Abstract
Hepatocellular carcinoma (HCC) is frequently associated with abnormalities in cell cycle regulation, leading to increased activity of cyclin-dependent kinases (Cdks) due to the loss, or low expression of, Cdk inhibitors. In this study, we showed that ibulocydine (an isobutyrate prodrug of the specific Cdk inhibitor, BMK-Y101) is a candidate anti-cancer drug for HCC. Ibulocydine has high activity against Cdk7/cyclin H/Mat1 and Cdk9/cyclin T. Ibulocydine inhibited the growth of HCC cells more effectively than other Cdk inhibitors, including olomoucine and roscovitine, whereas ibulocydine as well as the other Cdk inhibitors and BMK-Y101 minimally influenced the growth of normal hepatocyte cells. Ibulocydine induced apoptosis in HCC cells, most likely by inhibiting Cdk7 and Cdk9. In vitro treatment of HCC cells with ibulocydine rapidly blocked phosphorylation of the carboxyl-terminal domain (CTD) of the large subunit of RNA polymerase II, a process mediated by Cdk7/9. Anti-apoptotic gene products such as Mcl-1, survivin, and X-linked IAP (XIAP) are crucial for the survival of many cell types, including HCC. Following the inhibition of RNA polymerase II phosphorylation, ibulocydine caused rapid down-regulation of Mcl-1, survivin, and XIAP, thus inducing apoptosis. Furthermore, ibulocydine effectively induced apoptosis in HCC xenografts with no toxic side effects. These results suggest that ibulocydine is a strong candidate anti-cancer drug for the treatment of HCC.
Keywords: Anticancer Drug, Apoptosis, Cancer Therapy, Cdk (Cyclin-dependent Kinase), RNA Polymerase II, Transcription Regulation, Hepatocellular Carcinoma (HCC), Prodrug, Xenograft
Introduction
Hepatocellular carcinoma (HCC)3 is a leading cause of mortality in patients with liver cirrhosis (1). In the United States, the incidence of HCC has risen during the last few decades from 1.3/100,000 population between 1978 and 1980 to 3.3/100,000 population between 1998 and 2001 (2, 3). This is likely to continue, primarily due to progressive liver diseases attributable to the hepatitis C virus in individuals infected a few decades ago (4). However, as hepatitis B virus infection is more prevalent globally than hepatitis C virus, hepatitis B virus remains the single most important cause of HCC worldwide, and is particularly high in Asia and Africa (5). Treatment of HCC is clinically difficult, as HCC expresses multidrug-resistance genes and is insensitive to current chemotherapeutic agents (6). HCC cells are characterized by high cellular levels of cyclin-dependent kinases (Cdks). Up-regulation of Cdks results from the inactivation of p16Ink4, p21Waf1 and p27Kip1, Cdk inhibitory proteins, and abnormal activation of cyclins (7–10). For this reason, Cdk inhibitors are strong candidates for the treatment of HCC, and some (e.g. olomoucine, roscovitine and flavopiridol) have been tested in clinical trials (11).
Cdks regulate two biological processes essential for cancer cell survival: cell cycle progression and gene transcription (12). They control entry into each stage of the cell cycle by phosphorylating key substrates such as pRb (13, 14). Cdks usually form heterodimers with cyclins to create active complexes and, because cyclin levels oscillate throughout the cell cycle, this contributes to the temporal activation of specific Cdks. Cdks also regulate transcription by phosphorylating the carboxyl-terminal domain (CTD) of the large subunit of RNA polymerase II (15). In humans, the CTD contains 52 heptapeptide (YSPTSPS) repeats that can be phosphorylated on serines, threonines, and tyrosines (15). A number of Cdks phosphorylate these sites, including Cdk7/cyclin H/Mat1 (part of the TFIIH complex that initiates transcription) (16) and Cdk9/cyclin T (also called P-TEFb), which activates transcriptional elongation (17–21).
Mcl-1, an anti-apoptotic member of the Bcl-2 family (22), was first identified as a protein up-regulated in a human carcinoma cell line induced to differentiate along the monocyte lineage (23), and is essential for the survival of carcinoma cells (24–27). Mcl-1 is thought to act by antagonizing pro-apoptotic proteins such as Bim (28). Inhibitor of apoptosis protein (IAP) is a family of proteins that regulates cell death; X-linked IAP (XIAP) and survivin are two important members of this family in mammals. Both inhibit caspases and block apoptosis (29).
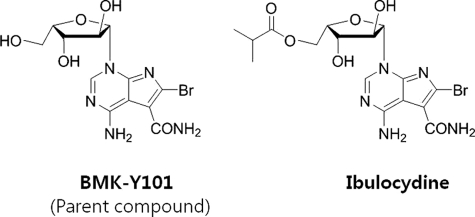
Ibulocydine, ((2S,3S,4S,5S)-5-(4-amino-6-bromo-5-carbamoyl-1H-pyrrolo[2,3-d]pyrimidin-1-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl isobutyrate, is an isobutyrate ester prodrug of a novel synthetic Cdk inhibitor, BMK-Y101 (4-amino-6-bromo-1-((2S,3S,4S,5S)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1H-pyrrolo[2,3-d]pyrimidine-5-carboxamide) (Fig. 1). Prodrugs are designed to improve absorption, distribution, metabolism, and excretion (ADME) profiles (30, 31), and conventional prodrug designs have been used to develop several anticancer drugs (32).
FIGURE 1.
The structure of BMK-Y101 and ibulocydine. BMK-Y101 is 4-amino-6-bromo-1-((2S,3S,4S,5S)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1H-pyrrolo[2,3-d]pyrimidine-5-carboxamide. Ibulocydine is ((2S,3S,4S,5S)-5-(4-amino-6-bromo-5-carbamoyl-1H-pyrrolo[2,3-d]pyrimidin-1-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl isobutyrate.
The aim of this study was to investigate the molecular mechanisms of cell death induced by ibulocydine in HCC cells (compared with its parent compound and other Cdk inhibitors) and to define its efficacy in a mouse model.
EXPERIMENTAL PROCEDURES
Cells and Reagents
SNU-354 (human HCC) cells were obtained from The Center for Functional Analysis of Human Genome in South Korea. SK-HEP-1, HepG2 (human HCC), and AML-12 (mouse hepatocyte) cells were purchased from the American Type Culture Collection. Ibulocydine and BMK-Y101 were synthesized in the Laboratory of Synthetic and Medicinal Chemistry at the Department of Chemistry, Seoul National University, South Korea. Olomoucine and roscovitine were purchased from Calbiochem (San Diego, CA), dissolved in dimethyl sulfoxide, and stored at −20 °C as 50 mm stocks.
