Abstract
The essence of neuronal function is to generate outputs in response to synaptic potentials. Synaptic integration at postsynaptic sites determines neuronal outputs in the CNS. Using immunohistochemical and electrophysiological approaches, we first reveal that steroidogenic factor 1 (SF-1) green fluorescent protein (GFP)-positive neurons in the ventromedial nucleus of the hypothalamus express P2X4 subunits that are activated by exogenous ATP. Increased membrane expression of P2X4 channels by using a peptide competing with P2X4 intracellular endocytosis motif enhances neuronal excitability of SF-1 GFP-positive neurons. This increased excitability is inhibited by a P2X receptor antagonist. Furthermore, increased surface P2X4 receptor expression significantly decreases the frequency and the amplitude of GABAergic postsynaptic currents of SF-1 GFP-positive neurons. Co-immunopurification and pulldown assays reveal that P2X4 receptors complex with aminobutyric acid, type A (GABAA) receptors and demonstrate that two amino acids in the carboxyl tail of the P2X4 subunit are crucial for its physical association with GABAA receptors. Mutation of these two residues prevents the physical association, thereby blocking cross-inhibition between P2X4 and GABAA receptors. Moreover, disruption of the physical coupling using competitive peptides containing the identified motif abolishes current inhibition between P2X4 and GABAA receptors in recombinant system and P2X4 receptor-mediated GABAergic depression in SF-1 GFP-positive neurons. Our present work thus provides evidence for cross-talk between excitatory and inhibitory receptors that appears to be crucial in determining GABAergic synaptic strength at a central synapse.
Keywords: ATP, GABA Receptors, Purinergic Receptor, Signal Transduction, Synapses, Trafficking, P2X
Introduction
ATP acts at cell surface receptors of two fundamentally distinct types: ligand-gated ion channels (P2X receptors) and G-protein-coupled receptors (P2Y receptors). ATP P2X receptors are widely distributed in excitable and non-excitable cells of vertebrates and are nonselective cation channels (1). Their activation mediates membrane depolarization and calcium influx (2). Among the seven P2X subunits, P2X4 receptors are most widely distributed in the CNS (3). It appears that P2X4-containing receptors are involved in purinergic synaptic transmission at central synapses since they are located at postsynaptic sites in the brain, including the hippocampus and the cerebellum (4). Although there is increasing evidence for their implications in various physiological and pathological conditions (5), the physiological role of P2X receptors at the synaptic level has been poorly defined, at least in part, due to the paucity of purinergic synaptic transmission in the CNS.
In the nervous system, ATP appears to be primarily a cotransmitter rather than a principal transmitter (6). ATP is released either with an inhibitory neurotransmitter, GABA4 (7–10) or with an excitatory neurotransmitter, glutamate in the CNS (4, 11). Recent studies have clearly demonstrated the interactions of P2X receptors with other ligand-gated channels, including 5-hydroxytryptamine, GABAA, or nicotinic receptors in recombinant expression and cell culture preparations (12–21). It thus appears that P2X receptors prefer to interact with other receptors rather than act independently. This cross-talk would play a critical role in finely tuning synaptic strength at central synapses.
The hypothalamus is the primary locus for integration of signals that influence energy balance. Among the hypothalamic nuclei, the ventromedial nucleus of the hypothalamus (VMH) is considered the brain's “satiety center” as early studies revealed that lesions of the VMH resulted in hyperphagia and obesity in a variety of species including humans (for review, see Ref. 22). The VMH expresses ATP P2X4 receptors (23) and contains anorexigenic steroidogenic factor 1 (SF-1)-expressing neurons that provide excitatory input onto feeding-related neurons in the hypothalamus (24). In the present study we examined the physiological consequences of increased membrane expression of P2X4 receptors on SF-1 green fluorescent protein (GFP)-positive neurons in the VMH. We then investigated the molecular and cellular mechanisms that mediate cross-inhibition between P2X4 and GABAA receptors in recombinant expression system. We further explored whether the physical interaction between these two distinct receptors is essential for synaptic integration that determines synaptic efficacy at a feeding-related synapse. As the VMH is an important center for ingestive behavior, it is likely that the mechanism by which P2X4 receptors regulate synaptic efficacy at a feeding-related synapse will be important for overall energy balance.
EXPERIMENTAL PROCEDURES
Animals
Experiments were performed on hypothalamic slices obtained from transgenic mice that selectively express enhanced GFP in SF-1-positive neurons in the VMH (a gift from Dr. Keith Parker). Oocytes were removed from Xenopus laevis. All experimental procedures involving animals were approved by the Albert Einstein College of Medicine Committee on Animal Care and Use or the committee of the Prefecture of Gironde.
Peptides
Peptides were synthesized by Tufts University Core Facility and GeneScript. Peptide 11C corresponds to the last 11 amino acid sequence of the mouse wild-type P2X4 subunit. As control peptides, we used mutated (3A) peptides. In peptide 3A, adaptor protein 2 binding domain residues YXXGL were replaced with three alanines. The biotinylated and non-biotinylated form of YV6 and YV6/ST peptides were used for pulldown assays and functional competition experiments, respectively. YV6 and YV6/ST correspond to 372YKYVED377 sequence of wild-type P2X4 subunit and 372YKSTED377 of mutated P2X4 subunit, respectively.
Constructs
All P2X or GABA subunit cDNAs used in this study were subcloned into pcDNA3 expression vector (Stratagene, La Jolla, CA). Deletions (Δ) of P2X4 subunits were generated as described (Toulmé et al. (21)). Point mutations or insertions were constructed using the QuikChange site-directed mutagenesis method (Stratagene) and verified by automatic sequencing.
Xenopus Oocyte Electrophysiology
cDNAs coding for wild-type, internalization-deficient, or mutated P2X4 subunits were injected with each of the GABA subunits to reach similar expression levels for both channels. As wild-type P2X4 receptor expression was low in comparison to GABAA receptors, most of the co-expression experiments were performed using internalization-deficient P2X4 subunits (P2X4FLAGIN or P2X4Y378A, which lack the endocytosis motif and has been shown to be defective in internalization) to facilitate characterization of the functional cross-talk. The amount of cDNA was adjusted to reach similar levels of expression and avoid overexpression to ensure that non-additivity was not due to inadequate voltage clamp. After nuclear injection of cDNAs, oocytes were incubated in Barth's solution containing 1.8 mm CaCl2 and gentamycin (10 μg/ml, Sigma) at 19 °C for 1–3 days before electrophysiological recordings. Peptides were dissolved in 10 mm HEPES buffer and injected into the cytoplasm of receptor-expressing oocytes to reach a final concentration of 150 μm. Two-electrode voltage-clamp recordings were carried out at room temperature using glass pipettes (1–2 megaohms) filled with 3 m KCl solution to ensure a reliable holding potential. Oocytes were voltage-clamped at −60 mV, and the membrane currents were recorded with an OC-725B amplifier (Warner Instruments) and digitized at 1 KHz on a Power PC Macintosh G4 using Axograph X software (Axograph). Oocytes were perfused at a flow rate of 10–12 ml/min with Ringer solution, pH 7.4 (97 mm NaCl, 3 mm NaOH, 2 mm KCl, 1.8 mm CaCl2, and 10 mm HEPES), and agonists were applied using a computer-driven valve system (Ala Scientific).
Biochemistry
Rat hypothalamus or injected oocytes were homogenized as previously described (19) using 0.8% Triton X-100. In biotinylation experiments, oocytes were incubated 20 min at room temperature after cytoplasmic injection of peptides and then treated and analyzed as previously described (19). Surface and total proteins were revealed by Western blotting using anti-P2X4 (Alomone Labs) or anti-FLAG antibodies (Sigma).
