Abstract
Endothelial nitric-oxide synthase (eNOS) plays a central role in cardiovascular regulation. eNOS function is critically modulated by Ca2+ and protein phosphorylation, but the interrelationship between intracellular Ca2+ mobilization and eNOS phosphorylation is poorly understood. Here we show that endoplasmic reticulum (ER) Ca2+ release activates eNOS by selectively promoting its Ser-635/633 (bovine/human) phosphorylation. With bovine endothelial cells, thapsigargin-induced ER Ca2+ release caused a dose-dependent increase in eNOS Ser-635 phosphorylation, leading to elevated NO production. ER Ca2+ release also promoted eNOS Ser-633 phosphorylation in mouse vessels in vivo. This effect was independent of extracellular Ca2+ and selective to Ser-635 because the phosphorylation status of other eNOS sites, including Ser-1179 or Thr-497, was unaffected in thapsigargin-treated cells. Blocking ERK1/2 abolished ER Ca2+ release-induced eNOS Ser-635 phosphorylation, whereas inhibiting protein kinase A or Ca2+/calmodulin-dependent protein kinase II had no effect. Protein phosphorylation assay confirmed that ERK1/2 directly phosphorylated the eNOS Ser-635 residue in vitro. Further studies demonstrated that ER Ca2+ release-induced ERK1/2 activation mediated the enhancing action of purine or bradykinin receptor stimulation on eNOS Ser-635/633 phosphorylation in bovine/human endothelial cells. Mutating the Ser-635 to nonphosphorylatable alanine prevented ATP from activating eNOS in cells. Taken together, these studies reveal that ER Ca2+ release enhances eNOS Ser-635 phosphorylation and function via ERK1/2 activation. Because ER Ca2+ is commonly mobilized by agonists or physicochemical stimuli, the identified ER Ca2+-ERK1/2-eNOS Ser-635 phosphorylation pathway may have a broad role in the regulation of endothelial function.
Keywords: Calcium, Endoplasmic Reticulum (ER), ERK, Nitric-oxide Synthase, Protein Phosphorylation
Introduction
NO is a chief signaling molecule in cardiovascular regulation. In addition to relaxing vascular tone, NO regulates cardiac contractility, platelet aggregation, angiogenesis, and vascular smooth muscle proliferation (1, 2). Thus, physiological NO formation is essential for cardiovascular homeostasis. Abnormalities of NO production, on the other hand, are found in almost all cardiovascular diseases. In endothelial cells, NO is primarily produced by endothelial NO synthase (eNOS).3 Because NO is a diffusible gas and cannot be stored in an intracellular compartment, the onset of NO signaling is triggered by eNOS activation (2, 3). The intensity and time span of NO signaling are largely dictated by the functional status of eNOS. Thus, understanding the mechanisms of eNOS activation and regulation has been the focus of cardiovascular NO research.
eNOS is activated by the binding of calmodulin (CaM) (4, 5). In a classic view, the binding between eNOS and CaM is initiated by the increased intracellular Ca2+ concentrations. A canonical eNOS activation process begins with the bindings between agonists and their respective receptors. Activation of these receptors results in the increases of intracellular free Ca2+. The increases of cytosolic Ca2+ concentrations elicited by some agonists, including acetylcholine, are dependent on the influx of extracellular Ca2+ (6). In other cases, such as the activation of purine receptors (P2Y) by ATP, Ca2+ is primarily released from the intracellular Ca2+ repertoire: endoplasmic reticulum (ER), although the refill of ER Ca2+ relies on extracellular Ca2+ supply (7, 8). Increased cytosolic Ca2+ binds with CaM, forming a Ca2+-CaM complex, which subsequently binds with eNOS. Binding with CaM induces changes in eNOS conformation (4, 5). This enables the electron transfer from the eNOS reductase domain to the oxygenase domain, where l-arginine, oxygen, and NADPH are converted to NO and l-citrulline.
In addition to Ca2+, eNOS is also regulated by protein phosphorylation (9). eNOS is known to be phosphorylated at several serine and threonine residues. The first site where phosphorylation was found to significantly influence eNOS function is the serine 1179/1177 (bovine/human and mouse) (10, 11). eNOS Ser-1179 was initially reported to be phosphorylated by Akt. Subsequent studies showed that other kinases, such as AMP-activated protein kinase, protein kinase G, and CaMKII, also phosphorylate eNOS Ser-1179. Ser-1179 phosphorylation enhances eNOS function. Various extracellular stimuli influence eNOS activity by modulating its Ser-1179 phosphorylation (2). eNOS is also phosphorylated at residue Ser-635 (12). Ser-635 phosphorylation augments eNOS activity, and this has been shown to be important in the response of endothelial cells to shear stress (13). So far, studies suggest that protein kinase A (PKA) phosphorylates eNOS Ser-635 (8, 12). In contrast to the stimulating effect of Ser-1179 and Ser-635 phosphorylation, Thr-497 phosphorylation inhibits eNOS activity (14). Beyond Ser-1179, Ser-635, and Thr-497, eNOS has been reported to be phosphorylated at residues Ser-116 and Ser-617. Compared with the extensively studied roles of eNOS Ser-1179, Ser-635, and Thr-497 phosphorylation in endothelial regulation and diseases, the in vivo significance of eNOS Ser-116 and Ser-617 phosphorylation remains to be established (2, 8).
Although both Ca2+ and protein phosphorylation play crucial roles in eNOS regulation, the interrelationship between Ca2+ mobilization and eNOS phosphorylation is understood in much less detail. Nevertheless, recent studies show that although CaM binding remains essential for eNOS activation, whether or not the binding between CaM and eNOS requires the increase of cytosolic Ca2+ is heavily affected by the phosphorylation status of eNOS. For example, phosphorylation of Ser-1179 or Ser-635 was reported to render eNOS activation in resting intracellular Ca2+ concentrations (10, 11, 15). This is apparently because phosphorylation of these two sites enhances the binding affinity of eNOS with CaM (16). On the other hand, how intracellular Ca2+ mobilization affects eNOS phosphorylation is largely unclear. In the present study, we report that discharging Ca2+ from the ER results in a selective up-regulation of eNOS phosphorylation at residue Ser-635. Our studies further identify ERK1/2 as the kinase that mediates the action of ER Ca2+ release on eNOS Ser-635 phosphorylation. Moreover, we demonstrate that ER Ca2+ release-elicited ERK1/2 activation and subsequent Ser-635 phosphorylation account for the regulation of ATP and bradykinin on eNOS in endothelial cells.
