Abstract
BACKGROUND
This study examined elderly stage II and III rectal cancer patients’ adjuvant chemoradiation therapy adherence, trends in adherence over time, and the relation of levels of adherence to mortality.
METHODS
The authors studied 2886 stage II and III rectal cancer patients who had surgical resection and who appeared in 1992–1999 linked SEER-Medicare claims data. The authors compared measures of adjuvant radiation and chemotherapy receipt and completion between stage II and III patients. Adjusted risk of cancer-related 5-year mortality was calculated by multivariate logistic regression for different levels of chemoradiation adherence among stage II and III patients.
RESULTS
Of the 2886 patients, 45.4% received both adjuvant radiation and chemotherapy. Stage III patients were more likely to receive chemoradiation than stage II patients. The receipt of chemoradiation by stage II patients increased significantly from 1992 to 1999. Stage III patients were more likely to complete radiation therapy (96.6%), chemotherapy (68.2%), and both modalities (67.5%) than stage II patients (91.5%, 49.8%, 47.6%, respectively). Only a complete course of both radiation and chemotherapy for both stage II (relative risk [RR] 0.74; 95% CI, 0.54, 0.97) and III (RR 0.80; 95% CI, 0.65, 0.96) decreased the adjusted 5-year cancer mortality risk compared with counterparts with no adjuvant therapy.
CONCLUSIONS
Even though stage II rectal cancer patients were less likely than stage III patients to receive and complete adjuvant chemoradiation, both patient groups in the general population had lower cancer-related mortality if they completed chemoradiation. These patients deserve support and encouragement to complete treatment.
Keywords: rectal cancer, adjuvant therapy, chemotherapy, radiation therapy, cancer mortality
Randomized controlled trials in the late 1980s demonstrated improved survival among stage II and III rectal cancer patients who received a concurrent course of adjuvant chemotherapy and radiation therapy, leading to a 1990 National Institutes of Health consensus statement recommending adjuvant chemoradiation for patients with lymph-node positive or transmural rectal cancer.1–4 Recent clinical trials have confirmed the survival benefit of this treatment recommendation.5–8
Prior observational studies examined initiation rates of combined chemoradiotherapy for stages II and III rectal cancer and the association between initiation of chemoradiation and mortality.9–13 However, randomized controlled trials demonstrating survival benefit were based on completion of a specified course of chemoradiation rather than initiation alone.1–4,7,8 We found no published population-based studies that evaluated the degree to which stage II and III rectal cancer patients complete adjuvant chemoradiation or the association between chemoradiotherapy completion and survival.
There are reasons to question whether patients, especially the elderly, are completing a recommended course of chemoradiotherapy, which includes the potential occurrence of acute toxicities.2,14,15 Recent studies among elderly patients with stage III colon cancer found that increased age and factors suggesting frailty are inversely associated with adjuvant chemotherapy completion.12,16 Incomplete therapy may be more dramatic among rectal cancer patients, who require both chemotherapy and radiation therapy.
In this study, we examined adherence to the recommended course of chemoradiation, trends in adherence over time, and the relation between different levels of treatment completion and mortality in a general population of elderly stage II and III rectal cancer patients.
MATERIALS AND METHODS
Data Source
This study used data from the Surveillance, Epidemiology, and End Results (SEER) cancer registries linked with Medicare claims for persons found in both files. The SEER-Medicare database is generated through the cooperative efforts of the Center for Medicare & Medicaid Services (CMS), the National Cancer Institute (NCI), and SEER registries. Our study included data for incident rectal cancer cases reported to SEER registries between 1992 and 1999. SEER-Medicare data allow examination of cancer treatment claims for elderly Americans in fee-for-service care within SEER program areas in 5 states and 8 metropolitan or county-based areas in 5 additional states as follows: Connecticut, Hawaii, Iowa, New Mexico, Utah, Atlanta and rural Georgia, Arizona Indians (which we group with New Mexico), Detroit, Los Angeles, San Francisco, San Jose, and Seattle/Puget Sound. SEER data provided diagnosis date, patient demographics, cancer type and stage, and tumor characteristics. Medicare data provided date and cause of death through December 2004, enrollment dates in parts A and B Medicare, HMO enrollment dates, dates and types of treatment, and diagnosis and procedure codes for services provided by hospitals (MedPAR files), physicians and clinics (Carrier file), and noninstitutional facilities (Outpatient file). The SEER-Medicare database provided US Census data for area socioeconomic status at the ZIP-code level.11
Study Population
We identified 5249 patients aged 66 years and older who were diagnosed with primary stage II and III rectal cancer between January 1, 1992 and December 31, 1999, allowing for at least 5 years of follow-up for vital status. Rectal cancers included all adenocarcinomas in the rectum and excluded rectosigmoid cancers. American Joint Committee on Cancer criteria were used to designate cancer stage.17
We sequentially excluded patients with prior colorectal cancer (n = 118), simultaneous stage IV colorectal cancer (n = 5), and autopsy or death certificate-based rectal cancer diagnosis (n = 4). To adequately measure baseline comorbidity, we then excluded patients without continuous Medicare Part A and Part B enrollment in fee-for-service Medicare in the 11 months preceding the month before diagnosis (n = 1317). We excluded an additional 658 patients who died and 68 with incomplete enrollment in Medicare Part A and Part B fee-for-service Medicare in the 12 months after diagnosis to ascertain adjuvant chemoradiotherapy receipt. Lastly, we excluded 193 patients without a surgical resection Medicare claim within 6 months of diagnosis. Our final study population included 2886 patients.