In co-immunoprecipitation studies, homogenates from hypothalamus or cells expressing P2X4 and α2β3 GABAA subunits (2–6 mg of proteins) were incubated overnight at 4 °C in the presence or absence of primary P2X4 antibodies (Alomone Labs) covalently immobilized to antibody coupling resin (Co-IP kit, Pierce). Co-immunopurification was also performed using protein extracts of oocytes expressing P2X4FLAGIN or P2X4FLAGINYV/ST and/or α2β3myc GABAA subunits with either immobilized monoclonal anti-myc antibodies (Genscript) coupled to resin (Co-IP kit, Pierce) or anti-FLAG M2 affinity gel (Sigma). Beads were washes 4 times with TBS (50 mm Tris, 150 mm NaCl) and eluted with low pH buffer or SDS sample buffer and subjected to SDS-PAGE. Co-precipitated proteins were detected by Western blotting analysis with primary antibodies against P2X4 (1:1000, Alomone Labs), GABA β3 (1:2000, Chemicon) or GABA α2 (1:500, Alomone Labs) and horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000, Jackson ImmunoResearch) or primary HRP-conjugated antibodies (anti-myc 1:2000, Genscript; anti-DDDDK tag 1:10000, Abcam) as specified.
For pulldown assays, protein extracts from oocytes expressing α2β3myc GABAA subunits were incubated with immobilized streptavidin bound to the indicated biotinylated peptides (100 μg) overnight at 4 °C (pulldown biotinylated protein-protein interaction kit, Pierce). Beads (40 μl) were washed 4 times with 375 mm acetate/NaCl buffer and eluted with 1 volume of SDS sample buffer. β3myc subunit was revealed by Western blot using monoclonal anti-myc antibody (1:1000, Genscript). Quantification of Western blots was performed using Image J software (National Institutes of Health). To quantitate changes in co-immunoprecipitation, we determined the coimmunoprecipitation fraction as the ratio of FLAG/myc or myc/FLAG signals, respectively, after immunoprecipitation with myc or FLAG antibodies.
Immunofluorescence Labeling
SF1-GFP transgenic mice were anesthetized with a mixture of ketamine and xylazine and perfused with 0.9% NaCl before fixation with by 4% paraformaldehyde. Brain sections (40 μm) obtained on a vibrating microtome were incubated with rabbit anti-P2X2 (1:200, Alomone), anti-P2X4 (1:200, Alomone), and/or mouse anti-GABAA β3 subunit antibodies (1:500, NeuroMab) for 48 h at 4 °C visualized with anti-rabbit secondary antibody conjugated to AlexaFluor 568 (1:1000) or anti-mouse secondary antibody conjugated to AlexaFluor 647 (1:200). Living cell surface and intracellular labeling of COS-7 cells transfected with HA-tagged P2X4, P2X4FLAGIN, and/or α2β3myc subunits was carried out as previously described (25). For internalization labeling, live COS cells transiently transfected with HA-P2X4WT or HA-P2X4FLAGIN were incubated in DMEM containing mouse anti-HA antibody for 30 min at 37 °C, and internalization was stopped on ice. Surface P2X4 receptors were stained with anti-mouse-FITC secondary antibody before cells were fixed with 4% paraformaldehyde, permeabilized in PBS containing 0.1% Triton X-100, and incubated with anti-mouse AlexaFluor 568 antibody to stain internalized receptors. Images were obtained from a Leica fluorescence microscope or DMR PCS SP2 AOBS confocal microscope (Leica, Heidelberg, Germany). Quantification of internalized and surface immunofluorescence was performed using the Image J software.
Brain Slice Electrophysiology and Analysis
Transverse brain slices were prepared from SF1-GFP mice at postnatal age 21–28 days. Animals were anesthetized with a mixture of ketamine and xylazine. After decapitation, the brain was transferred into a sucrose-based solution bubbled with 95%O2, 5% CO2 and maintained at ∼3 °C. This solution contained 248 mm sucrose, 2 mm KCl, 1 mm MgCl2, 1.25 mm KH2PO4, 26 mm NaHCO3, and 1 mm sodium pyruvate, and 10 mm glucose. Transverse coronal brain slices (200 μm) were prepared using a vibratome. Slices were equilibrated with an oxygenated artificial cerebrospinal fluid for >1 h at 32 °C before transfer to the recording chamber. The slices were continuously superfused with artificial cerebrospinal fluid at a rate of 1.5 ml/min containing 113 mm NaCl, 3 mm KCl, 1 mm NaH2PO4, 26 mm NaHCO3, 2.5 mm CaCl2, 1 mm MgCl2, and 5 mm glucose in 95% O2, 5% CO2 at 28 or 36 °C when indicated.
Brain slices were placed on the stage of an upright, infrared-differential interference contrast microscope (Olympus BX50WI) mounted on a Gibraltar X-Y table (Burleigh) and visualized with a 40× water immersion objective by infrared microscopy (DAGE MTI camera). Membrane currents and potentials were recorded at 28 °C with an Axopatch 200B patch clamp amplifier or a Multiclamp 700B. 6-Cyano-7-nitroquinoxaline-2,3-dione (10 μm), dl-amino-phosphonovaleric acid (50 μm), picrotoxin (100 μm), strychnine (1 μm), and 8-cyclopentyl-1,3-dipropylxanthine (1 μm) were continuously present in artificial cerebrospinal fluid. The internal solution contained 115 mm potassium acetate, 10 mm KCl, 2 mm MgCl2, 0.2 mm EGTA, 10 mm HEPES, 1 mm Na2ATP, 0.5 mm Na2GTP and 5 mm phosphocreatine or 125 mm CsCl (or KCl), 2 mm MgCl2, 0.2 mm EGTA, 10 mm HEPES, 1 mm Na2ATP, 0.5 mm Na2GTP, and 5 mm phosphocreatine. The cesium-based internal solution was used to record P2X receptor-mediated synaptic currents. Pipette resistance ranged from 2.5 to 4 megaohms.
SF-1 neuronal responsiveness was heterogeneous. We thus divided responding neurons from non-responding neurons based on the criterion that the change in membrane potential or firing rate induced by inclusion of 11C was ±3 times the standard deviation at the onset of recording. The membrane potential in actively spiking neurons was measured between spikes.
GABAergic spontaneous inhibitory postsynaptic currents (IPSCs) were recorded in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione, dl-amino-phosphonovaleric acid, and strychnine for at least 30 min, and autodetected events with amplitudes of more than −5 pA were also visually examined to correct for noise fluctuation (Mini analysis 6, Synaptosoft, Inc. and Clampfit 10, Molecular Devices, Inc.). Analysis of IPSC decay phase (Clampfit 10, Molecular Devices) was based on the following criteria; 1) single events only (i.e. no multiple events), 2) events having stable base lines 15 ms before the rise, and 3) smooth transition from 0 current to peak amplitude (<20% deviation in d(pA)/dt) during rise). Aligned IPSCs were averaged, and the decay was fit by single or double exponential functions, D(t) = Afaste(−t/tfast) + Aslowe(−t/tslow), where D(t) is the decay of the IPSCs as a function of time (t), Afast and Aslow are constants, and tfast and tslow are the fast and slow decay time constants, respectively. The weighted decay time constant was calculated tw = (Afasttfast + Aslowtslow)/(Afast + Aslow).
Drugs
All substances used in electrophysiological experiments were from Sigma, Tocris, and Ascent Scientific.
Statistics
Statistical analysis was performed using Student's paired t test, one-way ANOVA followed by a Bonferroni post hoc procedure for between-group comparison in some experiments in which there are more than two variables (as noted) or Kruskal-Wallis test with Dunn's post-hoc comparison (Origin 7.0 or Prism 4.0, Graphpad). Data were considered significantly different when the p value was less than 0.05. All statistical results are given as the mean ± S.E.