EXPERIMENTAL PROCEDURES
Materials
Cell culture materials were obtained from Invitrogen. The antibody against eNOS was purchased from the BD Transduction Laboratories. Antibodies against phospho-eNOS (Ser-1179, Ser-635, and Thr-497) were purchased from Upstate Biotechnology (Lake Placid, NY). Antibody against ERK1/2 was from Cell Signaling Technology (Beverly, MA). Antibodies against phospho-ERK1/2 and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). H89 and thapsigargin were purchased from Tocris Bioscience (Ellisville, MO). BAPTA-AM, KN93, KN92, and PKA peptide inhibitor 14-22 were from EMD Biosciences (San Diego, CA). PD98095, U0126, bradykinin, and other reagents were purchased from Sigma unless otherwise indicated.
Cell Culture and Transfection
Bovine aortic endothelial cells (BAECs) and human umbilical vein endothelial cells (HUVECs) were purchased from Cell Systems (Kirkland, WA) and grown in advanced Dulbecco's modified Eagle's medium supplemented with 2% fetal bovine serum, 1% penicillin-streptomycin, and 15 μg/ml endothelial cell growth supplement in a 37 °C humidified incubator containing 95% air and 5% CO2. Wild-type bovine eNOS and S635A eNOS in pcDNA3 were transfected into cells using Lipofectamine 2000 reagents (Invitrogen) according to the manufacturer's instructions. After 24 h of plasmid transfection, the cells were subjected to treatments and further analyzed.
Site-directed Mutagenesis
The Ser-635 of bovine eNOS was replaced with an Ala with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. A pair of primers (forward, 5′-CGG CGG AAG AGA AAG GAG GCC AGC AAC ACA GAC AGC-3′; reverse, 5′-GCT GTC TGT GTT GCT GGC CTC CTT TCT CTT CCG CCG-3′) containing the mutation of Ser(TCC) to Ala(GCC) were used. The mutation of eNOS Ser-635 to Ala was verified by DNA sequencing.
In Vitro Protein Phosphorylation Assay
Recombinant bovine eNOS was purified from an Escherichia coli expression system as previously reported (17). eNOS from this expression system is not phosphorylated because of the absence of relative kinases in E. coli. In vitro phosphorylation was performed in a 50-μl reaction system containing 50 mm Tris-HCl, pH 7.4, 10 mm MgCl2, 2 mm DTT, 1 mm EGTA, 0.01% Brij 35, 0.5 mm ATP, eNOS (0.1 μg), and ERK2 (100 units; New England Biolabs, Beverly, MA). After the 1-h incubation at 30 °C, the reactions were stopped by boiling in SDS/PAGE sample buffer. The phosphorylation status of eNOS was determined by Western blotting.
Western Blotting
The cells were lysed on ice for 30 min in modified radioimmunoprecipitation assay buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 50 mm NaF, 1 mm Na3VO4, 5 mm sodium pyrophosphate, and protease inhibitor tablet (Roche Applied Sciences). After protein content assays, the lysates were denatured, separated by SDS/PAGE, and transferred to nitrocellulose membranes. After blocking, the membranes were probed with the appropriate primary antibodies. Membrane-bound primary antibodies were detected using secondary antibodies conjugated with horseradish peroxidase. Immunoblots were developed on films using the enhanced chemiluminescence technique (SuperSignal West Pico; Pierce). Densitometry was performed using an AlphaImager 3300 gel documentation and image analysis system.
eNOS Activity Assay
eNOS activity was measured by the l-[14C]arginine to l-[14C]citrulline conversion assay. To measure the activity of phospho-eNOS, the assay was performed in the presence of 10 nm Ca2+ as previously reported (18). Briefly, the cells were harvested in the homogenate buffer (50 mm Tris-HCl, pH7.4, 2 mm DTT, 50 mm NaF, 1 mm Na3VO4, and protease inhibitor mixture) and homogenated by pulse sonication. After centrifugation (14,000 × g for 15 min at 4 °C), the pellets were recovered, washed, and resuspended in the homogenate buffer. The cell lysates (45 μg of protein) were added to the reaction mixture containing 50 mm Tris-HCl, pH 7.4, 0.5 mm NADPH, 10 nm CaCl2, 10 μg/ml CaM, 10 μm BH4, 1 μm l-[14C]arginine, and 20 μm l-arginine. After a 60-min incubation at 37 °C, the reactions were terminated by ice-cold stop buffer. l-[14C]Citrulline was separated by passing the reaction mixture through Dowex AG 50W-X8 (Na+ form; Sigma) cation exchange columns and quantitated by liquid scintillation counting.
Fura-2 Assay of Intracellular Ca2+
BAECs were seeded into a black-walled and clear-based 96-well plate at a density of 25,000 cells/well and cultured overnight prior to use. The cells were loaded with 2 μm Fura-2 (Molecular Probes, Eugene, OR) for 45 min at 37 °C. After washing, the cells were incubated in 150 μl of Tyrode's solution (145 mm NaCl, 2.5 mm KCl, 10 mm HEPES, 10 mm glucose, 1.2 mm MgCl2, and 1.5 mm CaCl2, pH 7.4) for 30 min. Fura-2 340- and 380-nm fluorescence pairs (512-nm emission) were recorded at 37 °C by using a FlexStation Scanning Fluorometer (Molecular Devices, Sunnyvale, CA). Thapsigargin was added to the cells automatically by the fluid transfer system incorporated in the fluorometer. Intracellular Ca2+ concentrations were expressed by the ratio of fluorescence at 340 nm to that at 380 nm.
Nitrite Assay
Total nitrite levels released in cell culture media were measured with a Griess reagent kit (Invitrogen). The reaction consisted of 20 μl of Griess reagent, 150 μl of medium, and 130 μl of deionized water. After incubation of the mixture for 30 min at room temperature, nitrite levels were measured at 548 nm using an M2 spectrophotometric microplate reader (Molecular Devices).
Statistics
The data are expressed as the means ± S.E. Comparisons are made using a two-tailed Student's paired or unpaired t test. Differences are considered statistically significant at p < 0.05.