Study Variables
Initiation and completion of adjuvant chemoradiotherapy
We defined chemotherapy initiation as at least 1 claim indicating administration of chemotherapy (Current Procedural Terminology [CPT] codes 96408; 96410; 96412; 96414; 96545; 96549; 96520; and 96530; International Classification of Diseases, 9th Edition [ICD-9] procedure code 99.25; ICD-9 diagnosis codes E 0781 and V58.1; and Health Care Common Procedure Codes [HCPCS] J0640, J9190, and Q0083-85). Radiation therapy initiation was defined as at least 1 claim indicating administration or management of radiation therapy (CPT codes 77331-4; 77336; 77370; 77399; 77402-17, 77419-31; and 77499; ICD-9 procedure codes 92.20, 92.23-6, and 92.29; ICD-9 diagnosis code V58.0; and Outpatient Revenue Center code 0333). We defined neoadjuvant chemotherapy and neoadjuvant radiation therapy as receipt of adjuvant chemotherapy or radiation therapy anytime starting in the month of diagnosis up to the day before surgical resection. Time to initiation of adjuvant therapy was defined as the number of weeks from the hospital admission date for surgical resection to the first radiation or chemotherapy administration or management claim. Prior research has documented a high sensitivity of Medicare claims in identifying chemotherapy and radiation initiation.18–21
We created variables to denote receipt of a complete course of adjuvant chemotherapy and adjuvant radiation therapy. During our study period (1992–1999), the standard of care for adjuvant chemotherapy shifted from 12 to 6 months, based on research evidence.22 To avoid underascertainment of completion, we accepted 6 months or cycles of a chemotherapy regimen as the standard for a complete course. Medicare data identified individual dates on which chemotherapy was administered. We defined 5 months of chemotherapy within which there was at least 1 chemotherapy administration claim per month as a complete course.16 This definition allows for missing claims in our database.
Published recommendations advise 6 weeks of radiation as the standard course of therapy.3 To allow for missing claims, we defined a complete course as 5 weeks and a week of radiation therapy as having at least 1 claim for radiation administration or management in the week.
We wanted to ensure that chemotherapy and radiotherapy claims used to determine completion of a course were not claims for treatment of a cancer recurrence. Therefore, we considered only chemotherapy or radiation claims that began with the first claim date for surgery, chemotherapy, or radiation therapy after diagnosis and ended1 with the claim date after which there were 3 months without any type of rectal cancer treatment,2 with a cancer recurrence (eg, evidence of metastasis or secondary malignancy according to CPT codes, or ICD-9 diagnosis, or procedure codes), or3 12 months after diagnosis, whichever came first. Our analyses confirmed that all patients who completed an adjuvant course of chemotherapy or radiation therapy did so within 12 months of diagnosis.
We identified 5 adjuvant therapy completion options as follows: 1) complete chemotherapy and radiation therapy; 2) complete chemotherapy, but no or incomplete radiation therapy; 3) complete radiation therapy, but no or incomplete chemotherapy; 4) some chemotherapy and/or radiation therapy, but neither therapy complete; and 5) no chemotherapy or radiation therapy. We included any neoadjuvant chemotherapy and radiotherapy that a patient received in the determination of completion.
The recommended chemoradiation treatment includes a 21-day overlap in receipt of chemotherapy and radiation therapy. Among individuals in our study who completed both radiation and chemotherapy, more than 95% had the recommended 21-day overlap. However, because of the potential for missing or incorrectly dated claims records, we did not require patients to have an overlap in therapies.
Mortality
We identified patients who died of cancer within 5 years of their diagnosis to calculate cancer-related mortality rates for the different adjuvant therapy completion groups.
Explanatory variables
Patient sociodemographic, clinical, and tumor characteristics
Age, race, marital status, sex, tumor (T) classification, number of positive nodes, and tumor grade were identified from SEER data. Comorbidity was identified from inpatient and outpatient claims in the 11 months preceding the month before diagnosis by using Romano’s adaptation of the Charlson comorbidity index,23,24 classifying individuals into scores of 0, 1, or 2 or more.
We constructed 2 variables representing clinical factors that may influence adjuvant chemoradiation therapy receipt or completion as follows: rehospitalizations during the postsurgical period (1–6 weeks) and rehospitalizations during the adjuvant treatment period (7 weeks until the end of the treatment period). We excluded hospitalizations focused on receipt of chemotherapy or radiation therapy (Diagnostic Related Groupings [DRGs] 409, 410). These variables are further described in our prior publication.16
Contextual variables
Median household income in the patient’s residence ZIP code was used as a proxy for socioeconomic status. Other contextual variables included the SEER registries to which patients were reported and residence location as defined by the Rural Urban Commuting Areas (RUCAs).25,26 RUCAs, based on the patients’ plurality residence ZIP code on the Medicare claims during the month of diagnosis or the most proximate claim, were designated as urban-focused, large rural city/town-focused, small rural town-focused, and isolated small rural town-focused.
