The surface expression and channel activation of transient receptor potential canonical 6 (TRPC6) were regulated by tyrosine phosphorylation and resultant binding with stimulatory PLC-γ1 and inhibitory nephrin. Disease-causing mutations made the TRPC6s insensitive to nephrin suppression, suggesting that the cell-type–specific regulation of TRPC6 might be involved in the pathogenesis.
Abstract
Transient receptor potential canonicals (TRPCs) play important roles in the regulation of intracellular calcium concentration. Mutations in the TRPC6 gene are found in patients with focal segmental glomerulosclerosis (FSGS), a proteinuric disease characterized by dysregulated function of renal glomerular epithelial cells (podocytes). There is as yet no clear picture for the activation mechanism of TRPC6 at the molecular basis, however, and the association between its channel activity and pathogenesis remains unclear. We demonstrate here that tyrosine phosphorylation of TRPC6 induces a complex formation with phospholipase C (PLC)-γ1, which is prerequisite for TRPC6 surface expression. Furthermore, nephrin, an adhesion protein between the foot processes of podocytes, binds to phosphorylated TRPC6 via its cytoplasmic domain, competitively inhibiting TRPC6–PLC-γ1 complex formation, TRPC6 surface localization, and TRPC6 activation. Importantly, FSGS-associated mutations render the mutated TRPC6s insensitive to nephrin suppression, thereby promoting their surface expression and channel activation. These results delineate the mechanism of TRPC6 activation regulated by tyrosine phosphorylation, and imply the cell type–specific regulation, which correlates the FSGS mutations with deregulated TRPC6 channel activity.
INTRODUCTION
Dynamic changes in calcium concentration trigger a plethora of cellular responses, including secretion, contraction, cell growth, survival, and differentiation by versatile regulatory mechanisms (Berridge et al., 2000). The increase in Ca2+ concentration is initiated by the opening of Ca2+-permeable channels on the plasma membrane or on the endoplasmic reticulum resulting from direct receptor activation by ligands or indirect activation through the intracellular signaling pathways (Putney, 1986, 2009). The mammalian homologues of Drosophila transient receptor potential canonical, the TRPCs, are potent plasma membrane channels that contribute to changes in the cytosolic free Ca2+ concentration (Birnbaumer et al., 1996), either by acting as Ca2+ entry pathways on the plasma membrane or by modulating the membrane-driving force for Ca2+ entry through changing the membrane potential (Nilius et al., 2007; Abramowitz and Birnbaumer, 2009; Kiselyov and Patterson, 2009). Among the seven mammalian TRPC channels, a subfamily of TRPC3, 6, and 7 can be defined by sequence similarity. These proteins form a nonselective cation channel that is activated by receptor stimulation or by the exogenous application of diacylglycerol analogues (Hofmann et al., 1999; Okada et al., 1999; Inoue et al., 2001; Trebak et al., 2003).
One of the mechanisms for the regulation of TRPC channel activity is insertion of channels into the plasma membrane. TRPC3, 4, and 6 are translocated to the plasma membrane upon stimulation of Gq-coupled receptors or receptor tyrosine kinases (Cayouette et al., 2004; Odell et al., 2005; Smyth et al., 2006). Phosphorylation of TRPC channels by protein kinase C, protein kinase G, or Src family tyrosine kinase also causes their membrane insertion, and a number of phosphorylation sites have been documented on these channels (Kiselyov and Patterson, 2009; Nishida et al., 2010). Src family kinase interacts with all TRPC channels (Kawasaki et al., 2006), and TRPC4 and 6 undergo tyrosine phosphorylation by Src family kinase upon epidermal growth factor (EGF) stimulation (Hisatsune et al., 2004; Odell et al., 2005). Fyn phosphorylates TRPC6 and increases its diacylglycerol-stimulated single channel activity (Hisatsune et al., 2004). Phosphorylation-independent interaction with phospholipase C (PLC)-γ also induces membrane insertion of TRPC3 (van Rossum et al., 2005). The precise mechanisms of the activation and regulation of TRPCs remain to be clarified, however.
It is widely recognized that dysregulation of TRPC channels can result in the pathogenesis of various diseases, such as cardiovascular, neurodegenerative, respiratory, and renal diseases (Nilius et al., 2005 , 2007; Abramowitz and Birnbaumer, 2009; Woudenberg-Vrenken et al., 2009). TRPC6 is the essential component of receptor-operated cation channels in vascular smooth muscle cells (Inoue et al., 2001), and has been implicated in hypoxia-induced pulmonary hypertension (Lin et al., 2004; Wang et al., 2006). TRPC3 and TRPC6 promote cardiac hypertrophy through activation of calcineurin and its downstream effector, nuclear factors of activated T-cells (Bush et al., 2006; Kuwahara et al., 2006; Nakayama et al., 2006; Onohara et al., 2006). Recently mutations in the TRPC6 gene have been linked to the human proteinuric kidney disease, focal segmental glomerulosclerosis (FSGS) (Reiser et al., 2005; Winn et al., 2005). In this disease, the glomerular epithelial cells (podocytes) and the specific cellular junctional structure between podocyte foot processes, called the slit diaphragm, lose their integrity, disrupting glomerular filtration barrier (Tryggvason et al., 2006; Patrakka and Tryggvason, 2009). TRPC6 is expressed in podocytes, and binds to nephrin and podocin (Reiser et al., 2005; Huber et al., 2006), which are the critical components of the slit diaphragm, forming an essential part of the glomerular permeability barrier in the kidney (Tryggvason et al., 2006). Although overexpression of TRPC6 in the mouse kidney resulted in the induction of proteinuria (Moller et al., 2007; Krall et al., 2010), how the channel activity of mutated TRPC6 is involved in the pathogenesis remains unclear. Some mutations (P112Q, R895C, E897K) enhanced angiotensin II receptor–mediated activation of TRPC6 when expressed in HEK293 cells, but neither the S270T nor the N143S missense mutations, nor a 57-amino-acid truncation (K874X) mutation, altered the channel activity (Reiser et al., 2005; Winn et al., 2005). These channel activities correlated well with the extent of downstream nuclear factor of activated T-cells activation (Schlondorff et al., 2009). In contrast, the P112Q mutation increased the plasma membrane expression of TRPC6 (Winn et al., 2005), suggesting that changes in surface expression may also contribute to the pathogenesis of the disease.