RESULTS
ER Ca2+ Release Triggers eNOS Ser-635 Phosphorylation
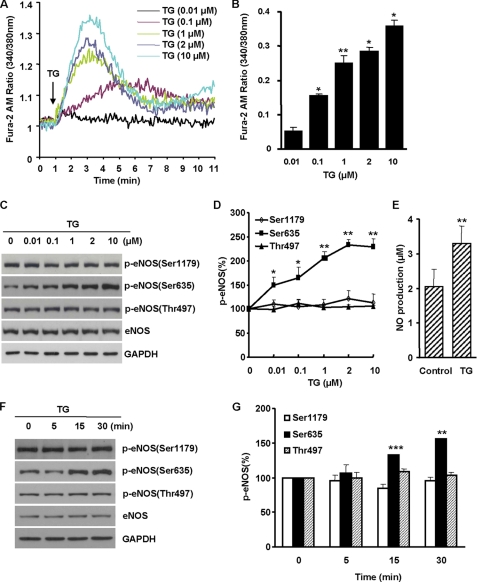
To determine the effects of ER Ca2+ release on eNOS phosphorylation and function, we treated BAECs with thapsigargin (TG), a compound known to induce Ca2+ release from ER via inhibiting Ca2+ pumps (6–8). As expected, TG resulted in a dose-dependent increase of intracellular Ca2+ (Fig. 1, A and B). In TG-treated cells, eNOS Ser-635 phosphorylation was enhanced. The increasing magnitudes of eNOS Ser-635 phosphorylation were parallel to the rise of cytosolic Ca2+ levels (Fig. 1, C and D). The enhancing effect was selective to Ser-635 because neither Ser-1179 nor Thr-497 phosphorylation was affected by the release of ER Ca2+. Ser-635 phosphorylation augmented eNOS function as evidenced by the significant increases of NO metabolites in the cell culture media (Fig. 1E).
FIGURE 1.
Effects of ER Ca2+ release on eNOS phosphorylation and function in BAECs. A, TG induced a dose-dependent increase of Fura-2 fluorescence in cells. Representative data are shown from three independent experiments. B, TG-induced increases of intracellular Ca2+ in cells. The data shown are the cytosolic Ca2+ levels after TG treatment for 2 min (means ± S.E.; *, p < 0.05 versus control; **, p < 0.01 versus control; n = 4). C, the dose-dependent effects of TG on eNOS phosphorylation. After 30 min of TG treatment, the changes of eNOS phosphorylation were measured. D, quantitative analyses of the effects of TG on eNOS Ser-1179, Ser-635, and Thr-497 phosphorylation (n = 5). E, increased NO formation from Ser-635-phospohorylated eNOS in cells. The levels of nitrite in cell culture medium were measured after 2 h of TG treatment. F, representative blots showing the time course effects of TG (2 μm) on eNOS phosphorylation. G, quantitation on the time course effects of TG on eNOS Ser-1179, Ser-635, and Thr-497 phosphorylation (n = 5).
We also characterized the time course effect of ER Ca2+ release on eNOS phosphorylation. As shown in Fig. 1 (F and G), ER Ca2+ release by TG (2 μm) induced a rapid increase of eNOS Ser-635 phosphorylation, which reached to the peak levels at 30 min. In contrast to the transient nature of TG-induced Ca2+ release in the cytosol, the increases of eNOS Ser-635 phosphorylation sustained up to 2 h (supplemental Fig. S1). Taken together, these data revealed that ER Ca2+ release induced a selective and sustained augmentation of eNOS Ser-635 phosphorylation leading to increased NO production.
ER Ca2+ Release Promotes Mouse eNOS Ser-633 Phosphorylation in Vivo
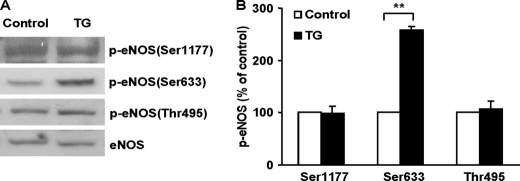
To ascertain whether the above cell culture findings occur in vivo, we injected TG (1 mg/kg, intraperitoneally) in mice and examined the alterations of eNOS phosphorylation in aortas. In agreement with the results from BAECs, TG treatment resulted in a selective increase of eNOS Ser-633 (equivalent to the Ser-635 of bovine eNOS) phosphorylation in mouse aortas (Fig. 2, A and B). The phosphorylation status of Ser-1177 or Thr-495 in TG-injected mice was not significantly changed. Thus, discharging ER Ca2+ also promoted eNOS Ser-633 phosphorylation in animals in vivo.
FIGURE 2.
ER Ca2+ release promoted eNOS Ser-633 phosphorylation in mouse aortas in vivo. A, effects of TG on eNOS phosphorylation in the aortas of mice. The mice were injected with TG (1 mg/kg, intraperitoneal) for 1 h, and then the phosphorylation status of eNOS in aortas was detected by respective antibodies. As shown, TG induced an elevation of eNOS Ser-633 phosphorylation without significantly altering the phosphorylation status of Ser-1177 or Thr-495. Representative blots are shown from five independent experiments. B, quantitative analyses of the effects of TG on eNOS phosphorylation in mouse aortas (means ± S.E.; **, p < 0.01 versus control; n = 5).
ER Ca2+ Release-induced eNOS Ser-635 Phosphorylation and Extracellular Ca2+
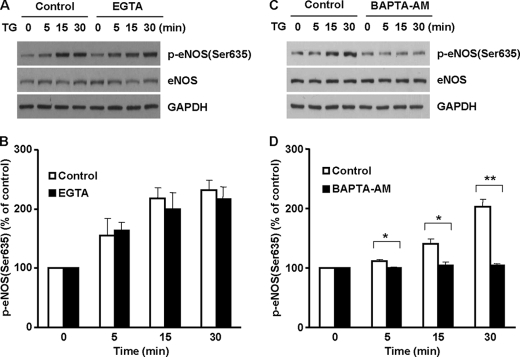
The release of ER Ca2+ is influenced by extracellular Ca2+, which is thought to be important for ER Ca2+ refill by the capacitative Ca2+ entry (6, 19). We therefore examined the role of extracellular Ca2+ in TG-induced eNOS Ser-635 phosphorylation. BAECs were stimulated by TG in normal media (containing 2 mm Ca2+) or Ca2+-free media (no Ca2+ and with 5 mm EGTA). As shown in Fig. 3 (A and B), TG-induced eNOS Ser-635 phosphorylation was not significantly affected by removing extracellular Ca2+. These results indicated that ER Ca2+ release-induced eNOS Ser-635 phosphorylation was independent of extracellular Ca2+.
FIGURE 3.