Analysis
We described demographic, clinical, tumor, and contextual characteristics of eligible rectal cancer patients by cancer stage using chi-square tests to identify significant differences. We used chi-square tests to compare the characteristics of therapies that study patients received by cancer stage. We calculated completion rates for adjuvant chemotherapy, adjuvant radiation therapy, and both overall and by year, then we tested for the association over time of adjuvant chemotherapy and radiation therapy receipt overall and in the neoadjuvant phase by cancer stage by using the Mantel-Haenszel chi-square test.
We report unadjusted 5-year cancer mortality rates among patients at the 5 levels of completeness of adjuvant therapy. We tested for unadjusted differences in mortality across these groups by using chi-square tests. We conducted multivariate logistic regression (SAS, version 9.1) to compute the adjusted relative risk of 5-year mortality among those with various levels of adjuvant therapy completeness compared with those with no adjuvant therapy. Recognizing that factors besides a complete course of adjuvant therapy could affect cancer mortality rates, we controlled for tumor extent, number of positive lymph nodes, and tumor grade, as well as patient’s age, race, sex, marital status, residence location, SEER registry, ZIP code-based median household income, and comorbidity. Because the outcomes were relatively common, odds ratios (ORs) were transformed to Relative Risks (RRs) with 95% confidence intervals (CIs) for the variables retained in the final model by using published methods.27
RESULTS
There were few significant differences in the characteristics of stage II and III rectal cancer patients (Table 1). Stage II patients were slightly older and were less likely to be rehospitalized at 7 or more weeks after surgery than stage III patients.
TABLE 1.
Sociodemographic, Clinical, Tumor, and Contextual Characteristics of Stage II and III Rectal Cancer
| Characteristic | % Stage II* | % Stage III* |
|---|---|---|
| n = 1524 | n = 1362 | |
| Age, y† | ||
| 66–70 | 23.3 | 27.0 |
| 71–75 | 26.4 | 30.2 |
| 76–80 | 23.7 | 21.9 |
| 81–85 | 16.5 | 14.1 |
| ≥86 | 10.2 | 6.8 |
| Race | ||
| Caucasian | 85.2 | 85.9 |
| African American | 3.9 | 4.6 |
| Asian or Pacific Islander | 5.1 | 4.4 |
| Hispanic | 4.4 | 4.5 |
| Other, unknown | 1.4 | 0.7 |
| Men | 52.9 | 55.7 |
| Marital status | ||
| Married | 55.7 | 58.6 |
| Divorced, separated, or single | 11.9 | 12.4 |
| Widowed | 31.0 | 27.8 |
| Unknown | 1.3 | 1.2 |
| Median household income in ZIP code of residence | ||
| ≤$25,000 | 13.0 | 13.6 |
| $25,001–$35,000 | 25.8 | 25.8 |
| $35,001–$45,000 | 27.6 | 25.6 |
| ≥$45,001 | 33.7 | 35.0 |
| Residence location | ||
| Isolated small rural town-focused | 6.1 | 6.5 |
| Small rural town-focused | 6.3 | 7.2 |
| Large rural city/town-focused | 7.2 | 5.4 |
| Urban-focused | 80.3 | 80.9 |
| Comorbidity score | ||
| 0 | 54.6 | 56.2 |
| 1 | 20.3 | 21.2 |
| ≥2 | 25.1 | 22.6 |
| Year of diagnosis | ||
| 1992 | 14.1 | 14.0 |
| 1993 | 14.6 | 12.9 |
| 1994 | 12.1 | 11.3 |
| 1995 | 12.8 | 12.3 |
| 1996 | 12.7 | 11.6 |
| 1997 | 11.6 | 12.4 |
| 1998 | 11.4 | 13.4 |
| 1999 | 10.8 | 12.1 |
| SEER registry | ||
| Atlanta/Rural Georgia | 4.1 | 5.2 |
| Connecticut | 16.1 | 14.8 |
| Detroit | 15.0 | 17.8 |
| Hawaii | 2.5 | 2.5 |
| Iowa | 17.3 | 17.1 |
| Los Angeles | 10.6 | 11.2 |
| New Mexico/Arizona Indians | 4.0 | 3.7 |
| San Francisco | 8.1 | 6.6 |
| San Jose | 5.9 | 4.7 |
| Seattle/Puget Sound | 10.8 | 11.2 |
| Utah | 5.5 | 5.1 |
| Hospital readmission in 1st–6th wk after surgery | 13.5 | 12.0 |
| Hospital readmission in 7th wk after surgery to end of treatment period‡ | 39.6 | 43.8 |
| Tumor extent†,§ | ||
| T1 | — | 3.5 |
| T2 | — | 17.6 |
| T3 | 91.9 | 73.9 |
| T4 | 8.1 | 5.0 |
| Tumor grade† | ||
| G1 (well differentiated) | 7.2 | 5.3 |
| G2 (moderately differentiated) | 74.4 | 67.2 |
| G3 (poorly differentiated) | 13.5 | 22.7 |
| G4 (undifferentiated) | 0.4 | 0.4 |
| Unknown | 4.5 | 4.5 |
| No. of positive nodes† | ||
| N0 (no nodes) | 81.1 | 0.6 |
| N1 (1–3) | — | 62.6 |
| N2 (4–96) | — | 31.9 |
| Nx (no nodes examined) | 18.9 | 1.8 |
| Ny (positive nodes, number not specified) | — | 3.1 |
All chi squares are overall, comparing stage II and stage III with different characteristics.