Cell Culture and Treatments
SK-HEP-1 and HepG2 cells were maintained at 37 °C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen) and antibiotics/antimycotics (Invitrogen). SNU-354 cells were maintained at 37 °C in 5% CO2 in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS and antibiotics/antimycotics. AML-12 cells were maintained at 37 °C in 5% CO2 in 1:1 mixture of DMEM and Ham's F-12 medium (Invitrogen) supplemented with insulin-transferrin-selenium-X (0.005 mg/ml of insulin, 0.005 mg/ml of transferrin, 5 ng/ml of selenium, 40 ng/ml of dexamethasone) (Invitrogen), 10% FBS, and antibiotics/antimycotics. Cells were treated with 0–50 μm (final concentration) of each drug (ibulocydine, BMK-Y101, olomoucine, or roscovitine) for 24 h.
In Vitro Kinase Assay
Cdk7/9 in vitro kinase assays were performed at 30 °C for 20 min in kinase assay buffer (50 mm Tris (pH 7.4), 10 mm MgCl2, 1 mm EGTA, 40 mm β-glycerophosphate, 0.1 mm Na3VO4, 1 mm DTT, 0.1 μg/ml of leupeptin, 0.1 μg/ml of pepstatin A, 0.1 μg/ml of antipain, and 1 mm phenylmethylsulfonyl fluoride) (final volume 50 μl) containing recombinant RNA Pol II-CTD-GST protein (Jena Bioscience, Germany; a specific substrate for each kinase), recombinant Cdk9/cyclin T1 or Cdk7/cyclin H/MAT1 (Upstate) as an enzyme source, 2.5 μCi of [γ-32P]ATP, and 100 μm MgAc/ATP. Samples were analyzed by 8% SDS-PAGE followed by autoradiography. The intensity of the bands was analyzed by densitometry (GS-800, Bio-Rad).
Western Blot Analysis
Cell pellets were lysed in lysis buffer (0.5% Triton X-100, 20 mm Tris-HCl (pH 7.5), 2 mm MgCl2, 1 mm DTT, 1 mm EGTA, 50 mm β-glycerophosphate, 25 mm NaF, 1 mm Na3VO4, 2 μg/ml of leupeptin, 2 μg/ml of pepstatin A, 100 μg/ml of phenylmethylsulfonyl fluoride, and 1 μg/ml of antipain) for 1 h at 4 °C. Lysates were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore Co., Bedford, MA). After blocking at room temperature for 1 h in phosphate-buffered saline (PBS) containing 5% skim milk and 0.1% Tween 20, the membranes were incubated with the following antibodies (diluted 1:1000): rabbit anti-poly(ADP-ribose) polymerase (PARP), rabbit anti-Mcl-1 (from Santa Cruz Biochemicals, Santa Cruz, CA), rabbit anti-survivin, rabbit anti-XIAP (Cell Signaling), rabbit anti-actin (Sigma), rabbit anti-RNA Pol II, rabbit anti-phospho-RNA Pol II (Ser-5), and rabbit anti-phospho-RNA Pol II (Ser-2) (Bethyl Laboratories, Inc.). Reactive bands were visualized with horseradish peroxidase-conjugated antibodies and ECL (GE Healthcare).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Assay
AML-12, SK-HEP-1, HepG2, and SNU-354 cells in log phase growth were aliquoted into 24-well plates (2 × 104 cells/well) and exposed to various concentrations of ibulocydine, BMK-Y101, olomoucine, or roscovitine. After 24 h, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (Sigma; 5 mg/ml in phosphate-buffered saline) was added to each well. After 3 h at 37 °C, the supernatants were removed, and the formazan crystals were dissolved in dimethyl sulfoxide. The absorbance was determined using an ELISA plate reader.
[3H]Thymidine Incorporation Assay
SNU-354 cells were plated onto 6-well plates (5 × 104 cells/well). Cells were serum deprived for 24 h, then serum stimulated in culture medium containing various drug concentrations (ibulocydine, BMK-Y101, olomoucine, or roscovitine) and 1 μCi/ml of tritiated thymidine ([3H]dT; GE Healthcare) for 18–24 h. Cells were washed twice with ice-cold PBS, fixed, and washed in ice-cold 5% trichloroacetic acid. DNA was solubilized in 10.25 n NaOH for 1 h at 37 °C. [3H]dT incorporation was measured by liquid scintillation counting.
Flow Cytometric Cell Cycle Assay
AML-12, SK-HEP-1, HepG2, and SNU-354 cells seeded in 90-mm dishes were treated with ibulocydine or BMK-Y101 for 24 or 12 h. Cells were harvested, pelleted, and resuspended in 300 μl of ice-cold PBS, fixed by gentle addition of 500 μl of ice-cold 70% ethanol, and incubated overnight at 4 °C. Cells were washed twice with 500 μl of PBS and incubated in 500 μl of PBS containing 50 μg/ml of propidium iodide and 50 μl of 1 mg/ml of RNase A in the dark for 30 min at room temperature. Cells were analyzed using a FACSCalibur flow cytometer and Cell Quest software (BD Biosciences).
SNU-354 Tumor Xenografts
Male athymic nude mice (5–6 weeks old) were obtained from the Central Lab, Animal Inc., and housed in the animal facilities at the Seoul National University College of Pharmacy. All animal work was conducted according to protocols approved by the Seoul National University Institute of Laboratory Animal Resources. Mice were inoculated subcutaneously under the right front leg with 200 μl of PBS containing 1 × 107 SNU-354 cells. Xenografts were grown to a mean tumor volume of 100 ± 30 mm3. Mice were randomized into three groups (n = 8) and treatment was initiated. Two groups were treated with ibulocydine (single daily intraperitoneal injection of 4 or 20 mg/kg for 3 weeks). The control group received intraperitoneal injections of carrier solution (5% ethanol and 30% polyethylene glycol 200 (Fluka, Netherlands)), following an identical schedule. Body weight was measured daily. Primary tumor volumes were calculated using the formula: V = a × b × c × π/6 (a = length, b = width, and c = depth). All mice were euthanized with ether. Ibulocydine-treated mice were euthanized 33 days after the first injection. Tumors and liver tissues were removed, measured, and prepared for TUNEL assays. Data represent mean ± S.E.
Immunohistochemistry
All tumors and tissues were fixed in 36% formaldehyde solution (Junsei Chemical Co. Ltd., Tokyo, Japan) and embedded in paraffin. Sections (5 μm) were stained with H&E, assayed for apoptosis by TUNEL, or assayed for cleaved caspase-3 using a rabbit monoclonal anti-cleaved caspase-3 antibody (1:50; Cell Signaling) and the ABC peroxidase labeling procedure (Dako Cytomation California, Inc.) for immunologic detection.
