Abstract
During implantation, the uterine stromal cells undergo terminal differentiation into decidual cells, which support the proper progression of maternal-embryo interactions to successful establishment of pregnancy. The decidual cells synthesize extracellular matrix (ECM) components, such as laminins and collagens, which assemble into a unique basal lamina-like network that surrounds these cells. The functional significance of this matrix during implantation is unknown. We previously showed that the transcription factor CCAAT enhancer-binding protein β (C/EBPβ) critically regulates decidualization in the mouse. We now provide evidence that C/EBPβ directly controls the Lamc1 gene, which encodes a predominant laminin constituent of the ECM produced by the decidual cells. Suppression of Lamc1 expression in mouse primary endometrial stromal cells prevented the assembly of this ECM and impaired stromal differentiation. Attenuation of expression of integrin β1, a major constituent of the integrin receptors targeted by decidual laminins, also inhibited this differentiation process. Disruption of laminin-integrin interactions led to impaired activation of the focal adhesion kinase, an integrin-mediated regulator of cytoskeletal remodeling during decidualization. To further analyze the role of the decidual ECM in modulating maternal-embryo interactions, we monitored trophoblast invasion into differentiating uterine stromal monolayers, using a co-culture system. Silencing of stromal Lamc1 expression, which prevented formation of the basal lamina-like matrix, resulted in marked reduction in trophoblast outgrowth. Collectively, our findings identified C/EBPβ as a critical regulator of the unique ECM that controls decidual cell architecture and differentiation, and it provided new insights into the mechanisms by which the uterine stromal microenvironment controls the progression of embryo implantation.
Keywords: C/EBP Transcription Factor, Cell Differentiation, Extracellular Matrix, Reproduction, Steroid Hormone Receptor
Introduction
Implantation of the embryo into the uterus involves a series of complex and reciprocal interactions between the maternal and embryonic tissues. The steroid hormones estrogen (E)2 and progesterone (P) coordinate the events surrounding implantation by controlling the proliferation and differentiation of uterine cells in a dynamic manner (1). The attachment of the embryo to the uterine epithelium triggers the hormone-driven transformation of the underlying fibroblast-like stromal cells into secretory decidual cells, a process known as decidualization (1–4). Terminally differentiated decidual cells undergo extensive remodeling to form a highly specialized tissue, which surrounds the implanted embryo and modulates its survival and growth during the initial stages of pregnancy. It is well documented that decidual cells synthesize diverse protein factors and hormones that signal to the growing embryo in a paracrine or juxtacrine manner (5–7). As the embryo invasion proceeds, a variety of extracellular matrix (ECM) proteins is deposited in the pericellular space by the differentiating stromal cells and assembled into a matrix network surrounding these cells (8–10). Prominent among these molecules are laminins, collagen type IV and desmin, which are components of a prototypical basement membrane or basal lamina. Although the basal lamina exists in the epithelial-mesenchymal boundary of a variety of tissues, the decidual tissue is indeed a unique setting for this matrix. The precise function of the decidual basal lamina during implantation is unknown.
The basal lamina is an intricate and dynamic extracellular structure that controls cellular functions by regulating the interactions between a cell and its microenvironment (11). Laminins, a large family of glycoproteins, are vital components of basal lamina in many different mammalian tissues and organs. The integrity of the multimolecular structure of basal lamina depends on the self-polymerization of laminin α, β, and γ subunits and the subsequent interactions of the laminin heterotrimers with other ECM molecules such as collagen type IV, nidogen, and fibronectin (11–14). Multiple distinct isoforms exist for laminin α, β, and γ subunits. At least 15 different laminin heterotrimers are derived from various combinations of single α, β, and γ subunits (11, 13, 15, 16). Laminin γ1, encoded by the Lamc1 gene, is shared among 10 of these combinations, making it the most critical subunit for laminin assembly. In mice, the Lamc1 null mutation is peri-implantation lethal because of a failure in the formation of the embryonic basement membrane (17). Previous studies reported abundant expression of laminins, collagen type IV, and fibronectin in stromal compartment of pregnant uteri (9, 10). It was also observed that uterine stromal cells cultured in vitro produced a basal lamina-like matrix (18, 19). These observations led to the speculation that the stromal ECM microenvironment functionally contributes to the differentiation of uterine stromal cells. Another possibility is that the uterine basal lamina, by interfacing with embryonic tissues, provides the guidance cues that an embryo needs for correct implantation (5, 10, 20, 21).
Our laboratory previously reported that the steroid hormone-regulated transcription factor CCAAT enhancer-binding protein β (C/EBPβ) is a critical regulator of uterine functions (22–24). Female mice lacking C/EBPβ are infertile and exhibit impaired decidualization. Our studies further revealed that C/EBPβ-null uterine stromal cells are unable to undergo proper proliferation and differentiation in response to a decidual stimulation (22, 25). Using a DNA microarray approach, we analyzed uterine stromal cells isolated from wild-type (WT) and C/EBPβ-null mice to identify candidate downstream targets of C/EBPβ during the decidualization process. Prominent among these targets were the Lama1 and Lamc1 genes encoding the laminin subunits α1 and γ1, respectively, which are well known components of the stromal ECM generated during decidualization. These findings suggested that C/EBPβ-regulated pathways play an important role in the formation of the basal lamina-like ECM during decidualization.
We therefore investigated how C/EBPβ regulates specific laminin components of the basal lamina-like ECM synthesized by mouse endometrial stromal cells (MESC). For this purpose, we utilized a primary culture system wherein MESCs express high amounts of basal lamina-like ECM as they undergo differentiation into decidual cells (26). We also evaluated the role of laminins in facilitating cross-talk between endometrial cells and the embryo. To study these interactions, we designed a co-culture system in which pre-implantation mouse blastocysts are allowed to attach to and invade a monolayer of differentiating MESC. These model systems offer the opportunity to conduct powerful in vitro functional analyses. By modulating the expression of C/EBPβ, we discovered that it directs formation of uterine stromal basal lamina-like ECM by controlling laminin γ1 expression. Furthermore, we directly tested and showed that ECM containing laminin γ1 is critical for proper progression of stromal differentiation and cytoskeletal remodeling, which in turn control embryo invasion into the stromal compartment.
EXPERIMENTAL PROCEDURES
Materials
Recombinant mouse laminin-111 was purchased from Trevigen® (Gaithersburg, MD). Laminin- and MatrigelTM-coated plates (BD-BioCoatTM Cellware) were purchased from BD Biosciences. Estrogen (17β-estradiol) and progesterone were purchased from Sigma.
Animal Experiments and Tissue Collection
All experiments involving animals were conducted in accordance with the National Institutes of Health standards for the use and care of animals. The animal protocols were approved by the University of Illinois Institutional Animal Care and Use Committee. Female mice (CD-1) were sacrificed on day 4 of gestation, and uteri were collected for isolation of MESC. Artificial decidualization was induced in WT and C/EBPβ-null mice as described previously (27) for 24 h, and uteri were collected for isolation of MESC.
Isolation of MESC and in Vitro Decidualization
Isolation of MESC was carried out as described previously (26). Cells (2.5 × 105) were seeded in 6-well cell culture plates. Alternatively, ∼4 × 104 cells were seeded in 2- or 4-well slide chamber dishes. Cells were allowed to undergo decidualization in the presence of 10 nm E and 1 μm P.
