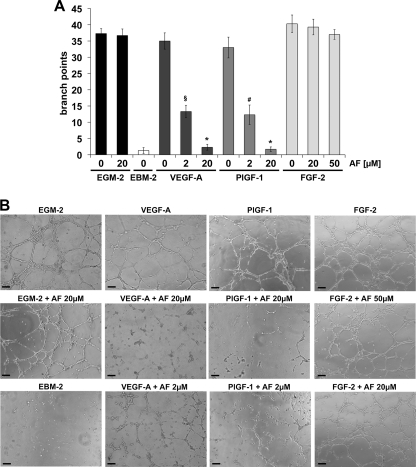
The antiangiogenic activity of AF was evaluated successfully by testing its capability to suppress the induction of capillary-like tube formation (CTF) of HUVECs grown on Matrigel (BD Biosciences) stimulated by PlGF-1, VEGF-A, or FGF2. Endothelial basal medium 2 and complete EGM-2 medium were used as negative and positive controls, respectively (Fig. 3). All growth factors and EGM-2 induced a visible network of capillary-like tube structures with no significant difference in terms of branch points. The suppression of PlGF-1- and VEGF-A-induced CTF by AF was virtually complete at 20 μm (p < 0.0005 versus VEGF-A, PlGF-1 and EGM-2), with a number of branch points similar to that of the negative control. At 2 μm AF, the great part of tube network was absent with a reduction of >50% of branch points (p < 0.003 versus VEGF-A, and EGM-2; p < 0.01 versus PlGF-1, and EGM-2). AF was not able to inhibit FGF-2-induced CTF when assayed at 20 and 50 μm, as well as the CTF induced by complete EGM-2 medium (Fig. 3). These latter data confirm that AF possesses a specific activity toward VEGFs and that it does not have a direct effect on endothelial cells.
FIGURE 3.
Amentoflavone inhibits capillary-like tube formation induced by VEGF-A or PlGF-1 but not by FGF-2. A, CTF stimulated with 100 ng/ml of VEGF-A, PlGF-1, or FGF-2 in presence or absence of AF, used at concentrations ranging between 2 and 50 μm. Branch points for almost three capillary-like structures have been quantified in three optical fields for each sample. Data are represented as the mean ± S.E. §, p < 0.003 versus VEGF-A, and EGM-2; #, p < 0.01 versus PlGF-1, and EGM-2; *, p < 0.0005 versus VEGF-A, PlGF-1, and EGM-2. B, representative pictures. The scale bar represents 100 μm.
In Vivo Antiangiogenic Activity of Amentoflavone
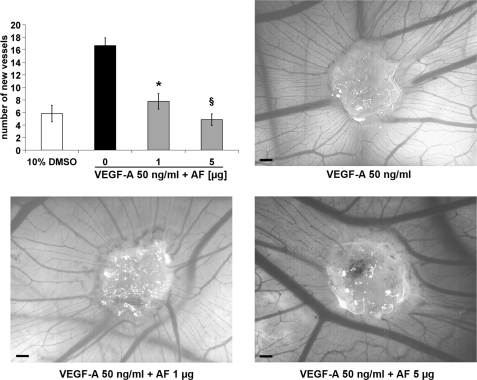
The antiangiogenic activity of AF was investigated in the model of chicken embryo chorioallantoic membrane assay. VEGF-A at 50 ng/ml determined a good increment of vessel formation compared with vehicle alone (Fig. 4). AF addressed VEGF-A-induced angiogenesis in all of the embryos tested showing a significant dose-response inhibition. One μg (1.9 nmol) of AF was sufficient to achieve ∼50% of inhibition (p < 0.01), whereas in the presence of 5 μg (9.5 nmol), the number of neovessels was similar to that observed with vehicle alone (p < 0.001) (Fig. 4). Therefore, AF was able to abolish the stimulation of VEGF-A without affecting pre-existing vessels. Finally, the antiangiogenic property of AF was evaluated in an orthotopic model of melanoma and a xenograft model of colon carcinoma.
FIGURE 4.
Amentoflavone inhibits VEGF-A-induced chorioallantoic membrane neovascularization. Inhibition of VEGF-A-induced neovessels formation exerted by AF (1 and 5 μg). The vehicle used was 10% dimethyl sulfoxide (DMSO; n = 8 per group). Data are represented as the mean ± S.E. *, p < 0.01 and §, p < 0.001 versus VEGF-A treated embryos. The scale bar represents 200 μm.