In the present study we investigated the molecular basis of the translocation of TRPC6 by using HEK293T cells and cultured podocytes. We present evidence that surface expression of TRPC6 is regulated by its phosphorylation at Y284 by Src family kinase and interaction with PLC-γ1. Notably, a slit diaphragm protein, nephrin, interacts with phosphorylated TRPC6, and suppresses its translocation by interfering with TRPC6–PLC-γ1 binding. Importantly, FSGS-causing mutations dramatically weaken the nephrin–TRPC6 interaction, resulting in an increased membrane expression and Ca2+ channel activity of TRPC6 in living cells. Collectively, our results provide a correlation between the FSGS-associated mutations with deregulated TRPC6 activity, which may underlie the pathogenesis of this disease.
RESULTS
Phosphorylation of TRPC6 Tyr-284 is necessary for its trafficking to the plasma membrane
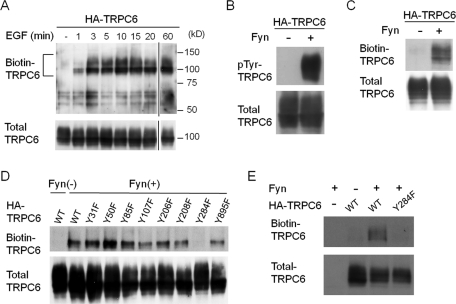
Because regulated translocation of TRPC channels to the plasma membrane has been proposed as a mechanism for their activation, we examined, by a surface biotinylation assay, whether phosphorylation of TRPC6 induces its translocation to the plasma membrane. HEK293T cells expressing hemagglutinin (HA)-tagged TRPC6 were stimulated with EGF for various times before biotinylation. Biotinylated TRPC6 was barely detectable under basal conditions (Figure 1A). TRPC6 appeared at the plasma membrane within 1 min after the stimulation, and its level reached a plateau at 3 min, which lasted for at least 60 min. The phosphorylation of TRPC6 by Src family protein tyrosine kinases (PTKs) has been implicated in physiological stimulation, because PP2, a specific inhibitor of Src family PTK, or a dominant-negative form of Fyn abrogates EGF-induced tyrosine phosphorylation of TRPC6 (Hisatsune et al., 2004). Indeed, tyrosine phosphorylation (Figure 1B) and increased cell surface localization of TRPC6 (Figure 1C) were induced by coexpression of a constitutively active Fyn.
FIGURE 1:
Phosphorylation of TRPC6 Y284 is necessary for its trafficking to the plasma membrane. (A) Induction of membrane trafficking of TRPC6 by EGF. HEK293T cells expressing HA-TRPC6 were stimulated by EGF (200 ng/ml) for the indicated times. The cells were surface biotinylated with Sulfo-NHS-SS-Biotin, and the streptavidin-agarose–bound proteins were analyzed by Western blotting with α-TRPC6 antibody. The positions of the molecular-weight-marker proteins in kilodaltons are shown on the right side of the panel. A portion of each lysate (2%) was examined by Western blot to confirm the expression level of TRPC6 (bottom panel). (B) HA-TRPC6 expressed in HEK293T cells with or without constitutively active Fyn was immunoprecipitated and probed with anti-phosphotyrosine antibody. (C) HEK293T cells expressing HA-TRPC6 with or without Fyn were processed as in A. (D and E) Fyn and each of a series of phenylalanine substitution mutants of HA-TRPC6 were expressed in HEK293T cells (D) or in cultured podocytes (E), and the surface biotinylation assay for TRPC6 was performed as in A. Representative data from three to five independent experiments are shown.
TRPC6 has 23 tyrosine residues in its cytoplasmic regions. To address which tyrosine residue of TRPC6 is critical for its membrane trafficking, we analyzed the effect of a single phenylalanine substitution for each of eight tyrosine residues (Y31, Y50, Y85, Y107, Y206, Y208, Y284, Y895) on the surface localization. Y107, Y206, Y208, and Y284 correspond to the previously reported phosphorylation sites Y49, Y148, Y150, and Y226 in TRPC3, respectively (Kawasaki et al., 2006). Y31, Y50, Y85, and Y895 were predicted to be binding sites for Src homology 2 (SH2)-containing proteins by the motif search program in ScanSite (http://scansite.mit.edu/). All of these eight tyrosine residues are conserved between several animal species (human, rat, mouse, dog). Among them, the Y284F mutation dramatically decreased the Fyn-induced surface expression of TRPC6 in HEK293T cells (Figure 1D) and in cultured podocytes (Figure 1E).