Co-culture of Blastocysts and MESC
Female mice (CD-1) were sacrificed on day 4 of gestation. Blastocysts were collected by flushing uterine horns and oviducts with sterile PBS. Blastocysts were cultured for ∼24 h in 30-μl microdrops of M16 embryo culture medium (Sigma) under mineral oil, at 37 °C in 5% CO2. MESC or mouse embryonic fibroblasts (MEF) were plated into 2-well slide chamber dishes and grown in fresh medium (DMEM/F12A; with 100 units/liter penicillin, 0.1 g/liter streptomycin, 1.25 mg/liter fungizone, 2% heat-inactivated fetal bovine serum) supplemented with E and P as described above. After 24 h, unhatched blastocysts were carefully transferred onto confluent layers of MESCs or MEFs in culture dishes. Co-cultures were continued for up to 96 h, and outgrowth was photographed with an Olympus CKX41 inverted microscope. The total area of embryo outgrowth and spreading was determined from cytokeratin-stained samples using ImageJ software analysis (28). The final value for each condition was derived from the average of 3–5 independent embryos.
Microarray Analysis
WT and C/EBPβ-null mice (n = 5 in each group) were subjected to decidual stimulation, and after 24 h, MESC were isolated from the uteri of these mice, and total RNA was prepared from these cells. Total RNA from WT and mutant MESC was hybridized to Affymetrix Mouse Genome Array 430A 2.0. The resulting signals were analyzed as per standard procedures described previously (29).
Adenoviral Transductions
An adenoviral vector, expressing the dominant negative A-C/EBP mutant and under the control of the CMV promoter, was a gift of Dr. Charles Vinson (30). This recombinant virus is termed Ad-DN in this paper. Control adenoviral vectors, Ad-CTRL, lacking an A-C/EBP insert were also obtained from this source. Concentrations of viral titers were determined in infective units/ml using the Adeno-XTM rapid titer kit (Clontech). MESC were infected with equal amounts of control or A-C/EBP adenoviral viruses at a multiplicity of infection (m.o.i.) ranging from 2 to 60 infective units/cell. After 24 h of infection, cells were induced to differentiate in virus-free medium containing E and P as defined above.
siRNA Transfections
siRNAs corresponding to mouse Lamc1 (AAAGGTGTTCAGGCGATTG), Itgb1 (TGCTTGTATACATTCTCCG), and scrambled siRNAs (negative control) were pre-designed and synthesized by Ambion Inc. Annealed siRNA duplexes were transfected in the MESCs following the manufacturer's protocol for SilentFectTM lipid reagent (Bio-Rad). Briefly, in 6-well plates, 4 μl of SilentFect transfection reagent was mixed with 20–40 nm of siRNA duplexes in normal differentiation medium (containing E and P) to form complexes and dispersed. In 2- or 4-well slide chambers, 2 μl of SilentFect transfection reagent was mixed with 20–40 nm of siRNA duplexes and dispersed. The transfection was repeated every 24 h for duration of culture.
Real Time PCR Analysis
Total RNA was isolated from MESCs by standard TRIzol-based protocols, converted to cDNA, and subjected to real time PCR analysis as described previously (29).
Immunocytochemistry
MESC and blastocysts were fixed (10% formalin) and blocked with a 5% solution of normal donkey serum (Jackson ImmunoResearch) in PBS. Sections were incubated with the following primary antibodies diluted in blocking solution (5% NDS, sterile PBS) overnight at 4 °C: laminin γ1 (Chemicon and Abcam); laminin α1 (Chemicon); C/EBPβ and ERα (Santa Cruz Biotechnology); PRP (Chemicon); integrin β1 (BD Biosciences); integrin α6 (Abcam); phospho-Tyr397 FAK (Cell Signaling Technology); and pan-cytokeratin (Sigma). Samples were washed and incubated with biotin-, Cy3-, and DyLightTM488-conjugated secondary antibodies (Jackson ImmunoResearch) for 60 min. For PRP immunostaining, MESC were incubated with the avidin-biotin complex (Vectastain kit; Vector Laboratories Inc.) for 30 min and subsequently stained with diaminobenzidine solution (Sigma) and counterstained with 3% methyl green (Sigma). For immunofluorescence, counterstaining was done using 4′,6′-diamidino-2-phenylindole (DAPI; Invitrogen). Negative controls included incubation with donkey serum and omission of the primary antibody for all samples.
Western Blotting
Whole cell lysates prepared from MESC were subjected to Western blotting as described previously (24) using primary antibodies directed against laminin γ1 (Abcam), phospho-Tyr397 FAK, and total FAK (Cell Signaling Technology), and calnexin (Santa Cruz Biotechnology).
Chromatin Immunoprecipitation (ChIP)
MESC were cultured for 24 h in medium supplemented with E and P, and the ChIP analysis was conducted using the EZ-ChIP kit (Upstate Biotechnology) as described previously (24). The DNA-protein complexes were immunoprecipitated using antibodies against RNA polymerase II, rabbit IgG, or C/EBPβ (Santa Cruz Biotechnology).
In Silico Promoter Analysis
Putative C/EBPβ-binding sites in the Lamc1 promoter region were determined by in silico analysis of the proximal promoter region (−1 to −1900 bp) using Consite (31) and TESS (32) software. Primers were designed to amplify specific regions containing putative C/EBPβ-binding sites as follows: −15 to −23 bp (5′-CTGTCATTTAACCGGGCAAG-3′ and 5′-AGGTCCGAAGAGGAGGATGT-3′); −94 to −105 bp (5′-GGATGTTCAAAGCGAGATGA-3′ and 5′-ACCTGGGTAAGCGATGACAG-3′); −499 to −501 bp (5′-GTAGCCACCACGGTCACATT-3′ and 5′-AGGATCGGCCTCGGGATAC-3′); and −1533 to −1544 bp (5′-CTGTCATTTAACCGGGCAAG-3′ and 5′-AGGTCCGAAGAGGAGGATGT-3′).
Statistical Analysis
All experiments were performed at least 2–3 times in independent trials as indicated in the figure legends. Real time PCRs for each gene and sample were performed in 3–4 replicates. Statistical significance was assessed by analysis of variance at a significance level of p < 0.05 and is indicated by an asterisk in the figures. NS indicates nonsignificant changes (p > 0.05).
RESULTS
Expression of Laminin Subunits during Decidualization Is Regulated by C/EBPβ in Vivo
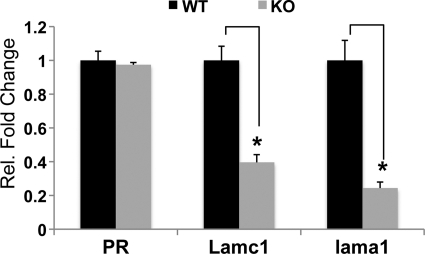
To identify C/EBPβ-regulated pathways controlling stromal differentiation and remodeling during decidualization, we used DNA microarray analysis to compare the uterine mRNA expression profiles of MESC isolated from WT and C/EBPβ-null uteri at 24 h following initiation of decidualization as described under “Experimental Procedures.” We identified several hundred genes whose expression was altered significantly in C/EBPβ-null uteri compared with WT uteri.3 Prominent among the down-regulated genes, which are potential targets of positive regulation by C/EBPβ, were Lamc1 and Lama1, encoding laminin subunits γ1 and α1, respectively. We validated these findings by assessing the Lamc1 and Lama1 mRNA levels in WT and C/EBPβ-null MESC using real time PCR analysis. As shown in Fig. 1, the expression of both Lamc1 and Lama1 mRNAs was markedly reduced in C/EBPβ-null MESC relative to WT cells, whereas the levels of progesterone receptor (PR) mRNA did not alter significantly. These results revealed that C/EBPβ is a regulator of the constituents of the stromal basal lamina-like ECM during decidualization.