First, we assessed whether AF affected per se the proliferation in vitro of the two cell lines used for tumor models, MeWo melanoma and HCT-116 colon carcinoma cells. As shown in supplemental Fig. S3, B and C, AF did not affect cell proliferation when assayed at concentrations ranging between 6.25 and 50 μm.
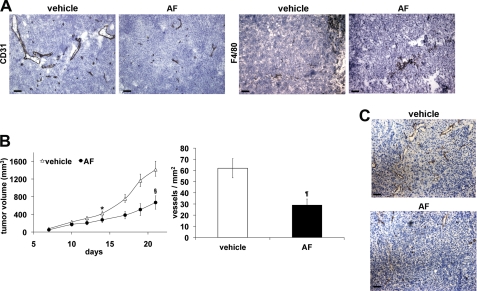
MeWo melanoma cells were injected intradermally in nude mice. Three days after, AF dissolved in 1% gum acacia was daily delivered intraperitoneally at 50 mg/10 ml/kg (27). After 14 days from cell injection, AF gave a significant and homogenous tumor volume reduction (−63%, p = 0.0005 versus vehicle) despite the short dimension of control tumors (∼60 mg), to which a significant reduction of vessel density corresponded (−52,5%, p = 0.0051 versus vehicle), as assessed by CD31 immunohistochemical analysis (Table 1 and Fig. 5A).
TABLE 1.
In vivo antitumor activity of AF on human MeWo melanoma
MeWo tumor cells (5 × 105) were injected i.d. in CD1 nude mice. Test compound was given i.p. starting 3 days after tumor implantation to day 13. TV (tumor volume) was evaluated at day 14. The vessel number and F4/80-positive area were determined in five optical fields for each tumor. Data represent the average ± S.E. BW, body weight.
| Group | Dose (mg/kg/BW) | Lethality | TV (mm3) | Vessels/mm2 | F4/80 positive area/mm2 |
|---|---|---|---|---|---|
| Vehicle | 0 | 0/9 | 57.2 ± 8.2 | 112.5 ± 14.5 | 8.1 ± 0.4 |
| AF | 50 | 0/9 | 21.4 ± 0.5a | 53.5 ± 11.0b | 4.8 ± 0.3c |
a p = 0.0005.
b p = 0.0051.
c p < 0.0001 versus vehicle.
FIGURE 5.
Amentoflavone inhibits tumor growth and associated neovascularization. A, representative pictures of CD31 and F4/80 staining of MeWo melanoma (see Table 1). B, HCT-116 xenograft tumor volume was measured three times a week, and data are represented as the mean ± S.E. *, p = 0.0109; §, p = 0.0078 versus vehicle. Vessel density was calculated analyzing five optical fields for each tumor, counting CD31-positive vessels. Data are represented as the mean ± S.E. ¶, p = 0.0070 versus vehicle. C, representative pictures of CD31 staining of HCT-116 tumors. The scale bar represents 50 μm.
The immunohistochemical analysis, performed to evaluate the tumor infiltration by F4/80 positive cells, clearly indicated a significant inhibition of their recruitment (−41%, p < 0.0001 versus vehicle) (Table 1 and Fig. 5A), as expected due to the crucial role of both VEGF-A and PlGF-1 in this biological activity exerted mainly via activation of VEGFR-1.
HCT-116 colon carcinoma cells were injected subcutaneously in nude mice. In this case, AF was delivered when tumors became measurable (50–100 mm3) after 7 days from cell inoculation using the same delivery schedule described for melanoma model. After 7 days of treatment with AF, a significant inhibition of tumor growth in mice became measurable (−34%, p = 0.0109 versus vehicle) (Fig. 5B). At day 21 from cell inoculation, the reduction of tumor growth increased to 53% (p = 0.0078 versus vehicle) and the immunohistochemical analysis for CD31 performed on explanted tumors allowed to associate a significant vessel density decrease (−53%, p = 0.0070) to reduced tumor growth (Fig. 5B, C). In course of the treatment in both tumor models, no side effects were noted except for a reduction of mice body weight (between 6–8% at the experimental end point).