Percentages do not always add to 100 due to rounding.
P ≤.001.
P ≤.05.
T1 is tumor confined to mucosa, muscularis mucosa, head or stalk of polyp, or submucosa. T2 is tumor with muscularis propria invaded or localized, not otherwise specified. T3 is invasion through muscularis propria, wall, or invasion through perimuscular or subserosal tissue, or extension into fat, adjacent tissue, or through peritoneum. T4 is extension into bladder, prostate, vagina, pelvic wall, uterus, ureter, colon, ductus deferens, or other structure.
Missing values: median household income (58 stage II, 41 stage III), residence location (4 stage II, 4 stage III), race (3 stage II included in American Indian/other category).
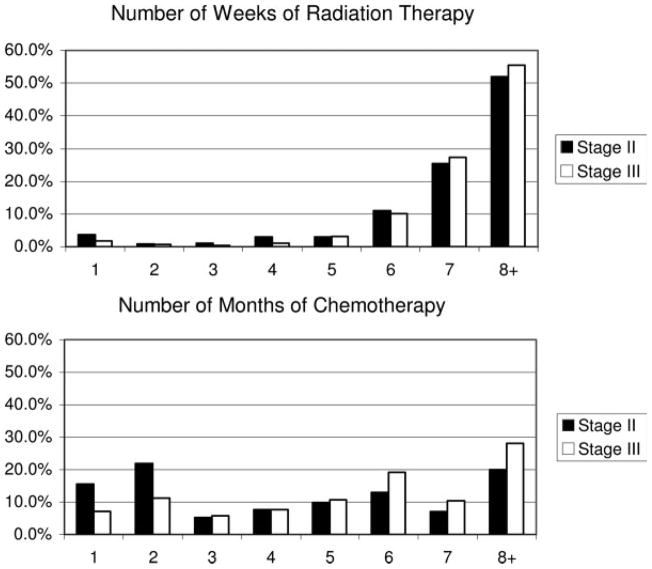
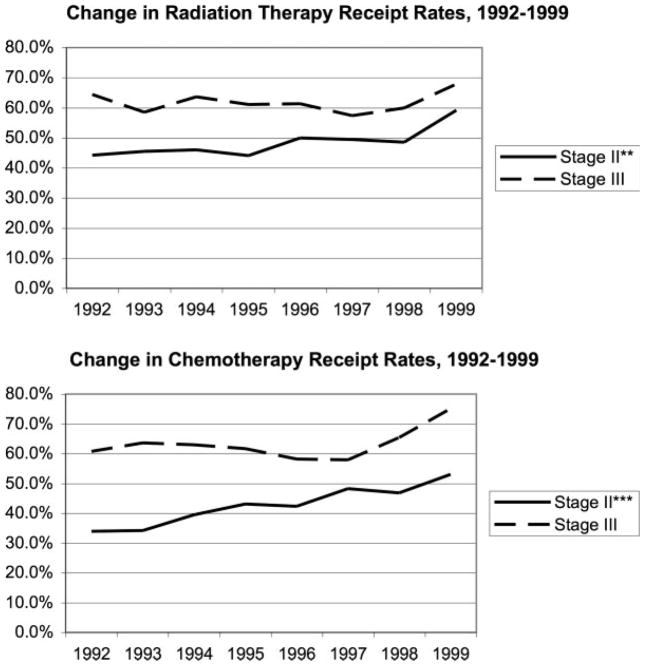
Of the study’s 2886 patients, 1766 (61.2%) received some adjuvant therapy (Table 2; Fig. 1). Stage III patients were significantly more likely to receive chemotherapy, radiation therapy, or both than stage II patients. Among stage II patients, but not stage III patients, the receipt rates of both adjuvant chemotherapy and radiation therapy increased significantly between 1992 and 1999 (Fig. 2). Notably, about half of both stage II (51.9%) and stage III (55.5%) patients received 8 weeks or more of radiation therapy (Table 2).
TABLE 2.
Description of Adjuvant Therapy Received by Stage II and III Rectal Cancer Patients
| Total |
Stage II |
Stage III |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Total | 2886 | 100.0 | 1524 | 100.0 | 1362 | 100.0 |
| Rates of | ||||||
| Radiation therapy receipt* | 1573 | 54.5 | 732 | 48.0 | 841 | 61.8 |
| Chemotherapy receipt* | 1502 | 52.0 | 641 | 42.1 | 861 | 63.2 |
| Both radiation therapy and chemotherapy receipt* | 1309 | 45.4 | 571 | 37.5 | 738 | 54.2 |
| Among those receiving adjuvant radiation therapy | ||||||
| Receiving neoadjuvant radiation therapy* | 306 | 19.4 | 191 | 26.1 | 115 | 13.7 |
| Initiation of radiation therapy before or within 8 wk of resection* | 1006 | 64.0 | 509 | 69.5 | 497 | 59.1 |
| Among those receiving adjuvant chemotherapy | ||||||
| Receiving neoadjuvant chemotherapy* | 198 | 13.2 | 127 | 19.8 | 71 | 8.2 |
| Initiation of chemotherapy before or within 8 wk of resection† | 1176 | 78.3 | 483 | 75.4 | 693 | 80.5 |
All chi-square overall, comparing stage II with stage III with different characteristics of therapy. (No statistics for testing duration of therapy.)