FIGURE 1.
C/EBPβ regulates Lamc1 and Lama1 expression during decidualization. WT and C/EBPβ-null mice were subjected to artificial decidualization as described previously (26). Uteri were collected 24 h after decidual stimulation, and MESC were isolated as described under “Experimental Procedures.” Total RNA was analyzed by real time PCR using specific primers for Lamc1, Lama1, and PR. The fold changes indicate gene expression levels in C/EBPβ-null cells (KO) relative to WT cells. All experiments were conducted in three independent trials. Statistically significant differences (p < 0.05) are indicated by *.
Analysis of C/EBPβ-regulated Pathways in an in Vitro Decidualization System
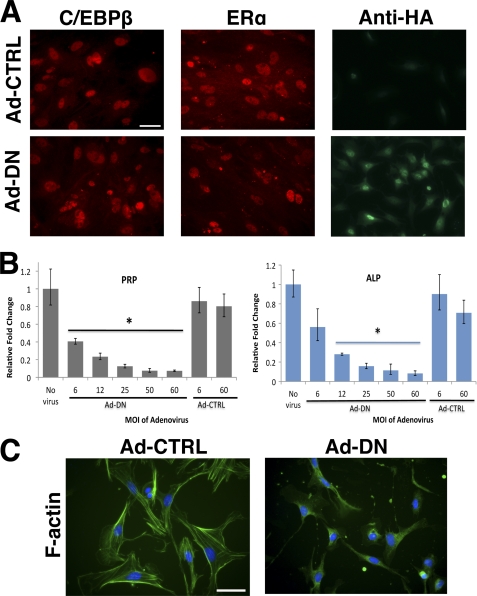
To analyze the mechanisms involved in the C/EBPβ-driven assembly of the stromal ECM during decidualization, we utilized a primary culture system in which undifferentiated MESC undergo proliferation and differentiation in a steroid hormone-dependent manner. The MESC expressed C/EBPβ protein during decidualization (Fig. 2A). To directly examine whether C/EBPβ plays a critical role in this in vitro differentiation, we employed a dominant negative mutant C/EBP (30). This mutant is able to heterodimerize with the endogenously expressed C/EBPβ, preventing its interaction with the target genes and thereby blocking its transcriptional activity. When MESC were transduced with an adenovirus expressing dominant negative C/EBP (Ad-DN) or a control adenovirus (Ad-CTRL), the levels of endogenous C/EBPβ and estrogen receptor (ERα) proteins remained unchanged in the transduced cells (Fig. 2A). However, Ad-DN efficiently suppressed the differentiation of MESC (Fig. 2B). This was evidenced by the decrease in mRNA levels of two well characterized biochemical markers of decidualization, PRP, and alkaline phosphatase (ALP) in an Ad-DN dose-dependent manner (Fig. 2B). No significant effect on the expression of PRP and ALP was observed when the cells were transduced with Ad-CTRL.
FIGURE 2.
C/EBPβ regulates uterine stromal differentiation in vitro. MESC isolated from day 4 pregnant uteri were transduced with adenovirus AD-CTRL or Ad-DN for 24 h. After removal of free adenovirus, MESC were treated with E and P for 72 h. A, adenovirus-treated MESC (m.o.i. = 1:50) was analyzed by immunofluorescence, using antibodies recognizing C/EBPβ and ERα. C/EBPβ levels are unaltered by introduction of the dominant negative mutant. Bars, 100 μm. B, relative expression levels of PRP and ALP mRNAs in Ad-DN-treated MESC compared with Ad-CTRL-treated cells. The m.o.i. is as indicated. Statistically significant differences (p < 0.05) are indicated by *. C, adenovirus-treated MESC (m.o.i. = 1:50) was analyzed by immunofluorescence using Alexa Fluor 488-conjugated phalloidin to label actin filaments (F-actin). All experiments were conducted in three independent trials. Bars, 100 μm.
Because the differentiation of MESC is also characterized by phenotypic changes in cellular morphology, we examined whether the loss of C/EBPβ function affects the organization of the actin cytoskeleton. During decidualization, the actin cytoskeleton organizes into short to intermediate filaments that lie parallel to the long axis of the stromal cell (19). The cells transduced with Ad-CTRL showed the expected cytoskeletal rearrangement and appeared flattened and cuboid-shaped, characteristic features of decidualized cells (Fig. 2C, left panel). In contrast, Ad-DN-treated MESC retained an undifferentiated fibroblastic phenotype and displayed a poorly formed, diffuse network of actin cytoskeleton (Fig. 2C, right panel). Collectively, these results demonstrated that C/EBPβ-regulated pathways critically regulate biochemical as well as morphological processes associated with in vitro decidualization.
C/EBPβ Directly Regulates Lamc1 Gene Expression during in Vitro Decidualization
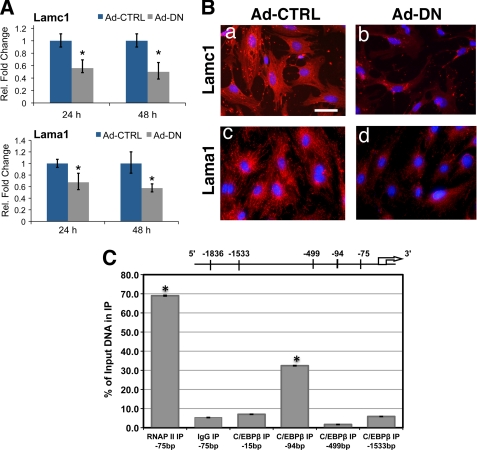
To examine whether the regulation of in vivo expression of Lamc1 and Lama1 by C/EBPβ is recapitulated in MESC, we monitored their mRNA levels in cells transduced with Ad-CTRL or Ad-DN. As shown in Fig. 3A, in Ad-DN-treated MESC, the levels of Lamc1 and Lama1 mRNAs were significantly reduced at 24 and 48 h of the differentiation process. There was also a corresponding decrease in the levels of laminin γ1 and laminin α1 proteins in Ad-DN-treated cells (Fig. 3B, panels b and d), confirming C/EBPβ regulation of Lamc1 and Lama1 during in vitro decidualization.
FIGURE 3.
Lamc1 is directly regulated by C/EBPβ during decidualization. MESC were transduced with either Ad-CTRL or Ad-DN (m.o.i. = 1:50) for 24 h. After removal of adenovirus, cells were treated with E and P for 24 or 48 h. A, relative expression levels of Lamc1 and Lama1 in adenovirus-treated MESC were determined by real time PCR analysis. Statistically significant differences (p < 0.05) are indicated by *. B, adenovirus-treated MESC were treated with E and P for 48 h and analyzed by immunofluorescence using antibodies against laminin γ1 and laminin α1. DAPI-stained nuclei are shown in blue. Bars, 100 μm. C, MESC treated with E and P for 24 h were subjected to ChIP as described under “Experimental Procedures,” using antibodies against C/EBPβ, RNA polymerase II (RNAP II), and rabbit IgG. Relative levels of recruitment at various sites of the Lamc1 promoter were determined by real time PCR and normalized to input DNA and RNA polymerase II values. All experiments were conducted in three independent trials except for ChIP experiments, which were performed in two independent trials. IP, immunoprecipitation.