Roles of extracellular and intracellular Ca2+ in TG-induced eNOS Ser-635 phosphorylation in cells. A, roles of extracellular Ca2+ in TG-elicited eNOS Ser-635 phosphorylation. As shown, removing extracellular Ca2+ (EGTA, 5 mm) had no significant effect on eNOS phosphorylation in BAECs stimulated by TG (2 μm). Representative blots are shown from three independent experiments. B, quantitative analyses of the effects of extracellular Ca2+ removal on TG-induced eNOS Ser-635 phosphorylation (means ± S.E., n = 3). C, effect of intracellular Ca2+ chelator BAPTA-AM (50 μm) on TG-initiated eNOS Ser-635 phosphorylation. D, quantitative analyses of the preventative effect of intracellular Ca2+ chelation on TG-induced eNOS Ser-635 phosphorylation (*, p < 0.05 versus control; **, p < 0.01 versus control; n = 5).
To further prove that TG-induced eNOS Ser-635 phosphorylation was due to intracellular Ca2+ release, we examined whether quenching intracellular Ca2+ could prevent TG from activating eNOS Ser-635 phosphorylation. Indeed, preloading the cells with the intracellular Ca2+ chelator BAPTA-AM abolished the enhancing effect of TG on eNOS Ser-635 phosphorylation (Fig. 3, C and D). These data reconfirmed that TG-induced eNOS Ser-635 phosphorylation was due to ER Ca2+ release.
Role of PKA and CaMKII in ER Ca2+ Release-induced eNOS Ser-635 Phosphorylation
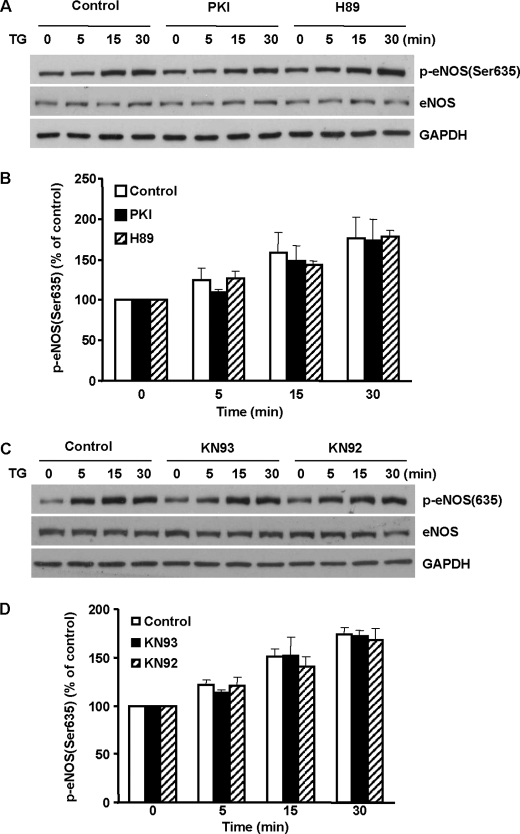
We then sought to identify the kinases that were responsible for ER Ca2+ release-induced eNOS Ser-635 phosphorylation. PKA was previously reported to phosphorylate eNOS Ser-635 in endothelial cells exposed to shear stress (12, 13). We thus first examined the possible role of PKA. Interestingly, blocking PKA with H89 (10 μm) did not affect TG-induced eNOS Ser-635 phosphorylation (Fig. 4, A and B). To corroborate these data obtained with H89, we also treated the cells with PKI (10 μm), a highly specific peptide inhibitor of PKA. PKI also failed to change the increases in eNOS Ser-635 phosphorylation in TG-stimulated cells. These studies suggested that ER Ca2+ release-induced eNOS Ser-635 phosphorylation was not mediated by PKA.
FIGURE 4.
Roles of PKA and CaMKII in ER Ca2+ release-induced eNOS Ser-635 phosphorylation. BAECs were pretreated with PKA or CaMKII inhibitors for 15 min and then subjected to TG stimulation. A, effects of PKA inhibition on ER Ca2+ release-induced eNOS Ser-635 phosphorylation. As shown, PKA inhibitor H89 (10 μm) or PKI (10 μm) had no significant effect on the increases of eNOS Ser-635 phosphorylation in TG-treated cells. Representative data are shown from three independent experiments. B, quantitative analyses of the effects of PKA inhibitors on ER Ca2+ release-induced eNOS Ser-635 phosphorylation. The data are shown as the means ± S.E. (n = 3). C, effects of CaMKII inhibitor KN93 (50 μm) and the inactive control compound KN92 (50 μm) on ER Ca2+ release-induced eNOS Ser-635 phosphorylation. D, quantitative analyses of the effects of CaMKII inhibition on ER Ca2+ release-induced eNOS Ser-635 phosphorylation (n = 3).
Another possible kinase that may be activated by the rise of intracellular Ca2+ is Ca2+-CaM-dependent protein kinase II (CaMKII) (20). To probe whether CaMKII mediated TG-induced eNOS Ser-635 phosphorylation, we treated the cells with the CaMKII blocker KN93 (50 μm). Equal amounts of KN92, an inactive compound structurally resembling KN93, were used as control. As shown in Fig. 4 (C and D), neither KN93 nor KN92 had a significant effect on the increases of eNOS Ser-635 phosphorylation in TG-stimulated cells. These data ruled out the possible role of CaMKII in the ER Ca2+ release-elicited eNOS Ser-635 phosphorylation.
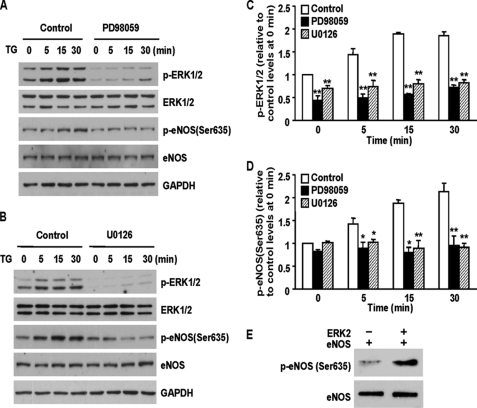
ERK1/2 Activation Mediates ER Ca2+ Release-induced eNOS Ser-635 Phosphorylation
ER Ca2+ release has been reported to induce ERK1/2 activation (21, 22). We therefore explored the role of ERK1/2 in ER Ca2+ release-initiated eNOS Ser-635 phosphorylation. Consistent with previous reports, releasing ER Ca2+ with TG induced ERK1/2 activation (Fig. 5A). Parallel increases of eNOS Ser-635 phosphorylation were also seen. Pretreatment of cells with ERK1/2 inhibitor PD98059 blocked ERK1/2 activation in TG-treated cells. Remarkably, ERK1/2 inhibition completely prevented the increases of eNOS Ser-635 phosphorylation induced by TG (Fig. 5, A, C, and D). U0126, another ERK1/2 inhibitor whose structure is different from that of PD98059, also prevented TG-induced ERK1/2 activation and subsequent eNOS Ser-635 phosphorylation (Fig. 5, B–D). These results suggested that ER Ca2+ release-induced eNOS Ser-635 phosphorylation was mediated by ERK1/2.