RESULTS
Blockade of P2X4 Receptor Internalization Using an Interference Peptide
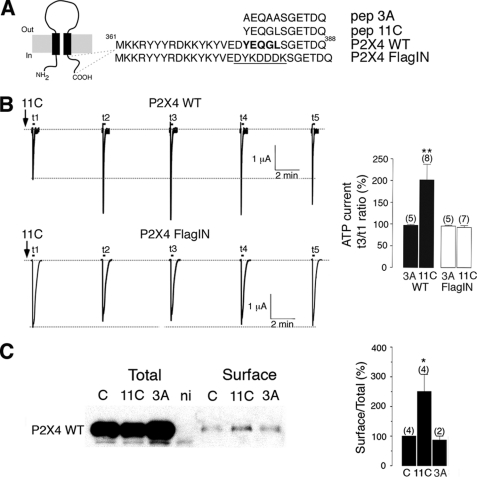
It is well described that P2X4 receptors cycle into and out of the plasma membrane, thereby modulating receptor density (26). Constitutive endocytosis of the P2X4 receptor requires an atypical tyrosine-based motif in the C-terminal domain of P2X4 receptor, which binds to adaptor protein 2, and mutation of the motif causes a decrease in receptor internalization and a dramatic increase in the surface expression of P2X4 receptors (27). To determine the potential electrophysiological impacts of P2X4 receptors at central synapses, we designed a site-specific interference peptide (11C) that includes the endocytosis motif YXXGL in the intracellular C terminus of P2X4 subunits in order to increase surface P2X4 expression (Fig. 1A). This motif is totally conserved among mammalian P2X4 sequences but is absent from other P2X subtypes or other neurotransmitter-gated channels (26–28).
FIGURE 1.
Blockade of P2X4 receptor internalization using an interference peptide. A, P2X receptor topology and sequence of the C-terminal domain of wild-type P2X4 and internalization-defective mutant P2X4FLAGIN is shown. Adaptor protein 2 binding domain is in bold, and the FLAG sequence is underlined. Sequences of the interference (11C) and control (3A) peptides are also indicated. B, representative traces show the effect of peptide 11C on the amplitude of ATP (100 μm) current evoked every 5 min in oocytes expressing P2X4 wild-type (top) or internalization-deficient P2X4FLAGIN (bottom) after injection of 11C (150 μm, black arrows). Right panel, the graph shows the amplitude of ATP current recorded 10 min after injection (t3) of the peptide 11C or control peptide (3A) relative to the initial ATP response recorded immediately after injection (t1) in P2X4 or P2X4FLAGIN expressing cells. n is between parentheses; **, p < 0.005. C, a representative Western blot using anti-P2X4 antibodies shows the effect of peptide 11C on the surface expression of P2X4 receptors expressed in Xenopus oocytes after biotinylation. Right panel, quantification of total and surface P2X4 proteins obtained in presence of peptides (11C or 3A) or in absence of peptides (C). Non- ni, injected oocytes; *, p < 0.05; 2–4 independent experiments.
To validate the effect of our designed peptide, we first injected 11C into Xenopus oocytes expressing wild-type P2X4 receptors. We found that 11C alone induced a rapid and long-lasting increase of ATP-induced current amplitude (Fig. 1B), whereas loading of control peptide (3A) had no effect on ATP response (Fig. 1B). The amplitude of ATP response was 202 ± 36% that of control 10 min after 11C injection (n = 8, p < 0.005). This effect appears to be specific, as injection of either 11C or control peptides in cells expressing non-internalized P2X4FLAGIN receptors, which lacks the endocytosis motif and has been shown to be defective in internalization (see supplemental Fig. S1), had no effect on the amplitude of ATP currents (Fig. 1B). Furthermore, biotinylation experiments revealed that 11C specifically increased the surface fraction of P2X4 receptors (250 ± 57% of control, n = 4, p < 0.05. 2–4 independent experiments; Fig. 1C). These results indicate that 11C alters P2X4 endocytosis, consistent with the prior study showing an essential role of the YXXGL motif in the internalization of P2X4 receptor (27).
Functional Expression of P2X4 Receptors in SF-1 GFP-positive Neurons
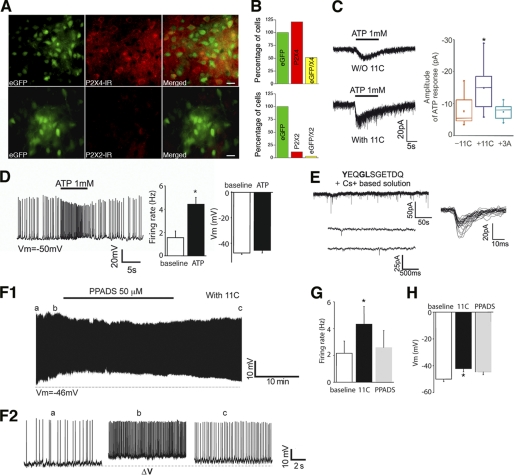
P2X4 mRNAs are found in the VMH (23). We, therefore, used a transgenic animal model expressing enhanced GFP in SF-1 neurons that are mainly located in the VMH to study P2X4-mediated transmission in these neurons (29). Immunostaining using anti-P2X2 and anti-P2X4 antibodies showed that P2X subunits were expressed in VMH neurons, including SF-1 GFP-positive neurons and non-SF-1 GFP-positive neurons (Fig. 2A and B). Overall, 51% of SF-1 GFP-positive neurons (n = 194) expressed P2X4 subunit, whereas only 3% of SF-1-GFP neurons (n = 100) had P2X2 subunits. In addition, SF-1 GFP-positive neurons responded to exogenous application of ATP (1 mm) with a mean amplitude of −7.6 ± 1.7 pA (n = 9 of 9 neurons), suggesting that SF-1 neurons may express other P2X subtypes. Importantly, inclusion of 11C, but not control peptide (3A, Fig. 1A), in the intracellular recording solutions significantly increased the mean amplitude of ATP responses (Fig. 2C, mean amplitude −14.9 ± 2.5 pA; n = 9 neurons; p < 0.05; control peptide (3A), −7.4 ± 0.8 pA; n = 9). Furthermore, application of ATP (1 mm) depolarized and increased action potential discharge of SF-1 GFP-positive neurons (Fig. 2D, mean firing rate, 1.6 ± 0.6 to 4.4 ± 0.6 Hz, p < 0.05; mean membrane potential, −47.7 ± 1 mV to −45.6 ± 2 mV, p > 0.05; n = 5 neurons).
FIGURE 2.
Blockade of P2X4 receptor internalization increases excitability of SF-1 GFP-positive neurons. A, representative images of immunofluorescence microscopy show the expression of P2X4 (top panel) and P2X2 subunits (bottom panel) in the VMH of SF-1 GFP-positive mice (scale bar = 15 μm). P2X4 subunits (red) were highly expressed in the VMH, including SF-1 GFP-positive neurons, whereas P2X2 subunit detection was scarce in the VMH. B, numbers of cells expressing P2X4 or P2X2 normalized to the number of SF1 GFP-positive cells (n = 100 or 194, respectively, for P2X4 and P2X2) are shown. 51% of SF1 neurons expressed P2X4 subunits. C, recording samples show the responses of SF-1 GFP-positive neurons to exogenous ATP with or without 11C (150 μm). SF-1 GFP-positive neurons readily respond to 1 mm ATP (left panel). HP = −70 mV. Pooled data are the mean amplitude of ATP responses. Inclusion of 11C, but not control peptide, increased the mean amplitude of ATP responses (right panel). D, application of exogenous ATP increased the firing rate of SF-1 GFP-positive neurons. A brief application (∼10 s) of ATP (1 mm) reversibly depolarized and increased the frequency of action potential discharge. Vm = −50 mV. Bar graphs represent changes in the firing rate and the membrane potential of SF-1 GFP-positive neurons (n = 5 neurons). E, samples of recording show sPSCs in the presence of 11C (top panel). Bottom panel, an expanded time scale is shown. A third of the neurons examined in the presence of 11C showed spontaneous synaptic events (n = 7 of 20 neurons). Left panel, superimposition of traces of sPSCs is shown. HP = −70 mV. F1 and F2, representative traces are recorded from SF-1 GFP-positive neurons in current clamp mode. Inhibition of the membrane internalization of P2X4 receptors increased spontaneous SF-1 neuron excitability. The frequency of action potential discharge was increased over time. Vm = −58 mV. F2, action potential discharge of the neuron is shown in F1 (a, b, and c) on an expanded time scale. PPADS reversed the effect of 11C (F1 and F2). G and H, pooled data of the firing rate and the membrane potential in the presence of 11C before and after application of PPADS (50 μm) are shown. 11C depolarized and increased the firing rate of SF-1 GFP-positive neurons (n = 6 neurons).