Measurement of Caspase-3 Activity
Cell pellets were lysed in cell lysis buffer (0.5% Triton X-100, 20 mm Tris-HCl (pH 7.5), 2 mm MgCl2, 1 mm DTT, 1 mm EGTA, 50 mm β-glycerophosphate, 25 mm NaF, 1 mm Na3VO4, 2 μg/ml of leupeptin, 2 μg/ml of pepstatin A, 100 μg/ml of phenylmethylsulfonyl fluoride, and 1 μg/ml of antipain). Protease assay buffer (20 mm HEPES (pH 7.5), 10% glycerol, 2 mm DTT) and substrate, Ac-DEVD-AMC (20 μm, final concentration, BD Biosciences), were added to the cell lysates and incubated for 1 h at 37 °C. Samples were quantified using a spectrofluorometer (TECAN, Switzerland) with an excitation wavelength of 380 nm and emission wavelength 460 nm.
RNA Isolation and Reverse Transcription-PCR
Total RNA was extracted from SNU-354 cells using standard protocols. For reverse transcription, 5 μg of RNA was used in standard assays with Moloney murine leukemia virus reverse transcriptase (Promega Corporation). Reverse transcription was carried out at 37 °C for 60 min followed by 5 min at 95 °C. PCR were performed in a final volume of 50 μl containing 5 μl of cDNA as template for 30 cycles with the indicated primers and Taq DNA polymerase as follows: 30 cycles at 95 °C for 1 min, 56 °C for 1 min, and 72 °C for 1 min. The final extension (72 °C; 10 min) was the same for all reactions. PCR products (10 μl) were analyzed on 1% agarose gels. Specific primers were: Mcl-1 (sense, 5′-CGG TAA TCG GAC TCA ACC TC-3′, and antisense 5′-CCT CCT TCT CCG TAG CCA A-3′), XIAP (sense, 5′-AGT GGT AGT CCT GTT TCA GCA TCA-3′, and antisense, 5′-CCG CAC GGT ATC TCC TTC A-3′), survivin (sense, 5′-TGC CTG GCA GCC CTT TC-3′, and antisense, 5′-CCT CCA AGA AGG GCC AGT TC-3′), GAPDH (sense, 5′-GAA GGT GAA GGT CGG AGT-3′, and antisense, 5′-GAA GAT GGT GAT GGG ATT TC-3′) (BIONICS, Seoul, Republic of Korea).
Pharmacokinetics
Sprague-Dawley rats were used for these tests. Briefly, after insertion of a catheter into the right jugular vein, 10 mg/kg of ibulocydine was injected into the femoral vein. For oral administration, 10 mg/kg of ibulocydine was used. For intraperitoneal injection, 5 mg/kg of ibulocydine was used. At the indicated times, plasma and urine samples were collected and analyzed by LC-MS/MS. Pharmacokinetic parameters of BMK-Y101 in plasma and urine after ibulocydine administration were calculated using standard non-compartmental methods and WinNonlin (Pharsight). Maximum plasma concentrations (Cmax) and the time to maximum plasma concentration (Tmax) were determined directly from the plasma concentration-time data. AUC from time 0 to infinity (AUC0-inf) was estimated from AUC0–8 + Ct/λz, where AUC0–8 was calculated from time 0 to 8 for the last quantifiable concentration (Ct) using the linear trapezoidal rule, and the terminal elimination rate constant (λz) was determined by linear regression analysis of the terminal phase of the plasma concentration-time data. MRT was calculated using AUMC/AUC, where AUMC = area under the moment curve computed to the observation. The absolute bioavailability of a drug was calculated using (AUCper oral/AUCintravenous) × 100.
RESULTS
Ibulocydine Inhibits HCC Cell Growth
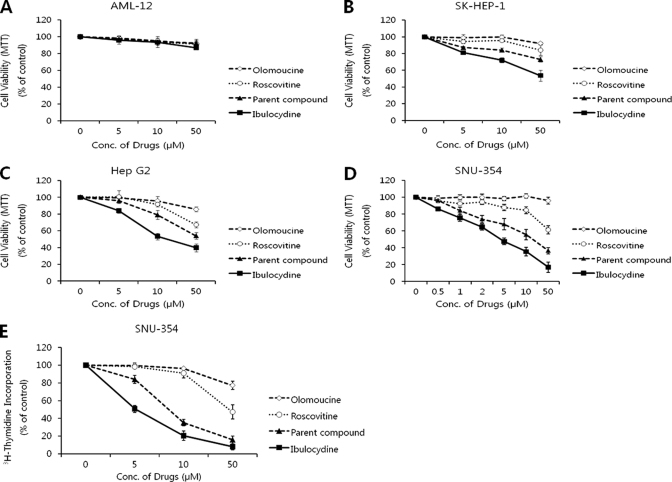
We determined the viability of HCC cells after treatment with ibulocydine using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and [3H]thymidine incorporation assays. The results showed that ibulocydine strongly inhibited cell growth in a dose-dependent manner in HCC cells (SK-HEP-1, HepG2, and SNU-354) (Fig. 2, B–E), whereas ibulocydine as well as the Cdk inhibitors olomoucine, roscovitine, and BMK-Y101 (parent compound) minimally influenced the growth of AML-12 cells (Fig. 2A). Thus, ibulocydine strongly inhibits cell growth of HCC cells, but not of normal hepatocyte cells. Furthermore, the inhibitory activity of ibulocydine was much greater than that of the comparison group (olomoucine < roscovitine < BMK-Y101 < ibulocydine).
FIGURE 2.
Ibulocydine inhibits the growth of HCC cells more effectively than olomoucine or roscovitine. HCC cells were treated in triplicate experiments with the indicated concentrations of each drug (ibulocydine, BMK-Y101, olomoucine, and roscovitine) for 24 h. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (A–D) and [3H]thymidine incorporation assays (E).