PLC-γ1 binds to phosphorylated TRPC6 and controls its surface expression
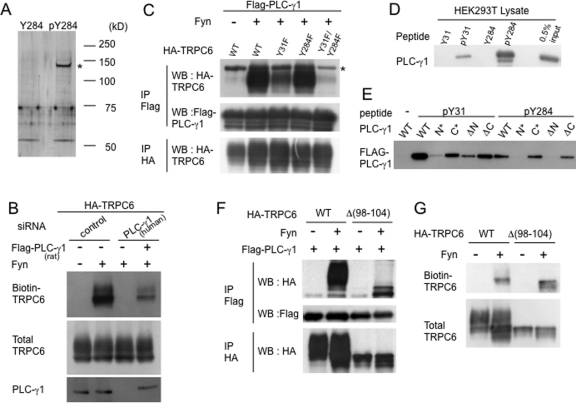
We hypothesized that the phosphorylation of TRPC6 Y284 provides a binding site for a protein that is crucial for TRPC6 trafficking. To identify such a protein, we performed in vitro binding assays using phosphorylated or nonphosphorylated TRPC6 peptide around Y284 (the same peptide used as an immunogen for anti–pY284 TRPC6 described in Materials and Methods). Both peptides were immobilized to a coupling gel and incubated with HEK293T cell lysates, and the bound proteins were subjected to SDS–PAGE followed by silver staining. As shown in Figure 2A, a protein with an apparent molecular weight of 140,000 specifically bound to the phosphorylated TRPC6 Y284 peptide (marked by an asterisk). Mass spectrometric analysis of tryptic digest of the protein identified it as PLC-γ1. The score for PLC-γ1 was 60.65, and peptides with a greater than 95% confidence covered 30.9% of the total PLC-γ1 sequence (Supplemental Figure S1). siRNA-mediated depletion of endogenous PLC-γ1 in HEK293T cells markedly reduced the phosphorylation-induced surface expression of TRPC6 on the plasma membrane, and this reduction was rescued by adding rat PLC-γ1, demonstrating that PLC-γ1 is a prerequisite for Fyn-induced TRPC6 trafficking (Figure 2B). The coimmunoprecipitation analysis shown in Figure 2C demonstrates that TRPC6 binds to PLC-γ1 in a Fyn-dependent manner. Whereas TPRC6 mutants with substitution (Y50F, Y85F, Y107F, Y206F, Y208F, Y284F, Y895F) bound to PLC-γ1 to the same extent as did wild-type TRPC6 (unpublished data), Y31F mutation partially abrogated the binding to PLC-γ1, and TRPC6 mutated both at Y31 and Y284 did not interact with PLC-γ1. Therefore, the TRPC6–PLC-γ1 interaction requires not only the phosphorylation of Y284, but also that of Y31. Indeed, a TRPC6 peptide around Y31 also bound to PLC-γ1 upon phosphorylation as did phosphorylated Y284 (Figure 2D). PLC-γ1 has two SH2 domains, N-SH2 and C-SH2, which are located side-by-side at the center of the molecule. Both pY31 and pY284 peptides preferentially bound to PLC-γ1 N-SH2, because point mutation (N*) (Plattner et al., 2003) or deletion (Δ N) (Bae et al., 2000) of N-SH2 strongly impaired the binding (Figure 2E). The phosphorylation-dependent TRPC6 trafficking was not affected by a PLC-γ inhibitor, U73122 (Supplemental Figure S2A), indicating that catalytic activity of PLC-γ1 is not necessary for the TRPC6 translocation.
FIGURE 2:
PLC-γ1 binds to phosphorylated TRPC6 Y31/Y284 and controls the surface expression of TRPC6. (A) Phosphorylated or nonphosphorylated TRPC6 Y284 peptide immobilized on a coupling gel was incubated with HEK293T cell lysates. The bound proteins were analyzed by SDS–PAGE followed by silver staining. A protein band (marked by an asterisk) was excised and subjected to tryptic digestion followed by analysis by LC-MS/MS. (B) Scrambled siRNA (control) or hPLC-γ1 siRNA was transfected into HEK293T cells, then HA-TRPC6, Fyn, and Flag-PLC-γ1 (rat) were transfected on the next day. One day after the transfection, the surface expression of TRPC6 was examined as in Figure 1A. (C) Flag-PLC-γ1, Fyn, and HA-TRPC6 (wild type, Y31F, Y284F, Y31F/Y284F) were transfected into HEK293T cells. Immunoprecipitation (IP) and Western blot (WB) were performed with the indicated antibodies. The protein band marked by an asterisk is a cross-reaction with overexpressed PLC-γ1. (D) Phosphorylated or nonphosphorylated peptides surrounding TRPC6 Y31 or Y284 were used to pull down PLC-γ1 from HEK293T cell lysates. A portion of the lysates (0.5% input) was applied for a recovery marker. (E) Peptide pull-down assays were performed as in C using wild type (WT) or SH2 point mutants (N*: R586K, C*: R694K) or SH2 deletion mutants (ΔN : Δ550–657, ΔC : Δ667–756) of PLC-γ1. (F) Coimmunoprecipitation was performed using HEK293T cells transfected with Flag-PLC-γ1, Fyn, and TRPC6 (wild type or Δ98–104). (G) The surface expression of TRPC6 lacking 98–104 (Δ98–104) was examined.
PLC-γ1 binds to the N-terminal amino acids 40–46 of TRPC3 (that correspond to residues 98–104 of TRPC6) to form a functional pleckstrin homology (PH) domain that binds to phosphatidylinositol bisphosphate, which is required for the agonist-induced surface expression of TRPC3 (van Rossum et al., 2005). We evaluated the role of these residues in TRPC6 by coimmunoprecipitation and biotinylation assays using TRPC6 mutant lacking residues 98–104. Because this mutant (Δ98–104) still bound to PLC-γ1 (Figure 2F) and translocated to the plasma membrane (Figure 2G) in a Fyn-dependent manner, as did the wild type, we conclude that Y31 and Y284, but not residues 98–104, are critical for the interaction with PLC-γ1 and trafficking to the plasma membrane.
TRPC6 Y31 and Y284 are phosphorylated by Src family kinase in isolated glomeruli
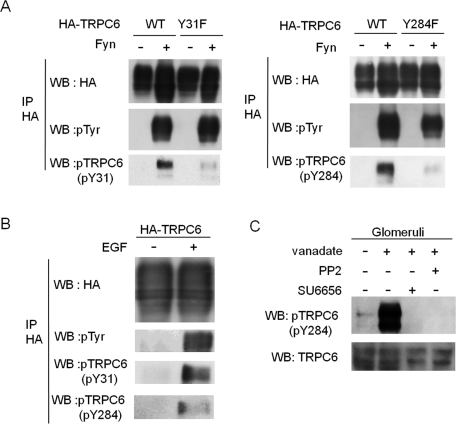
We raised phosphospecific anti-TRPC6 antibodies against pY31 and pY284 (Figure 3). Specificity of these antibodies was shown using Y31F and Y284F mutants of TRPC6 (Figure 3A). EGF also induced the phosphorylation of these sites (Figure 3B). In isolated rat kidney glomeruli, Y284 phosphorylation of endogenous TRPC6 was significantly augmented by treatment with vanadate, a nonspecific protein phosphatase inhibitor (Figure 3C). This phosphorylation was suppressed when glomeruli were pretreated with either SU6656 or PP2, specific Src family kinase inhibitors, before vanadate treatment. This finding suggests that Src family kinase is responsible for the reaction.