Although SF-1 GFP-positive neurons expressed functional P2X receptors, we were unable to detect any spontaneous synaptic currents in the presence of a mixture of glutamate, glycine, adenosine, and GABAA receptor antagonists (n = >30 neurons). We thus sought to determine whether specific blockade of constitutive P2X4 receptor endocytosis revealed spontaneous purinergic transmission. Under the same experimental conditions, introduction of 11C (80–150 μm) through the patch pipette into SF-1 GFP-positive neurons revealed spontaneous postsynaptic currents in a subset of SF-1 GFP-positive neurons (sPSCs; n = 7 of 20 neurons; Fig. 2E). However, the mean frequency of sPSCs was extremely low (mean frequency, 0.02 ± 0.01 Hz; range from 0.002 to 0.06 Hz; mean rise time, 3.6 ± 0.1 ms; mean amplitude, −16.8 ± 0.5 pA; mean decay time, 19.3 ± 0.6 ms; n = 7 neurons). It is very unlikely that P2X4 receptors are located at synaptic sites due to the slow rise time as well as the low frequency of sPSCs under these experimental conditions.
In contrast to the lack of P2X receptor-mediated synaptic currents, we noticed that P2X4 receptor blockade of internalization with 11C increased the excitability of SF-1 neurons (Fig. 2F). Examination of SF-1 neurons in current clamp mode demonstrated that blockade of endocytosis of P2X4 receptors by 11C significantly increased the mean membrane potential as well as the firing rate in 35% of the neurons examined (Fig. 2F; n = 6 of 17 neurons; mean membrane potential, −49.9 ± 2 mV versus −42.1 ± 2 mV; mean firing rate, 2.2 ± 0.9 Hz versus 4.3 ± 1.3 Hz; p < 0.005; p < 0.05; respectively). This enhancement was reversed by application of 50 μm pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) (Vm, −44.6 ± 2 mV, p > 0.05; firing rate, 2.6 ± 1.3 Hz, p < 0.05 between 11C and PPADS; n = 6 neurons; Fig. 2, F, G, and H). Together, these results indicated that, although purinergic synaptic transmission is scarce at central synapses, the activation of P2X4 receptors by ambient ATP may alter SF-1 neuronal activity.
Functional Cross-talk between P2X4 and GABAA Receptors
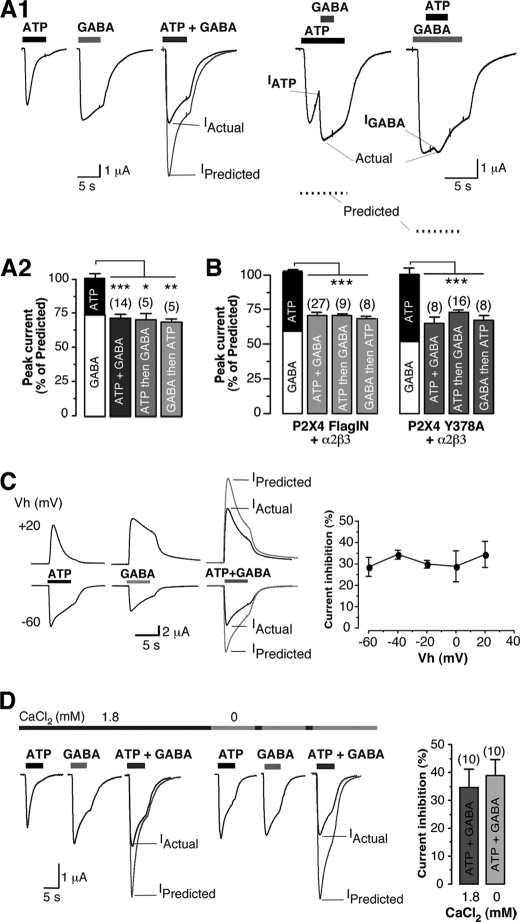
Several P2X receptors have been shown to functionally interact with GABAA receptors (17, 18). The next experiments were to determine whether P2X4 receptors interacted with GABAA receptors. α2 and β3 GABAA subunits are highly expressed in VMH neurons (30, 31); we thus co-injected cDNAs encoding P2X4 and α2, β3 GABAA subunits into Xenopus oocytes. In these experiments, the low surface expression of P2X4 subunits made co-expression difficult. Robust ATP and GABA currents were detected in 10% of cells recorded (n > 100) and allowed us to examine effect of agonist co-application. The mean amplitude of the currents evoked by concomitant application of ATP and GABA (Fig. 3, A1 and A2; mean amplitude: 1.88 ± 0.37 μA, n = 14) was significantly smaller (p < 0.001) than the predicted sum of the responses to separate application of ATP (mean amplitude, 0.84 ± 0.26 μA, n = 14) and GABA (mean amplitude, 1.82 ± 0.39 μA, n = 14). IATP+GABA represented 71.86 ± 2.90% of the predicted current (Fig. 3A2). This inhibition appears to be reciprocal as ATP application before GABA (or vice versa) induced a significant inhibition of the responses (Fig. 3A1). IATP then GABA and IGABA then ATP represented 70.52 ± 4.79% (n = 5) and 68.94 ± 2.37% (n = 5) of the predicted current, respectively (Fig. 3A2).
FIGURE 3.
Cross-inhibition between P2X4 and GABAA receptors in cells co-expressing both receptors. A, reciprocal cross-talk between wild-type P2X4 and α2β3 receptors expressed in Xenopus oocytes is shown. A1, co-application of ATP + GABA-induced currents (Actual) was significantly smaller than the arithmetic sum (Predicted) of the individual ATP (100 μm) and GABA (100 μm) responses (left panel). Sequential application of GABA during ATP application conversely indicated that current inhibition was reciprocal (right panel). Vm = −60 mV. A2, the bar graph shows the normalized peak current to the predicted response from individual cell; ***, p < 0.0005; **, p < 0.005; *, p < 0.05. B, shown is a summary of the mean current amplitudes recorded from oocytes co-expressing internalization-deficient P2X4 (P2X4FLAGIN or P2X4Y378A) receptors and α2β3 GABA receptors during co-application or sequential application of ATP and GABA (100 μm each). C, current inhibition was measured at several holding potentials. Representative traces recorded at −60 or +20 mV after application of ATP, GABA, or both agonists (left panel) is shown. Pooled data of the percentage of current inhibition recorded at holding potentials between −60 to 20 mV (right panel, n = 3–7) is shown. D, similar current inhibition was measured in the absence of extracellular CaCl2. Representative currents induced by application of ATP, GABA, or both agonists in normal ringer (1.8 mm CaCl2) or in calcium-free ringer are shown. Right panel, pooled data of the percentage of current inhibition recorded in the presence or absence of external CaCl2 (n = 10 for each conditions) are shown. Vh, holding potential.
To further analyze the effect of the co-activation of both receptor types, we co-injected cDNAs of internalization-deficient P2X4 mutants with α2β3 GABAA subunits to reach similar levels of expression of P2X4 mutants and GABAA receptors. In contrast to the weak co-expression of wild-type P2X4 and GABAA receptors, non-internalized P2X4 channel mutants, including a point mutation (i.e. P2X4Y378A (19, 26) or a substitution of the endocytosis motif (i.e. P2X4FLAGIN; supplemental Fig. S1), were readily co-expressed with GABAA receptors; IATP = 2.23 ± 0.4 and IGABA = 2.66 ± 0.37 μA, n = 27 for P2X4FLAGIN and α2β3 receptors. Under these conditions, the mean amplitude of the responses to GABA and ATP was also significantly smaller than the prediction (Fig. 3B). IATP+GABA represented 70.88 ± 2.05% (n = 27) and 65.20 ± 4.09% (n = 8) of the predicted current (Fig. 3B) for P2X4FLAGIN and P2X4Y378A, respectively (mean inhibition, 29.12 ± 2.05 and 34.80 ± 4.09%, respectively). A similar degree of inhibition (∼30%) was obtained during sequential application of agonists (i.e. ATP then GABA or GABA then ATP) (Fig. 3B). This cross-inhibition was not voltage-dependent as we observed similar cross-inhibition at holding potentials ranging from −60 to +20 mV (Fig. 3C). Moreover, changes in [Ca2+]internal through P2X4 receptors did not cause this inhibition. We found a similar extent of inhibition with or without external Ca2+ (Fig. 3D; mean inhibition, 34.55 ± 6.61% in normal ringer (n = 10) versus 38.99 ± 5.73% in absence of calcium (n = 10).