Because Lamc1 is an essential component of many laminin heterotrimers, we examined whether C/EBPβ directly controls its expression in MESC. We identified four putative binding sites for C/EBPβ in the Lamc1 promoter using in silico analysis as indicated under “Experimental Procedures.” We then performed ChIP analysis to examine C/EBPβ occupancy at these sites. As expected, a significant recruitment of RNA polymerase II was observed at the transcription initiation site of the Lamc1 gene (Fig. 3C). Little or no recruitment of C/EBPβ was seen at sites −15, −499, or −1533. In contrast, a significant recruitment of this transcription factor was observed at the −94 region of the Lamc1 promoter during differentiation of MESC (Fig. 3C). This observation is consistent with a direct regulation of Lamc1 by C/EBPβ.
Lamc1 Plays a Critical Role in Differentiation and Cytoskeletal Remodeling of Uterine Stromal Cells
The regulation of Lamc1 by C/EBPβ in MESC raised the possibility that this important ECM molecule also plays a regulatory role in the transformation and remodeling of the stromal tissue during decidualization. We examined this possibility by performing siRNA-mediated silencing of Lamc1 expression during in vitro decidualization. This intervention, which is expected to eliminate the assembly of laminin γ1-containing heterotrimers in MESC, would disrupt the ECM network that typically forms around the decidual cells.
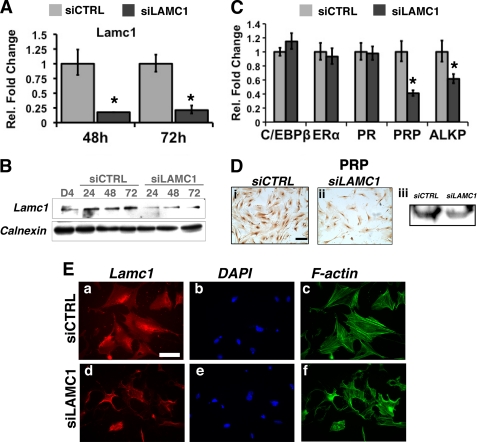
The MESC were transfected with control siRNA (siCTRL) or siRNA targeted to exon 3 of Lamc1 (siLAMC1). The levels of Lamc1 mRNA and Lamc1 protein were efficiently suppressed (>80%) upon administration of siLAMC1 (Fig. 4, A and B), whereas those of C/EBPβ, ERα, and PR remained unaffected (Fig. 4C). Interestingly, this reduction in Lamc1 expression, which did not alter MESC proliferation (data not shown), caused mRNA levels of the decidualization biomarkers PRP and ALP to decline significantly (Fig. 4C). A decrease in the level of PRP protein was also observed in siLAMC1-treated cells (Fig. 4D). The down-regulation of PRP and ALP strongly indicated that differentiation of MESC is impaired when Lamc1 expression is attenuated.
FIGURE 4.
siRNA-mediated silencing of Lamc1 expression inhibits stromal differentiation and cytoskeletal remodeling. MESC were transfected with 20 nm siCTRL or siRNA targeted against Lamc1 (siLAMC1) as described under “Experimental Procedures.” A, after 48 and 72 h, real time PCR was conducted using primers specific for Lamc1. Fold changes indicate gene expression levels in siLAMC1-treated MESCs relative to siCTRL-treated cells. B, Western blot analysis of Lamc1 protein in whole cell lysates of MESCs transfected with siCTRL or siLAMC1. C, relative expression of C/EBPβ, ERα, PR, PRP, and ALP mRNAs in siRNA-transfected MESC at 72 h. D, 72 h following transfection, siRNA-treated MESC were subjected to immunocytochemistry to monitor PRP protein levels. Bars, 200 μm. Western blotting of lysates of cells treated with siLAMC or siCTRL was performed using antibody against PRP. Calnexin staining indicated equal protein loading (data not shown). E, after 48 h, siRNA-treated MESC were analyzed by immunofluorescence using an antibody recognizing Lamc1 (panels a and d). DAPI-stained nuclei are shown in blue (panels b and e). Actin filaments (F-actin) were labeled with Alexa Fluor-phalloidin (panels c and f). Bars, 100 μm. All immunofluorescence experiments were conducted in two independent trials, and real time PCR measurements were conducted in three independent trials.
Concomitant with the decrease in differentiation markers, we observed that the actin cytoskeleton is disorganized in siLAMC1-treated MESC as compared with the siCTRL-treated cells (Fig. 4E, panels c and f). Specifically, the morphology of siLAMC1-treated cells was not representative of the typical decidual phenotype (Fig. 4E, compare panels c and f). These observations indicated that the siRNA-mediated suppression of Lamc1, which encodes a critical component of the stromal ECM, perturbs cellular remodeling and phenotypes associated with decidualization.
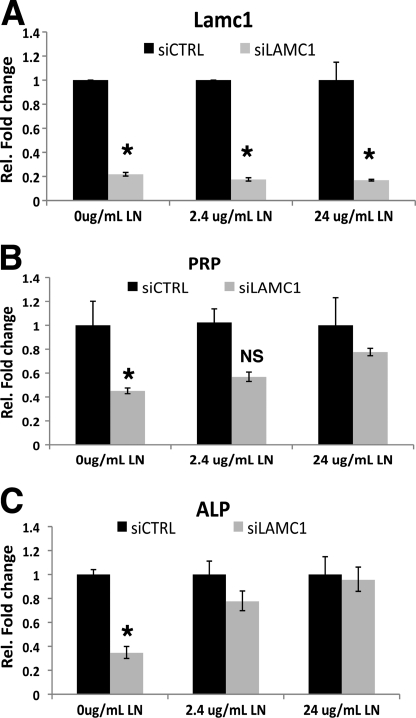
We next tested whether the decidualization defect could be rescued by restoring the level of laminin proteins in MESC in which Lamc1 mRNA expression was repressed. In this experiment, the cells were treated with siCTRL or siLAMC1 in the absence or presence of exogenous recombinant laminin-111 heterotrimer composed of α1, β1, and γ1. Addition of laminin-111 to siLAMC1-treated cells led to a remarkable dose-dependent recovery of PRP and ALP mRNA levels (Fig. 5, A–C), These results further confirmed the importance of the laminin-containing ECM in the decidualization of MESC.
FIGURE 5.
Recombinant laminin-111 (LN) rescues stromal differentiation in cells lacking Lamc1. MESC were transfected with siCTRL or siLAMC1 for 48 h. siRNAs were removed, and stromal culture was continued in the absence or presence of 2.4 or 24 μg/ml recombinant laminin-111 (LN) for an additional 24 h. Real time PCR analysis was conducted using primers specific to Lamc1 (A), PRP (B), and ALP (C). Experiments were performed in three independent trials. Statistically significant differences (p < 0.01) are indicated by *.
Integrin Receptor Complexes in Decidual Cells, Potential Targets of Laminins
The integrins are transmembrane cell surface receptors for laminins (11, 33, 34). The α and β subunits of integrins form heterodimers, which transmit “outside-in” signals from the laminin polymers in the ECM. We postulated that the laminins produced and secreted by the decidual cells interact directly with integrin receptors to activate intracellular signaling pathways that promote stromal differentiation.