Ibulocydine Inhibits Cdk7 and Cdk9 Kinases in Vitro and in Cultured HCC Cells
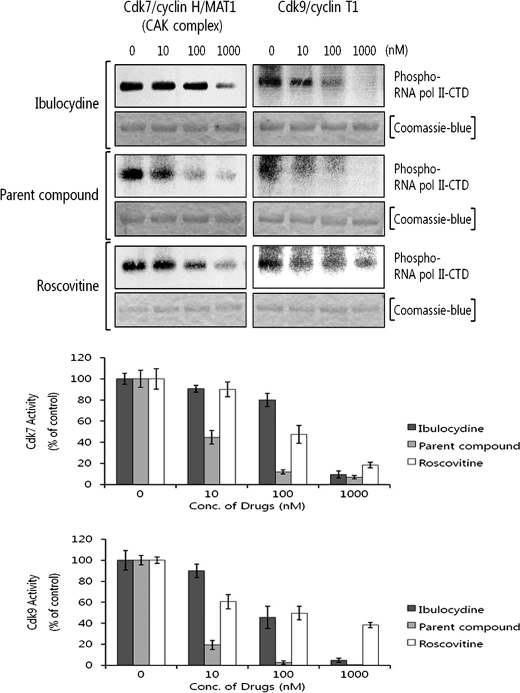
In vitro kinase assays employing specific RNA polymerase II-CTD-GST recombinant protein substrates showed that ibulocydine inhibited Cdk7 and Cdk9 at IC50 values of 530 and 85 nm, respectively (Fig. 3). The in vitro inhibitory activity of ibulocydine was much lower than that of BMK-Y101 (IC50 values were less than 10 nm for Cdk7 and Cdk9) and roscovitine (IC50 88 and 56 nm for Cdk7 and Cdk9, respectively).
FIGURE 3.
Ibulocydine inhibits Cdk7 and Cdk9 activity in vitro. Kinase assays were performed at 30 °C for 20 min in the reaction buffer containing RNA polymerase II-CTD-GST recombinant protein as a specific substrate for each kinase. Recombinant Cdk7 and Cdk9 proteins were used as the enzyme source. Radioactivity trapped in SDS-PAGE gels was measured using a phosphorimage analyzer in two independent experiments. Bar graphs show relative percentage (mean values) of inhibition of kinase activity by ibulocydine (dark gray columns), BMK-Y101 (gray columns), or roscovitine (white columns). Error bars represent mean ± S.E. of two independent experiments.
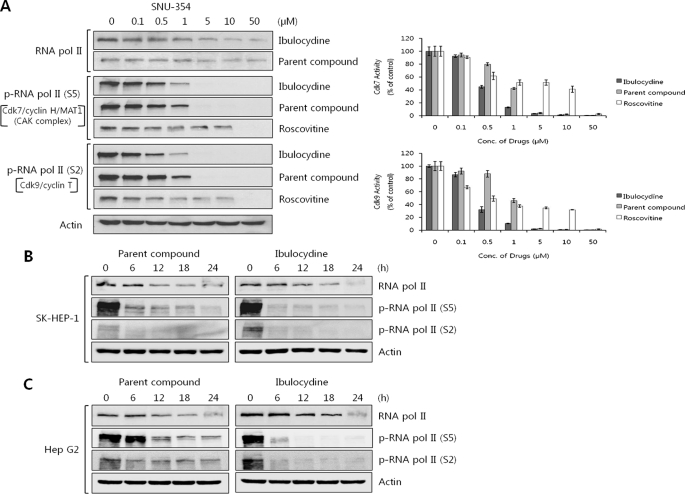
We next examined whether ibulocydine inhibited Cdk7 and Cdk9 in HCC cells by measuring cellular levels of RNA polymerase II phosphoforms at Ser-5 and Ser-2. These two serine residues are the specific phosphorylation sites for RNA polymerase II mediated by Cdk7 and Cdk9, respectively. The results indicated that ibulocydine significantly reduced the phosphorylation of RNA polymerase II at Ser-5 and Ser-2 in dose- and time-dependent manners in HCC cells. This effect was greater than that of either BMK-Y101 (Fig. 4, A–C) or roscovitine (Fig. 4A). In comparison, ibulocydine and BMK-Y101 minimally influenced the total protein levels of RNA polymerase II. In Fig. 4A, the IC50 values for ibulocydine-mediated inhibition of Cdk7 and Cdk9 were 0.45 and 0.33 μm, respectively, noticeably lower than those of BMK-Y101 (IC50 value 0.84 and 0.95 μm for Cdk7 and Cdk9, respectively) or roscovitine (IC50 value 8.5 and 1.2 μm for Cdk7 and Cdk9, respectively) in HCC SNU-354 cells. Furthermore, ibulocydine also inhibited phosphorylation of RNA polymerase II at Ser-5 and Ser-2 more effectively than BMK-Y101 in other HCC cells (SK-HEP-1 and HepG2) (Fig. 4, B and C). This higher inhibitory activity of ibulocydine compared with BMK-Y101 seen in cultured cells (but not in the assays) strongly suggests that the prodrug nature of ibulocydine is critical for effective delivery of the drug into cells.
FIGURE 4.
Ibulocydine inhibits Cdk7 and Cdk9 activity in HCC cell cultures. A, SNU-354 cells were treated with ibulocydine, BMK-Y101, or roscovitine for 24 h. B and C, SK-HEP-1 and HepG2 cells were treated with ibulocydine and BMK-Y101 for 24 h. Cell lysates were analyzed for Cdk7/9-specific phosphorylation of RNA polymerase II-CTD by Western blotting. Two independent experiments were conducted. In A, bar graphs show relative percentage (mean values) of inhibition of kinase activity by ibulocydine (dark gray columns), BMK-Y101 (gray columns), or roscovitine (white columns). Error bars represent mean ± S.E. of two independent experiments.
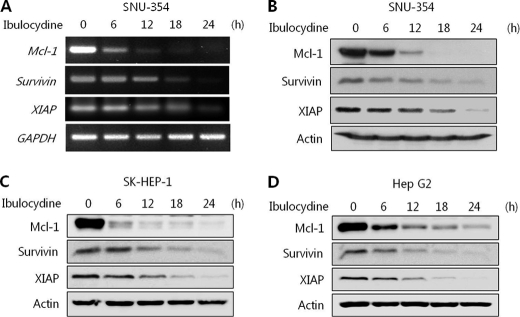
Ibulocydine Reduces Mcl-1, Survivin, and XIAP mRNA and Protein Levels in HCC Cells
Immunoblot and RT-PCR assays were carried out to assess the protein and mRNA levels of Mcl-1, survivin, and XIAP. Total protein (from SK-HEP-1, HepG2, and SNU-354) and RNA (from SNU-354) were prepared from HCC cells treated with either dimethyl sulfoxide or ibulocydine (10 μm). RT-PCR confirmed that the loss of Mcl-1, survivin, and XIAP proteins was due to a block in transcription. Ibulocydine caused a rapid reduction in Mcl-1, survivin, and XIAP mRNA and protein levels in HCC cells (Fig. 5, A–D). This suggests that the loss of Mcl-1, survivin, and XIAP proteins correlates with their transcriptional inhibition due to the blocking of RNA polymerase II phosphorylation by ibulocydine.
FIGURE 5.