Cellular and Molecular Mechanisms Underlying Cross-talk between P2X4 and GABAA Receptors
We investigated the cellular mechanisms that mediate this observed cross-talk between the two receptors. First, we tested the hypothesis that P2X4 and GABAA receptors were physically linked by performing co-immunoprecipitation. Our data revealed that GABAA receptors co-immunoprecipitated with P2X4 subunits in oocytes co-expressing wild-type P2X4 and α2β3 GABAA subunits or α2β3myc GABAA subunits (Fig. 4A, right panel) as well as P2X4FLAGIN and α2β3myc GABAA subunits (see also Fig. 6). Native GABAA and P2X4 receptors were also co-immunoprecipitated from hypothalamic tissue (Fig. 4A, left panel). Furthermore, double immunostaining using anti-P2X4 and anti-β3 antibodies revealed co-expression of P2X4 and β3 GABA subunits in SF-1 GFP-positive neurons (Fig. 4B). It is thus likely that the two distinct receptors physically interact with each other in situ.
FIGURE 4.
Association of P2X4 and GABAA receptors in rat hypothalamus and in vitro. A, Western blots with anti-β3, anti-α2, or anti-myc antibodies revealed co-immunopurification of wild-type α2β3 or α2 myc-tagged β3 GABAA receptors with P2X4 antibodies in oocytes co-expressing both receptor types (left panel) as well as from hypothalamic tissue (HT) (right panel). Left panel, Western blots are revealed with anti-myc and anti-P2X4 antibodies. Right panel, Western blots are revealed with anti-α2 and anti-P2X4 antibodies. No band was detected in oocytes expressing α2β3 receptors alone after P2X4 immunoprecipitation (left panel) or in the absence of P2X4 antibodies (IP−, left and right panel). Images are representative of four independent experiments. B, shown is an image of double immunofluorescence indicating the co-labeling of P2X4 and GABAA β3 subunits in SF-1 GFP-positive neurons (eGFP). Arrows indicate examples of triple-labeled cells (scale bar = 20 μm). IR, immunoreactivity.
FIGURE 6.
Physical association of P2X4 and GABAA receptors and maintenance of current inhibition is dependent on specific residues of P2X4 C terminus in recombinant expression system. A, shown are representative Western blots with anti-FLAG or anti-myc antibodies after immunoprecipitation with anti-myc (IP: myc) or anti-FLAG antibodies (IP: FLAG) from extracts of cells expressing GABAA α2β3myc, P2X4FLAGIN, or P2X4FLAGINYV/ST alone or in combination (A1). Bars represent the relative intensities of the signals observed after immunoprecipitation by anti-myc or FLAG antibodies for both receptor types (Co-IP, co-immunoprecipitation fraction). Data are from four independent experiments; * p < 0.05 (A2). B, sequences of the C-terminal domain of wild-type P2X4 subunits and designed peptides (YV6 and YV6/ST). Adaptor protein 2 binding domain is in bold. C, shown is a Western blot with anti-myc antibody of oocytes expressing α2β3myc receptors after pull down by immobilized peptides (YV6 or YV6/ST) or in the absence of peptides (resin) (C1). Bars indicate that association of α2β3myc is significantly stronger with YV6 than with YV6/ST. * p < 0.05, three independent experiments (C2). ns, not significant. D, inward currents evoked with 100 μm ATP, GABA, or both agonists (Actual) in oocytes co-expressing P2X4FLAGIN and GABAA α2β3 receptors in the absence (Control) or after injection of YV6 peptide (150 μm) (D1). Current inhibition is reduced in the presence of YV6 peptide but not with YV6/ST. **, p < 0.005, n = 5–13. Vm = −60 mV (D2).
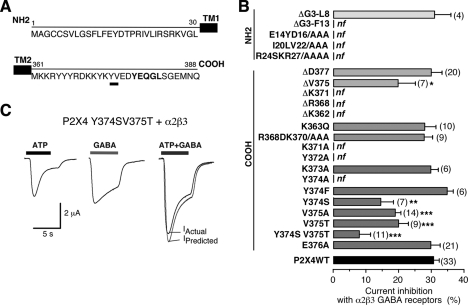
To assess a possible structural basis for the physical interaction between P2X4 and GABAA receptors, we sought to identify the intracellular domains of P2X4 subunit that are involved in the cross-inhibition. We constructed a number of mutated or truncated P2X4 channels of the N- and C-terminal domains and co-expressed functional mutants of P2X4 receptor with α2β3 receptors in oocytes (Fig. 5A). As summarized in Fig. 5B, we noted a similar degree of inhibition between α2β3 and ΔD377 (29.88 ± 3.24%, n = 20) as seen in co-expression of GABAA and wild-type P2X4. In contrast, current inhibition was significantly reduced when we co-expressed ΔV375 with α2β3 receptors (current inhibition = 19. 68 ± 5.39%, n = 7; p < 0.05). It thus appears that residues in the vicinity of amino acid Val375 are involved in the functional cross-talk. Point mutation analysis showed that indeed, two successive residues of the C-terminal domain of P2X4, including Tyr374 and Val375, appear to be required for the cross-inhibition. Single substitution of Tyr374 with serine or Val375 with alanine or threonine caused a significant reduction of the cross-inhibition (mean percentage of inhibition, 14.39 ± 3.96%, n = 7; 18.83 ± 1.74%, n = 14; 19.81 ± 3.05%, n = 9, respectively; Fig. 5B). Importantly, the double mutant P2X4 Y374S/V375T (P2X4YV/ST) showed highly reduced inhibition (7.83 ± 3.39%, n = 11) (Fig. 5, B and C). All other functional alanine mutants, including Lys373 or Glu376, did not alter current inhibition.
FIGURE 5.
Identification of two residues in the carboxyl tail of P2X4 subunits is required for the cross-inhibition. A, P2X4 subunit sequence of the N- and C-terminal tails is shown. Identified residues crucial for the cross-talk are underlined. B, a summary bar graph represents the percentage of current inhibition for wild-type and mutated P2X4 receptors (by deletion Δ or substitution) co-expressed with α2β3 GABA subunits recorded during co-application of ATP + GABA (100 μm each); *, p < 0.05; the number of cells is indicated between the parentheses; nf, non functional mutants. C, representative traces of additive currents recorded between mutant P2X4 Y374S/V375T and GABAA (α2β3) receptors (HP = −60 mV) show that Tyr374 and Val375 are essential for the molecular interaction between P2X4 and GABAA receptors.
We then investigated whether mutation, indeed, disrupted the physical interaction between P2X4 and GABAA receptors in oocytes. Under conditions where P2X4FLAGIN or P2X4FLAGINYV/ST expression levels were similar and they were co-expressed with GABAA α2β3myc receptor subunits, the formation of complexes between P2X4 and GABAA receptors was strongly reduced in the presence of the mutation. As illustrated in Fig. 6, A1 and A2, immunoprecipitation of P2X4FLAGIN-α2β3myc complexes by anti-myc or anti-FLAG antibodies was significantly higher than those observed between P2X4FLAGINYV/ST and α2β3myc complexes. The co-immunoprecipitated fraction for P2X4FLAGINYV/ST and α2β3myc represented 33.08 ± 20.64% (p < 0.05, n = 4 independent experiments) that of those obtained with P2X4FLAGIN and α2β3myc (Fig. 6A2).
Given this YV motif appears to be essential for the physical interaction, we examined the ability of this identified motif within the C-terminal domain of P2X4 subunit to bind to GABAA receptors by performing pulldown assays with YV6 peptide (Fig. 6B). Extracts of oocytes expressing GABA α2β3myc receptors were incubated in the presence or in the absence of immobilized peptides. As illustrated in Fig. 6C1, GABA β3myc subunit mainly co-precipitated with YV6 peptide. The fraction of GABA β3myc that was pulled down with the control peptide YV6YV/ST was similar to the residual fraction obtained in the absence of peptide (resin) (i.e. 34.04 ± 13.50 and 31.55 ± 2.15% that of the fraction detected with YV6 peptide, respectively; Fig. 6C2, p < 0.05, n = 3 independent experiments).