Structural Characterization of PlGF-1·AF Complex
To unveil the nature and the characteristics of AF binding to soluble proangiogenic factors, we started a structural characterization focusing our attention on the PlGF-1·AF complex because it showed the highest stability. We first performed a circular dichroism analysis. The near-UV circular dichroism spectroscopy demonstrated that binding of AF to PlGF-1 produces a large change in the spectrum of the protein, thus suggesting a rearrangement of its tertiary structure (supplemental data and Fig. S4, A and B). On the other hand, ESI-MS analyses suggested a noncovalent interaction because the acquired spectra for PlGF-1·AF complex was almost identical to that of PlGF-1 alone (supplemental Fig. S4C).
To identify the PlGF-1 protein regions involved in the interaction with AF, structural studies were performed starting from the noncovalent PlGF-1·AF complex. As a first analytical step, we attempted to transform the noncovalent interaction in a stable bond by UV cross-linking reaction. Since the phenol group was the unique functional group of AF reactive enough, we used the phenol reactive-NHS-diaziridine as photoactivable reagent. The complex stabilized by cross-linking was detectable by linear MALDI-TOF analysis (supplemental Fig. S5). Therefore, it was subjected to trypsin cleavage. The comparison analysis of digested peptides performed by MALDI-TOF/TOF analysis (supplemental Fig. S6, A and B) revealed that the peptide-(94–110) and peptide-(125–132) were modified after the cross-linking reaction (supplemental Table S2). We did not observe complex formation if the same experiment was performed using two structurally related compounds (catechin and quercetin), which showed no binding toward VEGFs (supplemental Fig. S7).
As a second approach, limited proteolysis experiments coupled with mass spectrometry analysis were carried out to probe the conformational changes of proteins induced by ligand binding and to map the interface regions protected from the access of protease by the molecular interaction (30). A comparison among the digestion patterns observed when PlGF-1 or PlGF-1·AF noncovalent complex underwent proteolytic digestion using different enzymes, demonstrated that the residues mainly protected by AF against proteolysis were as follows: Leu-2, which was miscleaved by chymotrypsin, Lys-119 and Arg-131 were protected from trypsin cleavage; Glu-123, which was not cleaved by endoproteinase Glu-C (Prot. V8); and residues Gln-27 and Arg-32, which were protected when endoproteinase PK was used (supplemental Table S3 and supplemental Fig. S8).
The peptide-(94–110) and residues Gln-27 and Arg-32, highlighted by cross-linking and limited proteolysis analyses, respectively, belong to the structural core of PlGF crucial for VEGFR-1 recognition (residues 18–117) (31). Some of these residues are involved in receptor binding (Table 2). Therefore, these results suggest that AF is effectively able to mask some PlGF-1 residues that are involved in receptor recognition.
TABLE 2.
Role of PlGF-1 core residues involved in the interaction with AF, as elicited by structural analyses and docking model
LP, Limited Proteolysis; UVcl, UV cross-linking; Dock: docking. For the nature of interactions, PlGF-1/VEGFR-1 and the data reported in the last column (the numbers in parentheses represent the number of contacts with the VEGFR-1 residues indicated), see Ref. 31. For the decrease affinity by mutation in Ala, see Ref. 25.
| PlGF residues | Type of analysis | Residues at 6 Å from AF (type of interaction) | Nature of interactions PlGF/VEGFR-1 | Affinity decrease by mutation in Ala | VEGFR-1D2 putative intermolecular contacts |
|---|---|---|---|---|---|
| Pro-25 | Dock | 25% | |||
| Phe-26 | Dock | aromatic π-π | Van der Waals | Pro-143(2), Leu-221(2)* | |
| Gln-27 | LP + Dock | polar | 50% | Glu-141(3), Ile-142, Pro-143(9), Leu-204, Asn-219(3) | |
| Gln-88 | Dock | H-π | Van der Waals | Ile-142 | |
| Arg-97 | UVcl + Dock | ||||
| Pro-98 | UVcl + Dock | H-bond (C7-OH group) | 50% | ||
| Ser-99 | UVcl + Dock | H-π | |||
| Tyr-100 | UVcl + Dock | aromatic π-π | Van der Waals + polar | 25% | Ile-142(10), Pro-143(2) |
DISCUSSION
Flavonoids are a class of natural compounds belonging to the large polyphenolic family widely distributed in the plant kingdom displaying a variety of biological effects like antioxidant, antiviral, and anti-inflammatory activities. In the past few years, the interest on these compounds has increased because they displayed potentiality not only as cancer-chemopreventive but also as cancer therapeutic natural agents. It has been reported that these compounds are able to modulate proteins activity acting on multiple key elements of signal transduction pathways involved in cell proliferation, differentiation, apoptosis, in angiogenesis, and inflammation, determining cancer growth inhibition. However, these mechanisms of action have not been fully characterized, and many features remain to be elucidated (32–34).