Ibulocydine reduces the level of Mcl-1, survivin, and XIAP protein and mRNA in HCC cells. HCC cells were exposed to ibulocydine for the indicated times. A, RT-PCR of SNU-354 cells treated with ibulocydine. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. B–D, cells were collected and whole cell lysates were prepared and separated on SDS-PAGE gels followed by immunoblotting with the indicated antibodies. Actin was used as the loading control. Two independent experiments were conducted.
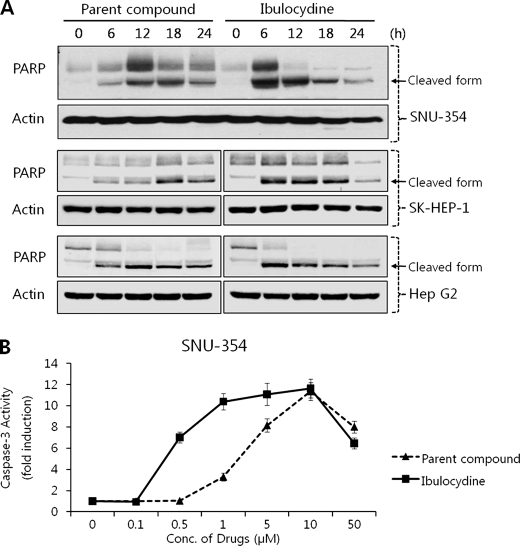
Ibulocydine Induces Apoptotic Cell Death in HCC Cells
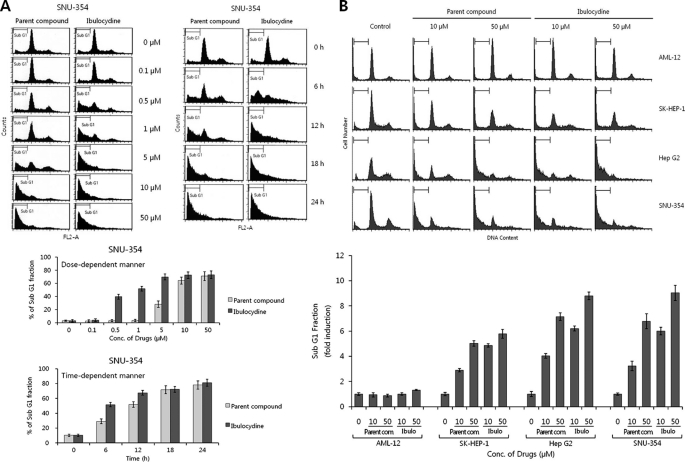
To see whether ibulocydine-mediated reduction of RNA polymerase II phosphorylation induced apoptosis in HCC cells, we measured the cleavage of PARP and caspase-3 activity in HCC cells. Ibulocydine (50 μm) strongly induced PARP cleavage after 6 h in HCC cells (Fig. 6A). Also, ibulocydine gradually induced caspase-3 activity in a dose-dependent manner in HCC SNU-354 cells (Fig. 6B). In both assays, ibulocydine was more effective than BMK-Y101. We then analyzed the cell cycle progression of HCC cells after treatment with ibulocydine or BMK-Y101 (various concentrations) for 24 h and after treatment with 50 μm of the same inhibitors in a time-dependent manner (Fig. 7A). After ibulocydine treatment the sub-G1 population (an index of apoptotic cell death) of HCC SNU-354 cells increased to a greater extent than after treatment with BMK-Y101. Moreover, we compared sub-G1 populations of normal hepatocyte and HCC cells after treatment with ibulocydine or BMK-Y101 (10 and 50 μm) for 12 h. The sub-G1 population increased in all three types of HCC cells, but not in AML-12 normal hepatocyte cells (Fig. 7B). Thus, ibulocydine induces apoptosis in HCC cells, but not in normal hepatocyte cells.
FIGURE 6.
Ibulocydine induces apoptosis in HCC cells. A, HCC cells were cultured with ibulocydine or BMK-Y101 for the indicated times. Cells were then processed for Western blotting. Native PARP was cleaved into a typical apoptotic fragment of 85 kDa in HCC cells treated with ibulocydine or BMK-Y101. Actin was used as the loading control. B, HCC SNU-354 cells were treated with ibulocydine or BMK-Y101 with the indicated doses for 24 h and caspase-3 activity was measured by determining the cleavage of the specific peptide substrate DEVD-AMC. Data represent mean ± S.E. of two independent triplicate experiments.
FIGURE 7.
Ibulocydine induces apoptotic cell death in HCC cells. A, HCC SNU-354 cells were treated with ibulocydine or BMK-Y101 with the indicated doses for 24 h and the indicated times. B, HCC (SK-HEP-1, HepG2, and SNU-354) and normal hepatocyte (AML-12) cells were treated with ibulocydine or BMK-Y101 with the indicated doses for 12 h. The DNA content (propidium iodide) and cell cycle were analyzed by flow cytometry. Apoptosis was measured according to the percentage of cells containing hypodiploid DNA (sub-G1 peak). Increased sub-G1 peaks indicate drug-induced apoptotic cell death. Two independent experiments were conducted. Error bars represent mean ± S.E. of two independent experiments.
Pharmacokinetics of Ibulocydine
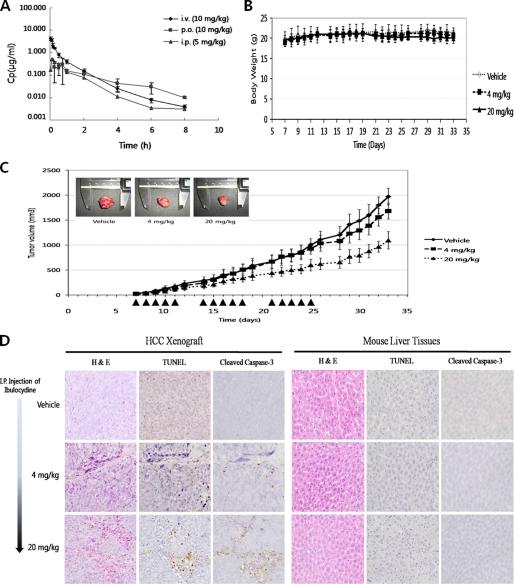
The pharmacokinetics of ibulocydine was evaluated in male Sprague-Dawley rats and the results are shown in Fig. 8A and Table 1. Ibulocydine is converted into BMK-Y101 by cytoplasmic hydrolytic enzymes. Therefore, we checked the pharmacokinetic parameters of BMK-Y101 in the plasma and urine of rats injected with ibulocydine. Following intravenous injection of ibulocydine (10 mg/kg, n = 3), the Cmax of BMK-Y101 averaged 4.3 ± 0.51 g/ml, with a t½ of 1.3 h. After oral administration of ibulocydine (10 mg/kg, n = 3), the average Cmax for BMK-Y101 was 0.36 ± 0.223 g/ml, with a t½ of 2.0 ± 0.74 h. Oral absorption exhibited a relatively slower uptake, reaching peak plasma concentrations after 66 min. Oral administration resulted in a bioavailability of 34%. When ibulocydine was injected intraperitoneally, the Cmax of BMK-Y101 averaged 0.51 ± 0.21 g/ml, with a t½ of 1 h and a bioavailability of 58% (Table 1).
