Abstract
Matrix metalloproteinase-9 (MMP-9) is important in numerous normal and pathological processes, including the angiogenic switch during tumor development and tumor metastasis. Whereas TNF-α and other cytokines up-regulate MMP-9 expression, interferons (IFNs) inhibit MMP-9 expression. We found that IFN-γ treatment or forced expression of the IFN-induced GTPase, mGBP-2, inhibit TNF-α-induced MMP-9 expression in NIH 3T3 fibroblasts, by inhibiting MMP-9 transcription. The NF-κB transcription factor is required for full induction of MMP-9 by TNF-α. Both IFN-γ and mGBP-2 inhibit the transcription of a NF-κB-dependent reporter construct, suggesting that mGBP-2 inhibits MMP-9 induction via inhibition of NF-κB-mediated transcription. Interestingly, mGBP-2 does not inhibit TNF-α-induced degradation of IκBα or p65/RelA translocation into the nucleus. However, mGBP-2 inhibits p65 binding to a κB oligonucleotide probe in gel shift assays and to the MMP-9 promoter in chromatin immunoprecipitation assays. In addition, TNF-α activation of NF-κB in NIH 3T3 cells is dependent on Rac activation, as evidenced by the inhibition of TNF-α induction of NF-κB-mediated transcription by a dominant inhibitory form of Rac1. A role for Rac in the inhibitory action of mGBP-2 on NF-κB is further shown by the findings that mGBP-2 inhibits TNF-α activation of endogenous Rac and constitutively activate Rac can restore NF-κB transcription in the presence of mGBP-2. This is a novel mechanism by which IFNs can inhibit the cytokine induction of MMP-9 expression.
Keywords: Gene Regulation, Interferon, Matrix Metalloproteinase, NF-κB, Transcription, GTPase, Guanylate-binding Protein, Rac, TNF
Introduction
Interferons (IFNs)2 are a family of cytokines that elicit a wide variety of cellular activities (reviewed in Refs. 1, 2). Best known for their antiviral activities, IFNs also possess anti-proliferative, immunomodulatory, and anti-angiogenic activities. IFNs can also alter cell adhesion and migration, in part by modulating the interactions of cells with their extracellular environment. This can occur by changing the expression levels of cell adhesion molecules, but can also be facilitated by modulating the expression of enzymes that alter the extracellular matrix. Both IFN-α/β (type I IFNs) and IFN-γ (type II IFN) down-regulate the expression of at least three members of the matrix metalloproteinase (MMP) family (3–8). MMPs are a family of Ca2+- and Zn2+-requiring endoproteases that can cleave most of the components of the ECM (9). These proteins are important in such processes as cell migration, proliferation, wound healing, and angiogenesis. The greater than 20 MMPs can be divided into subgroups based on substrate specificity (10). The gelatinases are MMPs with elevated activity against denatured collagens and contain only two members, MMP-2 and MMP-9 (9), both of which can be down-regulated by IFNs. MMP-9, also called gelatinase B, can degrade collagens type IV, V, XIV, aggrecan, elastin, entactin, laminin, and vitronectin (11). Because type IV collagen and laminin are common to all basement membranes, MMP-9 is important in metastasis, tumor growth, and angiogenesis (11–13). MMP-9 expression is frequently elevated in human tumors and correlates with increased metastasis (11, 14, 15). MMP-9 is also associated with the angiogenic switch, which contributes to tumor progression (16). The expression of MMP-9 can be modulated by a variety of cytokines and other agents, in addition to IFNs (12, 17–22).
Cytokines regulate MMP-9 transcription by activating a number of transcription factors that bind to specific promoter elements (12, 23). The promoters of both the human and murine MMP-9 genes have an NF-κB site, two AP-1 sites, an Sp-1 site, and an Ets site (12). These sites are involved in regulation by TNF-α (24), PMA, v-src, ras, and LPS (12, 22). More recently, investigators have demonstrated the importance of chromatin remodeling pathways, including binding of coactivators and histone acetylation, in regulating the expression of MMP-9 (23). The mechanisms by which IFNs inhibit MMP-9 expression are still actively under investigation.
The guanylate-binding proteins (GBPs) are a family of large, unique GTPases induced by both type I and type II IFNs (for review see Ref. 25). Human guanylate-binding protein-1 (hGBP-1) inhibits the expression of MMP-1 in growth factor-stimulated endothelial cells (26). We examined whether the putative murine ortholog, mGBP-2, also inhibits the expression of one or more MMPs. mGBP-2 has previously been shown to promote fibroblast proliferation (27), exhibit mild anti-viral activity (28), confer resistance to the chemotherapeutic drug paclitaxel (29), and inhibit cell spreading (30). In this report, we demonstrate that mGBP-2 inhibits both basal and TNF-α-induced expression of MMP-9 in NIH 3T3 fibroblasts. The inhibition of MMP-9 is primarily at the level of transcription, with mGBP-2 inhibiting NF-κB-mediated DNA binding and transcription. Inhibition of MMP-9 expression does not reflect enhanced stability of IkBα or a failure of p65/RelA to translocate into the nucleus. It is mediated, in part, by an inhibition of TNF-α activation of the small GTPase, Rac, which plays an important role in NF-κB activation by various stimuli. This represents a novel mechanism by which IFN-γ can inhibit the expression of MMP-9.
EXPERIMENTAL PROCEDURES
Cell Lines and Tissue Culture
NIH 3T3 cells (American Type Culture Collection) and control transfectant and mGBP-2-expressing NIH 3T3 cells were generated and cultured as described (27).
Eukaryotic Expression Plasmids
The generation of FLAG-mGBP-2 in pCMV2FLAG(NH) was described (31). The murine MMP-9 promoter in pGL3 (MMP-9 luc) was a kind gift from Dr. Yves St. Pierre (University of Quebec) (32). The plasmids pRK5, pRK5 Rac1(G12V), pRK5 Rac1(T17N), pRK5 Cdc42(G12V), pRK5 Cdc42 (T17N), pRK5 RhoA(G14V), and pRK5 RhoA(T19N) were a gift from Dr. Amy Wilson-Delfosse (Case Western Reserve University, Cleveland, OH). The actin promoter driven β-galactosidase (actin-β-gal) and NF-κB-Luciferase (NF-κB luc) constructs were gifts from Dr. Brian Ashburner (University of Toledo) (33). The NF-κB luc construct has 3 repeats of consensus p65/RelA binding sites from the major histocompatibility complex class I gene upstream of the luciferase reporter gene in pGL3. The NF-κB sites in this reporter plasmid are identical to the NF-κB site of the murine MMP-9 promoter.
Antibodies and Reagents
The following antibodies were obtained from: rabbit anti-mouse MMP-9 antibody (Chemicon International, Temecula, CA), rabbit polyclonal anti-MMP-9 (ab38898: Abcam), rabbit polyclonal anti-p65 NF-κB IgG (sc-372) and anti-IkBα (sc-371) (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-actin antibody (Sigma). Recombinant mouse interferon-γ (rmIFN-γ) was purchased from PBL Biomedical Laboratories (Piscataway, NJ) and recombinant mouse tumor necrosis factor-α (rmTNF-α) was purchased from R&D Systems (Minneapolis, MN).
Indirect Immunofluorescence
Cells were serum starved for 2 h, treated with 10 ng/ml TNF-α for the times listed, fixed, and stained as described previously (31). The antibodies used were rabbit polyclonal anti-p65/Rel A (1:600), and Alexa 488-conjugated goat anti-rabbit (Invitrogen). Images were captured with a Leica DM IRB HC inverted microscope with a cooled Retiga EX Monochrome Digital camera.
Zymography
For zymography, 5 × 105 cells were incubated in serum-free media (SFM) ± rmIFN-γ (500 U/ml) for 24 h. Seven hours before collection, rmTNF-α (10 ng/ml) was added where appropriate. Conditioned media (CM) was cleared of debris by centrifugation at 17,000 × g for 5 min at 4 °C (34). Cleared CM (16 μl each) was resolved on 10% SDS-PAGE gels containing 1 mg/ml Bovine Skin Gelatin (Sigma) under non-reducing conditions. After electrophoresis, the gels were re-natured for 1 h in 50 mm Tris-HCl (pH 7.5) containing 100 mm NaCl and 2.5% Triton X-100, washed three times in distilled water, and incubated for 24 h in developing buffer (renaturing buffer with 10 mm CaCl2) (35). Gels were stained with Coomassie Blue R-250 and destained to visualize enzymatically cleared areas.