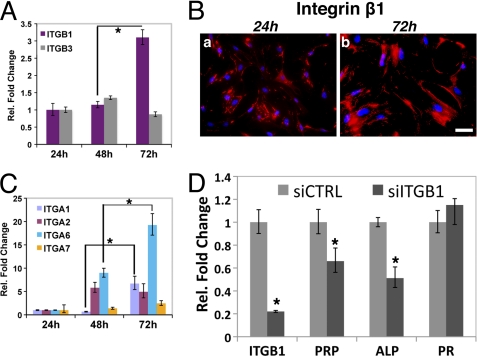
Integrin β1 is the most prevalent constituent of the cell surface integrin receptor complexes recognized by laminins (11, 35). Integrin β3 is also bound by laminins but to a lesser extent (11). We therefore surveyed the expression levels of integrin β1 and β3 and several of their potential α partners in differentiating MESC. As shown in Fig. 6A, the level of integrin β1 mRNA was markedly elevated during decidualization. We also localized the integrin β1 protein in the decidual cell membrane, consistent with its potential role as a part of the integrin receptor targeted by laminins (Fig. 6B). We observed that the levels of the mRNAs corresponding to integrins α1, α2, and α6 rose significantly during in vitro decidualization. The level of integrin α2 mRNA increased at 48 h, although that of α1 rose only at 72 h. Interestingly, the level of integrin α6 mRNA continued to increase steadily as differentiation progressed from 24 to 72 h (Fig. 6C). These findings point to the possibility that integrins α1β1, α2β1, and α6β1 represent the cell surface integrin receptors via the stromal basal lamina-like matrix signals to mediate distinct functions during decidualization.
FIGURE 6.
Role of integrins during in vitro decidualization. A, MESC were subjected to in vitro decidualization for 24, 48, and 72 h, and the levels of integrin β1 and integrin β3 mRNAs were determined using real time PCR. B, MESC undergoing decidualization was analyzed by immunofluorescence using antibodies recognizing integrin β1. DAPI-stained nuclei are shown in blue. Bar, 100 μm. C, relative expression of mRNAs encoding integrins α1, α2, α6, and α7 in decidualizing MESC. D, MESC were transfected with 40 nm siCTRL or siRNA against integrin β1 siRNA (siITGB1). Relative expressions of integrin β1, PRP, ALP, and PR mRNAs are shown. Fold changes indicate gene expression levels in siITGB1-treated MESC relative to siCTRL-treated MESC after 72 h. All real time PCR measurements were performed in three independent trials, and statistically significant differences (p < 0.01) are indicated by *.
Functional Laminin-Integrin Interactions Are Critical for Stromal Differentiation
To determine whether a functional interaction between the laminin ECM and integrin β1 complex is necessary for decidualization, we attenuated integrin β1 expression in MESC, using a siRNA targeted to its transcript. This intervention, which drastically reduced the level of integrin β1 mRNA, is expected to prevent the assembly of integrin complexes that act as targets of laminins. As shown in Fig. 6D, siRNA-mediated silencing of integrin β1 mRNA expression led to a decrease in the levels of the stromal differentiation markers, PRP and ALP. Taken together, these results are consistent with the concept that functional interactions between the laminin components of the stromal basal lamina-like matrix and their target integrin receptors in the cell surface contribute to the efficient progression of the decidualization process.
Basal Lamina-like ECM Controls FAK Signaling Pathway in Decidual Cells
To further test our hypothesis that the association between the laminins in the stromal ECM and the integrin β1 complex at the cell surface is important for decidualization, we examined the activation of a classical integrin-dependent signaling molecule, FAK. FAK is an important regulator of actin cytoskeleton remodeling, making it a suitable candidate for further investigation into the role of laminin-integrin interaction in decidualization (36, 37). FAK is activated through phosphorylation of multiple residues among which a major site is at Tyr397. We first examined the levels of phospho-Tyr397-FAK in MESC undergoing decidualization following treatment with siCTRL or siLAMC1. Although the cells treated with siCTRL displayed a punctate distribution of phospho-Tyr397-FAK at the plasma membrane (Fig. 7A, panel a, arrows) at 72 h into the differentiation program, the levels of phospho-FAK were reduced in siLAMC1-treated cells (Fig. 7A, panel b). This finding was confirmed by Western blotting of MESC lysates lacking Lamc1 (Fig. 7B). These observations suggested that FAK activation within the decidual cell is dependent on the proper assembly of the laminin ECM that interacts with the integrin receptor on the cell surface.
FIGURE 7.
Lamc1 expression controls FAK activity during stromal differentiation. MESC were transfected with siCTRL or siLAMC1 and subjected to in vitro decidualization for 72 h. A, siRNA-transfected MESC were analyzed by immunofluorescence using antibodies recognizing phosphorylated Tyr397-FAK. Arrows indicate punctate localization pattern of phospho-FAK at the cell membrane at 72h of decidualization. Nuclei are stained with DAPI. Bar, 100 μm. B, Western blot showing protein levels of Lamc1, phosphorylated Tyr397-FAK, total FAK, and calnexin in lysates prepared from undifferentiated MESC (day 4) and siRNA-transfected MESC at 48 h and 72 h following the initiation of decidualization. Western blot assays were performed in two separate experiments.
A Co-culture System to Study Maternal-Embryo Interactions
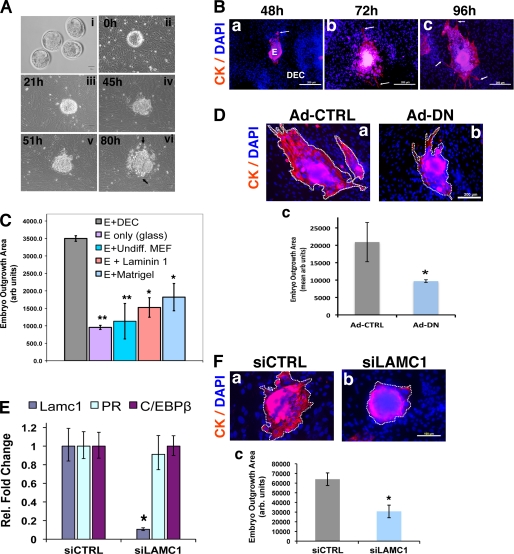
Implantation is highly dependent on the ability of the differentiating uterine stromal cells to interact with the invading trophoblasts. It is thought that the ECM proteins produced by the decidual cells assist in the establishment of a maternal-fetal interphase that aids in successful embryo invasion and embryo survival (5, 6). Previously described in vitro models were able to capture some of the functional features of implantation in vivo (5, 20, 21). To better understand the role of stromal ECM during embryo invasion into the decidua, we employed a strategy of co-culturing blastocysts and primary uterine stromal cells. In this approach, viable blastocysts collected from uteri of day 4 pregnant mice were placed on a confluent bed of MESC obtained from the same tissue (Fig. 8A, panels i and ii). We monitored the outgrowth of embryos by examining the migration of the outer trophoblast cell layer into the surrounding decidual cell layers for a period of time ranging between 21 and 80 h (Fig. 8A). The blastocysts underwent hatching, as evidenced by the shedding of the protective zona pellucida (Fig. 8A, panel ii), and attached to the monolayer of MESC by 21 h of co-culture (Fig. 8A, panel iii). The overall area occupied by the embryonic tissue continued to increase steadily, especially at 51 and 80 h of co-culture (Fig. 8A, panels iv–vi). To further characterize the embryonic outgrowth, we labeled trophoblast cells with antibodies against cytokeratin, a distinctive biochemical marker of these cells (Fig. 8B). We observed that cytokeratin-positive trophectoderm spreads and penetrates into the MESC monolayer over time (Fig. 8B, arrows). By 72–96 h of co-culture, the migrating trophoblasts were interfaced closely with the stromal layer (Fig. 8B, panels b and c, arrows).