P ≤.001.
P ≤.05.
FIGURE 1.
Length of therapy.
FIGURE 2.
Adjuvant therapy receipt among stage II and III rectal cancer patients, 1992–1999. Mantel Haenszel chi-square test of trend in radiation and chemotherapy receipt over the study period, *P ≤ .05, **P ≤ .01, ***P ≤ .001.
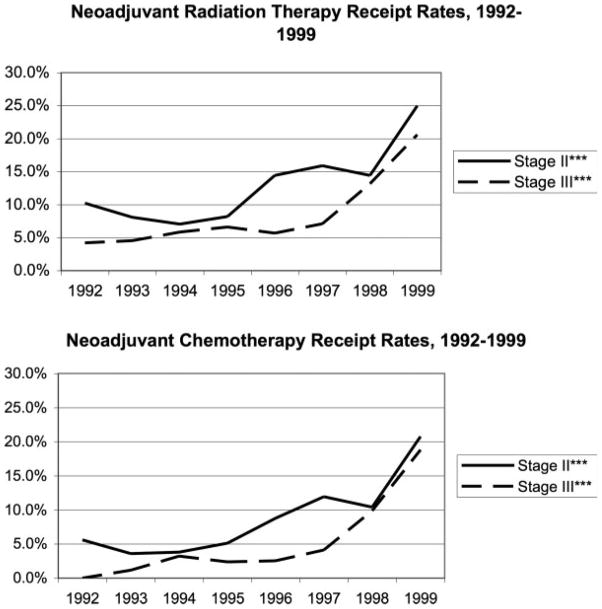
Stage II patients were more likely to receive neoadjuvant chemotherapy and neoadjuvant radiation therapy than stage III patients (Table 2). Both neoadjuvant therapies increased significantly between 1992 and 1999 for stage II and III patients (Fig. 3). Overall, including those who received neoadjuvant therapy, 64.0% initiated radiation therapy and 78.3% initiated chemotherapy before or within 8 weeks of surgery, the standard timeframe for these treatments (Table 2).
FIGURE 3.
Neoadjuvant therapy receipt among stage II and III rectal cancer patients, 1992–1999. Mantel-Haenszel chi-square test of trend in radiation and chemotherapy receipt over the study period, *P ≤ .05, **P ≤ .01, ***P ≤ .001.
Among those who initiated adjuvant radiation, 91.5% of stage II and 96.6% of stage III patients completed it (Table 3). A much lower proportion of patients who initiated adjuvant chemotherapy completed treatment, 49.8% of stage II and 68.2% of stage III patients. Nearly all patients who used adjuvant chemotherapy also completed a course of adjuvant radiation therapy. Stage III patients were significantly more likely than stage II patients to complete adjuvant radiation, adjuvant chemotherapy, and the combination of both.
TABLE 3.
Completion Rates for Stage II and III Rectal Cancer Patients Who Initiated Adjuvant Radiation Therapy and/or Chemotherapy
| Stage II |
Stage III |
|||
|---|---|---|---|---|
| No. of patients initiating treatment | Completion rate | No. of patients initiating treatment | Completion rate | |
| Radiation therapy* | 732 | 91.5% | 841 | 96.6% |
| Chemotherapy* | 641 | 49.8% | 861 | 68.2% |
| Both radiation therapy and chemotherapy* | 571 | 47.6% | 738 | 67.5% |
All chi squares overall comparing stage II with stage III for completion of therapies.
P ≤.001.
Stage II and III rectal cancer patients who completed both adjuvant radiation and chemotherapy had significantly lower adjusted 5-year cancer-related mortality than those with no adjuvant therapy (stage II adjusted relative risk [RR] 0.74 [95% CI, 0.54,0.97], stage III adjusted RR 0.80 [95% CI, 0.65, 0.96]) (Table 4). Stage III patients who completed adjuvant chemotherapy but who received incomplete radiation therapy had a reduced risk of cancer-related mortality that approached statistical significance (adjusted RR 0.76 [95% CI, 0.53,1.02]) compared with those with no therapy. Adjusted 5-year mortality rates among patients who received incomplete courses of chemotherapy and/or radiation therapy were comparable to the 5-year mortality rates of patients who received no chemotherapy and radiation therapy.
TABLE 4.