Here, we have reported the screening of a large collection of plant extracts based on the idea to identify small molecules able to prevent the initial event needed for the proangiogenic activity of the VEGF family members, the interaction of VEGF-A, PlGF-1, and VEGF-B with VEGFR-1 and VEGFR-2 receptors.
As a result, we were able to isolate AF from an extract of the Malian plant C. senegalensis as an antiangiogenic bioactive molecule. Indeed, we have demonstrated that AF is able to bind VEGF-A and PlGF-1 preventing the interaction and consequent phosphorylation of VEGFR-1 and VEGFR-2, as well as endothelial cell migration and CTF induced by either protein. It also inhibits the in vitro VEGF-B/VEGFR-1 interaction. Interestingly, AF did not inhibit the activity of other homodimeric proteins involved in neovessel formation and stabilization such as PDGF, structurally related to VEGFs and FGF because it fails to block in vitro PDGF-B/PDGFR-β interaction and FGF-2-induced chemotaxis and CTF. These results indicate how AF possesses a binding specificity for VEGF family members acting at the low micromolar concentration range, therefore widely distant from its cytotoxic concentration.
In vivo, AF is able to inhibit VEGF-induced chorioallantoic membrane neovascularization. Finally, we evaluated its anti-tumor effects on two different cancer experimental models: an orthotropic model of melanoma and a xenograft model of colon carcinoma. AF, daily delivered intraperitoneally at 50 mg/kg, was able to inhibit tumor growth and associated neoangiogenesis in a significant manner in both models.
AF belongs to a unique class of naturally occurring biflavonoids and is able to exert the general properties of flavonoids (35–38). More recently, its antiangiogenic effect has been described in an assay of tumor-directed capillary formation using a melanoma model generated via subcutaneous injection of B16-F10 melanoma cells. AF delivered simultaneously with tumor cell inoculation for 5 days, determined a significant inhibition of tumor directed neovessels, as observed 9 days after cell injection. The authors reported an altered proinflammatory circulating cytokines production as well as reduction of circulating VEGF-A as possible explanation of AF action (27).
These results may be fully accounted for by considering the mechanism of action that we have discovered, which has never been reported before. Indeed, the AF ability to interact with proangiogenic VEGF family members preventing their binding to VEGF receptors is crucial for the observed inhibition of new vessel formation, as direct effect on endothelial cells proliferation, migration, and differentiation, in which both proangiogenic VEGF growth factors and VEGF receptors are involved.
Moreover, inflammation is strictly associated with tumor angiogenesis and growth and is able to sustain neoangiogenesis. PlGF and VEGF-A play a crucial role in the recruitment of inflammatory cells at neoangiogenic site (17), mainly interacting with VEGFR-1 expressed on their surfaces (39, 40), as demonstrated by the inhibition of F4/80 positive cells recruitment observed in AF treated melanoma. This reduction may be responsible, almost in part, for the reported decrease of general proinflammatory circulating cytokines. Moreover, this reduction, together with reduced tumor growth, also explains the decrease of circulating VEGF-A because it is mainly produced by tumor cells and by recruited myeloid cells such as monocyte macrophages. Nonetheless, we cannot exclude that AF may exert its action also modulating proinflammatory cytokines by alternative pathways.
From a structural point of view, AF is an apigenin dimer. Many data have been reported on the antiangiogenic and antitumoral action of apigenin, in particular for its ability to interfere with hypoxia-inducible factor-1α activity and expression (41, 42). Interestingly, neither apigenin nor related molecules as quercetin, naringenin, and catechin are able to interact with VEGFs, as assessed by surface plasmon resonance assays. Consequently, the binding properties and activity of AF strictly depends on their own structural features and not on general flavonoid characteristics.