FIGURE 8.
Analysis of maternal-embryo interactions in co-cultures of blastocysts and MESC. A, blastocysts (panel i) and MESC were harvested from pregnant (day 4) mouse uteri as described under “Experimental Procedures.” Blastocysts were placed onto a confluent bed of MESC (panel ii) and cultured together for durations of 21 h (panel iii), 45 h (panel iv), 51 h (panel v), and 80 h (panel vi). Live cell images were captured at each time point. B, blastocyst and MESC were co-cultured for 48 h (panel a), 72 h (panel b), and 96 h (panel c), fixed, and analyzed by immunofluorescence using an antibody recognizing cytokeratin (CK). DAPI-stained nuclei are shown in blue. Migrating trophoblasts are indicated by arrows. E, embryo; DEC, decidual cells. Bar, 100 μm. C, blastocysts were cultured for 48 h on MESC (E+DEC), glass alone (E only), MEFs (E+Undiff. MEF), laminin-111-coated glass (E+laminin 1), and Matrigel-coated glass (E+matrigel). Columns represent the area of embryonic outgrowth determined as described under “Experimental Procedures.” D, MESC was transduced with Ad-CTRL or Ad-DN for 24 h. After removal of adenovirus, blastocysts were placed on MESC treated with Ad-CTRL (panel a) or Ad-DN (panel b). Co-cultures were subjected to immunofluorescence using an antibody against cytokeratin (CK). Bar, 100 μm. Areas of embryonic outgrowth, indicated by dashed line, were determined using ImageJ software (panel c). Embryo outgrowth experiments were conducted in five independent trials. E, relative expression of Lamc1, PR, and C/EBPβ mRNAs in MESC transfected with siCTRL or siLAMC1 prior to co-culture with blastocysts. Real time PCR experiments were performed twice. F, MESC were transfected with 40 nm of siCTRL or siLAMC1. After removal of siRNAs, blastocysts were placed on MESC treated with siCTRL (panel a) or siLAMC1 (panel b). Cells were analyzed by immunofluorescence using an antibody against cytokeratin. Bar, 100 μm. Areas of embryonic outgrowth, indicated by dashed line, were determined using ImageJ software (panel c). Embryo outgrowth experiments were conducted in five independent trials. Statistically significant differences are indicated as follows: *, p < 0.05; **, p < 0.01.
To determine the specificity of embryo spreading on MESC and the requirement of a decidual ECM for this process, we compared the efficiency of different ECM substrates in promoting trophoblast outgrowth. When cultured on a glass surface without any matrix coating, blastocysts were able to attach but did not grow (Fig. 8C). In contrast, they spread very effectively on differentiating MESC. When placed on undifferentiated MEF, blastocysts showed very little outgrowth. On plates coated with laminin alone, the spreading was about ∼40% as effective as on decidual cells (Fig. 8C). We also tested embryo outgrowth on Matrigel, a solubilized tissue basement membrane containing a variety of ECM molecules, such as laminins, collagen type IV, and heparan sulfate proteoglycan. Surprisingly, blastocyst outgrowth on this matrix was only ∼60% as effective as on the decidual cells (Fig. 8C). These data strongly suggested that the decidualizing MESC present an ECM of unique composition that facilitates embryo development and spreading in the most effective manner. The absence of properly reconstituted ECM clearly limits embryo outgrowth. These results suggested that we have succeeded in creating an in vitro system to analyze the functions of the basal lamina-like stromal ECM in regulating embryo growth.
Blockade of C/EBPβ Function in MESC Prevents Trophoblast Outgrowth
We next tested whether maternal contributions to embryo outgrowth can be studied in this co-culture system by manipulating gene function in MESC. For this purpose, MESC were transduced with Ad-CTRL or Ad-DN followed by removal of the free adenovirus particles from the culture and placement of blastocysts on virus-treated stromal cells. We then measured the extent of embryo outgrowth on these cells. After 48 h of co-culture, blastocysts placed on Ad-CTRL-treated MESC exhibited an efficient spreading into the decidual cells (Fig. 8D, panels a and c). However, blastocysts placed on MESC in which C/EBPβ function is inhibited by Ad-DN displayed markedly reduced (∼60% less) trophoblast outgrowth (Fig. 8D, panels b and c). These data showed that C/EBPβ-directed differentiation of stromal cells is necessary to support trophoblast outgrowth in the co-culture model.
Disruption of Stromal Basal Lamina-like ECM Inhibits Trophoblast Outgrowth
We then investigated whether trophoblast outgrowth is controlled by the basal lamina-like ECM in decidual cells. MESC were transfected with siCTRL or siLAMC1 for 24 h, and the siRNAs were removed prior to the placement of blastocysts on these cells (Fig. 8E). The co-cultures were monitored for 48 h following which they were analyzed for trophoblast outgrowth. As expected, blastocysts placed on cells treated with siCTRL displayed efficient trophoblast spreading into the decidual cells (Fig. 8F, panels a and c). In contrast, a pronounced reduction in trophoblast spreading was observed when blastocysts were placed on MESC in which Lamc1 expression was suppressed by siLAMC1 (Fig. 8F, panels b and c). These results demonstrated that blastocysts spread less efficiently on MESC lacking Lamc1 than on cells expressing Lamc1 (Fig. 8F, panel c). Collectively, these results strongly supported a critical role of the basal lamina-like stromal ECM in promoting trophoblast outgrowth and migration.
DISCUSSION
C/EBPβ Is a Key Regulator of Uterine Stromal ECM Remodeling
Our earlier studies using in vivo approaches indicated a central role of C/EBPβ in controlling the decidualization program in mice, humans, and non-human primates (22, 38, 39). We recently reported that the stromal cells of C/EBPβ-null uteri fail to undergo mitotic expansion because of lack of expression or activity of key molecules controlling G2-M transition of the cell cycle (25). In addition, a striking lack of expression of the several biomarkers of stromal cell differentiation, such as ALP and connexin 43, was observed in C/EBPβ-null uteri subjected to artificial decidualization, indicating that stromal differentiation is also inhibited in these mutant mice.4 We postulated that C/EBPβ regulates the transcription of genes encoding key downstream factors that determine the differentiated phenotype of the stromal cells. This concept was supported by the results of our microarray analysis, which revealed that C/EBPβ regulates the expression of the ECM components, Lama1 and Lamc1, during the initial phase of the decidualization process. In the presence of a dominant negative C/EBPβ, stromal differentiation was repressed, and the synthesis of these laminin subunits was drastically reduced. Most importantly, we demonstrated that C/EBPβ is recruited to the Lamc1 promoter, strongly indicating that it directly regulates the synthesis of this critical laminin subunit during stromal differentiation. This study therefore established a novel regulatory link between C/EBPβ and the generation of a basal lamina-like ECM by MESC during decidualization.