Relation Between 5-Year Cancer-specific Mortality and Level of Adjuvant Radiation Therapy and Chemotherapy Completion by Stage II and III Rectal Cancer Patients
| Stage II, n = 1524 |
Stage III, n = 1362 |
|||||
|---|---|---|---|---|---|---|
| No. (%) | Unadjusted cancer-related mortality rate,* % | Adjusted RR of cancer-related death† (95% CI) | No. (%) | Unadjusted cancer-related mortality rate, % | Adjusted RR of cancer-related death† (95% CI) | |
| Radiation therapy and chemotherapy completed | 272 (17.9) | 22.1 | 0.74 (0.54,0.97) | 498 (36.6) | 41.0 | 0.80 (0.65,0.96) |
| Chemotherapy completed, no radiation therapy or incomplete course | 47 (3.1) | 25.5 | 0.94 (0.54,1.48) | 89 (6.5) | 40.4 | 0.76 (0.53,1.02) |
| Radiation therapy completed, no chemotherapy or incomplete course | 398 (26.1) | 33.9 | 0.92 (0.73,1.14) | 314 (23.1) | 48.7 | 0.95 (0.78,1.13) |
| Initiated radiation therapy and/or chemotherapy, without completion of either | 85 (5.6) | 32.9 | 1.02 (0.68,1.45) | 62 (4.6) | 46.8 | 0.90 (0.62,1.21) |
| Neither radiation therapy nor chemotherapy initiated | 722 (47.4) | 29.1 | 1.00 | 398 (29.2) | 47.7 | 1.00 |
RR indicates relative risks; 95% CI, confidence interval.
Missing values for logistic regressions: median household income (58 stage II, 41 stage III), residence location (4 stage II, 4 stage III), missing date of death (1 stage III).
Chi squares for unadjusted mortality by 5 levels of completion.
P ≤.05.
Adjusted for patients’ age, race, sex, marital status, residence location, SEER registry, median household income, comorbidity, extent of tumor, grade of tumor, and number of positive nodes.
DISCUSSION
This study demonstrated that fewer than half of stage II (37.5%) and just over half of stage III (54.2%) rectal cancer patients diagnosed from 1992 through 1999 initiated combined chemoradiation therapy. This is consistent with earlier studies of stage II and III rectal cancer patients according to 1992–1996 SEER-Medicare data that reported chemoradiation initiation rates of 37% and 42%.12,13
For stage II patients, we found significantly increasing rates of adjuvant chemotherapy from 1992 to 1999 (from 34.0% to 53.0%) and consistently higher rates among stage III compared with stage II patients. Adjuvant radiation therapy use also significantly increased for stage II patients from 44.2% to 59.1% over this study period. Although no statistically significant trend could be detected, adjuvant chemotherapy use by stage III patients increased from 60.7% in 1992 to 75.2% in 1999, and adjuvant radiation therapy increased from 64.4% to 67.9% over that period. This is consistent with a report that used the SEER registry from 2000, wherein about ⅓ of patients with nonmetastatic advanced rectal cancer did not receive adjuvant radiation therapy.28
The most dramatic change over the course of our study was the increase in use of neoadjuvant therapy for both stage II and III patients. Although still infrequently received, by 1999, neoadjuvant chemotherapy rates had more than tripled to approximately 20%, and neoradiation therapy more than doubled for stage II patients and increased 4-fold for stage III patients, both to more than 20%. During this period, oncologists recognized that neoadjuvant treatment was more easily tolerated than adjuvant treatment,29,30 and there were indications that neoadjuvant therapy offered improved disease control.31,32 Recent studies have confirmed that neoadjuvant therapy offers more favorable local control and an increased likelihood of a sphincter-sparing surgical procedure.30,33,34 Although neoadjuvant therapy has beneficial aspects, it does not constitute a complete course of adjuvant therapy.
Our data suggest that completion of therapy had a significant impact on survival for stage II and III patients. These findings confirm the results of clinical trials that have demonstrated that the addition of chemotherapy to external-beam pelvic radiation provides optimal post-treatment survival.8 The adjusted relative risk of cancer-related death for patients initiating but not completing chemoradiation was comparable to that of patients with no adjuvant therapy, emphasizing the importance of completing adjuvant treatment once it has begun.
Stage III patients in our study who received a complete course of chemotherapy but an incomplete course of radiation therapy had a lower risk of cancer-related death than those with no therapy, but this finding did not achieve statistical significance, perhaps because of the small number in this group (n = 89). Future work with a larger population should explore whether treatment with surgery and only complete chemotherapy is necessary for improved survival of stage III rectal cancer patients. However, this does not take into account the importance of preventing local recurrence, where the role of radiation therapy is, perhaps, most important.33,35
Whereas some have argued that meticulous surgical technique may be adequate to provide excellent local rectal cancer control,36,37 the Dutch rectal cancer trial demonstrated that even with optimal surgical technique, radiation therapy significantly decreased local recurrence rates.35 Our data do not allow us to examine surgical technique or local recurrence, the outcome most affected by inadequate radiation treatment. Therefore, our findings cannot be interpreted as rationale for changing adjuvant radiation therapy recommendations for these patients.
Our data indicated that only 54.2% of stage III patients and 37.5% of stage II patients initiated both chemotherapy and radiation therapy as indicated by NIH guidelines. Complying with recommended therapy for rectal cancer is challenging because the course for chemotherapy and radiation is lengthy, and there is significant toxicity. The majority of patients who initiated radiation therapy completed the recommended course, but fewer patients who started chemotherapy completed treatment. Of those who initiated chemoradiation, 47.6% of stage II patients and 67.5% of stage III patients completed both treatments. Given the relatively low rates of treatment initiation and significant attrition from therapy completion, a strikingly low proportion of all rectal cancer patients completed chemoradiation, 22.6% of all stage II and 43.6% of all stage III patients in 1999.