PAGE Gels and Western Analysis
CM (65 μl) was separated on SDS-PAGE gels, followed by transfer to PVDF membrane. Membranes were blocked for one hr in TBST (0.025 m Tris pH 7.5, 0.5 m NaCl, 0.05% Tween 20) containing 3% nonfat dry milk, followed by incubation with anti-MMP-9 antibody (1:2000). After washing, the membranes were incubated with HRP-conjugated donkey anti-rabbit antibody (1:2000; Jackson ImmunoResearch Laboratories, West Grove, PA). Chemiluminescence was detected using ECL plus (Amersham Biosciences, Piscataway, NJ). For analysis of MMP-9 expression in CM, 6 ng of purified murine MMP-9 (CC069, Chemicon) was used as a positive control (not shown). Western blots were also performed with anti-IκBα (1:1000), anti-p65 NF-κB (1:400), anti-mGBP-2 (1851; 1:800), and anti-actin (1:3000).
Small Interfering RNA (siRNA) Transfections and Analyses
NIH 3T3 cells (2 × 106) were suspended in Amaxa Cell Line Nucleofector Kit R solution per manufacturer's instructions (Lonza, Basel, Switzerland). Cells were added to 350 nm nontarget siRNA or mGBP-2 SMART pool siRNA (Dharmacon RNA Technologies, Lafayette, CO) in manufacturer supplied cuvettes. Transfections were performed using program U-030 on the Amaxa Nucleofector. The cells were equally divided between four 6-cm dishes. After adhering for at least 3 h, cells were treated with 500 units/ml IFN-γ for 24 h. TNF-α (5 ng/ml) was added for the final 7 h of the IFN-γ treatment, where appropriate. Cells were then lysed and processed for Western blot as described.
Luciferase Assays
Cells (2 × 105/well) were plated overnight in 6-well dishes in complete media. For analysis of IFN-γ and TNF-α effects on the murine MMP-9 minimal promoter, cells were transfected with 0.5 μg each of MMP-9 luc and actin-β-gal per well using FuGene 6 (Roche Applied Science, Indianapolis, IN). After 24 h, the cells were shifted to SFM ± IFN-γ (500 units/ml) for 24 h. After 20 h, 10 ng/ml TNF-α was added, and 4 h later the cells were lysed and processed. To analyze the effects of mGBP-2 and TNF-α on MMP-9 promoter activity, cells were transfected with 0.5 μg of mGBP-2 plasmid (or empty control vector), 0.33 μg actin β-gal plasmid, and 0.33 μg MMP-9 luc per well. To analyze the role of Rho family members on NF-κB transcription, NIH 3T3 cells were transfected with 0.5 μg of mGBP-2-containing plasmid (or empty control vector), 0.5 μg of dominant negative (DN) or constitutively active (CA) Rho family members, 0.33 μg of actin β-gal plasmid, and 0.33 μg of NF-κB luc. After 18 h, 10 ng/ml TNF-α was added for 6 h (DN and CA Rho family) or for 9 h (± mGBP-2 with NF-κB luc). The cells were washed with PBS and lysed in CCLR (25 mm Tris-phosphate (pH 7.8) 2 mm DTT, 2 mm 1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 10% glycerol, 1% Triton X-100; Promega, Madison, WI). Cleared lysates (20 μl) were read on a Lmax luminometer (Molecular Devices, Sunnyvale, CA) after the injection of 100 μl of Luciferase Assay Reagent (Promega) by the P injector. Luminescence was measured for 10 s after a 1.6 s delay and analyzed with the Softmax Pro for Lmax software (Version 1.1L, Molecular Devices). The values were recorded as relative light units (RLUs).
To control for variations in transfection efficiencies, the cells were co-transfected with a β-galactosidase expression plasmid driven by the β-actin promoter. The lysates (5 μl) were added to 50 μl of 1× CCLR and 50 μl of 2× β-gal assay buffer (335.7 mm Na2HPO4, 154.4 mm NaH2PO4, 4.9 mm MgCl2, 256.6 mm β-mercaptoethanol, and 3.3 mg ONPG/ml). Mixtures were incubated at 37 °C for 10–15 min. The reaction was terminated by the addition of 150 μl of 1 m Tris. Absorbance was read at 420 nm using a Vmax Plate Reader (Molecular Devices, Sunnyvale, CA). The results are graphically represented as RLU/OD/μl where the RLU per μl was divided by the OD β-gal/μl. In some experiments the results are expressed as percentage of control where the RLU/OD/μl for each sample is compared with control values.
Quantitative Real Time PCR
Cells (1 × 106/well) were plated in 6-cm dishes and allowed to adhere for 3 h before incubation ± 500 units/ml of IFNγ for 18 h. Total RNA was extracted using 500 μl Trizol reagent (Invitrogen, Carlsbad, CA). SuperScript II First Strand synthesis kit (Invitrogen) was used to generate cDNA and real Time PCR was carried out using 1 μl of the resulting cDNA, 12.5 μl of iQTM Sybr Green (Bio-Rad), and 200 nm each MMP-9 sense and antisense primers (sense-GAGGAAGCCCATTACAGGGCCCCTTC, antisense- CACGCCCCTTGCTGAACAGCAGAG), or 50 nm murine GAPDH sense and antisense primers (sense-CCAGGTTGTCTCCTGCGACT, antisense-ATACCAGGAAATGAGCTTGACAAAGT). PCR was performed utilizing a Bio-Rad iCycler iQ Real Time PCR detection system with an initial incubation of 95 °C for 3.5 min, then 40 cycles of 95 °C for 30 s, 60 °C for 30 s, 74 °C for 30 s. The relative quantity of MMP-9 mRNA was determined using the comparative threshold cycle (Ct) method in which the relative quantity of MMP-9 was normalized to GAPDH by the equation 1.5−ΔΔCt, where ΔΔCt results from the mean Ct of the sample-mean Ct of GAPDH for that sample. The mean Ct is the average of the sample in triplicate. Values are expressed as % GAPDH, which is represented by 1.5−ΔΔCT × 100 (36).
For the time course analysis, cells were treated with 10 ng/ml TNF-α for the time points indicated. RNA was isolated using RNeasy kit (Qiagen) and qRT-PCR was performed using iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad). Primers used were: β-actin (sense-5′-AGTGTGACGTTGACATCCGTA-3′, antisense-5′-GCCAGAGCAGTAATCTCCTTCT-3′) MMP-9 (sense-CTGGACAGCCAGACACTAAAG, antisense-CTCGCGGCAAGTCTTCAGAG) IL-6 (sense-TAGTCCTTCCTACCCCAATTTCC, antisense-TTGGTCCTTAGCCACTCCTTC) CXCL11 (sense-GGCTTCCTTATGTTCAAACAGGG, antisense-GCCGTTACTCGGGTAAATTACA). The mRNA levels were normalized to β-actin expression and presented as average ± S.E. (n = 6).
RT-PCR Analysis for MMP-1
In mice there are two MMP-1-related genes, MMP1A and MMP-1B (37). To determine whether NIH 3T3 cells express MMP-1A or MMP-1B, PCR was performed. cDNA from control and mGBP-2-expressing cells were synthesized as described above. Genomic DNA was extracted as described (38). PCR primers were designed to span an intron to allow detection of genomic DNA. For MMP-1A, the primers were sense-GGATCCAGGTTATCCCAGATTAACAGCAG and antisense-GAGGAAGCCCATTACAGGGCCCTTC, which would amplify a region that spans the intron (129 bp) between exons 9 and 10. For MMP-1B, the primers were sense-GGATTTCCCATGGACATCCAGAGTTTC and antisense- CTGGGGTAACCTGGATCCATGG and would amplify a genomic fragment that spanned the 640 bp intron between exons 8 and 9. The GAPDH primers were described above.