FIGURE 5.
ERK1/2 mediated ER Ca2+ release-induced eNOS Ser-635 phosphorylation. BAECs were pretreated with PD98059 or U0126 for 15 min and then subjected to TG stimulation. A, effect of ERK1/2 inhibition (PD98059, 20 μm) on ERK1/2 activation and eNOS Ser-635 phosphorylation in TG-treated cells. B, U0126 (10 μm), another MEK inhibitor that is structurally different from PD98059, also prevented ERK1/2 activation and eNOS Ser-635 phosphorylation in TG-stimulated cells. C, quantitative analyses of the effects of PD98059 and U0126 on ERK1/2 activation. The data are shown as the means ± S.E. (**, p < 0.01; n = 5). D, quantitative analyses of the effects of ERK1/2 inhibition on ER Ca2+ release-induced eNOS Ser-635 phosphorylation (*, p < 0.05; **, p < 0.01; n = 5). E, in vitro protein phosphorylation assays showing that active ERK2 directly phosphorylated eNOS Ser-635 residues. Representative blots are shown from three triplicate experiments.
To unequivocally demonstrate that ERK1/2 can phosphorylate the eNOS Ser-635 residue, we performed in vitro protein phosphorylation assays with purified eNOS and ERK1/2. As shown in Fig. 5E, incubating eNOS with active ERK2 resulted in a dramatic increase of eNOS Ser-635 phosphorylation. These data proved that ERK1/2 can phosphorylate eNOS Ser-635.
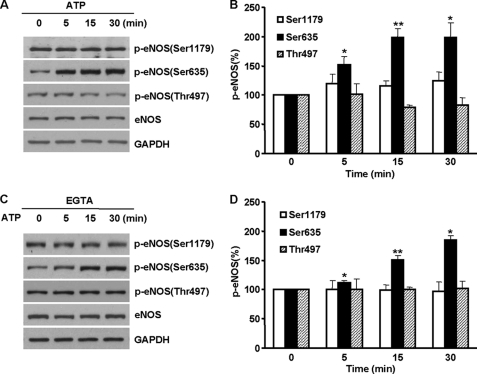
ATP Triggers eNOS Ser-635 Phosphorylation in Endothelial Cells
The above results collectively demonstrated an eNOS activation pathway in which ER Ca2+ release induced eNOS Ser-635 phosphorylation via ERK1/2. This finding was obtained by using TG to directly induce ER Ca2+ release. An important question remains regarding whether or not this pathway has functional significance in a physiological setting. Because purine receptor activation by ATP primarily induces ER Ca2+ release (7, 8), we therefore sought to explore whether the ER Ca2+ release-ERK1/2-eNOS Ser-635 phosphorylation pathway mediates the regulation of eNOS function by ATP. We first characterized the effect of ATP on eNOS phosphorylation. As shown in Fig. 6A, ATP stimulated a time-dependent increase of eNOS Ser-635 phosphorylation. The increases of eNOS Ser-635 phosphorylation in ATP-treated cells persisted up to 2 h (supplemental Fig. S2). Similar to the action of TG, the effect of ATP was specific to Ser-635 because eNOS Ser-1179 or Thr-497 phosphorylation was not altered by ATP (Fig. 6, A and B). This effect was also independent of extracellular Ca2+ (Fig. 6, C and D). Thus, ATP activated eNOS by specifically promoting its Ser-635 phosphorylation.
FIGURE 6.
ATP triggered eNOS Ser-635 phosphorylation in BAECs. A, time course effects of ATP (20 μm) on eNOS phosphorylation. Representative blots are shown from five independent experiments. B, quantitative analyses of the effects of ATP on eNOS Ser-1179, Ser-635, and Thr-497 phosphorylation (means ± S.E.; *, p < 0.05; **, p < 0.01; n = 5). C, roles of extracellular Ca2+ in ATP-stimulated eNOS phosphorylation. As shown, removing extracellular Ca2+ had no significant effect on ATP-induced eNOS Ser-635 phosphorylation. D, quantitative analyses of the effect of extracellular removal on ATP-induced eNOS phosphorylation.
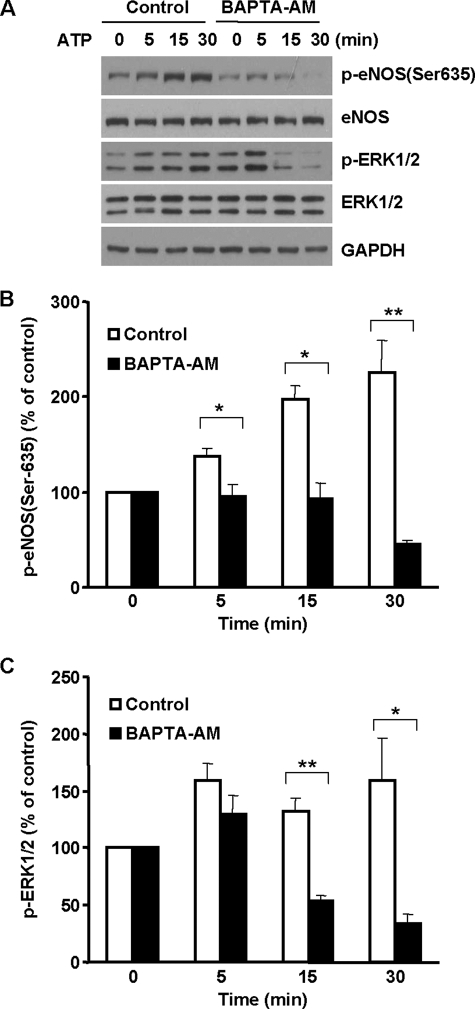
ATP Activates eNOS Ser-635 Phosphorylation by ER Ca2+ Release-induced ERK1/2 Activation
To demonstrate whether ER Ca2+ release-induced ERK1/2 activation was involved in the ATP-induced up-regulation of eNOS Ser-635 phosphorylation, we pretreated cells with BAPTA-AM. As shown in Fig. 7A, ATP activated ERK1/2 in endothelial cells. A correlating increase of eNOS Ser-635 phosphorylation was seen. Quenching the rise of intracellular Ca2+ with BAPTA-AM prevented ATP from activating ERK1/2 (Fig. 7, A and C). Consequently, the increases of eNOS Ser-635 phosphorylation in ATP-treated cells were blunted. These data strongly suggested that ATP triggered eNOS Ser-635 phosphorylation via ER Ca2+ release-induced ERK1/2 activation.