FIGURE 3:
Tyrosine phosphorylation of TRPC6 in living cells and kidney glomeruli. (A) Lysates from HEK293T cells transfected with the indicated plasmids were probed with α-pY31 or α-pY284 antibody. (B) HEK293T cells expressing HA-TRPC6 were stimulated by EGF. α-HA immunoprecipitates were probed with α-phosphospecific TRPC6 antibodies. (C) Isolated rat glomeruli treated with or without 1 mM sodium vanadate for 30 min were blotted with α-pY284. For the indicated samples, glomeruli were pretreated with 10 mM PP2 for 15 min or 5 mM SU6656 for 60 min before treatment with vanadate.
Tyrosine-phosphorylated TRPC6 interacts with nephrin
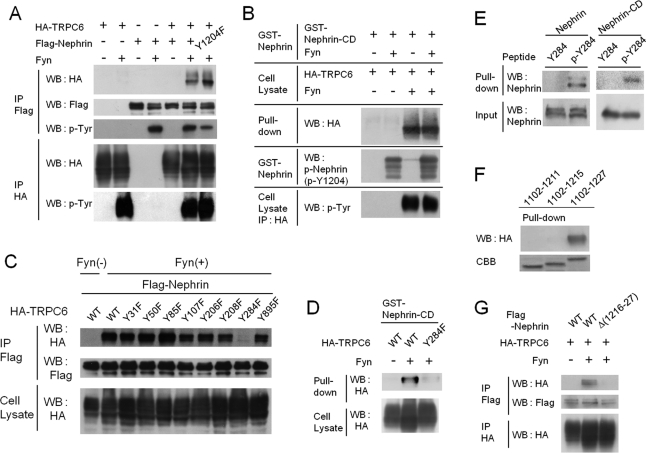
Given that TRPC6 is a slit diaphragm component (Reiser et al., 2005; Huber et al., 2006), we next sought to determine whether binding to other slit diaphragm components affected its phosphorylation-dependent membrane translocation. First, we performed coimmunoprecipitation assays using HEK293T cells expressing nephrin, Neph1, Neph2, or podocin, in addition to TRPC6 and Fyn. We found that nephrin bound to TRPC6 only when coexpressed with Fyn (Figure 4A). We did not detect any interaction between TRPC6 and other slit diaphragm components (unpublished data). Because nephrin is also tyrosine phosphorylated by Src family kinase (Verma et al., 2003, 2006; Jones et al., 2006), we examined which phosphorylation is necessary for the TRPC6-nephrin interaction. Upon phosphorylation nephrin Y1204 binds to PLC-γ1 (Harita et al., 2009). This phosphorylation site, however, was not involved in the binding with TRPC6, because Y1204F mutant also bound to TRPC6 (Figure 4A). We next performed a pull-down assay using glutathione S-transferase (GST)-nephrin-cytoplasmic domain (nephrin-CD, residues 1102–1252) with or without prior in vitro phosphorylation by Fyn (Harita et al., 2008, 2009). As shown in Figure 4B, TRPC6 from HEK293T cells coexpressing Fyn, but not from cells without Fyn, bound to GST-nephrin-CD, irrespective of nephrin-CD phosphorylation by Fyn in vitro. Thus, phosphorylation of TRPC6, but not that of nephrin, is required for the TRPC6–nephrin interaction. nephrin phosphorylation could also be induced by clustering its extracellular domain (Lahdenpera et al., 2003; Jones et al., 2006; Verma et al., 2006). A fusion protein construct in which the CD8 extracellular domain and the transmembrane domain were coupled to nephrin-CD (CD8/nephrin-CD) was expressed in HEK293T cells, and a mouse anti-CD8 antibody and a secondary anti–mouse IgG antibody were added to the culture medium (Harita et al., 2009). TRPC6 phosphorylation and surface expression, however, were not observed by cross-linking experiments (unpublished data), suggesting, that clustering-induced nephrin phosphorylation may not be enough for TRPC trafficking. Temporal or spatial regulation of SFK activation may be required.
FIGURE 4:
TRPC6 Y284 and nephrin 1216–1227 are critical for their tyrosine-phosphorylation–dependent interaction. (A) Lysates from HEK293T cells expressing the indicated plasmids were processed for immunoprecipitation and Western blotting with indicated antibodies. (B) Lysates from HEK293T cells expressing HA-TRPC6 with or without Fyn were pulled down with GST-nephrin-CD (residues 1102–1252) or GST-nephrin-CD prephosphorylated in vitro by Fyn. Bound proteins were probed with α-HA antibody. Phosphorylation of GST-nephrin and TRPC6 was confirmed (bottom two panels). (C) Flag-nephrin, Fyn, and HA-TRPC6 (wild type or one of a series of single phenylalanine mutants) were expressed in HEK293T cells. Immunoprecipitates and total cell lysates were blotted with indicated antibodies. (D) Lysates from HEK293T cells transiently expressing wild-type HA-TRPC6 or TRPC6 (Y284F) with or without Fyn were pulled down with GST-nephrin-CD, and probed with α-HA. (E) Lysates from HEK293T cells expressing nephrin or recombinant nephrin-CD were pulled down with phosphorylated/nonphosphorylated TRPC6 Y284 peptide immobilized on beads, and bound proteins were analyzed by Western blot with α-nephrin. (F) A series of deletion mutants of GST-nephrin-CD (residues 1102–1211, 1102–1215, 1102–1227) was used to pull down tyrosine-phosphorylated HA-TRPC6 from lysates of HEK293T cells expressing HA-TRPC6 and Fyn. CBB, Coomassie Brilliant Blue staining showing nephrin-CD. (G) HEK293T cells were transfected with HA-TRPC6, Fyn, and Flag-nephrin (wild type or nephrin lacking 1216–1227), and HA/Flag immunoprecipitates were blotted with indicated antibodies.