Stromal ECM Plays a Critical Role in Decidualization
In a wide variety of mammalian tissues, the basal lamina ECM interacts with the cell surface. The basal lamina plays a dynamic regulatory role in diverse cellular processes, such as proliferation, differentiation, morphogenesis, and apoptosis (11, 40, 41). The laminin αβγ heterotrimers serve a core function in the assembly of the basal lamina ECM by providing a continuous polymerized layer upon which other ECM components, such as collagen type IV, nidogen, entactin, and perlecan, are assembled (11, 42, 43). The level of laminin-111, composed of Lama1, Lamb1, and Lamc1 protein subunits, in decidual matrix is regulated in a dynamic fashion during in vitro decidualization. The expression of Lama1 and Lamc1 is markedly elevated during the first 48 h of the differentiation process. A constitutive level of Lamb1 is also present throughout this process. Among the various laminin subunits, Lamc1 is clearly the most important because it is an essential functional component of most of the laminin heterotrimers present in the basal lamina (41). In the absence of Lamc1 expression in MESC, the basal lamina-like ECM failed to assemble. The stromal cells underwent proliferation but failed to execute proper differentiation. Addition of exogenous laminin-111 to cells lacking Lamc1 rescued stromal differentiation, strongly supporting the concept that the assembly of a basal lamina-like ECM, composed of Lamc1 and other laminin subunits, dictates the proper progression of the stromal cells through the differentiation process.
Signaling Mechanisms Controlled by Laminins during Decidualization
Laminin heterotrimers in the ECM are attached to the stromal cells via extracellular domains of integrins. The intracellular part of an integrin controls signaling pathways, which alter the actin cytoskeleton to influence cell shape, and modulates cellular functions during differentiation. Integrin β1 is consistently the most abundant subunit among the integrin receptors targeted by laminins (11). A survey of the expression levels of the individual integrin subunits in the decidual cells indicated that several α subunits, such as α1, α2, and α6, may function as the heterodimeric partner of the β1 subunit in an integrin receptor complex recognized by laminins during stromal differentiation (Fig. 6, A and B). In our study, the disruption of laminin-integrin interactions in MESC by attenuation of integrin β1 expression impaired the differentiation process of these cells. This observation supported a critical role of integrin complexes harboring the β1 subunit as laminin receptors during decidualization.
Attenuation of Lamc1 expression in MESC, which led to a block in decidualization, was also accompanied by a down-regulation of FAK activity (Fig. 7). FAK is a critical component of the integrin-mediated signaling network. In a variety of cell types, upon activation via phosphorylation at multiple sites, including Tyr397, FAK modulates actin cytoskeleton remodeling via Src kinase and/or MAPK pathways (35–37). Interruption of laminin γ1 expression in MESC prevented laminin-integrin interactions and suppressed FAK activation at Tyr397. As a result, the stromal cells were unable to assemble actin cytoskeleton properly, failing to form focal adhesions. Therefore, our studies uncovered a signaling mechanism by which the stromal basal lamina-like ECM transmits information to the interior of the cell via the integrin receptor to promote differentiation and cellular remodeling.
Stromal Basal Lamina Plays a Vital Role in Mediating Maternal-Embryo Interactions during Implantation
Previous work, employing in vitro models, suggested that specific components of the uterine ECM mediate maternal-embryo interactions during implantation (5, 6). In these studies, blastocysts were cultured on surfaces coated with laminin or collagen IV or fibronectin and subsequently, trophoblast behavior was assessed. It was proposed that, by directly interacting with the mural trophoblast layer of the blastocyst, the ECM components of the decidualized matrix modulate trophoblast outgrowth and infiltration into the stromal compartment (5). It was shown that, in the peri-implantation period, the trophoblast cells express integrin receptors, such as integrin α7β1, which are likely targets of recognition by maternal ECM components (44, 45). In this study, we analyzed peri-implantation events by examining how blastocysts behave when cultured together with differentiating stromal cells harvested from the same uterine tissue (Fig. 8). This co-culture model, which provides an improved recapitulation of the in vivo implantation events, is similar to the one developed by Mardon and co-workers (46, 47) who reported that human blastocysts can successfully invade into endometrial stromal monolayers in vitro. Interestingly, we noted that trophoblast outgrowths occur more effectively on decidual cell matrix than on proliferating mouse embryonic fibroblasts or surfaces coated with certain of the ECM components, underlining the specific requirement of a unique maternally derived matrix in modulating embryo invasion.
The co-culture system allowed us to analyze the contributions of the stromal matrix to the process of trophoblast invasion into the decidual tissue. As differentiation progresses, expression of C/EBPβ promotes the production of critical laminins, which are incorporated into the basal lamina-like matrix in the pericellular space. The resulting ECM controls embryo spreading into the stromal tissue by direct or indirect mechanisms. In support of this concept, we observed that if stromal differentiation is inhibited upon blocking of C/EBPβ function, the trophoblast cells fail to acquire an invasive behavior. We interpret this defect as a cumulative outcome of several molecular mechanisms disrupted by the loss of C/EBPβ function. These include an interruption of production of Lama1 and Lamc1 subunits, resulting in impaired stromal ECM formation, and the loss of functional interactions between trophoblasts and decidual cells. This idea was further bolstered when we witnessed that silencing of Lamc1 expression led to a significant impairment in embryo spreading.
In summary, our findings strongly support the premise that the stromal basal lamina-like ECM plays an important role in modulating trophoblast invasion. Most importantly, this study provides new insights into the molecular pathways that act downstream of C/EBPβ, a key regulator of the decidualization process, to create the stromal microenvironment that controls critical interactions between maternal and embryonic tissues during implantation.
Acknowledgments
We thank Dr. Sunghee Park and Dr. Benita Katzenellenbogen for kindly providing the MEFs and Katya Dribinsky for excellent technical assistance. We also thank Sandeep Pawar for assistance with preparation of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Eunice Kennedy Shriver NICHD Cooperative Agreement U54 HD055787 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
W. Wang, I. C. Bagchi, and M. K. Bagchi, unpublished data.
W. Wang and M. K. Bagchi, unpublished data.
- E
- estrogen
- P
- progesterone
- C/EBPβ
- CCAAT enhancer-binding protein β
- PRP
- prolactin-related protein
- ALP
- alkaline phosphatase
- ECM
- extracellular matrix
- FAK
- focal adhesion kinase
- MESC
- mouse endometrial stromal cells
- MEF
- mouse embryonic fibroblast
- PR
- progesterone receptor
- ER
- estrogen receptor
- Ad-DN
- adenovirus expressing dominant negative
- Ad-CTRL
- control adenovirus.