FIGURE 7.
ER Ca2+ release mediated the effect of ATP on eNOS Ser-635 phosphorylation in BAECs. A, effects of BAPTA-AM (50 μm) on ATP-induced ERK1/2 activation and eNOS Ser-635 phosphorylation. BAECs were preloaded with BAPTA-AM for 30 min and then exposed to ATP. As shown, ATP (20 μm) stimulated ERK1/2 activation and corresponding eNOS Ser-635 phosphorylation, and these effects were prevented by intracellular Ca2+ chelation. Representative blots are shown from three independent experiments. B, quantitative analyses of the effects of BAPTA-AM on ATP-elicited eNOS Ser-635 phosphorylation. C, quantitative analyses of the effects of BAPTA-AM on ERK1/2 activation in ATP-treated cells (means ± S.E.; *, p < 0.05 versus control; **, p < 0.01 versus control; n = 3).
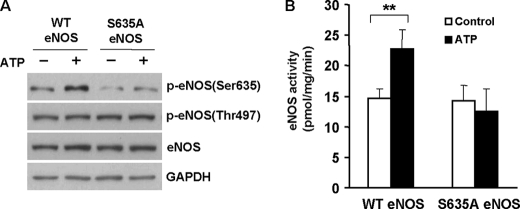
Mutation of Ser-635 to Alanine Prevents ATP from Activating eNOS in Cells
We used a loss-of-function approach to prove the essential role of ER Ca2+ release-induced Ser-635 phosphorylation in the regulation of eNOS function by ATP inside endothelial cells. We constructed a S635A eNOS mutant in which the Ser-635 residue was changed to a nonphosphorylatable Ala. The cells were transfected with WT and S635A eNOS vectors and then challenged by ATP. As expected, ATP induced Ser-635 phosphorylation of WT eNOS, leading to increased enzymatic activity (Fig. 8, A and B). In contrast, mutating Ser-635 to Ala prevented ATP from activating eNOS Ser-635 phosphorylation. As a result, ATP-induced increases of eNOS activity were abolished in these cells. Thus, a loss of Ser-635 phosphorylation disabled ATP from activating eNOS. These results underscored the importance of the ER Ca2+ release-ERK1/2-eNOS Ser-635 phosphorylation cascade in the regulation of eNOS function by ATP in cells.
FIGURE 8.
Mutation of Ser-635 to nonphosphorylatable alanine prevented ATP from activating eNOS in cells. A, effects of ATP (20 μm) on eNOS Ser-635 phosphorylation in WT eNOS and S635A eNOS-transfect cells. Representative blots are shown from three independent experiments. B, effects of ATP on eNOS activity in WT eNOS and S635A eNOS-transfected cells. As shown, ATP-stimulated increases of eNOS activity were lost by mutating the Ser-635 to alanine (means ± S.E.; **, p < 0.01 versus control; n = 3).
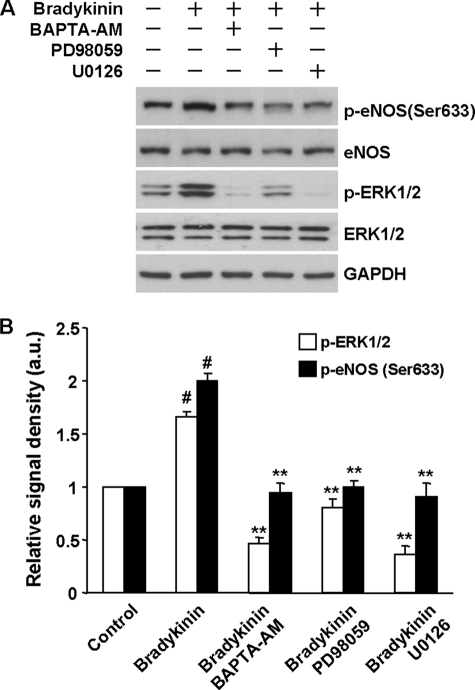
ER Ca2+ Release-induced ERK1/2 Activation Mediates the Effect of Bradykinin on eNOS Ser-633 Phosphorylation in Human Endothelial Cells
Our studies insofar have been performed on bovine cells or mice treated with TG or ATP. To ascertain whether these findings have general significance, we examined the role of the ER Ca2+ release-ERK1/2 pathway in the regulation of eNOS phosphorylation in human endothelial cells by another agonist, bradykinin. As shown in Fig. 9A, bradykinin (10 μm) stimulated ERK1/2 activation and eNOS Ser-633 phosphorylation in HUVECs. Quenching the rise of intracellular Ca2+ prevented bradykinin from activating ERK1/2 and eNOS Ser-633. ERK1/2 inhibition using PD98059 or U0126 also blocked eNOS Ser-633 phosphorylation in bradykinin-stimulated cells (Fig. 9, A and B). These data demonstrated the importance of the ER Ca2+ release-ERK1/2-eNOS Ser-633 cascade in modulating human endothelial function by vasoactive substances.
FIGURE 9.
Roles of ER Ca2+ release and ERK1/2 activation in the effect of bradykinin on eNOS Ser-633 phosphorylation in HUVECs. A, as shown, bradykinin activated ERK1/2 and eNOS Ser-633 phosphorylation in HUVECs. Quenching Ca2+ released from ER by BAPTA-AM (50 μm) blocked ERK1/2 activation and prevented bradykinin from stimulating eNOS Ser-633 phosphorylation. ERK1/2 inhibition also abolished the increases of eNOS Ser-633 phosphorylation in bradykinin-stimulated cells. B, quantitative analyses of the effects of BAPTA-AM and ERK1/2 inhibitors on eNOS Ser-633 phosphorylation in bradykinin-stimulated HUVECs (means ± S.E.; #, p < 0.001 versus control; **, p < 0.01 versus the group treated by bradykinin only; n = 3).