FIGURE 8.
Ibulocydine inhibits the growth of HCC xenografts. The pharmacokinetic properties of ibulocydine were evaluated in male Sprague-Dawley rats (A). Oral and intraperitoneal (i.p.) administration of ibulocydine resulted in 34 and 58% bioavailability, respectively. Ibulocydine-mediated suppression of HCC cell growth was assessed in Balb/C-nu/nu mice. SNU-354 tumors were allowed to develop for 7 days. Tumor-bearing mice were randomized into three groups: two groups (n = 8) were treated with ibulocydine (single daily intraperitoneal injections at 4 or 20 mg/kg, for three 5-day series (arrowheads in C). The control group (n = 8) received intraperitoneal injections of vehicle. Tumors were observed for up to 33 days. On day 34, tumors were excised and analyzed. Body weight (B) and tumor size (C) were measured daily. Ibulocydine treatment resulted in a dose- and time-dependent reduction in tumor volume compared with controls. D, tumor and normal liver tissues (treated with ibulocydine; 4 or 20 mg/kg/day) were removed and measured. Tissue sections were stained with H&E and examined for evidence of apoptosis using in situ TUNEL assays or immunohistochemistry. Ibulocydine induced apoptosis in HCC SNU-354 cells, but not in normal liver tissues. p.o., per oral; i.v., intravenous.
TABLE 1.
Pharmacokinetic parameters of BMK-Y101 after ibulocydine administration
| Parametersa | Intravenous (10 mg/kg) | Per Oral (10 mg/kg) | Intraperitoneal (5 mg/kg) |
|---|---|---|---|
| Cmax (μg/ml) | 4.3 ± 0.51 | 0.36 ± 0.223 | 0.51 ± 0.208 |
| tmax (h) | 0.05 ± 0.029 | 1.1 ± 0.80 | 0.14 ± 0.048 |
| tλ1/2 (h) | 1.3 ± 0.31 | 2.0 ± 0.74 | 1.0 ± 0.05 |
| AUC0–8h (μg h/ml) | 1.76 ± 0.29 | 0.59 ± 0.064 | 0.51 ± 0.169 |
| AUC0-∞ (μg h/ml) | 1.77 ± 0.29 | 0.62 ± 0.074 | 0.51 ± 0.171 |
| CL (liter/kg/h) | |||
| Vss (liter/kg) | |||
| MRT (h) | 0.8 ± 0.04 | 2.2 ± 0.51 | 1.1 ± 0.10 |
| Ae in urine8h (% of dose) | 2.8 ± 0.73 | 0.67 ± 0.205 | 1.9 ± 0.787 |
| A in Gl8h (% of dose) | 4.9 ± 6.2 | 1.1 ± 0.62 | 4.5 ± 1.57 |
| F (%) | 33.9 | 57.7 |
a Cmax, maximum plasma concentration; tmax, time for Cmax; tλ1/2, terminal half-life; AUC0–8h, area under the plasma concentration-time curve up to 8 hours; AUC0–∞, area under the plasma concentration-time curve from time 0 to infinity; CL, total clearance from plasma; Vss, steady-state volume of distribution; MRT, mean residence time; Ae in urine8h, total amount of drug excreted in urine from time 0 to 8 h; A in Gl8h, total amount of drug recovered from entire gastrointestinal tract from time 0 to 8 h; F, bioavailability (the ratio of AUC values between per oral and intravenous).
Ibulocydine Inhibits Growth of the HCC Xenografts in Balb/C Nude Mice
We subsequently investigated the effect of ibulocydine on tumor growth in vivo using nude mice harboring SNU-354 xenografts. Animals bearing small tumors were injected intraperitoneally with ibulocydine (4 or 20 mg/kg/day five times/week for 3 weeks). Animals treated with ibulocydine or vehicle solution alone showed no apparent change in body weight (Fig. 8B). However, tumor growth was significantly inhibited in ibulocydine-injected mice in a time- and dose-dependent manner compared with controls (Fig. 8C). Injection of 20 or 4 mg/kg ibulocydine (over 33 days) suppressed the growth of SNU-354 xenografts by 47 and 15%, respectively. To assess whether this ibulocydine-induced tumor suppression resulted from apoptosis of the grafted cells, we conducted a TUNEL assay using excised tumor tissues in parallel with immunohistochemical analyses of cleaved caspase-3. The results showed that injection of ibulocydine induced apoptosis in the xenografted HCC cells in a dose-dependent manner (Fig. 8D). In contrast, minimal apoptosis was detected in HCC tumors excised from controls. Furthermore, ibulocydine did not induce apoptosis in normal liver tissues (Fig. 8D). To assess whether administration of ibulocydine induced apoptosis in other normal tissues, we injected ibulocydine intraperitoneally daily into the control non-tumor-bearing ICR mice with a dose of 200 mg/kg/day for 15 days. There were no apparent signs of apoptosis in the liver or other tissues. Body weight and the weights of the liver and spleen were also unaffected (data not shown). Thus, ibulocydine is a good candidate anti-cancer drug for the treatment of hepatocellular carcinoma.
DISCUSSION
This study shows that ibulocydine, a novel prodrug inhibitor of Cdk7/9, effectively inhibits the growth of HCC. Ibulocydine is converted to its parent compound (BMK-Y101) by cytoplasmic hydrolytic enzymes, such as lipases (33). The polar nature of BMK-Y101 (ClogP = −2.40) is expected to reduce cell penetration, leading to ineffective cell growth inhibition. Therefore, we designed ibulocydine, a butyrated analog of BMK-Y101, to improve its anticancer activity against HCC cells. We found that the less polar ibulocydine (ClogP = −1.32) suppressed HCC cell growth more effectively than BMK-Y101 and other Cdk inhibitors, such as olomoucine and roscovitine, whereas ibulocydine as well as the other Cdk inhibitors minimally influenced the growth of normal hepatocyte cells (Fig. 2).