REFERENCES
- 1. Carson D. D., Bagchi I., Dey S. K., Enders A. C., Fazleabas A. T., Lessey B. A., Yoshinaga K. (2000) Dev. Biol. 223, 217–237 [DOI] [PubMed] [Google Scholar]
- 2. Gu Y., Gibori G. (1999) in Encyclopedia of Reproduction (Knobil E., Neill J. D. eds) pp. 836–842, Academic Press, San Diego [Google Scholar]
- 3. Dey S. K., Lim H., Das S. K., Reese J., Paria B. C., Daikoku T., Wang H. (2004) Endocr. Rev. 25, 341–373 [DOI] [PubMed] [Google Scholar]
- 4. Irwin J. C., Giudice L. C. (1999) in Encyclopedia of Reproduction (Knobil E., Neill J. D. eds) pp. 823–835, Academic Press, San Diego [Google Scholar]
- 5. Armant D. R. (2005) Dev. Biol. 280, 260–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bischof P., Meisser A., Campana A. (2000) Placenta 21, S55–S60 [DOI] [PubMed] [Google Scholar]
- 7. Ramathal C. Y., Bagchi I. C., Taylor R. N., Bagchi M. K. (2010) Semin. Reprod. Med. 28, 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wewer U. M., Faber M., Liotta L. A., Albrechtsen R. (1985) Lab. Invest. 53, 624–633 [PubMed] [Google Scholar]
- 9. Farrar J. D., Carson D. D. (1992) Biol. Reprod. 46, 1095–1108 [DOI] [PubMed] [Google Scholar]
- 10. Senior P. V., Critchley D. R., Beck F., Walker R. A., Varley J. M. (1988) Development 104, 431–446 [DOI] [PubMed] [Google Scholar]
- 11. Colognato H., Yurchenco P. D. (2000) Dev. Dyn. 218, 213–234 [DOI] [PubMed] [Google Scholar]
- 12. Timpl R., Brown J. C. (1996) BioEssays 18, 123–132 [DOI] [PubMed] [Google Scholar]
- 13. Timpl R., Brown J. C. (1994) Matrix Biol. 14, 275–281 [DOI] [PubMed] [Google Scholar]
- 14. Utani A., Nomizu M., Timpl R., Roller P. P., Yamada Y. (1994) J. Biol. Chem. 269, 19167–19175 [PubMed] [Google Scholar]
- 15. Burgeson R. E., Chiquet M., Deutzmann R., Ekblom P., Engel J., Kleinman H., Martin G. R., Meneguzzi G., Paulsson M., Sanes J. (1994) Matrix Biol. 14, 209–211 [DOI] [PubMed] [Google Scholar]
- 16. Aumailley M., Bruckner-Tuderman L., Carter W. G., Deutzmann R., Edgar D., Ekblom P., Engel J., Engvall E., Hohenester E., Jones J. C., Kleinman H. K., Marinkovich M. P., Martin G. R., Mayer U., Meneguzzi G., Miner J. H., Miyazaki K., Patarroyo M., Paulsson M., Quaranta V., Sanes J. R., Sasaki T., Sekiguchi K., Sorokin L. M., Talts J. F., Tryggvason K., Uitto J., Virtanen I., von der Mark K., Wewer U. M., Yamada Y., Yurchenco P. D. (2005) Matrix Biol. 24, 326–332 [DOI] [PubMed] [Google Scholar]
- 17. Smyth N., Vatansever H. S., Murray P., Meyer M., Frie C., Paulsson M., Edgar D. (1999) J. Cell Biol. 144, 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glasser S. R., Lampelo S., Munir M. I., Julian J. (1987) Differentiation 35, 132–142 [DOI] [PubMed] [Google Scholar]
- 19. Babiarz B., Romagnano L., Afonso S., Kurila G. (1996) Dev. Dyn. 206, 330–342 [DOI] [PubMed] [Google Scholar]
- 20. Klaffky E. J., Gonzáles I. M., Sutherland A. E. (2006) Dev. Biol. 292, 277–289 [DOI] [PubMed] [Google Scholar]
- 21. Sutherland A. E., Calarco P. G., Damsky C. H. (1988) J. Cell Biol. 106, 1331–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mantena S. R., Kannan A., Cheon Y. P., Li Q., Johnson P. F., Bagchi I. C., Bagchi M. K. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1870–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bagchi M. K., Mantena S. R., Kannan A., Bagchi I. C. (2006) Cell Cycle 5, 922–925 [DOI] [PubMed] [Google Scholar]
- 24. Ramathal C., Bagchi I. C., Bagchi M. K. (2010) Mol. Cell. Biol. 30, 1607–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang W., Li Q., Bagchi I. C., Bagchi M. K. (2010) Endocrinology 151, 3929–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q., Kannan A., Wang W., Demayo F. J., Taylor R. N., Bagchi M. K., Bagchi I. C. (2007) J. Biol. Chem. 282, 31725–31732 [DOI] [PubMed] [Google Scholar]
- 27. Cheon Y. P., DeMayo F. J., Bagchi M. K., Bagchi I. C. (2004) J. Biol. Chem. 279, 10357–10363 [DOI] [PubMed] [Google Scholar]
- 28. Rasband W. S. (2010) ImageJ, National Institutes of Health, Bethesda, MD, http://imagej.nih.gov/ij [Google Scholar]
- 29. Kim J., Sato M., Li Q., Lydon J. P., Demayo F. J., Bagchi I. C., Bagchi M. K. (2008) Mol. Cell. Biol. 28, 1770–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J. W., Tang Q. Q., Vinson C., Lane M. D. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandelin A., Wasserman W. W., Lenhard B. (2004) Nucleic Acids. Res. 32, W249–W252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schug J. (2008) Curr. Protoc. Bioinformatics, Chapter 2, Unit 2.6 [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez A. M., Gonzales M., Herron G. S., Nagavarapu U., Hopkinson S. B., Tsuruta D., Jones J. C. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16075–16080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kikkawa Y., Sanzen N., Fujiwara H., Sonnenberg A., Sekiguchi K. (2000) J. Cell Sci. 113, 869–876 [DOI] [PubMed] [Google Scholar]
- 35. Miranti C. K., Brugge J. S. (2002) Nat. Cell Biol. 4, E83–E90 [DOI] [PubMed] [Google Scholar]
- 36. Parsons J. T., Martin K. H., Slack J. K., Taylor J. M., Weed S. A. (2000) Oncogene 19, 5606–5613 [DOI] [PubMed] [Google Scholar]
- 37. Giancotti F. G., Ruoslahti E. (1999) Science 285, 1028–1032 [DOI] [PubMed] [Google Scholar]
- 38. Plante B. J., Kannan A., Bagchi M. K., Yuan L., Young S. L. (2009) Reprod. Biol. Endocrinol. 7, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kannan A., Fazleabas A. T., Bagchi I. C., Bagchi M. K. (2010) Reprod. Sci. 17, 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Colognato H., Winkelmann D. A., Yurchenco P. D. (1999) J. Cell Biol. 145, 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li S., Edgar D., Fässler R., Wadsworth W., Yurchenco P. D. (2003) Dev. Cell 4, 613–624 [DOI] [PubMed] [Google Scholar]
- 42. Murray P., Edgar D. (2001) J. Cell Sci. 114, 931–939 [DOI] [PubMed] [Google Scholar]
- 43. Yurchenco P. D., Quan Y., Colognato H., Mathus T., Harrison D., Yamada Y., O'Rear J. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 10189–10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klaffky E., Williams R., Yao C. C., Ziober B., Kramer R., Sutherland A. (2001) Dev. Biol. 239, 161–175 [DOI] [PubMed] [Google Scholar]
- 45. Miner J. H., Li C., Mudd J. L., Go G., Sutherland A. E. (2004) Development 131, 2247–2256 [DOI] [PubMed] [Google Scholar]
- 46. Mardon H., Grewal S., Mills K. (2007) Semin. Reprod. Med. 25, 410–417 [DOI] [PubMed] [Google Scholar]
- 47. Grewal S., Carver J. G., Ridley A. J., Mardon H. J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 16189–16194 [DOI] [PMC free article] [PubMed] [Google Scholar]