Data generated by cross-linking and limited proteolysis clearly indicated the capability of AF to interact in the area of PlGF-1 involved in the recognition of the receptor. Indeed, the residue Gln-27 highlighted with limited proteolysis analysis is involved in polar interaction with VEGFR-1 (31). The relevance of this residue in the PlGF-1·VEGFR-1 complex stabilization was demonstrated previously also with mutagenesis studies, which indicated that its mutation in Ala was sufficient to decrease to 50% the PlGF-1 binding activity to the receptor (25). Among the residues of the peptide-(94–110) evidenced by UV cross-linking, the residue Tyr-100 is involved in both van der Waals and polar interactions with VEGFR-1. Mutagenesis studies indicated that its mutation in Ala, as well as that of the residue Pro-98, determined a decrease of 25 or of 50% of PlGF-1 binding activity to the receptor, respectively (Table 2) (25, 31).
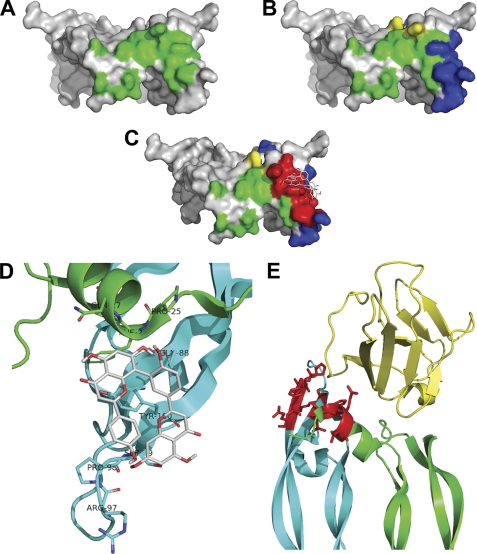
To better understand the mechanism of interaction between AF and PlGF-1, these data have been used to generate a preliminary model of PlGF-1·AF complex using the docking approach (Fig. 6C) (43, 44). The atomic coordinates and three-dimensional structure of recombinant PlGF-1 homodimer used for calculations were based on crystallographic structure of PlGF-1 (Protein Data Bank code 1FZV) (31).
FIGURE 6.
Docking analysis of PlGF-1·amentoflavone complex. Shown is a surface plot of PlGF-1 showing the residues involved in Flt-1 recognition in green (A), the peptide-(94–110), identified by UV cross-linking analysis, and the residues Gln-27 and Arg-32, evidenced with limited proteolysis analysis, highlighted in blue and yellow, respectively (B). C, surface plot of the model of the PlGF-1·AF complex showing the lowest binding energy. Red indicates the area within 6 Å from the ligand. A high overlapping with blue and green residues was observed. D, magnification of interaction area between AF and PlGF-1. The PlGF-1 residues of the two monomers (green and cyan) involved in AF interaction are indicated. AF and side chains of PlGF-1 residues involved in contacts are represented as sticks with colored atoms (white, hydrogen; red, oxygen; blue, nitrogen). E, the PlGF-1·VEGFR-1 D2 complex (Protein Data Bank code 1RV6) is shown with the two monomers of PlGF-1 homodimer represented in green and cyan, and the VEGFR-1 D2 domain is represented in yellow, whereas the PlGF-1 residues and corresponding side chains at 6 Å from AF inhibitor are highlighted in red.
PlGF-1 residues Pro-25, Phe-26, and Gln-27 from one monomer and Gln-88, Arg-97, Pro-98, Ser-99, and Tyr-100 from the other monomer all localized in the area involved in VEGFR-1 recognition (Fig. 6, D and E), were found at 6 Å from the inhibitor (25, 31, 45). According to this model, the interaction between PlGF-1 and AF is mainly hydrophobic (Table 2). Docking of the nonactive flavonoid catechin performed with identical parameters showed an interaction involving a protein region clearly different from that observed for AF and not implicated in the VEGFR-1 recognition (supplemental Fig. S9).