We demonstrated that ibulocydine-mediated dose-dependent growth inhibition of HCC cells was evident between 0.5 and 50 μm, whereas olomoucine and roscovitine inhibited cell growth only at doses >10 μm. Furthermore, HCC cell growth inhibition by ibulocydine was more effective than that of BMK-Y101. Earlier studies suggested that Cdk7 and Cdk9 play important roles in the initiation and elongation of transcription, respectively, via direct phosphorylation of the CTD of RNA polymerase II (19–21). Cdk7 and Cdk9 phosphorylate Ser-5 and Ser-2, respectively. Phosphorylation of the CTD of RNA polymerase II at these sites is critical not only to transcription, but also for mRNA capping, splicing, cleavage, and polyadenylation (34). Interestingly, our study showed that ibulocydine strongly inhibited Cdk7 and Cdk9 activity in HCC cell cultures (Fig. 4), although it was less effective than either BMK-Y101 or roscovitine in in vitro assays. However, ibulocydine induced apoptosis at considerably lower concentrations, and more quickly, than BMK-Y101 in HCC cells (Figs. 6 and 7). This discrepancy suggests that ibulocydine crosses the cell membrane more efficiently than BMK-Y101. The apoptotic effects of ibulocydine in HCC cells appear to relate to changes in the cellular levels of anti-apoptotic proteins. Based upon our results, changes in the cellular levels of anti-apoptotic proteins in HCC cells after treatment with ibulocydine appear to be gradual. Fig. 5 shows that both cellular protein and mRNA levels of Mcl-1, XIAP, and survivin are down-regulated by ibulocydine in a time-dependent manner in HCC cells. It has been suggested that prolonged inhibition of Cdk7 and Cdk9 within cells induces apoptosis (35). The prolonged inhibition of Cdk7 and Cdk9 eventually affected all transcripts produced by RNA polymerase II, and subsequently their proteins. This has an immediate effect upon transcripts and proteins with inherently rapid turnover rates (35), such as Mcl-1, XIAP, survivin, etc. Thus, studies have speculated that prolonged Cdk7/9-mediated inhibition of transcription decreases Mcl-1, XIAP, and survivin expression, releasing their ability to block primed cells from undergoing apoptosis (36). Therefore, our results support earlier studies suggesting that prolonged inhibition of Cdk7/9 leads to induction of apoptosis by down-regulating the cellular levels of anti-apoptotic proteins such as Mcl-1 and XIAP (36). The effects of ibulocydine on Cdk7 and Cdk9 suggest that one potential mechanism for ibulocydine-induced cell death may operate by inhibiting transcription. Moreover, our results show good agreement with a previous report indicating that roscovitine down-regulates IAP family proteins such as survivin and XIAP in other human tumor types (37). Taken together, our results suggest that ibulocydine induces apoptosis via the prolonged inhibition of Cdk7/9 activity.
We were also curious as to whether ibulocydine affected the activity of other Cdk families. This prompted us to assess ibulocydine-mediated inhibition of Cdk1 and Cdk2 in in vitro assays and HCC cell cultures (SNU-354). Our supplementary “Methods” indicate that ibulocydine inhibited the Cdk1- or Cdk2-mediated phosphorylation of histone H1 in vitro, with an IC50 value of ∼50 nm. Results from kinase assays using recombinant protein as an enzyme source indicate that the enzymatic activity of Cdk1 and Cdk2 was reduced by ibulocydine in a dose-dependent manner (supplemental Fig. 1A). Because it was not possible to directly determine the inhibitory activity within the cells using recombinant protein kinase assays, we measured the cellular levels of the Rb and nucleolin phosphoforms as an index of intracellular Cdk2 and Cdk1 kinase activity, respectively (38, 39), after treatment with ibulocydine. The results showed that 50 μm ibulocydine inhibited >50% of the nucleolin and Rb phosphorylation in HCC SNU-354 cells within 12 and 6 h, respectively (supplemental Fig. 1B). This indicated that ibulocydine strongly inhibited Cdk1 and Cdk2 activity in vitro and in HCC SNU-354 cell cultures. Moreover, inhibition of Cdk1 and Cdk2 by ibulocydine was accompanied by a significant inhibition of HCC SUN-354 cell growth. However, it is not clear whether ibulocydine inhibits other Cdk families (e.g. Cdk3–6).
Observing the strong inhibition by ibulocydine of HCC cell growth, we were curious if ibulocydine could affect the growth and death of normal cells. In Figs. 2A and 7B, our data indicate that ibulocydine and BMK-Y101 minimally influenced the cell growth and cell death of normal hepatocyte cells.
Preliminary pharmacokinetic studies indicated that ibulocydine was rapidly converted to the parent compound in the plasma after intravenous or intraperitoneal injection. The pharmacokinetic profile after oral administration indicated that BMK-Y101 was present in plasma even after 8 h, suggesting that ibulocydine has potential as an oral drug, although its bioavailability needs to be improved (34%).
We assessed whether ibulocydine preferentially induced apoptosis in HCC xenografts. Injection of ibulocydine, five times/week intraperitoneally at doses of 20 or 4 mg/kg/day for 3 weeks (total dose of 300 or 60 mg/kg), significantly suppressed tumor growth by 47 and 15%, respectively, in nude mice bearing SNU-354 tumors. These results indicate that ibulocydine also inhibits HCC cell growth in vivo.
We next examined whether ibulocydine (injected intraperitoneally at 20 or 4 mg/kg/day for 3 weeks) was cytotoxic to normal liver tissues using H&E stains, TUNEL assays, and immunohistochemical analysis of cleaved caspase-3. Ibulocydine did not induce apoptosis in normal liver tissues at either of the concentrations tested. Moreover, additional toxicity tests on tissues from ICR mice injected intraperitoneally with ibulocydine (200 mg/kg/day for 15 days) indicated that the prodrug has no toxic effects on the liver, kidney, colon, stomach, and spleen, and did not cause weight loss (data not shown). These results indicate that ibulocydine selectively induces apoptosis in human HCC xenografts, but has no cytotoxic effects on normal tissues. Taken together, the results of this study suggest that ibulocydine is a strong candidate for use as an anti-cancer drug for the treatment of hepatocellular carcinoma.
Supplementary Material
This work was supported by Fund for Functional Analysis of the Human Genome Grant M108KB010025-08K0201-02510, managed by The Center for Functional Analysis of Human Genome, from the 21st Century Frontier Research and Development program of the National Research and Development programs of the Korea Science and Engineering Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods” and Fig. S1.
- HCC
- hepatocellular carcinoma
- Cdk
- cyclin-dependent kinase
- CTD
- carboxyl-terminal domain
- IAP
- inhibitor of apoptosis protein
- PARP
- poly(ADP-ribose) polymerase
- AUC
- area under curve
- XIAP
- X-linked IAP.