Phosphorylation of Y31 and Y284 of TRPC6 is necessary for the interaction with PLC-γ1 (Figure 2C). Similarly, we evaluated the ability of a series of TRPC6 mutants with a phenylalanine substitution to bind to nephrin, to determine the critical tyrosine residue for the TRPC6–nephrin interaction (Figure 4C). We found that Y284 is the critical residue, because Y284F mutation almost completely abolished the interaction between TRPC6 and nephrin, whereas other substitutions including Y31F did not affect the interaction. The role of Y284 phosphorylation was further confirmed by a pull-down analysis using GST-nephrin-CD (Figure 4D). Although not as efficient as full-length TPRC6, a TRPC6 peptide surrounding Y284, when phosphorylated, could pull down nephrin from HEK293T cells (Figure 4E, left) and GST-nephrin-CD (Figure 4E, right), demonstrating the direct binding of nephrin to TRPC6.
To define the region in nephrin-CD that is required for the binding to TRPC6, we constructed a series of deletion mutants of GST-nephrin-CD. A GST pull-down assay using these deletion mutants showed that residues 1216–1227 are necessary for the interaction (Figure 4F) and that the deletion of this region from nephrin abolished the binding (Δ(1216–1227) in Figure 4G).
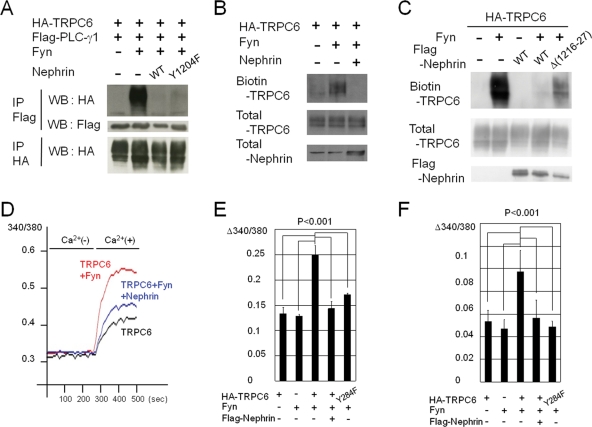
Nephrin suppresses the phosphorylation-dependent surface expression and channel activation of TRPC6
Because both PLC-γ1 and the nephrin-CD bind to the same tyrosine residue on TRPC6 (Y284), they may compete with each other for binding. As shown in Figure 5A, coexpression of nephrin in HEK293T cells completely inhibited the TRPC6–PLC-γ1 interaction. Because phosphorylated nephrin Y1204 provides the binding site for PLC-γ1 (Harita et al., 2009), and Y1204 is close to the TRPC6 binding region on nephrin (1216–1227), it is possible that the nephrin inhibition of TRPC6–PLC-γ1 complex formation is due to the binding of PLC-γ1 to phosphorylated nephrin Y1204. The nephrin Y1204F mutant that does not bind to PLC-γ1 still could abrogate the TRPC6–PLC-γ1 interaction (Figure 5A), however, indicating that nephrin interferes with the TRPC6–PLC-γ1 interaction irrespective of the nephrin–PLC-γ1 interaction.
FIGURE 5:
Nephrin negatively regulates the phosphorylation-dependent surface expression and channel activation of TRPC6. (A–C) Indicated plasmids were transfected into HEK293T cells (A and C) or cultured podocytes (B), and a coimmunoprecipitation (A) or surface biotinylation (B and C) assay was performed. (D–F) Fura-2–loaded HEK293T cells (D and E) and cultured podocytes (F) transfected with the indicated plasmids were superfused in succession with Ca2+-free HBS and HBS with CaCl2. [Ca2+]i was recorded from a cell population (n > 400) as F340/F380 ratio of Fura-2 fluorescence, and mean values are plotted (D). The peak ratio amplitude from baseline was calculated for each cell, and the bar graphs show the mean of the data from five independent experiments, with standard errors (E and F).
Because PLC-γ1 has a key role in membrane trafficking of TRPC6 (Figure 2B), nephrin may inhibit the trafficking of TRPC6 by interfering with the TRPC6–PLC-γ1 interaction. We performed a surface biotinylation assay, and found that coexpression of nephrin in cultured podocytes reduced the Fyn-induced surface expression of TRPC6 (Figure 5B). This result was also reproduced with HEK293T cells (Figure 5C). The phosphorylation status of TRPC6 was not attenuated by the addition of nephrin, and vice versa (Supplemental Figure S3), indicating that this inhibition did not result from altered Fyn kinase activity. A nephrin deletion mutant (Δ1216–1227), which does not bind to TRPC6, showed a much lower suppression compared to wild-type nephrin (Figure 5C). These results demonstrate that nephrin inhibition of TRPC6 trafficking is due to the inhibition of the TRPC6–PLC-γ1 interaction. A PLC-γ inhibitor U73122 did not affect the nephrin inhibition of TRPC6 trafficking (Supplemental Figure S2B).
Nephrin also suppressed TRPC6 channel activity when monitored by Ca2+ influx in HEK293T cells (Figure 5, D and E) and cultured podocytes (Figure 5F) expressing TRPC6 and Fyn. Fyn augmented Ca2+ influx in cells expressing TRPC6, whereas Fyn-dependent increase in the Ca2+ entry was significantly suppressed when nephrin was coexpressed (p < 0.001, Mann–Whitney test), consistent with its inhibitory effect on TRPC6 surface expression. Fyn-induced increase of Y284F mutant was marginal in the Ca2+ assays.