NF-κB DNA Binding Activity Assays
Nuclei were extracted with 20 mm Tris-HCl, pH 7.85, 250 mm sucrose, 0.4 m KCl, 1.1 mm MgCl2, 5 mm β-mercaptoethanol, 1 mm NaF, 1 mm Na3VO4, 1 mm PMSF, 5 mg/ml soybean trypsin inhibitor, 5 mg/ml leupeptin, and 1.75 mg/ml benzamidine and extracts were frozen and stored at −80 °C (39). For electrophoretic mobility shift assays (EMSA), the nuclear extracts were incubated with a [32P]-labeled κB probe (5′-AGTTGAGGGGACTTTCCCAGG-3′) derived from an NF-κB binding sequence in the immunoglobulin gene promoter (40). To define the presence of specific NF-κB proteins, nuclear extracts were pre-incubated with a 1:50 dilution of anti-p65/RelA antibodies at 25 °C for 0.5 h and then subjected to EMSA. Gels were subjected to PhosphorImage autoradiography.
Chromosome Immunoprecipitation Assays for the p65 NF-κB subunit
Cells (∼5 × 106) were treated with 10 ng/ml mouse TNF-α for 30 min. Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP-ITTM Express kit (Active Motif, Carlsbad, CA) according to the manufacturer's instructions. In brief, chromatin from cells was cross-linked with 1% formaldehyde (10 min at 22 °C), and sheared to an average size of ∼500 bp by sonication (Branson Sonifier 250, 20 s pulse × 20 pulses at ∼20% power). Sheared chromatin was incubated with 3 μg of control IgG or anti-p65 (Santa Cruz Biotechnology) and 50 μl of protein G magnetic beads overnight at 4 °C. Immunoprecipitated DNA was purified with the MiniElute PCR Purification kit (Qiagen) and qPCR was performed using iQTM SYBR® Green supermix (Bio-Rad) with the following parameters (enzyme activation: 95 °C 5 min; 40 cycles: 95 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s; and extension: 72 °C for 10min). The following sense and antisense primers for the putative NF-κB site present within the Mmp-9 promoter were designed using Primer 3 plus program: Sense-TCTTTCCTTCCCCAAGGAGT and Antisense-CCATCCCCACACTGTAGGTT. The ifi203 promoter which does not have an NF-κB site was chosen as our control sequence. The primers used for this site were: forward ATTCCCCATTACTGGCTGTG and reverse CCTCAGAGTCTGTTGTTCCTTG. The PCR products of the endogenous MMP-9 and Ifi203 promoter regions are 167 bp and 128 bp, respectively. The levels of anti-p65 bound DNA fragment was normalized to that bound by control IgG for each sample and expressed as average ± S.E. (n = 3).
Rac Activity Assays
Cells (5 × 106) were serum starved for 2 h and treated with 10 ng/ml TNF-α (Chemicon International, Temecula CA) for 5 min. Cells were lysed in 50 mm Tris, pH 7.5, 10 mm MgCl2, 1% IGEPAL CA-630 (Sigma), 150 mm NaCl, 1 mm sodium vanadate, 1 μl/ml protease inhibitor mixture (Sigma), and 1 mm PMSF. Cell lysates (500 μg) were added to 30 μg Pak1 PBD/GST beads in a final volume of 500 μl, and the samples were rotated at 4 °C for 45 min. Rac levels were determined by immunoblot analysis using Kodak one-dimensional Scientific Imaging software. Relative optical intensities for active Rac levels were performed by calculating the pixel intensity of each region of interest with an identical size and shape box for each band. The background was subtracted from the pixel intensities. Total cell lysates (20 μg) were also included. The levels of active Rac from the pulldowns were normalized to total cellular Rac and set to 100% for the control cells for each blot.
RESULTS
IFN-γ and mGBP-2 Inhibit the Basal Expression of MMP-9
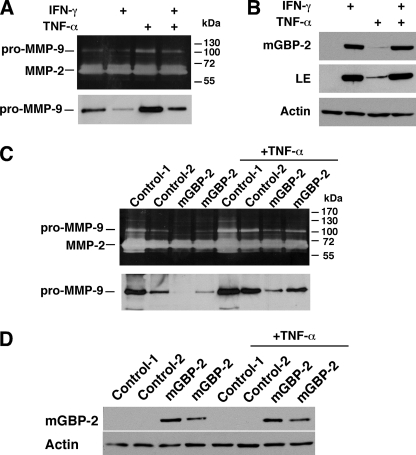
IFNs regulate the expression of the gelatinase MMPs, MMP-2, and MMP-9 (3, 6, 41). To characterize the gelatinases produced by NIH 3T3 cells, gelatin zymography was performed on conditioned media (CM). Enzymatic activities corresponding to pro-MMP-9 (105 kDa) and two different forms of MMP-2 (70 and 65 kDa) were observed (Fig. 1A, top panel). After 24 h of IFN-γ treatment, only the activity of the 105 kDa molecular species was reduced. Western blot analyses of the CM confirmed that the 105-kDa band is MMP-9 and that IFN-γ treatment reduced the level of secreted pro-MMP-9 (Fig. 1A, bottom panel). The amount of pro-MMP-9 secreted by cells is inversely correlated with the amount of mGBP-2 expressed (Fig. 1, A and B). No mGBP-2 was detected in untreated NIH 3T3 cells but mGBP-2 was robustly induced by 24 h IFN-γ treatment (Fig. 1B). To determine whether IFN-γ-induced mGBP-2 expression was responsible for the inhibition of MMP-9, the CM from control and mGBP-2-expressing cells was examined by zymography (Fig. 1C, top panel) and immunoblotting (Fig. 1C, lower panel). While there was some variation in the amounts of pro-MMP-9 secreted by the control transfectants and mGBP-2-expressing cells, mGBP-2 expression correlated with reduced pro-MMP-9 secretion (Fig. 1D). The intracellular levels of MMP-9 were also reduced by IFN-γ treatment or by expression of mGBP-2 (Fig. 2A).
FIGURE 1.
IFN-γ and mGBP-2 expression inhibit MMP-9 expression. A, NIH 3T3 cells were incubated in serum free media (SFM) for 24 h. IFN-γ (500 units/ml) was added with the SFM and 7 h before collection, TNF-α (10 ng/ml) was added. Top panel, CM was harvested and analyzed by zymography. Bottom panel, CM was analyzed by immunoblotting for MMP-9. The blots shown are representative of three experiments. B, cell lysates (20 μg) were analyzed for mGBP-2 expression by immunoblotting. The membranes were stripped and reprobed for actin to verify equal loading. A blot is shown at both short and long (LE) exposures and is representative of three experiments. C, two control transfectant and two mGBP-2-expressing cell lines were incubated in SFM for 24 h, and treated with TNF-α as above. CM was collected and analyzed by zymography (top panel) and Western analysis (bottom panel). Gels shown are representative of three experiments. D, the cells treated in C were analyzed for mGBP-2 expression as described above. A representative blot of three experiments is shown.
FIGURE 2.
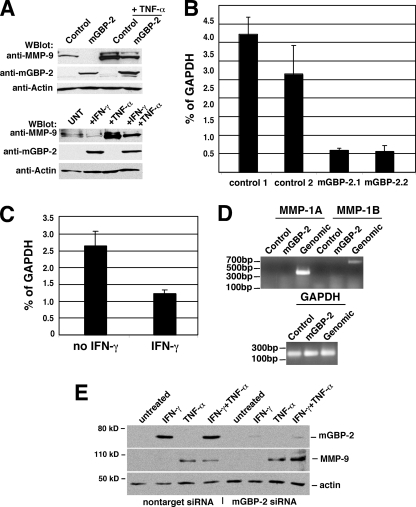
IFN-γ and mGBP-2 expression reduced steady state MMP-9 RNA. A, control transfectants and mGBP-2-expressing cells ± TNF-α (10 ng/ml) for 7 h were lysed and analyzed by immunoblotting for MMP-9, mGBP-2, and actin (top panel). NIH 3T3 cells ± IFN-γ (500 units/ml) for 24 h, TNF-α (10 ng/ml) for 7 h, or the combination of TNF and IFN where TNF is added for the final 7 h of the IFN treatment were analyzed as described above (bottom panel). B, real-time RT-PCR analysis of MMP-9 steady state RNA levels. Total RNA was extracted from control transfectant and mGBP-2-expressing NIH 3T3 cells. Real time-RT-PCR results are presented as percent of GAPDH. The analyses were performed three times, and the graph shows a representative experiment. The bars represent mean percent GAPDH ± standard errors for the triplicate samples calculated as described. C, total RNA was extracted from NIH 3T3 cells ± IFN-γ treatment (500 units/ml for 18 h) and analyzed as described above. D, the presence of either of the two related murine MMP-1 related proteins was examined by RT-PCR with primers specific for each isoform. Amplification from genomic DNA confirms that the primers work and amplification of GAPDH confirms that the first strand cDNA synthesis was good. D, NIH 3T3 cells were transfected with nontarget or mGBP-2-specific siRNAs as described under “Experimental Procedures.” Cells were treated ± IFN-γ (500 units/ml) for 24 h with 10 ng/ml of TNF-α added for the last 7 h, where appropriate. Total cell lysates were analyzed by sequential immunoblotting for MMP-9, mGBP-2, and actin. A representative result is shown.