Do these findings reflect physician skepticism about the necessity of these treatments, particularly for elderly patients for whom the survival benefit may be outweighed by treatment toxicity? Although it is difficult to answer this question with the current data set, the higher completion rates for patients with stage III compared with stage II disease suggest that patients and physicians may be using their judgment on the level of disease advancement to decide how vigorously to pursue treatment completion. An alternative explanation is that stage III patients have less treatment-related toxicity than stage II patients, but research suggests similar toxicities for both groups.14,30
This study has several limitations. First, this is a retrospective study based on administrative data. We did not have access to detailed clinical records that could provide additional variables influencing treatment, such as severity of comorbidity. Although we used logistic regression modeling techniques to adjust for all confounding variables identifiable in these data, including age, sex, stage of disease, comorbidity, and year of treatment, there may be unmeasured confounders.
Second, we do not have data on treatment rates for populations younger than 65 years of age. Future work could explore treatment in these younger populations, as well as treatment among patients with different characteristics (eg, sex, marital status, residence location) to determine whether there are subpopulations that are more and less likely to receive adjuvant chemoradiation therapy for rectal cancer.
Third, our findings of a more favorable mortality rate associated with completion of therapy may be related to other unexplored factors. Recent studies suggest that mortality is related to tumor characteristics, such as size, number of nodes, location of the primary tumor, surgical method, and complete pathologic response to neoadjuvant therapy.38–44 Although we controlled for tumor extent (T classification) and number of nodes, we were unable to control for other tumor and treatment factors. Needed is future analysis of the association of these variables with cancer-specific mortality. The lower mortality rate associated with completion of therapy may also be related to patient selection rather than to therapeutic benefit, although we used cancer-specific survival to minimize potential confounding from noncancer-related medical conditions.
Our results demonstrate clear, cancer-specific, survival benefit for a general population of elderly stage II and III rectal cancer patients who complete a full course of recommended therapy yet who have low rates of chemoradiation completion. Although the past decade has brought refinements in surgical technique and a move toward neoadjuvant therapy, this study underscores the importance of a complete course of adjuvant chemoradiotherapy for patients with stage II and III rectal cancer. This study’s findings provide large-scale, real-world confirmation of the clinical trial data that has shaped our practice patterns in rectal cancer therapy and should serve as encouragement for primary care physicians, medical oncologists, radiation oncologists, and oncology nurses to make all possible efforts to support rectal cancer patients through a full course of adjuvant therapy.
Acknowledgments
This research was supported by grant R01CA089544 from the National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
The authors would like to thank Yong Cai, PhD and Pam Green, PhD for their expertise and role on the study database design and development team and for their assistance in creating several the database variables; Denise Lishner for her assistance in literature review and manuscript editing; Marie Topor and Angela Fahey at Information Management Services for their assistance in understanding the linked SEER-Medicare database; and Jason Dominitz, MD, William Barlow, PhD, and George Wright, PhD for their insight into the analytical questions.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Cancer Institute.
References
- 1.Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 3.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 4.Douglass HO, Jr, Moertel CG, Mayer RJ, et al. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315:1294–1295. doi: 10.1056/NEJM198611133152014. [DOI] [PubMed] [Google Scholar]
- 5.Tepper JE, O’Connell M, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control—final report of intergroup 0114. J Clin Oncol. 2002;20:1744–1750. doi: 10.1200/JCO.2002.07.132. [DOI] [PubMed] [Google Scholar]
- 6.Radiation therapy and fluorouracil with or without semus-tine for the treatment of patients with surgical adjuvant adenocarcinoma of the rectum. Gastrointestinal Tumor Study Group. J Clin Oncol. 1992;10:549–557. doi: 10.1200/JCO.1992.10.4.549. [DOI] [PubMed] [Google Scholar]
- 7.O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 8.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 9.Cronin DP, Harlan LC, Potosky AL, Clegg LX, Stevens JL, Mooney MM. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am J Gastroenterol. 2006;101:2308–2318. doi: 10.1111/j.1572-0241.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 10.Dharma-Wardene MW, de Gara C, Au HJ, Hanson J, Hatcher J. Ageism in rectal carcinoma? Treatment and outcome variations. Int J Gastrointest Cancer. 2002;32:129–138. doi: 10.1385/IJGC:32:2-3:129. [DOI] [PubMed] [Google Scholar]
- 11.Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20:1192–1202. doi: 10.1200/JCO.2002.20.5.1192. [DOI] [PubMed] [Google Scholar]