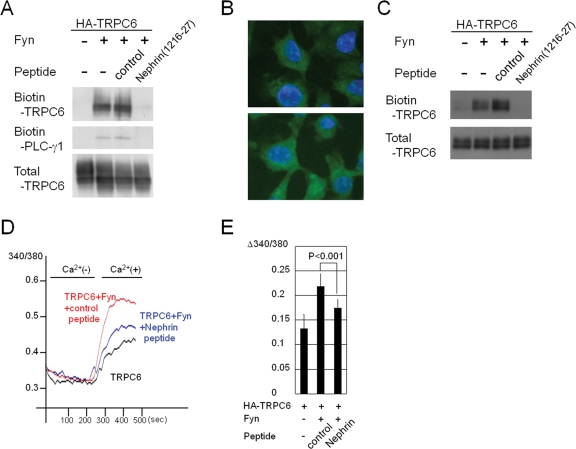
A nephrin cytoplasmic peptide mimics the inhibitory effect of nephrin on TRPC6 activity
Given that nephrin competitively inhibits TRPC6–PLC-γ1 complex formation and that residues 1216–1227 of nephrin are necessary for the interaction with TRPC6, we then introduced a nephrin peptide (residues 1216–1227) into HEK293T cells by a commercially available delivery kit and assessed Fyn-induced translocation of TRPC6 ( Figure 6A). Introduction of the nephrin peptide (1216–1227) blocked the Fyn-dependent membrane trafficking of TRPC6 in HEK293T cells. PLC-γ1 was detected in the biotinylated samples from cells cotransfected with Fyn, and the nephrin peptide (1216–1227) also inhibited PLC-γ1 from targeting to the plasma membrane (Figure 6A). As an alternative and convenient peptide-delivery approach, polyarginine-fusion (11R) peptides were transduced into cultured podocytes (Figure 6B). Almost all the cells incorporated the peptides. Treatment with the 11R-nephrin peptide (1216–1227) strongly inhibited the Fyn-induced membrane expression of TRPC6 in podocytes (Figure 6C). In agreement with its ability to suppress the membrane trafficking of TRPC6, the nephrin peptide (1216–1227) was able to mimic the inhibitory effect of nephrin on TRPC6 channel activity (p < 0.001, Figure 6, D and E).
FIGURE 6:
A nephrin peptide mimics the inhibitory effect of nephrin on TRPC6 activity. (A) A nephrin peptide (1216–1227) or a control peptide was transfected into HEK293T cells 4 h after the transfection of the indicated plasmids, and a surface biotinylation assay was performed 24 h later. (B) Cultured podocytes were incubated with an FITC-conjugated 11R-nephrin peptide (top panel) or an 11R-control peptide (bottom panel) and examined with a fluorescence microscope. (C) An 11R-nephrin peptide or an 11R-control peptide was delivered into cultured podocytes expressing HA-TRPC6 with or without Fyn, and the surface biotinylation assay was performed. (D) Indicated plasmids and peptides were introduced in HEK293T cells. Changes in [Ca2+]i were recorded as in Figure 5D. The representative data from five independent experiments are shown. (E) The data from five experiments were processed as in Figure 5E.
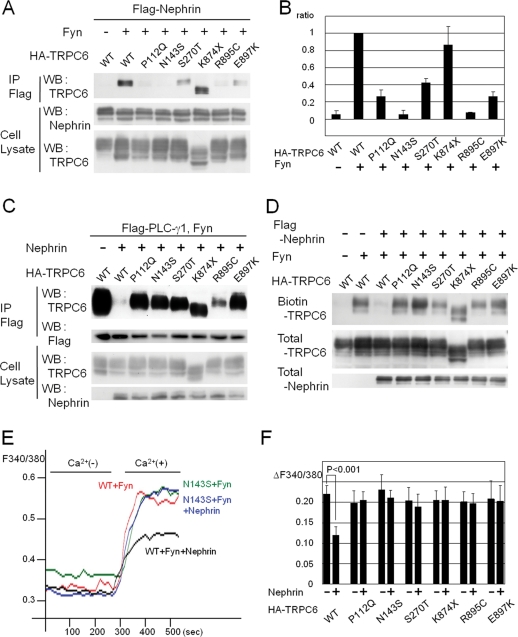
The FSGS mutants of TRPC6 escape the inhibitory effect of nephrin
Since the identification of TRPC6 mutations as the cause of FSGS, the activity of mutated TRPC6 channels has been measured in cultured cells (Reiser et al., 2005; Winn et al., 2005). The results, however, do not clearly demonstrate a relationship between channel activity and the pathogenesis of FSGS: Some TRPC6 mutations (P112Q, R895C, E897K) enhance channel activity, whereas others (N143S, S270T, K874X) do not. These apparently contradictory results led us to hypothesize that there may be a podocyte-specific regulation of TRPC6.
On the basis of this hypothesis, we analyzed the interaction between disease-causing TRPC6 mutants (P112Q, N143S, S270T, K874X, R895C, and E897K) and nephrin by coimmunoprecipitation (Figure 7, A and B). Five disease-causing mutations (all except K874X, p < 0.001) decreased the phosphorylation-dependent interaction between TRPC6 and nephrin. Then, we analyzed the inhibitory effect of nephrin on the TRPC6–PLC-γ1 interaction and on the surface expression of TRPC6 mutants. Interestingly, nephrin did not suppress the complex formation between TRPC6 mutants and PLC-γ1, contrasting with wild-type TRPC6 (Figure 7C). Fyn-induced membrane translocation was observed in all TRPC6 mutants as well as in wild-type TRPC6 in the absence of nephrin (unpublished data). Surprisingly, nephrin did not inhibit the surface expression of any disease-causing TRPC6 mutant, in striking contrast to wild-type TRPC6 (Figure 7D).