REFERENCES
- 1. Parkin D. M., Bray F., Ferlay J., Pisani P. (2005) CA Cancer J. Clin. 55, 74–108 [DOI] [PubMed] [Google Scholar]
- 2. Bolondi L., Sofia S., Siringo S., Gaiani S., Casali A., Zironi G., Piscaglia F., Gramantieri L., Zanetti M., Sherman M. (2001) Gut 48, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El-Serag H. B., Rudolph K. L. (2007) Gastroenterology 132, 2557–2576 [DOI] [PubMed] [Google Scholar]
- 4. El-Serag H. B., Davila J. A., Petersen N. J., McGlynn K. A. (2003) Ann. Intern. Med. 139, 817–823 [DOI] [PubMed] [Google Scholar]
- 5. Mendizabal M., Reddy K. R. (2009) Med. Clin. N. Am. 93, 885–900, viii [DOI] [PubMed] [Google Scholar]
- 6. Huang C. C., Wu M. C., Xu G. W., Li D. Z., Cheng H., Tu Z. X., Jiang H. Q., Gu J. R. (1992) J. Natl. Cancer Inst. 84, 262–264 [DOI] [PubMed] [Google Scholar]
- 7. Senderowicz A. M. (2003) Oncogene 22, 6609–6620 [DOI] [PubMed] [Google Scholar]
- 8. Swanton C. (2004) Lancet Oncol. 5, 27–36 [DOI] [PubMed] [Google Scholar]
- 9. Senderowicz A. M. (2004) Curr. Opin. Cell. Biol. 16, 670–678 [DOI] [PubMed] [Google Scholar]
- 10. Ito Y., Takeda T., Sakon M., Monden M., Tsujimoto M., Matsuura N. (2000) Oncology 59, 68–74 [DOI] [PubMed] [Google Scholar]
- 11. Malumbres M., Pevarello P., Barbacid M., Bischoff J. R. (2008) Trends Pharmacol. Sci. 29, 16–21 [DOI] [PubMed] [Google Scholar]
- 12. Nurse P. (2000) Cell 100, 71–78 [DOI] [PubMed] [Google Scholar]
- 13. Nurse P. M. (2002) Biosci. Rep. 22, 487–499 [DOI] [PubMed] [Google Scholar]
- 14. Kimura K., Hirano M., Kobayashi R., Hirano T. (1998) Science 282, 487–490 [DOI] [PubMed] [Google Scholar]
- 15. Bregman D. B., Pestell R. G., Kidd V. J. (2000) Front. Biosci. 5, D244–257 [DOI] [PubMed] [Google Scholar]
- 16. Yankulov K. Y., Bentley D. L. (1997) EMBO J. 16, 1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chao S. H., Fujinaga K., Marion J. E., Taube R., Sausville E. A., Senderowicz A. M., Peterlin B. M., Price D. H. (2000) J. Biol. Chem. 275, 28345–28348 [DOI] [PubMed] [Google Scholar]
- 18. Peterlin B. M., Price D. H. (2006) Mol. Cell. 23, 297–305 [DOI] [PubMed] [Google Scholar]
- 19. Oelgeschläger T. (2002) J. Cell. Physiol. 190, 160–169 [DOI] [PubMed] [Google Scholar]
- 20. MacCallum D. E., Melville J., Frame S., Watt K., Anderson S., Gianella-Borradori A., Lane D. P., Green S. R. (2005) Cancer Res. 65, 5399–5407 [DOI] [PubMed] [Google Scholar]
- 21. Cho S. J., Lee S. S., Kim Y. J., Park B. D., Choi J. S., Liu L., Ham Y. M., Kim B., Lee S. K. (2010) Cancer Lett. 287, 196–206 [DOI] [PubMed] [Google Scholar]
- 22. Coultas L., Strasser A. (2003) Semin. Cancer Biol. 13, 115–123 [DOI] [PubMed] [Google Scholar]
- 23. Yang T., Buchan H. L., Townsend K. J., Craig R. W. (1996) J. Cell. Physiol. 166, 523–536 [DOI] [PubMed] [Google Scholar]
- 24. Zhang B., Gojo I., Fenton R. G. (2002) Blood 99, 1885–1893 [DOI] [PubMed] [Google Scholar]
- 25. Derenne S., Monia B., Dean N. M., Taylor J. K., Rapp M. J., Harousseau J. L., Bataille R., Amiot M. (2002) Blood 100, 194–199 [DOI] [PubMed] [Google Scholar]
- 26. Gojo I., Zhang B., Fenton R. G. (2002) Clin. Cancer Res. 8, 3527–3538 [PubMed] [Google Scholar]
- 27. Michels J., O'Neill J. W., Dallman C. L., Mouzakiti A., Habens F., Brimmell M., Zhang K. Y., Craig R. W., Marcusson E. G., Johnson P. W., Packham G. (2004) Oncogene 23, 4818–4827 [DOI] [PubMed] [Google Scholar]
- 28. Gomez-Bougie P., Bataille R., Amiot M. (2004) Eur. J. Immunol. 34, 3156–3164 [DOI] [PubMed] [Google Scholar]
- 29. Salvesen G. S., Duckett C. S. (2002) Nat. Rev. Mol. Cell Biol. 3, 401–410 [DOI] [PubMed] [Google Scholar]
- 30. Silverman R. B. (2004) The Organic Chemistry of Drug Design and Drug Action, 2nd Ed., pp. 497–558, Elsevier Academic Press, New York [Google Scholar]
- 31. Wu K. M. (2009) Pharmaceuticals 2, 77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sinhababu A. K., Thakker D. R. (1996) Adv. Drug. Del. Rev. 19, 241–273 [Google Scholar]
- 33. Svendsen A. (2000) Biochim. Biophys. Acta 1543, 223–238 [DOI] [PubMed] [Google Scholar]
- 34. Hirose Y., Manley J. L. (2000) Genes Dev. 14, 1415–1429 [PubMed] [Google Scholar]
- 35. Lam L. T., Pickeral O. K., Peng A. C., Rosenwald A., Hurt E. M., Giltnane J. M., Averett L. M., Zhao H., Davis R. E., Sathyamoorthy M., Wahl L. M., Harris E. D., Mikovits J. A., Monks A. P., Hollingshead M. G., Sausville E. A., Staudt L. M. (2001) Genome Biol. 2, RESEARCH0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen R., Wierda W. G., Chubb S., Hawtin R. E., Fox J. A., Keating M. J., Gandhi V., Plunkett W. (2009) Blood 113, 4637–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hahntow I. N., Schneller F., Oelsner M., Weick K., Ringshausen I., Fend F., Peschel C., Decker T. (2004) Leukemia 18, 747–755 [DOI] [PubMed] [Google Scholar]
- 38. Knudsen E. S., Wang J. Y. (1996) J. Biol. Chem. 271, 8313–8320 [DOI] [PubMed] [Google Scholar]
- 39. Zarkowska T., Mittnacht S. (1997) J. Biol. Chem. 272, 12738-12746 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.