Docking results furtherly support the role of PlGF-1 residues highlighted with cross-linking and limited proteolysis analyses. In addition, the three residues Pro-25, Phe-26, and Gln-27 are part of the PlGF-1 α1-helix, a structural element crucial for the stabilization of PlGF-1 dimer (31, 45). Moreover, the residues Phe-26 and Gln-88 are involved in van der Waals interactions with the VEGFR-1 receptor, whereas mutagenesis of Pro-25 in Ala determined a 25% reduction of PlGF-1 binding activity to the receptor (Table 2 and supplemental Fig. S10) (25, 31).
Altogether, these data suggest how AF is able to interact with PlGF-1 residues that play a crucial role in receptor recognition. Moreover, additional structural studies are needed to identify the residues of VEGF-A and VEGF-B involved in AF binding to correctly evaluate how this interaction may affect the receptor recognition.
In conclusion, the data here reported demonstrate that AF possesses antiangiogenic activity due to its ability to selectively bind proangiogenic VEGF family members, preventing their interaction with VEGF receptors. Therefore, we propose that AF may be considered as a promising new chemical scaffold to develop, by medicinal chemistry approaches, powerful new small molecules for the inhibition of pathological neoangiogenesis.
Supplementary Material
Acknowledgments
We thank Professor D. Collen, Chairman of the D. Collen Research Foundation, for support; Vincenzo Mercadante and all the staff of IGB animal house for technical assistance; Andrea Lorentzen for assistance in acquisition of data on the 4800 instrument; and Anna Maria Aliperti for manuscript editing.
This work was supported by Associazione Italiana Ricerca sul Cancro Grant 4840 and Telethon Italy Grant GGP08062 (to S. D. F).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” “Results,” Tables S1–S3, and Figs. S1–S10.
- PlGF-1
- placental growth factor 1
- VEGFR-1
- VEGF receptor 1
- CTF
- capillary-like tube formation
- HUVEC
- human umbilical vein endothelial cell
- AF
- amentoflavone
- RP
- reverse phase.
REFERENCES
- 1. Ferrara N., Gerber H. P., LeCouter J. (2003) Nat. Med. 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P. (2005) Nature 438, 932–936 [DOI] [PubMed] [Google Scholar]
- 3. De Falco S., Gigante B., Persico M. G. (2002) Trends Cardiovasc. Med. 12, 241–246 [DOI] [PubMed] [Google Scholar]
- 4. Ferrara N., Hillan K. J., Novotny W. (2005) Biochem. Biophys. Res. Commun. 333, 328–335 [DOI] [PubMed] [Google Scholar]
- 5. Gragoudas E. S., Adamis A. P., Cunningham E. T., Jr., Feinsod M., Guyer D. R. (2004) N. Engl. J. Med. 351, 2805–2816 [DOI] [PubMed] [Google Scholar]
- 6. Rosenfeld P. J., Brown D. M., Heier J. S., Boyer D. S., Kaiser P. K., Chung C. Y., Kim R. Y. (2006) N. Engl. J. Med. 355, 1419–1431 [DOI] [PubMed] [Google Scholar]
- 7. Ellis L. M., Hicklin D. J. (2008) Nat. Rev. Cancer 8, 579–591 [DOI] [PubMed] [Google Scholar]
- 8. Wu Y., Zhong Z., Huber J., Bassi R., Finnerty B., Corcoran E., Li H., Navarro E., Balderes P., Jimenez X., Koo H., Mangalampalli V. R., Ludwig D. L., Tonra J. R., Hicklin D. J. (2006) Clin Cancer Res. 12, 6573–6584 [DOI] [PubMed] [Google Scholar]