FIGURE 7:
Impairment of nephrin interaction and dysregulated surface expression of TRPC6 by FSGS-associated TRPC6 mutations. (A) Flag-nephrin, Fyn, and each of the HA-TRPC6 mutants were coexpressed in HEK293T cells, and α-Flag or α-HA immunoprecipitates were analyzed by Western blot with indicated antibodies. (B) TRPC6-nephrin binding was quantified by densitometric tracing of A (WB: TRPC6), and the mean values were shown in the bar graph, with standard errors (n = 3). Values were normalized to wild-type TRPC6. (C) The effect of nephrin expression on the TRPC6–PLC-γ1 interaction was examined with HEK293T cells expressing wild type or each of the TRPC6 mutants, in addition to Flag-PLC-γ1 and Fyn. (D) Surface expression of TRPC6 was analyzed as in Figure 1A by using HEK293T cells transfected with a series of disease-causing TRPC6 mutants, with or without nephrin/Fyn. (E) Nephrin, Fyn, and either TRPC6 or TRPC6 N143S mutant were expressed in HEK293T cells. Changes in [Ca2+]i were recorded as in Figure 5D. The representative data from five independent experiments are shown. (F) The channel activities of FSGS-associated TRPC6 mutants were evaluated as in E, and the data from five experiments were processed as in Figure 5E.
Finally, we investigated the impact of nephrin on the Fyn-dependent channel activation of the TRPC6 disease-causing mutants (Figure 7, E and F). Consistent with the membrane localization results, nephrin suppressed the Fyn-induced augmentation of the wild-type channel, but the TRPC6 N143S mutant was almost insensitive to nephrin. The other five TRPC6 mutants also escaped the inhibitory effects of nephrin (Figure 7F). The result that nephrin could not suppress the activity of all the FSGS-associated mutants could lead to uncontrolled TRPC6 surface expression and exaggerated TRPC6 activation in patients’ podocytes.
DISCUSSION
Recently PLC-γ has been shown to regulate cell-surface expression of ion channels and transporters through protein–protein interaction, independent of its catalytic activity (Patterson et al., 2002). For example, the PH-c domain of PLC-γ1 binds to TRPC3 (amino acids 40–46), which functions in the membrane trafficking of the PLC-γ1-TRPC3 complex (van Rossum et al., 2005). The PH-c domain of PLC-γ1 also binds to a Na+/H+ antiporter, NHE3, mediating calcium regulation of Na+/H+ exchange activity (Zachos et al., 2009). In this study, we have shown TRPC6 to be another TRPC member for which the membrane trafficking is controlled by a tyrosine phosphorylation–dependent interaction with PLC-γ1. The critical role of PLC-γ1 is shown by siRNA-mediated depletion of PLC-γ1 abolishing membrane translocation of TRPC6 and by the rescue control (Figure 2B). This phosphorylation-dependent membrane insertion of TRPC6 was not mediated by the interaction through the PH domain of PLC-γ1, because the deletion TRPC6 mutation (Δ98–104, which correspond to residues 40–46 of TRPC3) did not affect the phosphorylation-dependent interaction with PLC-γ1 (Figure 2F) and membrane trafficking of TRPC6 (Figure 2G). Rather, the N-terminal SH-2 domain of PLC-γ1 was necessary for the binding with TRPC6 through the critical tyrosine residue essential for its surface localization (Figure 2, D and E). Taken together, our results indicate that PLC-γ1 regulates TRPC channels through multiple interactions depending on a variety of stimulations.
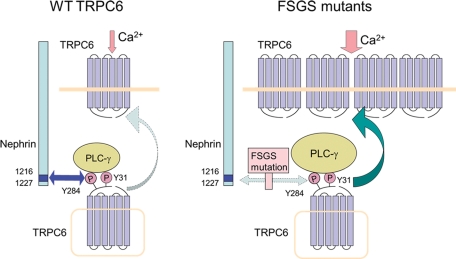
Nephrin is highly expressed in podocytes and plays a major role in the slit diaphragm (Wartiovaara et al., 2004). Nephrin directly binds to TRPC6 upon phosphorylation of Y284 and inhibits the TRPC6–PLC-γ1 interaction, leading to suppression of TRPC6 surface expression. Although PLC-γ1 interacts with phosphorylated Y1204 of nephrin (Harita et al., 2009), this interaction is not involved in the suppression of TRPC6 membrane insertion by nephrin, because nephrin mutant (Y1204F) suppressed TRPC6 trafficking to the same extent as did the wild type (Figure 5A). Recently a similar inhibitory mechanism for TRPC3 has been reported. A transcription factor TFII-I binds to the SH2 and PH-c domains of PLC-γ1 and displaces TRPC3 from PLC-γ1, which prevents membrane expression of the channel and associated calcium entry (Caraveo et al., 2006). This interference by TFII-I or nephrin on the TRPC–PLC-γ1 interaction reveals a multitiered regulatory mechanism for TRPC activity. The mode of regulation of TRPC6 trafficking by PLC-γ1 and nephrin is schematically summarized in Figure 8.
FIGURE 8:
Schematic illustration of the interaction among nephrin, TRPC6, and PLC-γ1.
Nephrin is thought to continuously turn over by endocytosis and exocytosis in podocytes to maintain constant reorganization of the foot process (Quack et al., 2006; Qin et al., 2009; Tossidou et al., 2010). In contrast, TRPCs are known to reside on vesicles beneath the plasma membranes, and these vesicles fuse with the plasma membranes to expose the channel upon growth factor stimuli (Bezzerides et al., 2004). TRPC3, which is a close member of TRPC6, is known to undergo constitutive recycling in and out of the plasma membranes (Smyth et al., 2006). Although we do not have quantitative data on the relative amount of nephrin on plasma membranes and endocytic vesicles in vivo, nephrin–TRPC6 interaction may occur at various membrane compartments.