Whether the reduction in secreted and intracellular pro-MMP-9 protein reflected changes in the steady state levels of MMP-9 RNA was determined by real-time RT-PCR. In cells expressing mGBP-2 there is a significant reduction in MMP-9 RNA compared with control transfectants (Fig. 2B). As expected, treatment with IFN-γ also resulted in reduced levels of MMP-9 RNA (Fig. 2C).
IFN-γ and mGBP-2 Inhibit TNF-α Induction of MMP-9
TNF-α induces the expression of MMP-9 in many cell types (21, 32, 42, 43), which can be inhibited by IFN-γ treatment (8, 41). NIH 3T3 cells treated with TNF-α showed greater secreted and intracellular pro-MMP-9 than untreated cells (Figs. 1A and 2A). Pretreatment with IFN-γ inhibited TNF-α induction of pro-MMP-9 (Figs. 1A and 2A). While TNF-α induces the expression of several GBPs in NIH 3T3 cells, mGBP-2 is only slightly induced (Fig. 1B LE). In addition to inhibiting basal expression of MMP-9, mGBP-2 expression also inhibited TNF-α induction of MMP-9 (Figs. 1C and 2A).
mGBP-2 is the putative ortholog of hGBP-1, which down-regulates MMP-1 expression (26). A RT-PCR approach was used to determine MMP-1 expression in NIH 3T3 cells (Fig. 2D). No MMP-1 related RNAs could be detected in NIH 3T3 cells. Confirming that the primers worked, appropriately sized PCR products were amplified from genomic DNA.
Knockdown of mGBP-2 in IFN-γ-treated Cells Restores TNF-α Induction of MMP-9
To confirm the significance of mGBP-2 in the IFN-γ-mediated inhibition of MMP-9 induction by TNF-α, NIH 3T3 cells were transfected with nontarget or mGBP-2-specific siRNA and the ability of TNF-α to induce MMP-9 expression after exposure to IFN-γ was examined (Fig. 2E). Nontarget siRNAs had no effect on the induction of mGBP-2 by IFN-γ and, as expected, pretreatment with IFN-γ inhibited TNF-α induction of MMP-9. The mGBP-2-specific siRNAs reduced the expression of mGBP-2 by IFN-γ by over 85–90% and rescued the expression of MMP-9. These findings confirm a role for mGBP-2 in IFN-γ-mediated inhibition of MMP-9 induction by TNF-α.
mGBP-2 Inhibits Basal and TNF-α-induced Transcription Driven by the Murine MMP-9 Promoter
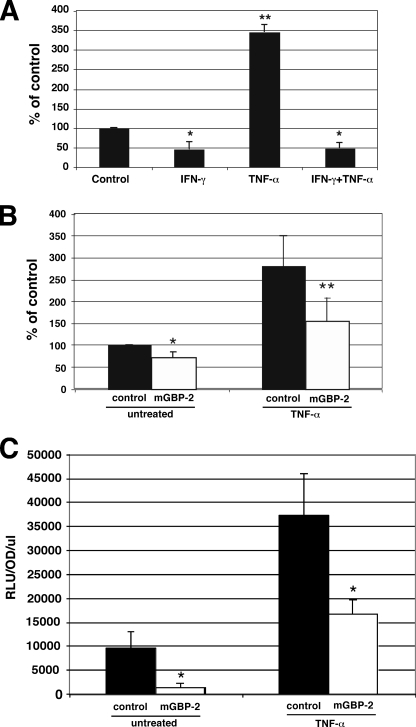
Many normal cells do not express detectable MMP-9, so little is known about the regulation of basal MMP-9 expression. To determine whether IFN-γ and/or mGBP-2 could regulate the murine MMP-9 minimal promoter when expressed in NIH 3T3 cells, luciferase assays were performed. After treatment with IFN-γ for 24 h, the activity of the MMP-9 minimal promoter was reduced to 46 ± 18% of untreated control (Fig. 3A). In similar experiments, mGBP-2 reduced the luciferase expression driven by the MMP-9 promoter to 71 ± 13% of control values (Fig. 3B).
FIGURE 3.
IFN-γ and mGBP-2 expression inhibit MMP-9 transcription. A, NIH 3T3 cells were transfected with MMP-9 luc and actin β-gal. After 24 h the cells were incubated in SFM for 24 h ± IFN-γ (500 units/ml). Where used, TNF-α (10 ng/ml) was added for the final 4 h. Cells were lysed and processed for luciferase activity. Results are presented as percent of control activity after normalization for β-galactosidase activity (n = 4; *, p < 0.05; **, p < 0.01 compared with control). B, NIH 3T3 cells were transfected with actin β-gal, MMP-9 luc, and control vector or mGBP-2. After 24 h, cells were incubated in SFM for 1 h before addition of TNF-α (10 ng/ml) for an additional 6 h. MMP-9 promoter activity was analyzed by luciferase activity. Results are presented as percent of untreated control activity after normalization for β-galactosidase activity (n = 4; *, p < 0.05 compared with untreated control; **, p < 0.05 compared with TNF-α-treated control). C, control transfectants and mGBP-2-expressing cells were transfected with NF-κB luc and actin β-gal as described. After 24 h, cells were left untreated or were treated with TNF-α (10 ng/ml) for 9 h and processed for luciferase activity as described. Results are presented as the mean RLU/OD/μl ± S.D. (n = 3; *, p < 0.05 compared with control).
TNF-α induction of the murine MMP-9 promoter has been previously demonstrated in rat C6 cells (32). We found that TNF-α could also induce MMP-9 promoter activity in NIH 3T3 cells to 346.4 ± 19.8% of the level of activity in untreated cells (Fig. 3A). Pretreatment with IFN-γ completely inhibited the ability of TNF-α to induce the MMP-9 promoter (48.2 ± 14.7% of control; Fig. 3A). mGBP-2 expression was also able to significantly inhibit the induction of MMP-9 promoter activity by TNF-α (Fig. 3B).
mGBP-2 Inhibits NF-κB-mediated Transcription
TNF-α induction of MMP-9 proceeds in part through NF-κB activation (42, 44, 45), and inhibiting NF-κB reduces MMP-9 production (45). Therefore, a NF-κB-dependent reporter construct was used to determine the role of NF-κB in the inhibition of the MMP-9 by mGBP-2. mGBP-2 expression inhibited NF-κB mediated transcription under basal conditions by about 85% (Fig. 3C). As expected, TNF-α increased NF-κB mediated transcription greater than 3.5-fold in the absence of mGBP-2 (Fig. 3C). The presence of mGBP-2 inhibited the ability of TNF-α to promote NF-κB-mediated transcription by greater than 2-fold (Fig. 3C).