Our electrophysiological analysis further supported a critical role of the YV motif in the cross-talk. In fact, current inhibition of ATP and GABA responses recorded between P2X4FLAGIN- and α2β3-expressing oocytes was abolished after injection of YV6 peptide (Fig. 6, D1 and D2; mean inhibition; control, 21.65 ± 2.89%, n = 13 versus YV6, 5.78 ± 2.19%; n = 8). In contrast, YV6/ST did not cause a significant reduction of the cross-inhibition (17.42 ± 4.5%, n = 5). These results showed that the physical coupling between P2X4 and GABAA receptors results in the functional cross-inhibition.
P2X4 and GABAA Receptor Interaction in SF-1 GFP-positive Neurons
To determine whether native P2X4 receptors interact with GABAA receptors at the synaptic level and whether this interaction regulates synaptic strength in situ, we examined the ability of P2X4 receptors to modulate spontaneous GABAA receptor-mediated IPSCs in SF-1 GFP-positive neurons. We first isolated sIPSCs, which were blocked by the GABAA receptor antagonist picrotoxin (100 μm). We found that introduction of 11C into the SF-1 GFP-positive neurons significantly decreased the frequency and the amplitude of sIPSCs (Fig. 7, A–C and Table 1). This depression developed over time and persisted during our recording period (>15 min). The mean frequency of IPSCs after infusion of 11C represented 41 ± 5% of the onset, which is significantly different from the variation observed in the presence of 3A (85 ± 6% of the onset) or in the absence of peptide (Fig. 7D; 102 ± 3% of the onset; n = 10, 10, and 5 neurons; p < 0.05, one-way ANOVA test). Similarly, the mean amplitude of sIPSC over time decreased to 85 ± 7% that of the onset in the presence of 11C peptide, whereas there was no change in the presence of peptide 3A (108 ± 7% of the onset) or in absence of peptide (105 ± 5% of the onset) (Fig. 7E; n = 10, 10, and 5 neurons; p < 0.05, one-way ANOVA test). In addition, the effect of 11C on sIPSCs was significantly reduced in the presence of the P2X antagonist TNP-ATP (30 μm) (Fig. 7F) (mean frequency; base line, 13.8 ± 2.5 Hz; 11C, 7.1 ± 2.3 Hz; TNP-ATP, 9.4 ± 2.3 Hz; p < 0.05 between 11C and TNP-ATP, n = 4 neurons; mean amplitude; base line, −98.6 ± 34.1 pA; 11C, −49.9 ± 15.2 pA; TNP-ATP, −63.7 ± 21.6 pA; p < 0.05, n = 4 neurons).
FIGURE 7.
Increased P2X4 receptor expression alters GABAergic synaptic efficacy in SF-1 GFP-positive neurons. A–E, blockade of P2X4 receptor internalization depressed GABAergic transmission A, and recordings show GABAergic transmission over time in the presence of 11C (150 μm) (A1). A2, shown are sample traces on an expanded time scale. HP = −70 mV. B, under the same conditions, infusion of control 3A peptide (150 μm) did not alter GABAergic transmission over time (B1). B2, shown are sample traces on an expanded time scale. HP = −70 mV. C, normalized IPSC frequency versus time in the presence of 11C (n = 10) and 3A (n = 10). D and E, changes in the frequency and amplitude of sIPSCs with 11C or 3A at two time points are shown. There was a significant difference among these parameters (p < 0.05, one-way ANOVA test). F, G, and H, 11C-induced GABAergic depression was inhibited by the P2X receptor antagonist TNP-ATP (30 μm). F, recording samples of sIPSCs before and after application of TNP-ATP in the presence of 11C are shown. TNP-ATP reversed the effect of 11C on sIPSCs. G and H, graphs show normalized changes in sIPSC frequency and amplitude of the neurons before and after the application of TNP-ATP. HP = −70 mV.
TABLE 1.
Summary of the effects of 11C and 3A on GABAergic sIPSCs
Mean values ± S.E. of the properties of GABAergic sIPSCs were recorded in the presence of 11C or 3A peptides or in the absence of peptide over time, respectively, from 10, 11, and 5 neurons. Significant statistic differences between 2 and 14 min are indicated.
| 11C |
3A |
No peptide |
||||
|---|---|---|---|---|---|---|
| 2 min | 14 min | 2 min | 14 min | 2 min | 14 min | |
| Frequency (Hz) | 5.2 ± 1.5 | 2.0 ± 0.6a | 5.6 ± 2.3 | 5.2 ± 2.2a | 6.4 ± 2 | 6.8 ± 2.2 |
| Amplitude (−pA) | 58.9 ± 10.2 | 50.0 ± 9.3a | 65.3 ± 13.3 | 68.5 ± 15 | 72.2 ± 11.2 | 74.3 ± 9.7 |
| tw (ms) | 13.5 ± 0.8 | 16.2 ± 0.9a | 14.6 ± 1.6 | 14.3 ± 1.1 | 10.4 ± 1.4 | 10.7 ± 1.5 |
a, p < 0. 05.
The effect of 11C on sIPSCs was also observed under experimental conditions, where [Ca2+]external was lowered from 2.5 to 0.3 mm (see supplemental Fig. S2). It thus appears that the physical interaction rather than increased [Ca2+]internal negatively modulates GABAergic transmission.
As the YV motif appears to be essential for the physical interaction between P2X4 and GABAA receptors, we further examined the effect of the YV6 peptide on P2X4 receptor-mediated depression of GABAergic sIPSCs onto SF-1 GFP-positive neurons. We found that inclusion of both 11C and YV6 peptides (Fig. 8A) into SF-1 GFP-positive neurons prevented the depression of GABAergic sIPSC induced by 11C peptide alone. The frequency revealed a modest enhancement (172 ± 40%, n = 11; Fig. 8D). Furthermore, the effect of 11C was still effective in the presence of the control peptide (YV6/ST) (Fig. 8, B–D; frequency variation. 51 ± 7%; n = 7 neurons). The significant difference of IPSCs frequency was observed under different experimental conditions (with 11C alone, 11C + YV6, and 11C + YV6/ST; n = 10, 11, and 7 neurons, respectively; p < 0.05 one-way ANOVA test; Fig. 8D), suggesting that complex formation between P2X4 and GABAA results in the modulation of GABAergic transmission.
FIGURE 8.
Molecular interaction between P2X4 and GABAA receptors modulates GABAergic synaptic transmission. A, shown is a sample recording of GABAergic sIPSCs in the presence of both 11C and YV6 in the patch pipette (upper panel). The bottom panel is on the expanded time scale. HP = −70 mV. SF-1 GFP-positive neurons showed no change in the frequency and amplitude of sIPSCs. B, sample traces of GABAergic sIPSCs are shown in the presence of 11C and YV6/ST. 11C depressed GABAergic transmission onto SF-1 GFP-positive neurons (upper panel). The bottom panel is on the expanded time scale. HP = −70mV. C, frequency versus time in the presence of 11C with YV6 (150 μm) or YV6/ST (150 μm) revealed that 11C induced depression of IPSCs were abolished with YV6 but not with YV6/ST. D, pooled data of frequency from 11 (11C + YV6) and 7 (11C + YV6/ST) neurons are shown. p < 0.05, one-way ANOVA test.
DISCUSSION
Our present work demonstrates that P2X4 receptor trafficking plays an essential role in the control of neuronal as well as synaptic excitability. More importantly, we provide cellular and physiological evidence that ATP P2X4 receptors directly interact with GABAA receptors, which appears to be critical for the regulation of synaptic strength at the synaptic level. By performing a series of experiments, we first show that P2X4 receptors co-immunoprecipitate with GABAA receptors. And then we demonstrate that these two receptors are co-expressed in SF-1 GFP-positive neurons in the VMH. Our electrophysiological experiments further reveal that there is a negative interaction between the two receptors in recombinant expression system and brain slice preparations. Finally, we provide evidence that two residues (Tyr374, Val375) in the C terminus of P2X4 receptor are required for the physical interaction between P2X4 and GABAA receptors. This motif is absent from other P2X subtypes, including GABAA receptor-interacting P2X2 or P2X3 subunits (18, 21), suggesting that P2X subunit-specific molecular determinants are involved in functional interactions with other ligand-gated channels.