DISCUSSION
The key finding in the current study is that ER Ca2+ release triggers eNOS Ser-635 phosphorylation. In the conventional paradigm, Ca2+ has always been thought to activate eNOS by facilitating CaM binding. The present findings extended the role of Ca2+ in eNOS regulation. In addition to facilitating CaM binding to eNOS, Ca2+ release from the ER also promotes eNOS phosphorylation. This appears to be a selective action on Ser-635 because the status of other major eNOS phosphorylation sites, including Ser-1179 and Thr-497, remains largely unchanged. The augmenting effect of ER Ca2+ release on eNOS Ser-633 phosphorylation was also seen in mouse vessels in vivo. The finding that ER Ca2+ release promotes eNOS Ser-635 phosphorylation may shed new light on a prior puzzle regarding the discrepancy between intracellular Ca2+ dynamics and the time course of NO production in endothelial cells (23, 24). It has been reported that the stimulating effects of agonists or shear stress on intracellular Ca2+ levels are transient, but the NO production lasts much longer (25, 26). Indeed, we also observed that discharging ER Ca2+ only results in a transitory elevation of cytosolic Ca2+ (less than 5 min); however, the increases of eNOS Ser-635 phosphorylation sustain for hours. Because Ser-635-phosphorylated eNOS is capable of producing NO at resting intracellular Ca2+ concentrations (15), the persistent Ser-635 phosphorylation may provide a plausible explanation for the continuous NO production by eNOS even after the increases of Ca2+ are sequestered. Thus, after the transient Ca2+ elevation, increased Ser-635 phosphorylation is likely the major mechanism that governs NO production from eNOS.
The increases of Ca2+ levels in the cytosol involve both ER and extracellular Ca2+. It has been well established that in nonexcitable cells, such as endothelial cells, agonist-induced Ca2+ increases consist of a quick burst followed by a plateau phase (6–8). The burst of Ca2+ is derived from the ER and independent of extracellular Ca2+. However, the plateau phase of Ca2+ release relies on the refill of external Ca2+ through the store-operated channels (19, 27). We found that ER Ca2+ release-induced eNOS Ser-635 phosphorylation was not affected by the removal of extracellular Ca2+. These results suggest that the burst of Ca2+ release is sufficient to initiate eNOS Ser-635 phosphorylation. Moreover, ER Ca2+ release-induced eNOS Ser-635 phosphorylation lasts for hours. The time course of eNOS Ser-635 phosphorylation was not affected by the removal of extracellular Ca2+ (supplemental Figs. S1 and S2). These results indicate that ER Ca2+, rather than external Ca2+, plays a dominant role in promoting eNOS Ser-635 phosphorylation in endothelial cells.
It was reported that overexpression of a constitutively active catalytic subunit of PKA resulted in eNOS Ser-635 and Ser-1179 phosphorylation in BAECs (15). Early studies using pharmacological inhibitors also suggested that PKA might phosphorylate eNOS Ser-635 in endothelial cells exposed to shear stress (12, 13). However, direct evidence demonstrating that shear stress activates PKA remains lacking. We found that PKA inhibition had no significant effect on ER Ca2+ release-elicited eNOS Ser-635 phosphorylation. The lack of involvement of PKA in ER Ca2+ release-induced eNOS Ser-635 phosphorylation is probably not a surprise. Studies showed that, at least in pulmonary endothelial cells, cytosolic Ca2+ elevation actually inhibits cAMP formation (28). Thus, PKA could be suppressed when ER Ca2+ was discharged into the cytosol. Interestingly, blocking CaMKII, a Ca2+-sensitive kinase, also did not affect eNOS Ser-635 phosphorylation in TG-stimulated cells. On the other hand, inhibiting ERK1/2 prevented ER Ca2+ release from activating eNOS Ser-635 phosphorylation, indicating a major contribution of ERK1/2. ERK1/2 activation was indeed seen in cells after ER Ca2+ was released. The in vitro protein phosphorylation assay further proved that ERK1/2 can phosphorylate eNOS Ser-635. Because the protein phosphorylation experiments were carried out with purified eNOS and ERK2, these findings also suggest that ERK1/2 can phosphorylate the eNOS Ser-635 residue directly.
To establish the significance of ER Ca2+ release-induced eNOS Ser-635 phosphorylation in a physiological setting, we studied the effect of ATP on eNOS phosphorylation and function in endothelial cells. ATP is known to stimulate P2Y receptors leading to phospholipase C activation and phosphatidylinositol 1,4,5-trisphosphate formation (2, 7). Phosphatidylinositol 1,4,5-trisphosphate binds with its receptors on the ER and discharges Ca2+. We thus predicted that ATP may modulate eNOS function through the ER Ca2+-ERK1/2-Ser-635 phosphorylation cascade. Consistent with such a prediction, ATP induces eNOS Ser-635 phosphorylation. More importantly, the effect of ATP on eNOS Ser-635 phosphorylation was completely blocked by either intracellular Ca2+ quenching or ERK1/2 inhibition, suggesting that ATP induces eNOS Ser-635 phosphorylation by ER Ca2+ release-induced ERK1/2 activation. We noticed that in a recent study with human umbilical vein endothelial cells, ATP was reported to activate eNOS Ser-1177 phosphorylation via PKC (29). The present study showed that ATP primarily affects eNOS Ser-635 phosphorylation in BAECs. Moreover, the loss-of-function experiments showed that a single mutation of Ser-635 to Ala abolished the stimulating effects of ATP on eNOS activity. This result strongly indicates the predominant role of Ser-635 phosphorylation in the regulation of eNOS by ATP. Although the present study focuses on ATP, we speculate that the ER Ca2+-ERK1/2-eNOS Ser-635 phosphorylation pathway may function in the actions of other agonists. Indeed, as an example, we demonstrated that ER Ca2+ release-induced ERK1/2 activation also mediated the effect of bradykinin on eNOS Ser-633 phosphorylation in human endothelial cells.