mGBP-2 Inhibits p65/RelA binding in Vitro and in Vivo
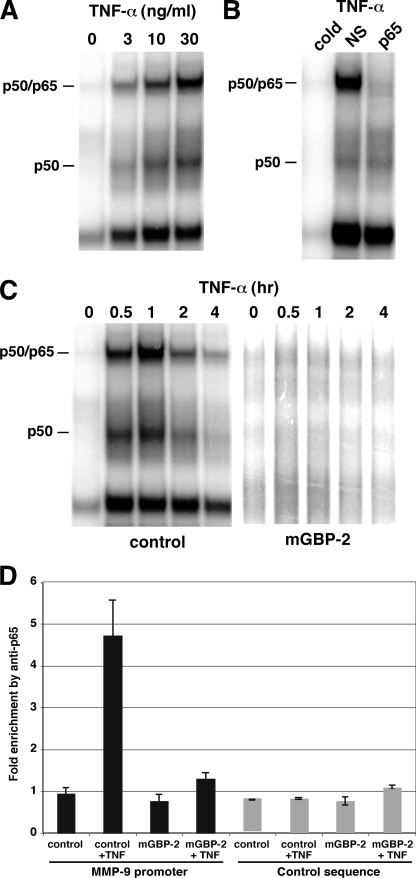
To further explore the inhibition of NF-κB by mGBP-2, electrophoretic mobility shift assays (EMSAs) were used to examine the ability of nuclear extracts of mGBP-2-expressing cells to bind to a synthetic NF-κB oligonucleotide probe (Fig. 4, A–C). In the absence of mGBP-2, NIH3T3 cells treated with TNF-α showed dose-dependent binding of p65 with maximum binding at 30 ng/ml (Fig. 4A). Supershift analysis with anti-p65 demonstrated the presence of p65 in TNF-treated nuclear extracts, consistent with our previous studies (40). Treatment of control transfectants with 10 ng/ml of TNF-α induced NF-κB binding within 30 min of addition, and the level of NF-κB binding peaked at about 1 h and then declined through 4 h (Fig. 4C). In contrast, in cells expressing mGBP-2, no binding to the oligonucleotide probe was observed (Fig. 4C).
FIGURE 4.
mGBP-2 expression inhibits NF-κB p65/RelA binding. A, nuclear extracts were prepared from control transfectants and mGBP-2-transfected cells treated with various concentrations of TNF-α for 30 min and subjected to EMSA with a consensus NF-κB oligonucleotide probe. B, nuclear extracts were prepared from TNF-treated cells (30 min, 10 ng/ml) and incubated with anti-p65 antibody, control IgG (NS) or a 50-fold excess of unlabeled κB oligonucleotide probe (cold) prior to EMSA. C, nuclear extracts were prepared from control transfectants and mGBP-2-transfected cells treated with TNF-α (10 ng/ml) for 30 min and were analyzed for p65/RelA binding to the MMP-9 promoter by ChIP. The binding of p65 to the endogenous MMP-9 promoter is greatly reduced in the presence of mGBP-2. The levels of anti-p65 bound DNA fragments were normalized to that bound by control IgG in each sample and expressed as average ± S.E. (n = 3).
To determine whether mGBP-2 expression inhibits p65/RelA binding to the MMP-9 promoter in vivo, ChIP assays were performed on chromatin prepared from control transfectants and mGBP-2-expressing cells treated with TNF-α. As shown in Fig. 4D, treatment of control transfectants with TNF-α (30 min) induced the binding of p65/RelA to the MMP-9 promoter, while there was no detectable p65/RelA binding to the promoter in untreated control cells. In contrast, in cells expressing mGBP-2, only low levels of TNF-induced p65 binding was observed.
IκBα Degradation after TNF-α Treatment Is Not Inhibited by mGBP-2
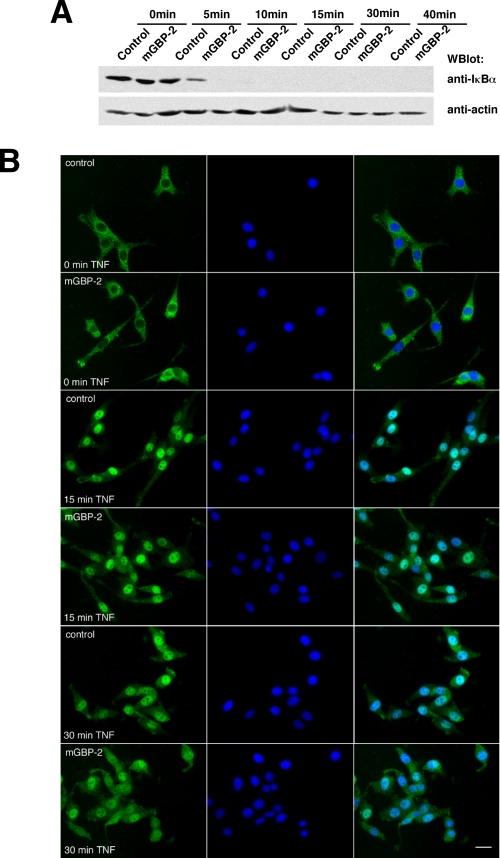
NF-κB is normally latent in the cytoplasm bound to the IκB complex. Various stimuli, including TNF-α treatment, activate the IKK complex, which results in the phosphorylation and degradation of IkBα. We asked whether mGBP-2 inhibited NF-κB-mediated transcription by inhibiting the degradation of the inhibitor IκBα, thereby inhibiting NF-κB release (Fig. 5A). Treatment with TNF-α resulted in a rapid loss of IκBα, becoming undetectable within 10 min. Expression of mGBP-2 did not affect TNF-induced levels of IκBα. Therefore, inhibition of p65 binding to DNA in mGBP-2-expressing cells was not a consequence of inhibiting IκBα degradation.
FIGURE 5.
mGBP-2 expression does not inhibit IκBα degradation or movement of p65/Rel A to the nucleus. A, control transfectants and mGBP-2-expressing cells were incubated in SFM for 18 h followed by treatment with TNF-α (10 ng/ml) for 0, 5 10, 15, 30, or 40 min. Cell lysates (20 μg) were analyzed for IκBα by Western blot. Membranes were stripped and reprobed with anti-actin to verify equal protein per lane. The results of a representative experiment (n = 3) are shown. B, control transfectants and mGBP-2-expressing cells were serum-starved for 2 h, treated with TNF-α (10 ng/ml) for 0, 15, and 30 min, and analyzed for the distribution of p65 by indirect immunofluorescence. Left panel p65 stained cells, middle panel contain cells with DAPI stain, and the right panel is the overlay. A representative of two experiments is shown.
mGBP-2 Does Not Block Movement of p65/RelA into the Nucleus upon TNF-α Treatment
Once released from IκBα, NF-κB translocates from the cytoplasm into the nucleus to bind DNA and activate transcription. To examine the movement of p65 into the nucleus of cells after TNF-α treatment, control transfectants and mGBP-2-expressing cells were treated with TNF-α and the intracellular distribution of p65 was examined by indirect immunofluorescence (Fig. 5B). Within 15 min, p65 translocated into the nuclei of all TNF- treated cells. mGBP-2 expression did not inhibit p65 translocation into the nucleus.
mGBP-2 Inhibits the TNF-α Activation of NF-κB-regulated Genes
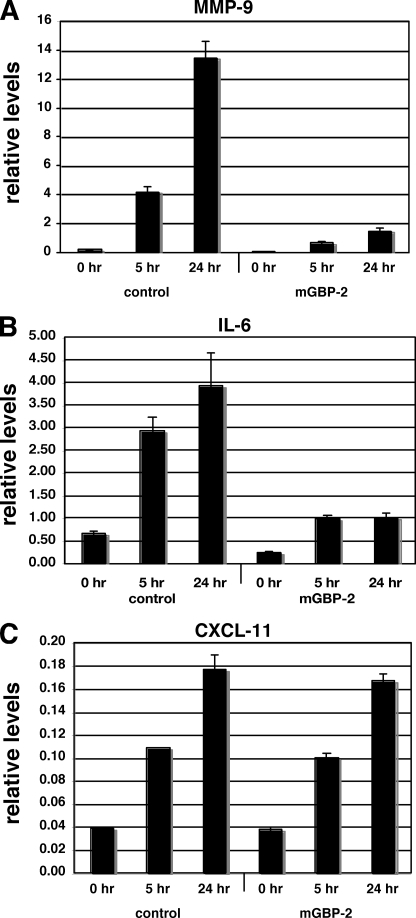
To determine whether mGBP-2 inhibited TNF-α induction of other genes that were regulated by NF-κB, control transfectants and mGBP-2-expressing cells were treated with TNF-α for the times indicated, total RNA prepared, and gene expression was determined by qPCR. As predicted, TNF-α treatment induced the transcription of MMP-9 in control transfectants (Fig. 6A) and induction was severally inhibited by mGBP-2 expression. The cytokine IL-6 is also induced by TNF-α treatment and the primary transcription factor involved in this induction is NF-κB (46, 47). IL-6 transcription is induced by TNF-α in control cells but the induction is significantly inhibited in the presence of mGBP-2 (Fig. 6B). The chemokine CXCL11 (also known as beta-R1, I-TAC, or H-174) is a TNF-α responsive gene that contains an NF-κB site in its promoter (48, 49). In control transfectants, CXCL11 gene expression is induced by TNF-α but its expression is unaffected by mGBP-2 expression (Fig. 6C). This suggests that mGBP-2 expression inhibits the TNF-α induction of a subset of NF-κB responsive genes.