- 9. Fischer C., Jonckx B., Mazzone M., Zacchigna S., Loges S., Pattarini L., Chorianopoulos E., Liesenborghs L., Koch M., De Mol M., Autiero M., Wyns S., Plaisance S., Moons L., van Rooijen N., Giacca M., Stassen J. M., Dewerchin M., Collen D., Carmeliet P. (2007) Cell 131, 463–475 [DOI] [PubMed] [Google Scholar]
- 10. Van de Veire S., Stalmans I., Heindryckx F., Oura H., Tijeras-Raballand A., Schmidt T., Loges S., Albrecht I., Jonckx B., Vinckier S., Van Steenkiste C., Tugues S., Rolny C., De Mol M., Dettori D., Hainaud P., Coenegrachts L., Contreres J. O., Van Bergen T., Cuervo H., Xiao W. H., Le Henaff C., Buysschaert I., Kharabi Masouleh B., Geerts A., Schomber T., Bonnin P., Lambert V., Haustraete J., Zacchigna S., Rakic J. M., Jiménez W., Noël A., Giacca M., Colle I., Foidart J. M., Tobelem G., Morales-Ruiz M., Vilar J., Maxwell P., Vinores S. A., Carmeliet G., Dewerchin M., Claesson-Welsh L., Dupuy E., Van Vlierberghe H., Christofori G., Mazzone M., Detmar M., Collen D., Carmeliet P. (2010) Cell 141, 178–190 [DOI] [PubMed] [Google Scholar]
- 11. Carmeliet P., Moons L., Luttun A., Vincenti V., Compernolle V., De Mol M., Wu Y., Bono F., Devy L., Beck H., Scholz D., Acker T., DiPalma T., Dewerchin M., Noel A., Stalmans I., Barra A., Blacher S., Vandendriessche T., Ponten A., Eriksson U., Plate K. H., Foidart J. M., Schaper W., Charnock-Jones D. S., Hicklin D. J., Herbert J. M., Collen D., Persico M. G. (2001) Nat. Med. 7, 575–583 [DOI] [PubMed] [Google Scholar]
- 12. Luttun A., Tjwa M., Moons L., Wu Y., Angelillo-Scherrer A., Liao F., Nagy J. A., Hooper A., Priller J., De Klerck B., Compernolle V., Daci E., Bohlen P., Dewerchin M., Herbert J. M., Fava R., Matthys P., Carmeliet G., Collen D., Dvorak H. F., Hicklin D. J., Carmeliet P. (2002) Nat. Med. 8, 831–840 [DOI] [PubMed] [Google Scholar]
- 13. Rakic J. M., Lambert V., Devy L., Luttun A., Carmeliet P., Claes C., Nguyen L., Foidart J. M., Noël A., Munaut C. (2003) Invest. Ophthalmol. Vis. Sci. 44, 3186–3193 [DOI] [PubMed] [Google Scholar]
- 14. Kaplan R. N., Riba R. D., Zacharoulis S., Bramley A. H., Vincent L., Costa C., MacDonald D. D., Jin D. K., Shido K., Kerns S. A., Zhu Z., Hicklin D., Wu Y., Port J. L., Altorki N., Port E. R., Ruggero D., Shmelkov S. V., Jensen K. K., Rafii S., Lyden D. (2005) Nature 438, 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gigante B., Morlino G., Gentile M. T., Persico M. G., De Falco S. (2006) FASEB J. 20, 970–972 [DOI] [PubMed] [Google Scholar]
- 16. Cao Y. (2009) Sci. Signal 2, re1. [DOI] [PubMed] [Google Scholar]
- 17. Tarallo V., Vesci L., Capasso O., Esposito M. T., Riccioni T., Pastore L., Orlandi A., Pisano C., De Falco S. (2010) Cancer Res. 70, 1804–1813 [DOI] [PubMed] [Google Scholar]
- 18. Fischer C., Mazzone M., Jonckx B., Carmeliet P. (2008) Nat. Rev. Cancer 8, 942–956 [DOI] [PubMed] [Google Scholar]
- 19. Newman D. J., Cragg G. M., Snader K. M. (2003) J. Nat. Prod. 66, 1022–1037 [DOI] [PubMed] [Google Scholar]
- 20. Ponticelli S., Braca A., De Tommasi N., De Falco S. (2008) Planta Med. 74, 401–406 [DOI] [PubMed] [Google Scholar]
- 21. Ponticelli S., Marasco D., Tarallo V., Albuquerque R. J., Mitola S., Takeda A., Stassen J. M., Presta M., Ambati J., Ruvo M., De Falco S. (2008) J. Biol. Chem. 283, 34250–34259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim H. P., Park H., Son K. H., Chang H. W., Kang S. S. (2008) Arch. Pharm. Res. 31, 265–273 [DOI] [PubMed] [Google Scholar]