Among P2X receptors, P2X4-containing receptors constitutively cycle into and out of the membrane by a dynamin-dependent mechanism. Consequently, P2X4 receptors are predominantly retained in intracellular compartments and may be up-regulated to the surface in response to physiological or pathological stimuli (Bobanovic 2002). Such a regulation of trafficking of GABA, N-methyl-d-aspartate, or AMPA receptors has been shown to be important in regulation of synaptic transmission (32–34). In addition, incubation with the agonist ATP was shown to accelerate the rate of retrieval of P2X4 (but not P2X2) receptors from the membrane in cultured neurons (26). Given that ATP is released from both neurons and glial cells, including astrocytes (35), both constitutive and/or agonist-regulated cycling of P2X4 may lead to lower occurrence of spontaneous synaptic currents. If this is the case, blockade of P2X4 receptor endocytosis would unmask purinergic transmission. However, inhibition of the membrane trafficking of P2X4 receptors had a minor or no effect on synaptic transmission in our preparations. This was in contrast to an increase in the mean amplitude of ATP responses in the presence of 11C. It thus seems likely that P2X4 subunit-containing receptors may be present at peri- and/or extrasynaptic areas of the neurons in the brain, including SF-1 neurons in our case. Indeed, P2X4 immunogold labeling revealed that the majority of P2X4 receptors were located at the periphery of the synapse of glutamatergic neurons in the brain (4).
If P2X4 subunit-containing receptors are located mainly at peri/extrasynaptic areas, what is the electrophysiological impact of ATP? Exogenous application of ATP depolarized and increased the firing rate of SF-1 GFP-positive neurons. Furthermore, increased membrane expression of P2X4 subunits after inclusion of 11C enhanced SF-1 GFP-positive neuron excitability that was abolished by the P2X receptor antagonist. Hence, there is no doubt about the excitatory effect of ATP. The generation of action potentials would be determined not only by activation of synaptic ligand-gated channels, including glutamate and GABA receptors, but also by activation of extra and/or perisynaptic receptors. Our present data support the idea that, although purinergic synaptic transmission is scarce at central synapses, the activation of P2X4 receptors by ambient ATP would play an important role in the alterations of neuronal activity. If this scenario is true, P2X4 receptors would act as an important means for creating communication between neuronal and glial cells in the brain as ATP is released from glial cells, including astrocytes (35).
In our current work, cross-inhibition between recombinant P2X4 and GABAA receptors was voltage- and ion-independent, which is consistent with the previous studies of other P2X subtypes and cys loop receptor members (12–19, 21). In addition, our biochemical and immunohistochemical experiments in transfected cells and SF-1 GFP-positive neurons further provide the cellular mechanisms that mediate cross-inhibition between the two distinct receptors.
In oocytes, P2X4 activation strongly reduces the amplitude of a subsequent GABA current, whereas the dominant effect of the up- regulation of P2X4 receptors in SF-1 neurons is to reduce GABAergic IPSCs frequency. This may imply a pre-synaptic mechanism of action of P2X4 receptor activation. This inhibition, however, is associated with a significant decrease in the mean amplitude of GABAergic IPSCs. Furthermore, there is a significant change in the mean decay time in the presence of 11C. It should also be noted that we directly introduce the 11C peptide into SF-1 neurons through the patch pipette. Direct infusion of 11C, but not 3A, modulates GABAergic synaptic transmission, and co-injection of 11C and YV6 into SF-1 neurons completely blocks this inhibition. Therefore, it appears that a physical interaction between P2X4 and GABAA receptors at the post-synaptic level may result in this depression of GABAergic currents. Alternatively, it is possible that the final outcome of P2X4 activation by exogenous ATP in oocytes and of up-regulation of P2X4 receptors in SF-1 neurons may be different. In fact, we injected cDNAs coding for P2X4 subunits with each of the GABAA receptor subunits to reach similar levels for both channels in oocytes. However, the expression levels of P2X4 and GABAA receptors in SF-1 neurons appear to be different as the mean amplitude of the responses to P2X4 and GABAA receptor agonists are largely different (−8 ± 2 pA versus −952.5 ± 89 pA, n = 9 and 5 neurons, respectively).5 It is thus plausible that only a subset of GABAA receptors on the membrane may interact with P2X4 receptors.
It has been well described that ligand-gated channels, particularly those permeable to calcium, can trigger signaling cascades, which in turn indirectly affects the function of other receptors. For instance, Ca2+ influx through N-methyl-d-aspartate channels that results in activation of kinases thereby leads to modification of the conductance properties of AMPA receptors (36) or the amplitude of GABAA receptors in in vitro preparations (37, 38. Although P2X4 receptor is considered the most calcium permeable ligand-gated channel (39), our present data showed that calcium entry via P2X4 receptors did not regulate GABAA receptors. In fact, removing calcium from the external solution did not block current inhibition in our both preparations. More importantly, mutation of two identified residues within the C-terminal of P2X4 disrupted the functional and physical association of the two receptors in recombinant expression system. We showed that the peptide containing the identified motif (YV6) was able to pull down GABAA subunits. This peptide readily abolished the cross-inhibition between P2X4 and GABAA receptors, which implies that the peptide interferes with the P2X4 and GABAA complex in recombinant system. Moreover, 11C was no longer effective on GABAergic transmission in the presence of the interference peptide YV6. These are consistent with the fact that physical coupling between intracellular domain of P2X4 and GABAA receptors results in the modulation of synaptic GABAA receptors. Such a physical interaction of the two distinct receptors may represent a novel form of short term synaptic plasticity in which synaptic efficacy depends on two active postsynaptic receptors.
Despite the co-transmission by GABAA and ATP P2X receptor-mediated synapses at hypothalamic synapses, purinergic and GABAergic transmission can be modulated independently from one another (9). This prior study clearly demonstrated that the independent modulation by cholinergic receptors of ATP and GABA release at hypothalamic synapses is a new level of synaptic flexibility in which individual neurons utilize more than one neurotransmitter but retain independent control over their synaptic activity. In addition to the differential regulation of two neurotransmitters at the presynaptic level, our current study further reveals that the reciprocal interaction between ATP P2X4 and GABAA receptors at the post-synaptic level also contributes to the regulation of synaptic strength, thereby regulating neuronal outputs. Of particular interest is that, as ambient ATP can be released from glial cells, our current work suggests that ATP is an important component for neuron-glial communication in the brain. This cross-talk between excitatory and inhibitory receptors may provide a novel mechanism of synaptic plasticity at central synapses.
Supplementary Material
Acknowledgments
We thank Laura Cardoit and Qui Xia for technical help, Christel Poujol and the Bordeaux Imaging Center for confocal imaging, and Marie-Christine Gerbod-Giannone for sharing observation of cross-inhibition between P2X4Y378A and GABAA receptors. We thank Dr. Streamson Chua for critical comments on the manuscript. We thank Dr. François Rassendren for providing mouse P2X4 construct and Dr. Keith Parker for providing the SF-1 GFP mice.
This work was supported by CNRS, Conseil Regional Aquitaine, Agence Nationale de la Recherche Grant ANR-JC-05-0057), and a junior faculty award from the American Diabetes Association (to Y.-H. J.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Y.-H. Jo, E. Donier, A. Martinez, M. Garret, E. Toulmé, and E. Boué-Grabot, unpublished data.
- GABA
- aminobutyric acid
- GABAA
- GABA type A
- SF-1
- steroidogenic factor 1
- VMH
- ventromedial nucleus of the hypothalamus
- sPSC
- spontaneous post-synaptic current
- IPSC
- inhibitory postsynaptic current
- sIPSC
- spontaneous IPSC
- PPADS
- pyridoxal phosphate-6-azophenyl-2′,4′-disulfonate
- TNP-ATP
- trinitrophenyl adenosine-5′-triphosphate
- ANOVA
- analysis of variance.