FIGURE 6.
mGBP-2 expression inhibits the TNF-α induction of a subset of TNF-α responsive genes. Control transfectants and mGBP-2-expressing cells were treated with 10 ng/ml TNF-α for the times indicated. Total RNA was isolated and analyzed by qRT-PCR as described under “Experimental Procedures.” A, treatment with TNF-α significantly induces MMP-9 RNA levels over 24 h. This induction is severally attenuated in the presence of mGBP-2. B, treatment with TNF-α induces the transcript of the IL-6 gene but this is severally diminished in the presence of mGBP-2. C, TNF-α treatment induces the expression of CXCL11 RNA, and this is not affected by the presence of mGBP-2. mRNA levels were normalized to β-actin and presented as average ± S.E. (n = 6).
Rac Is Required for TNF-α Induction of NF-κB-mediated Transcription in NIH 3T3 Cells
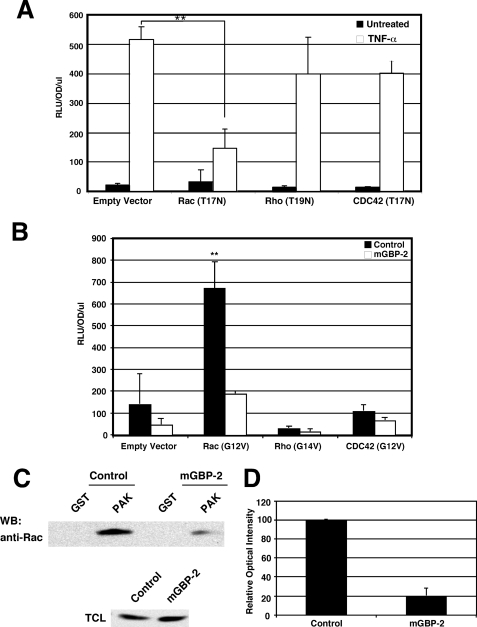
The small GTPase Rac is a component of a TNF-α signal transduction cascade (42, 50). In fact, Rac is required for the activation of NF-κB by a variety of stimuli (51–54). To determine whether Rac is required for TNF-α activation of NF-κB-mediated transcription in NIH 3T3 cells, cells were co-transfected with the NF-κB-driven luciferase reporter construct and dominant-negative (DN) constructs of Rac (T17N), Rho (T19N), and Cdc 42 (T17N). While DN-Rho and DN-Cdc42 did not significantly inhibit TNF-α-induced NF-κB-dependent transcription, DN-Rac1 markedly inhibited TNF-induced NF-κB activity (Fig. 6A). This demonstrates a requirement for Rac1 in NF-κB activation by TNF-α.
mGBP-2 Inhibits Rac Activation of NF-κB
We asked whether mGBP-2 inhibits NF-κB activation in NIH 3T3 cells by inhibiting Rac activity. NIH 3T3 cells were transfected with constitutively active forms of RhoA, Rac1, and Cdc42 in the presence or absence of mGBP-2 and monitored for NF-κB activation (Fig. 7B). Only Rac1(G12V) activated NF-κB above control levels and was able to restore NF-κB transcription to control levels in the presence of mGBP-2 (Fig. 7B). This suggests that mGBP-2 inhibits NF-κB activity in part by acting at the level of Rac. To confirm that mGBP-2 inhibits TNF-α activation of Rac, the relative levels of active Rac were measured in control transfectants and mGBP-2-expressing cells following TNF-α treatment. mGBP-2 lowered the level of active Rac in TNF-α treated cells by about 80% (Fig. 7, C and D).
FIGURE 7.
mGBP-2 expression inhibits Rac activation by TNF-α. A, NIH 3T3 cell were transfected with NF-κB luc, actin-β-gal, and the vectors listed. After 18 h the cells were treated with 10 ng/ml TNF-α for 6 h and cell lysates were analyzed for luciferase activity. Results presented are the mean relative fluorescence after normalization for β-gal activity ± S.D. (n = 3; **, p < 0.01 compared with TNF-α-treated cells with empty vector). B, NIH 3T3 cells were transfected with NF-κB luciferase, actin β-gal, mGBP-2, or pCMV vector, and Rac1 (G12V), RhoA (G14V), Cdc42 (G14V), or pRK5 plasmid. After 24 h the cells were incubated in SFM for 24 h, lysed, and processed for luciferase activity as described. Results presented are the mean relative fluorescence after normalization for β-gal activity ± S.D. (n = 4; **, p < 0.01 compared with basal luciferase in absence of mGBP-2 or Rho family member). C, control and mGBP-2-expressing cells were serum-starved for 2 h followed by treatment with 10 ng/ml TNF-α for 5 min. Cell lysates were analyzed for relative levels of active Rac as described under “Experimental Procedures.” A representative blot from three experiments is shown. D, relative optical intensities were determined, and the results are presented as means ± S.D. (n = 3).
This is the first report of a novel mechanism for IFN-γ-mediated inhibition of MMP-9 expression, the induction of mGBP-2. mGBP-2 inhibits MMP-9 transcription, at least in part, by inhibition of NF-κB p65/RelA binding to the MMP-9 promoter. This inhibition is not the consequence of failure to transport p65 into the nucleus. The coincident inhibition of Rac1 by mGBP-2 suggests that post-translational modifications of p65 necessary for optimal DNA binding and transcription are inhibited.
DISCUSSION
MMPs are important proteins in tissue remodeling during both normal and pathological processes. They also modulate the availability and activity of growth factors. In particular, MMP-9 is believed to direct the “angiogenic switch,” and its up-regulation in tumor cells correlates with progression and metastasis (reviewed in Refs. 9, 10, 12). Consistent with this, MMP-9 null mice show less angiogenesis and metastasis of LLC- or B16-derived tumors from their primary sites (55). Despite the biological importance of MMP-9, much remains unclear about its regulation. A variety of cytokines, growth factors, ras, and c-src can up-regulate MMP-9 expression, but few repressors of MMP-9 expression have been identified. The metastasis suppressor, KiSS-1, inhibits MMP-9 expression by inhibiting NF-κB translocation to the nucleus subsequent to increasing IκBα levels (56). Interestingly, KiSS-1 is unable to inhibit TNF-α-induced expression of MMP-9 (56). Recently, transgelin (SM22) was identified as a MMP-9 inhibitor (57). Transgelin is a small actin-binding protein that inhibits AP-1-dependent transcription of MMP-9 (57). In addition, type I IFNs (IFN-α/β) and type II IFN (IFN-γ) can inhibit cytokine-induced MMP-9 expression. This repression appears to be complicated, with multiple IFN-stimulated proteins involved.
An early event following IFN binding to its receptor is the activation of STAT1 (1). Treatment of HeLa cells with IFN-γ suppresses PMA induction of MMP-9 by activating STAT1α, which inhibits MMP-9 transcription by binding to and inhibiting the recruitment of the CBP/p300 co-activator to the MMP-9 promoter (58), which in turn inhibits the formation of the transcription complex required to initiate MMP-9 transcription. Sp-1, NF-κB, and AP-1 binding to the MMP-9 promoter are not inhibited by IFN-γ but recruitment of CBP/p300 and histone acetylation was (58). This ultimately resulted in reduced recruitment of RNA polymerase II. The inhibition by STAT1α is a relatively early event after IFN-γ exposure, requiring less than 4 h. In addition, activated STAT1α can induce the expression of a variety of genes, including the transcription factor interferon-responsive factor-1 (IRF-1). IRF-1 is a transcriptional activator and tumor suppressor (59, 60). IRF-1 inhibits MMP-9 transcription in EW-1 cells by competing with the p65 subunit of NF-κB for binding to the MMP-9 promoter (41). In both the mouse and human MMP-9 promoter, the NF-κB site contains a modified interferon-stimulated response element (ISRE), called an IRE. In addition to IRF-1, another IFN-induced protein down-regulates TNF-α-induced MMP-9 expression in antigen presenting cells, the class II major histocompatibility complex transactivator (CIITA) (61). CIITA expression is induced by IFN-activated STAT1α, and like STAT1α, CIITA binds to CBP and inhibits both its recruitment to the MMP-9 promoter and histone acetylation.