- 12.Neugut AI, Fleischauer AT, Sundararajan V, et al. Use of adjuvant chemotherapy and radiation therapy for rectal cancer among the elderly: a population-based study. J Clin Oncol. 2002;20:2643–2650. doi: 10.1200/JCO.2002.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Schrag D, Gelfand SE, Bach PB, Guillem J, Minsky BD, Begg CB. Who gets adjuvant treatment for stage II and III rectal cancer? Insight from surveillance, epidemiology, and end results—Medicare. J Clin Oncol. 2001;19:3712–3718. doi: 10.1200/JCO.2001.19.17.3712. [DOI] [PubMed] [Google Scholar]
- 14.Miller RC, Martenson JA, Sargent DJ, Kahn MJ, Krook JE. Acute treatment-related diarrhea during postoperative adjuvant therapy for high-risk rectal carcinoma. Int J Radiat Oncol Biol Phys. 1998;41:593–598. doi: 10.1016/s0360-3016(98)00084-4. [DOI] [PubMed] [Google Scholar]
- 15.Kollmorgen CF, Meagher AP, Wolff BG, Pemberton JH, Martenson JA, Illstrup DM. The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Ann Surg. 1994;220:676–682. doi: 10.1097/00000658-199411000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6. New York: Springer-Verlag; 2002. [Google Scholar]
- 18.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(8 suppl):IV-49–54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 19.Du X, Freeman JL, Goodwin JS. Information on radiation treatment in patients with breast cancer: the advantages of the linked Medicare and SEER data. Surveillance, Epidemiology and End Results. J Clin Epidemiol. 1999;52:463–470. doi: 10.1016/s0895-4356(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 20.Lamont EB, Lauderdale DS, Schilsky RL, Christakis NA. Construct validity of Medicare chemotherapy claims: the case of 5FU. Med Care. 2002;40:201–211. doi: 10.1097/00005650-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 suppl):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 22.O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion, 1081–1090. [DOI] [PubMed] [Google Scholar]
- 25.Morrill R, Cromartie J, Hart LG. Metropolitan, urban, and rural commuting areas: toward a better depiction of the US settlement system. Urban Geogr. 1999;20:727–748. [Google Scholar]
- 26.WWAMI Rural Health Research Center. [Accessed February 20, 2007];Rural-urban commuting area codes (version 2.0) Available at: http://depts.washington.edu/uwruca/
- 27.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 28.Baxter NN, Rothenberger DA, Morris AM, Bullard KM. Adjuvant radiation for rectal cancer: do we measure up to the standard of care? An epidemiologic analysis of trends over 25 years in the United States. Dis Colon Rectum. 2005;48:9–15. doi: 10.1007/s10350-004-0792-8. [DOI] [PubMed] [Google Scholar]
- 29.Minsky BD, Cohen AM, Kemeny N, et al. Combined modality therapy of rectal cancer: decreased acute toxicity with the preoperative approach. J Clin Oncol. 1992;10:1218–1224. doi: 10.1200/JCO.1992.10.8.1218. [DOI] [PubMed] [Google Scholar]
- 30.Osti MF, Valeriani M, Masoni L, Tombolini V, Enrici RM. Neoadjuvant chemoradiation for locally advanced carcinoma of the rectum. Tumori. 2004:309. doi: 10.1177/030089160409000308. [DOI] [PubMed] [Google Scholar]
- 31.Pahlman L, Glimelius B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg. 1990;211:187–195. doi: 10.1097/00000658-199002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmad NR, Marks G, Mohiuddin M. High-dose preoperative radiation for cancer of the rectum: impact of radiation dose on patterns of failure and survival. Int J Radiat Oncol Biol Phys. 1993;27:773–778. doi: 10.1016/0360-3016(93)90448-5. [DOI] [PubMed] [Google Scholar]
- 33.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 34.Valero G, Lujan JA, Hernandez Q, et al. Neoadjuvant radiation and chemotherapy in rectal cancer does not increase postoperative complications. Int J Colorectal Dis. 2003;18:495–499. doi: 10.1007/s00384-003-0520-1. [DOI] [PubMed] [Google Scholar]
- 35.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 36.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 37.Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335–346. [PubMed] [Google Scholar]
- 38.Bulow S, Christensen IJ, Harling H, Kronborg O, Fenger C, Nielsen HJ. Recurrence and survival after mesorectal excision for rectal cancer. Br J Surg. 2003;90:974–980. doi: 10.1002/bjs.4137. [DOI] [PubMed] [Google Scholar]
- 39.Habr-Gama A, Perez RO, Kiss DR, et al. Preoperative chemoradiation therapy for low rectal cancer. Impact on downstaging and sphincter-saving operations. Hepatogastroenterology. 2004;51:1703–1707. [PubMed] [Google Scholar]
- 40.Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–163. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- 41.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 42.Greene FL, Stewart AK, Norton HJ. New tumor-node-metastasis staging strategy for node-positive (stage III) rectal cancer: an analysis. J Clin Oncol. 2004;22:1778–1784. doi: 10.1200/JCO.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Jeong SY, Chessin DB, Schrag D, Riedel E, Wong WD, Guillem JG. Re: Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2005;97:1705–1706. doi: 10.1093/jnci/dji383. author reply 1706–1707. Comment on: J Natl Cancer Inst2004;96:1408–1409 and J Natl Cancer Inst 2004;96:1420–1425. [DOI] [PubMed] [Google Scholar]
- 44.Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–836. doi: 10.1097/01.sla.0000161980.46459.96. discussion 836–828. [DOI] [PMC free article] [PubMed] [Google Scholar]