REFERENCES
- 1. Surprenant A., North R. A. (2009) Annu. Rev. Physiol. 71, 333–359 [DOI] [PubMed] [Google Scholar]
- 2. Illes P., Alexandre Ribeiro J. (2004) Eur. J. Pharmacol. 483, 5–17 [DOI] [PubMed] [Google Scholar]
- 3. Séguéla P., Haghighi A., Soghomonian J. J., Cooper E. (1996) J. Neurosci. 16, 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubio M. E., Soto F. (2001) J. Neurosci. 21, 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burnstock G. (2008) Nat. Rev. Drug. Discov. 7, 575–590 [DOI] [PubMed] [Google Scholar]
- 6. Burnstock G. (2004) Curr. Opin. Pharmacol. 4, 47–52 [DOI] [PubMed] [Google Scholar]
- 7. Hugel S., Schlichter R. (2000) J. Neurosci. 20, 2121–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jo Y. H., Schlichter R. (1999) Nat. Neurosci. 2, 241–245 [DOI] [PubMed] [Google Scholar]
- 9. Jo Y. H., Role L. W. (2002) J. Neurophysiol. 88, 2501–2508 [DOI] [PubMed] [Google Scholar]
- 10. Jo Y. H., Role L. W. (2002) J. Neurosci. 22, 4794–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mori M., Heuss C., Gähwiler B. H., Gerber U. (2001) J. Physiol. 535, 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barajas-López C., Espinosa-Luna R., Zhu Y. (1998) J. Physiol. 513, 671–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodrigues R. J., Almeida T., de Mendonça A., Cunha R. A. (2006) J. Mol. Neurosci. 30, 173–176 [DOI] [PubMed] [Google Scholar]
- 14. Khakh B. S., Zhou X., Sydes J., Galligan J. J., Lester H. A. (2000) Nature 406, 405–410 [DOI] [PubMed] [Google Scholar]
- 15. Karanjia R., García-Hernández L. M., Miranda-Morales M., Somani N., Espinosa-Luna R., Montaño L. M., Barajas-López C. (2006) Eur. J. Neurosci. 23, 3259–3268 [DOI] [PubMed] [Google Scholar]
- 16. Boué-Grabot E., Barajas-López C., Chakfe Y., Blais D., Bélanger D., Emerit M. B., Séguéla P. (2003) J. Neurosci. 23, 1246–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boué-Grabot E., Emerit M. B., Toulmé E., Séguéla P., Garret M. (2004) J. Biol. Chem. 279, 6967–6975 [DOI] [PubMed] [Google Scholar]
- 18. Boué-Grabot E., Toulmé E., Emerit M. B., Garret M. (2004) J. Biol. Chem. 279, 52517–52525 [DOI] [PubMed] [Google Scholar]
- 19. Toulmé E., Soto F., Garret M., Boué-Grabot E. (2006) Mol. Pharmacol. 69, 576–587 [DOI] [PubMed] [Google Scholar]
- 20. Sokolova E., Nistri A., Giniatullin R. (2001) J. Neurosci. 21, 4958–4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toulmé E., Blais D., Léger C., Landry M., Garret M., Séguéla P., Boué-Grabot E. (2007) J. Neurochem. 102, 1357–1368 [DOI] [PubMed] [Google Scholar]
- 22. King B. M. (2006) Physiol. Behav. 87, 221–244 [DOI] [PubMed] [Google Scholar]
- 23. Lein E. S., Hawrylycz M. J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A. F., Boguski M. S., Brockway K. S., Byrnes E. J., Chen L., Chen L., Chen T. M., Chin M. C., Chong J., Crook B. E., Czaplinska A., Dang C. N., Datta S., Dee N. R., Desaki A. L., Desta T., Diep E., Dolbeare T. A., Donelan M. J., Dong H. W., Dougherty J. G., Duncan B. J., Ebbert A. J., Eichele G., Estin L. K., Faber C., Facer B. A., Fields R., Fischer S. R., Fliss T. P., Frensley C., Gates S. N., Glattfelder K. J., Halverson K. R., Hart M. R., Hohmann J. G., Howell M. P., Jeung D. P., Johnson R. A., Karr P. T., Kawal R., Kidney J. M., Knapik R. H., Kuan C. L., Lake J. H., Laramee A. R., Larsen K. D., Lau C., Lemon T. A., Liang A. J., Liu Y., Luong L. T., Michaels J., Morgan J. J., Morgan R. J., Mortrud M. T., Mosqueda N. F., Ng L. L., Ng R., Orta G. J., Overly C. C., Pak T. H., Parry S. E., Pathak S. D., Pearson O. C., Puchalski R. B., Riley Z. L., Rockett H. R., Rowland S. A., Royall J. J., Ruiz M. J., Sarno N. R., Schaffnit K., Shapovalova N. V., Sivisay T., Slaughterbeck C. R., Smith S. C., Smith K. A., Smith B. I., Sodt A. J., Stewart N. N., Stumpf K. R., Sunkin S. M., Sutram M., Tam A., Teemer C. D., Thaller C., Thompson C. L., Varnam L. R., Visel A., Whitlock R. M., Wohnoutka P. E., Wolkey C. K., Wong V. Y., Wood M., Yaylaoglu M. B., Young R. C., Youngstrom B. L., Yuan X. F., Zhang B., Zwingman T. A., Jones A. R. (2007) Nature 445, 168–176 [DOI] [PubMed] [Google Scholar]
- 24. Tong Q., Ye C., McCrimmon R. J., Dhillon H., Choi B., Kramer M. D., Yu J., Yang Z., Christiansen L. M., Lee C. E., Choi C. S., Zigman J. M., Shulman G. I., Sherwin R. S., Elmquist J. K., Lowell B. B. (2007) Cell Metab. 5, 383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frugier G., Coussen F., Giraud M. F., Odessa M. F., Emerit M. B., Boué-Grabot E., Garret M. (2007) J. Biol. Chem. 282, 3819–3828 [DOI] [PubMed] [Google Scholar]
- 26. Bobanovic L. K., Royle S. J., Murrell-Lagnado R. D. (2002) J. Neurosci. 22, 4814–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Royle S. J., Bobanović L. K., Murrell-Lagnado R. D. (2002) J. Biol. Chem. 277, 35378–35385 [DOI] [PubMed] [Google Scholar]
- 28. Royle S. J., Qureshi O. S., Bobanović L. K., Evans P. R., Owen D. J., Murrell-Lagnado R. D. (2005) J. Cell Sci. 118, 3073–3080 [DOI] [PubMed] [Google Scholar]
- 29. Stallings N. R., Hanley N. A., Majdic G., Zhao L., Bakke M., Parker K. L. (2002) Endocr. Res. 28, 497–504 [DOI] [PubMed] [Google Scholar]
- 30. Laurie D. J., Seeburg P. H., Wisden W. (1992) J. Neurosci. 12, 1063–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tobet S. A., Henderson R. G., Whiting P. J., Sieghart W. (1999) J. Comp. Neurol. 405, 88–98 [DOI] [PubMed] [Google Scholar]
- 32. Jacob T. C., Moss S. J., Jurd R. (2008) Nat. Rev. Neurosci. 9, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Groc L., Bard L., Choquet D. (2009) Neuroscience 158, 4–18 [DOI] [PubMed] [Google Scholar]
- 34. Derkach V. A., Oh M. C., Guire E. S., Soderling T. R. (2007) Nat. Rev. Neurosci. 8, 101–113 [DOI] [PubMed] [Google Scholar]
- 35. Zhang Z., Chen G., Zhou W., Song A., Xu T., Luo Q., Wang W., Gu X. S., Duan S. (2007) Nat. Cell Biol. 9, 945–953 [DOI] [PubMed] [Google Scholar]
- 36. Soderling T. R., Derkach V. A. (2000) Trends Neurosci. 23, 75–80 [DOI] [PubMed] [Google Scholar]
- 37. Aguayo L. G., Espinoza F., Kunos G., Satin L. S. (1998) Pflugers Arch. 435, 382–387 [DOI] [PubMed] [Google Scholar]
- 38. Chen Q. X., Wong R. K. (1995) J. Physiol. 482, 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Egan T. M., Khakh B. S. (2004) J. Neurosci. 24, 3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.