Little is known about the functions of mGBP-2. Whereas mGBP-2 is not expressed in NIH 3T3 cells prior to IFN exposure (27, 62), mGBP-2 is induced to detectable levels by about 4 h of IFN-γ treatment (62). mGBP-2 is therefore expected to be important in inhibiting MMP-9 at later times after IFN exposure than either STAT1 or IRF-1.
However, as with STAT1 and IRF-1, the target of mGBP-2 is NF-κB-mediated transcription of MMP-9. In the presence of mGBP-2, TNF-α induced IkBα degradation, release of NF-κB and translocation into the nucleus are not inhibited. However, mGBP-2 expression does inhibit the binding of p65 to the NF-κB element in the MMP-9 promoter. This inhibition of NF-κB is accompanied by inhibition of Rac activation.
In the present study we show that TNF-induced NF-κB activation requires Rac activity, which has been shown for a variety of other stimuli (63, 64). However, the precise molecular mechanism whereby Rac activates NF-κB remains unclear. Other studies suggest that Rac activation of NF-κB may be the consequence of NIK-mediated IKKβ activation (65), PAK activation (66, 67), and activation of the PI3-K/Akt pathway (68). Other studies suggest that TLRs transactivation of NF-κB requires Rac but is independent of IκBα degradation (54). Rac-induced reactive oxygen species (ROS) may be involved in NF-κB activation (52, 69), as well as Rac-activated MAPKs (70), but the pathway involved in NF-κB activation remains unclear.
Taken together our results identify a novel mechanism for IFN-induced repression of MMP-9 expression. The induction of mGBP-2 by IFN-γ results in the inhibition of Rac activation by TNF-α, which inhibits the full activation of NF-κB. Not unexpectly the inhibition of NF-κB resulted in the inhibition of other NF-kB-dependent, TNF-α induced genes, besides MMP-9. Recently IFN-γ-induction of mGBP-2 was also shown to inhibit Rac activation downstream of both integrin engagement during cell spreading and after PDGF treatment (30). This suggests that the IFN-γ-mediated inhibition of Rac by mGBP-2 is important in inhibiting the induction of MMP-9 and may constitute a more universal mechanism for IFN-γ to inhibit/dampen responses to subsequent exposures to cytokines, growth factors, and integrins.
Acknowledgments
We thank Dr. Brian Ashburner for advice on luciferase assays and for the use of his luminometer. We would also like to thank Dr. Alan Wolfman and members of the Vestal Laboratory for helpful discussions.
This work was supported by a grant from the American Cancer Society (RPM-CIM-88031) (to D. J. V.), National Institutes of Health Grant CA73753, and funds from the Muirhead Chair Endowment and the University of Tennessee Health Science Center (to L. M. P.).
- IFN
- interferon
- GBP
- guanylate-binding protein
- MMP
- matrix metalloproteinase
- SFM
- serum-free media
- TNF
- tumor necrosis factor.
REFERENCES
- 1. Stark G. R., Kerr I. A., Williams B. R., Silverman R. H., Schreiber R. D. (1998) Annu. Rev. Biochem. 67, 227–264 [DOI] [PubMed] [Google Scholar]
- 2. Sen G. C., Ransohoff R. M. (1993) Adv. Virus Res. 42, 57–102 [DOI] [PubMed] [Google Scholar]
- 3. Ma Z., Qin H., Benveniste E. N. (2001) J. Immunol. 167, 5150–5159 [DOI] [PubMed] [Google Scholar]
- 4. Karabudak R., Kurne A., Guc D., Sengelen M., Canpinar H., Kansu E. (2004) J. Neurol. 251, 279–283 [DOI] [PubMed] [Google Scholar]
- 5. Yaushchenko M., Mäder M., Elitok E., Bitsch A., Dressel A., Tumani H., Bogumil T., Kitze B., Poser S., Weber F. (2003) J. Neurol. 250, 1224–1228 [DOI] [PubMed] [Google Scholar]
- 6. Wu A. J., Lafrenie R. M., Park C., Apinhasmit W., Chen Z. J., Birkedal-Hansen H., Yamada K., Stetler-Stevenson W. G., Baum B. J. (1997) J. Cell. Physiol. 171, 117–124 [DOI] [PubMed] [Google Scholar]
- 7. Bauvois B., Dumont J., Mathiot C., Kolb J.-P. (2002) Leukemia 2002, 791–798 [DOI] [PubMed] [Google Scholar]
- 8. Zhou M., Zhang Y., Ardans J. A., Wahl L. M. (2003) J. Biol. Chem. 278, 45406–45413 [DOI] [PubMed] [Google Scholar]
- 9. Vu T. H., Werb Z. (2000) Genes Dev. 14, 2123–2133 [DOI] [PubMed] [Google Scholar]
- 10. Egeblad M., Werb Z. (2002) Nat. Rev. Cancer 2, 161–174 [DOI] [PubMed] [Google Scholar]
- 11. Sternlicht M. D., Werb Z. (2001) Annu. Rev. Cell Dev. Biol. 17, 463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van den Steen P. E., Dubois B., Nehlissen I., Rudd P. M., Dwek R. A., Opdenakker G. (2002) Crit. Rev. Biochem. Mol. Biol. 37, 375–536 [DOI] [PubMed] [Google Scholar]
- 13. Wang T. N., Albo D., Tuszynski G. P. (2002) Surgery 132, 220–225 [DOI] [PubMed] [Google Scholar]
- 14. Kupferman M. E., Fini M. E., Muller W. J., Weber R., Cheng Y., Muschel R. J. (2000) Amer. J. Pathol. 157, 1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bachmeier B. E., Nerlich A. G., Lichtinghagen R., Sommerhoff C. P. (2001) Anticancer Res. 21, 3821–3828 [PubMed] [Google Scholar]
- 16. Bergers G., Brekken R., McMahan G., Vu T. H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. (2000) Nat. Cell Biol. 2, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsuruda T., Costello-Boerrigter L. C., Burnett J. C. (2004) Heart Failure Reviews 9, 53–61 [DOI] [PubMed] [Google Scholar]
- 18. Nguyen J., Knapnougel P., Lesavre P., Bauvois B. (2005) FEBS Letters 579, 5487–5493 [DOI] [PubMed] [Google Scholar]
- 19. Nelissen I., Ronsse I., Van Damme J., Opdenakker G. (2002) J. Leukoc. Biol. 71, 89–98 [PubMed] [Google Scholar]
- 20. Migita K., Maeda Y., Abiru S., Nakamura M., Komori A., Yokoyama T., Takii Y., Mori T., Yatsuhashi H., Eguchi K., Ishibashi H. (2006) Life Sciences 78, 2510–2515 [DOI] [PubMed] [Google Scholar]
- 21. Leber T. M., Balkwill F. R. (1998) Br. J. Cancer 78, 724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woo C. H., Lim J. H., Kim J. H. (2004) J. Immunol. 173, 6973–6980 [DOI] [PubMed] [Google Scholar]
- 23. Ma Z., Shah R. C., Chang M. J., Benveniste E. N. (2004) Mol. Cell. Biol. 24, 5496–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moon S. K., Cha B. Y., Kim C. H. (2004) J. Cell. Physiol. 198, 417–427 [DOI] [PubMed] [Google Scholar]
- 25. Vestal D. J. (2005) J. Interferon Cytokine Res. 25, 435–443 [DOI] [PubMed] [Google Scholar]
- 26. Guenzi E., Töpolt K., Lubeseder-Martellato C., Jörg A., Naschberger E., Benelli R., Albini A., Stürzl M. (2003) EMBO J. 22, 3772–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gorbacheva V. Y., Lindner D., Sen G. C., Vestal D. J. (2002) J. Biol. Chem. 277, 6080–6087 [DOI] [PubMed] [Google Scholar]