- 23. Maglione D., Guerriero V., Viglietto G., Ferraro M. G., Aprelikova O., Alitalo K., Del Vecchio S., Lei K. J., Chou J. Y., Persico M. G. (1993) Oncogene 8, 925–931 [PubMed] [Google Scholar]
- 24. Dal Piaz F., Vassallo A., Lepore L., Tosco A., Bader A., De Tommasi N. (2009) J. Med. Chem. 52, 3814–3828 [DOI] [PubMed] [Google Scholar]
- 25. Errico M., Riccioni T., Iyer S., Pisano C., Acharya K. R., Persico M. G., De Falco S. (2004) J. Biol. Chem. 279, 43929–43939 [DOI] [PubMed] [Google Scholar]
- 26. Marcellini M., De Luca N., Riccioni T., Ciucci A., Orecchia A., Lacal P. M., Ruffini F., Pesce M., Cianfarani F., Zambruno G., Orlandi A., Failla C. M. (2006) Am J. Pathol. 169, 643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guruvayoorappan C., Kuttan G. (2008) Biochemistry 73, 209–218 [DOI] [PubMed] [Google Scholar]
- 28. Gillet A., Sanner M., Stoffler D., Olson A. (2005) Structure 13, 483–491 [DOI] [PubMed] [Google Scholar]
- 29. Patel D., Shukla S., Gupta S. (2007) Int. J. Oncol. 30, 233–245 [PubMed] [Google Scholar]
- 30. Renzone G., Salzano A. M., Arena S., D'Ambrosio C., Scaloni A. (2007) Curr. Proteomics 4, 1–16 [Google Scholar]
- 31. Iyer S., Leonidas D. D., Swaminathan G. J., Maglione D., Battisti M., Tucci M., Persico M. G., Acharya K. R. (2001) J. Biol. Chem. 276, 12153–12161 [DOI] [PubMed] [Google Scholar]
- 32. Yang C. S., Landau J. M., Huang M. T., Newmark H. L. (2001) Annu. Rev. Nutr. 21, 381–406 [DOI] [PubMed] [Google Scholar]
- 33. Albini A., Noonan D. M., Ferrari N. (2007) Clin. Cancer Res. 13, 4320–4325 [DOI] [PubMed] [Google Scholar]
- 34. Ramos S. (2008) Mol. Nutr. Food Res. 52, 507–526 [DOI] [PubMed] [Google Scholar]
- 35. Cholbi M. R., Paya M., Alcaraz M. J. (1991) Experientia 47, 195–199 [DOI] [PubMed] [Google Scholar]
- 36. Kim H. K., Son K. H., Chang H. W., Kang S. S., Kim H. P. (1998) Arch. Pharm. Res. 21, 406–410 [DOI] [PubMed] [Google Scholar]
- 37. Lin Y. M., Flavin M. T., Schure R., Chen F. C., Sidwell R., Barnard D. L., Huffman J. H., Kern E. R. (1999) Planta Med. 65, 120–125 [DOI] [PubMed] [Google Scholar]
- 38. Ma S. C., But P. P., Ooi V. E., He Y. H., Lee S. H., Lee S. F., Lin R. C. (2001) Biol. Pharm. Bull 24, 311–312 [DOI] [PubMed] [Google Scholar]
- 39. Clauss M., Weich H., Breier G., Knies U., Röckl W., Waltenberger J., Risau W. (1996) J. Biol. Chem. 271, 17629–17634 [DOI] [PubMed] [Google Scholar]
- 40. Sawano A., Iwai S., Sakurai Y., Ito M., Shitara K., Nakahata T., Shibuya M. (2001) Blood 97, 785–791 [DOI] [PubMed] [Google Scholar]
- 41. Osada M., Imaoka S., Funae Y. (2004) FEBS Lett. 575, 59–63 [DOI] [PubMed] [Google Scholar]
- 42. Fang J., Xia C., Cao Z., Zheng J. Z., Reed E., Jiang B. H. (2005) FASEB J. 19, 342–353 [DOI] [PubMed] [Google Scholar]
- 43. Lengauer T., Rarey M. (1996) Curr. Opin. Struct. Biol. 6, 402–406 [DOI] [PubMed] [Google Scholar]
- 44. van Dijk A. D., Boelens R., Bonvin A. M. (2005) FEBS J. 272, 293–312 [DOI] [PubMed] [Google Scholar]
- 45. Christinger H. W., Fuh G., de Vos A. M., Wiesmann C. (2004) J. Biol. Chem. 279, 10382–10388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.