The findings in the present study also suggest that Ca2+ and protein phosphorylation may play sequential roles in the course of eNOS regulation. In the initial response to agonists, cytosolic Ca2+ levels control the activation of eNOS. Subsequently, Ca2+-initiated protein phosphorylation likely plays a major role in regulating eNOS function. The change from Ca2+ control to phosphorylation modulation may offer several advantages. First, Ca2+ is one of the most multifaceted intracellular messengers. Besides eNOS, various signaling processes depend on the mobilization of ER Ca2+ (30). Disengaging the dependence on Ca2+ concentrations will allow the ER to be refilled and ready for other signaling pathways. Second, long-term elevation of cytosolic Ca2+ may induce detrimental effects because Ca2+ overload is a common cause of cell death (31). Prolonged Ca2+ elevation must be avoided. An alternative mechanism is needed to sustain eNOS activity if continuous NO formation is required. Ser-635 phosphorylation renders eNOS activity in resting Ca2+ concentrations, thus fulfilling such a requirement. Finally a phosphorylation-based mechanism may enhance the versatility of eNOS regulation. ERK1/2 interacts with many other signaling pathways. Through the networks, eNOS function can be modulated by a variety of means under physiological conditions.
In summary, the present study revealed a novel relationship between intracellular Ca2+ mobilization and eNOS phosphorylation in endothelial cells. ER Ca2+ release selectively enhances eNOS Ser-635 phosphorylation and function via ERK1/2 activation. Because ER Ca2+ mobilization occurs commonly upon agonist or physicochemical stimulation, the identified ER Ca2+-ERK1/2-eNOS Ser-635 phosphorylation pathway may have a broad role in the regulation of endothelial function.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL77575 and HL86965.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- eNOS
- endothelial nitric-oxide synthase
- ER
- endoplasmic reticulum
- TG
- thapsigargin
- PKA
- protein kinase A
- CaM
- calmodulin
- CaMKII
- Ca2+-CaM-dependent protein kinase II
- BAEC
- bovine aortic endothelial cell
- BAPTA-AM
- 1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis (acetoxymethyl ester)
- HUVEC
- human umbilical vein endothelial cell.
REFERENCES
- 1. Moncada S., Palmer R. M., Higgs E. A. (1991) Pharmacol. Rev. 43, 109–142 [PubMed] [Google Scholar]
- 2. Dudzinski D. M., Michel T. (2007) Cardiovasc. Res. 75, 247–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bredt D. S., Snyder S. H. (1994) Annu. Rev. Biochem. 63, 175–195 [DOI] [PubMed] [Google Scholar]
- 4. Nathan C., Xie Q. W. (1994) Cell 78, 915–918 [DOI] [PubMed] [Google Scholar]
- 5. Griffith O. W., Stuehr D. J. (1995) Annu. Rev. Physiol. 57, 707–736 [DOI] [PubMed] [Google Scholar]
- 6. Nilius B., Droogmans G. (2001) Physiol. Rev. 81, 1415–1459 [DOI] [PubMed] [Google Scholar]
- 7. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 8. Bootman M. D., Berridge M. J., Roderick H. L. (2002) Curr. Biol. 12, R563–R565 [DOI] [PubMed] [Google Scholar]
- 9. Mount P. F., Kemp B. E., Power D. A. (2007) J. Mol. Cell. Cardiol. 42, 271–279 [DOI] [PubMed] [Google Scholar]
- 10. Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. (1999) Nature 399, 601–605 [DOI] [PubMed] [Google Scholar]
- 12. Michell B. J., Harris M. B., Chen Z. P., Ju H., Venema V. J., Blackstone M. A., Huang W., Venema R. C., Kemp B. E. (2002) J. Biol. Chem. 277, 42344–42351 [DOI] [PubMed] [Google Scholar]
- 13. Boo Y. C., Hwang J., Sykes M., Michell B. J., Kemp B. E., Lum H., Jo H. (2002) Am. J. Physiol. Heart. Circ. Physiol. 283, H1819–H1828 [DOI] [PubMed] [Google Scholar]
- 14. Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. (2001) Circ. Res. 88, E68–E75 [DOI] [PubMed] [Google Scholar]
- 15. Boo Y. C., Sorescu G. P., Bauer P. M., Fulton D., Kemp B. E., Harrison D. G., Sessa W. C., Jo H. (2003) Free Radic. Biol. Med. 35, 729–741 [DOI] [PubMed] [Google Scholar]
- 16. McCabe T. J., Fulton D., Roman L. J., Sessa W. C. (2000) J. Biol. Chem. 275, 6123–6128 [DOI] [PubMed] [Google Scholar]
- 17. Xia Y., Tsai A. L., Berka V., Zweier J. L. (1998) J. Biol. Chem. 273, 25804–25808 [DOI] [PubMed] [Google Scholar]
- 18. Wei Q., Xia Y. (2006) J. Biol. Chem. 281, 21652–21659 [DOI] [PubMed] [Google Scholar]
- 19. Parekh A. B., Putney J. W., Jr. (2002) Physiol. Rew. 85, 757–810 [DOI] [PubMed] [Google Scholar]
- 20. De Koninck P., Schulman H. (1998) Science 279, 227–230 [DOI] [PubMed] [Google Scholar]
- 21. Oh-hashi K., Kaneyama M., Hirata Y., Kiuchi K. (2006) Neurosci. Lett. 405, 100–1005 [DOI] [PubMed] [Google Scholar]
- 22. Hung C. C., Ichimura T., Stevens J. L., Bonventre J. V. (2003) J. Biol. Chem. 278, 29317–29326 [DOI] [PubMed] [Google Scholar]
- 23. Kuchan M. J., Frangos J. A. (1994) Am. J. Physiol. 266, C628–C636 [DOI] [PubMed] [Google Scholar]
- 24. Ayajiki K., Kindermann M., Hecker M., Fleming I., Busse R. (1996) Circ. Res. 78, 750–758 [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto K., Korenaga R., Kamiya A., Qi Z., Sokabe M., Ando J. (2000) Am. J. Physiol. Heart. Circ. Physiol. 279, H285–H292 [DOI] [PubMed] [Google Scholar]
- 26. Toma I., Bansal E., Meer E. J., Kang J. J., Vargas S. L., Peti-Peterdi J. (2008) Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1769–R1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tiruppathi C., Minshall R. D., Paria B. C., Vogel S. M., Malik A. B. (2002) Vascul. Pharmacol. 39, 173–185 [DOI] [PubMed] [Google Scholar]
- 28. Moore T. M., Chetham P. M., Kelly J. J., Stevens T. (1998) Am. J. Physiol. 275, L203–L222 [DOI] [PubMed] [Google Scholar]
- 29. da Silva C. G., Specht A., Wegiel B., Ferran C., Kaczmarek E. (2009) Circulation. 119, 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clapham D. E. (1995) Cell 80, 259–268 [DOI] [PubMed] [Google Scholar]
- 31. Reed J. C. (1994) J. Cell Biol. 124, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.