- 28. Carter C. C., Gorbacheva V. Y., Vestal D. J. (2005) Arch. Virol. 150, 1213–1220 [DOI] [PubMed] [Google Scholar]
- 29. Balasubramanian S., Nada S., Vestal D. (2006) Cell. Mol. Biol. 52, 43–49 [PubMed] [Google Scholar]
- 30. Messmer-Blust A. F., Balasubramanian S., Gorbacheva V. Y., Jeyaratnam J. A., Vestal D. J. (2010) Mol. Biol. Cell 15, 2514–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vestal D. J., Gorbacheva V. Y., Sen G. C. (2000) J. Interferon Cytokine Res. 20, 991–1000 [DOI] [PubMed] [Google Scholar]
- 32. Estève P. O., Chicoine E., Robledo O., Aoudjit F., Descoteaux A., Potworowski E. F., St-Pierre Y. (2002) J. Biol. Chem. 277, 35150–35155 [DOI] [PubMed] [Google Scholar]
- 33. Ashburner B. P., Westerheide S. D., Baldwin A. S., Jr. (2001) Mol. Cell. Biol. 21, 7065–7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liao J., Wolfman J. C., Wolfman A. (2003) J. Biol. Chem. 278, 31871–31878 [DOI] [PubMed] [Google Scholar]
- 35. Yang Z., Kyraikides T. R., Bornstein P. (2000) Mol. Biol. Cell 11, 3353–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfaffl M. W. (2001) Nucleic Acids Res. 29, 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balbín M., Fueyo A., Knäuper V., López J. M., Alvarez J., Sánchez L. M., Quesada V., Bordallo J., Murphy G., López-Otín C. (2001) J. Biol. Chem. 276, 10253–10262 [DOI] [PubMed] [Google Scholar]
- 38. Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., K. S. (eds) (1989) Preparation and Analysis of DNA, Greene Publishing Associates and Wiley-Interscience, New York [Google Scholar]
- 39. Yang C. H., Shi W., Basu L., Murti A., Constantinescu S. N., Blatt L., Croze E., Mullersman J. E., Pfeffer L. M. (1996) J. Biol. Chem. 271, 8057–8061 [DOI] [PubMed] [Google Scholar]
- 40. Yang C. H., Murti A., Pfeffer L. M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 5568–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sancéau J., Boyd D. D., Seiki M., Bauvois B. (2002) J. Biol. Chem. 277, 35766–35775 [DOI] [PubMed] [Google Scholar]
- 42. Han Y. P., Tuan T. L., Hughes M., Wu H., Garner W. L. (2001) J. Biol. Chem. 276, 22341–22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siwik D. A., Chang D. L., Colucci W. S. (2000) Circ. Res. 86, 1259–1265 [DOI] [PubMed] [Google Scholar]
- 44. Cheong R., Bergmann A., Werner S. L., Regal J., Hoffmann A., Levchenko A. (2006) J. Biol. Chem. 281, 2945–2950 [DOI] [PubMed] [Google Scholar]
- 45. Bond M., Chase A. J., Baker A. H., Newby A. C. (2001) Cardiovascular Res. 50, 556–565 [DOI] [PubMed] [Google Scholar]
- 46. You M., Flick L. M., Yu D., Feng G. S. (2001) J. Exp. Med. 193, 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanden Berghe W., Vermeulen L., De Wilde G., De Bosscher K., Boone E., Haegeman G. (2000) Biochem. Pharmacol. 60, 1185–1195 [DOI] [PubMed] [Google Scholar]
- 48. Tensen C. P., Flier J., Rampersad S. S., Sampat-Sardjoepersad S., Scheper R. J., Boorsma D. M., Willemze R. (1999) Biochim. Biophys. Acta. 1446, 167–172 [DOI] [PubMed] [Google Scholar]
- 49. Yang C. H., Wei L., Pfeffer S. R., Du Z., Murti A., Valentine W. J., Zheng Y., Pfeffer L. M. (2007) J. Immunol. 178, 986–992 [DOI] [PubMed] [Google Scholar]
- 50. Kim B. C., Lee M. N., Kim J. Y., Lee S. S., Chang J. D., Kim S. S., Lee S. Y., Kim J. H. (1999) J. Biol. Chem. 274, 24372–24377 [DOI] [PubMed] [Google Scholar]
- 51. Boyer L., Travaglione S., Falzano L., Gauthier N. C., Popoff M. R., Lemichez E., Fiorentini C., Fabbri A. (2004) Mol. Biol. Cell 15, 1124–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sulciner D. J., Irani K., Yu Z. X., Ferrans V. J., Goldschmidt-Clermont P., Finkel T. (1996) Mol. Cell. Biol. 16, 7115–7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kheradmand F., Werner E., Tremble P., Symons M., Werb Z. (1998) Science 280, 898–902 [DOI] [PubMed] [Google Scholar]
- 54. Arbibe L., Mira J. P., Teusch N., Kline L., Guha M., Mackman N., Godowski P. J., Ulevitch R. J., Knaus U. G. (2000) Nat. Immunol. 1, 533–540 [DOI] [PubMed] [Google Scholar]
- 55. Hiratsuka S., Nakamura K., Iwai S., Murakami M., Itoh T., Kijima H., Shipley J. M., Senior R. M., Shibuya M. (2002) Cancer Cell 2, 289–300 [DOI] [PubMed] [Google Scholar]
- 56. Yan C., Wang H., Boyd D. D. (2001) J. Biol. Chem. 276, 1164–1172 [DOI] [PubMed] [Google Scholar]
- 57. Nair R. R., Solway J., Boyd D. D. (2006) J. Biol. Chem. 281, 26424–26436 [DOI] [PubMed] [Google Scholar]
- 58. Ma Z., Chang M. J., Shah R. C., Benveniste E. N. (2005) J. Leuk. Biol. 78, 512–523 [DOI] [PubMed] [Google Scholar]
- 59. Kröger A., Dallügge A., Kirchhoff S., Hauser H. (2003) Oncogene 22, 1045–1056 [DOI] [PubMed] [Google Scholar]
- 60. Yim J. H., Ro S. H., Lowney J. K., Wu S. J., Connett J., Doherty G. M. (2003) J. Interferon Cytokine Res. 23, 501–511 [DOI] [PubMed] [Google Scholar]
- 61. Nozell S., Ma Z., Wilson C., Shah R., Benveniste E. N. (2004) J. Biol. Chem. 279, 38577–385589 [DOI] [PubMed] [Google Scholar]
- 62. Vestal D. J., Buss J. E., McKercher S. R., Jenkins N. A., Copeland N. G., Kelner G. S., Asundi V. K., Maki R. A. (1998) J. Interferon Cytokine Res. 18, 977–985 [DOI] [PubMed] [Google Scholar]
- 63. Perona R., Montaner S., Saniger L., Sanchez-Perez I., Bravo R., Lacal J. C. (1997) Genes Dev. 15, 463–475 [DOI] [PubMed] [Google Scholar]
- 64. Montaner S., Perona R., Saniger L., Lacal J. C. (1998) J. Biol. Chem. 273, 12779–12785 [DOI] [PubMed] [Google Scholar]
- 65. Cammarano M. S., Minden A. (2001) J. Biol. Chem. 276, 25876–25882 [DOI] [PubMed] [Google Scholar]
- 66. Frost J. A., Swantek J. L., Stippec S., Yin M. J., Gaynor R., Cobb M. H. (2000) J. Biol. Chem. 275, 19693–19699 [DOI] [PubMed] [Google Scholar]
- 67. Friedland J. C., Lakins J. N., Kazanietz M. G., Chernoff J., Boettiger D., Weaver V. M. (2007) J. Cell Sci. 120, 3700–3712 [DOI] [PubMed] [Google Scholar]
- 68. Chen B. C., Kang J. C., Lu Y. T., Hsu M. J., Liao C. C., Chiu W. T., Yeh F. L., Lin C. H. (2009) Mol. Immunol. 46, 1179–1188 [DOI] [PubMed] [Google Scholar]
- 69. Deshpande S. S., Angkeow P., Huang J., Ozaki M., Irani K. (2000) FASEB J. 14, 1705–1714 [DOI] [PubMed] [Google Scholar]
- 70. Williams L. M., Lali F., Willetts K., Balague C., Godessart N., Brennan F., Feldmann M., Foxwell B. M. (2008) Mol. Immunol. 45, 2446–2454 [DOI] [PubMed] [Google Scholar]