Recent findings that mutations in TRPC6 cause FSGS highlight the importance of Ca2+ signaling in podocytes (Reiser et al., 2005; Winn et al., 2005). How the channel activity of mutated TRPC6 is involved in the pathogenesis remains unclear, however, because the channel activity measured in cultured cells is increased by some mutations (P112Q, R895C, E897K) or unchanged by others (S270T, N143S, K874X) (Reiser et al., 2005; Winn et al., 2005). In HEK293T, the surface expression of wild-type TRPC6 and all the disease-causing mutant TRPC6s was equally increased by tyrosine phosphorylation by Fyn. Whereas the Fyn-induced increase in wild-type TRPC6 was significantly suppressed by nephrin, that of the mutant TRPC6s was insensitive to nephrin inhibition (Figure 7D). This insensitivity may be due to the much lower binding affinity of mutant TRPC6s (P112Q, N143S, S270T, R885C, E897K) to nephrin (Figure 7, A and B). Because of its decreased affinity, nephrin could not inhibit mutant TRPC6s from binding to PLC-γ1 (Figure 7C) and being expressed at the plasma membrane (Figure 7D). The channel activity of wild type and mutant TRPC6s (Figure 7, E and F) correlated well with the results obtained by the biotinylation assay (Figure 7D). Again, Fyn stimulated the Ca2+ channel activity of both the wild type and the mutants; the former stimulation was suppressed by nephrin, but the latter was not. These results suggest increased calcium signaling even in the patients’ podocytes with mutations that do not enhance the channel activity when measured in usual cell culture systems. In this regard, whereas one mutation (K874X) bound to nephrin, Fyn-induced translocation of the K874X mutant was insensitive to nephrin. The K874X mutant may have a structural defect such that interaction with nephrin somehow cannot suppress its interactions with molecules that promote its trafficking, including PLC-γ1 (Figure 7C).
Phosphorylated Y284 and the amino acids at the mutated positions (P112, N143, S270, R885, E897) of TRPC6 are both necessary for the interaction with nephrin (Figure 7A), indicating that these residues constitute the binding site. On nephrin itself, a 12-amino-acid sequence (residues 1216–1227) is essential for the binding to TRPC6, and this sequence is sufficient to suppress TRPC6 activation. It has been shown that TRPC channels form homo- or heterotetramers with other TRPC channels (Hofmann et al., 2002). Indeed, biochemical (Lepage et al., 2006) and cryo-electron microscopic (Mio et al., 2007) analyses have demonstrated that the N-terminal region is in close contact with the C-terminal region. In such quaternary structures, the N-terminal (including P112, N143, S270, Y284) and C-terminal (R885, E897) regions of TRPC6 may be in close proximity to each other, and these amino acids may be necessary for the interaction with nephrin. Nephrin and nephrin peptide may inhibit the membrane trafficking of TRPC6 by changing the tertiary structure through binding to TRPC6.
Whereas TRPC6 Y31F mutant decreased the levels of TRPC6–PLC-γ1 complex (Figure 2C), its membrane translocation was not significantly impaired (Figure 1D). In contrast, Y284F mutation robustly reduced the membrane trafficking with a marginal decrease in TRPC6–PLC-γ1 complex level. We interpret these results as follows: Because both the pY31 and pY284 peptides bind preferentially to N-SH2 of PLC-γ1 (Figure 2E), and C-SH2 of PLC-γ1 binds to pY783 of PLC-γ1 intramolecularly (Poulin et al., 2005), there may be two kinds of TRPC6–PLC-γ1 complexes, the interaction of which is mediated by N-SH2 of PLC-γ1 and either by pY31 or pY284 of TRPC6. Only the latter complex may adopt a conformation that is necessary for membrane trafficking. Although the majority of the complex is mediated by pY31, the membrane trafficking may shift the equilibrium between the two complexes in the cytoplasm to reproduce the complex mediated by pY284. Nephrin competitively binds to pY284 and may inhibit TRPC6–PLC-γ1 complex formation. Because nephrin does not have an SH2 or phosphotyrosine binding domain, its mode of interaction is currently unknown.
The mode of regulation of TRPC6 presented here may also have relevance to nonrenal diseases, because TRPC6 has been linked to pulmonary hypertension, cardiac hypertrophy, cardiac fibrosis, neuronal outgrowth, and eryptosis in human or animal models (Dietrich et al., 2005; Nilius et al., 2007; Abramowitz and Birnbaumer, 2009). Modulation of TRPC6 activity by small molecules or peptides may be an effective therapeutic strategy for treating proteinuric diseases or a wide variety of diseases.
MATERIALS AND METHODS
Antibodies and reagents
Rabbit α-phospho-TRPC6 antibodies (α-pY31, α-pY284) were raised against synthetic oligopeptides of 12 amino acids, CNESQDpYLLMDEL (pY31) and CLASPApYLSLSSE (pY284) (the first cysteine residues are not part of the TRPC6 sequence), respectively. The antisera were affinity purified using the immunogen coupled to a SulfoLink (Pierce, Rockford, IL) and absorbed with nonphosphorylated peptides. α-FLAG, 0.5 μg/ml (M2; Sigma, St. Louis, MO), α-HA, 50 ng/ml (3F10; Roche Diagnostics, Indianapolis, IN), α-phosphotyrosine, 0.5 μg/ml (4G10; Upstate, Lake Placid, NY), rabbit α-TRPC6, 0.5 μg/ml (Alomone Labs, Jerusalem, Israel), and rabbit α-PLC-γ1, 0.1 μg/ml (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies were obtained commercially. Rabbit α-nephrin and α-phospho-nephrin (pY1204) were previously described (Harita et al., 2009). Western blotting was performed with these antibodies diluted at 1:2000. PP2 (Merck KGaA, Darmstadt, Germany), SU6656 (Merck KGaA), and EGF (BD Biosciences, Bedford, MA) were purchased.
Cell culture, transfection, and RNAi
Human embryonic kidney HEK293T cells were purchased from the American Type Culture Collection (Manassas, VA). The cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. A temperature-sensitive rat podocyte cell line, 2DNA1D7, was described previously (Harita et al., 2008). Transfections were performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). The sequences of siRNA used were nontargeting control (D-001210–0120; Dharmacon, Lafayette, CA) and human PLC-γ1 (5′- CCUUGUUGACCUCAUCAGCUACUAU -3′) containing two mismatches with rat PLC-γ1. These siRNA duplexes were transfected into HEK293T cells using Lipofectamine RNAiMAX (Invitrogen).
Eukaryotic expression constructs
Mammalian expression plasmids encoding full-length rat nephrin, full-length nephrin-Flag, Y1204F nephrin, and Y1204F nephrin-Flag were previously described (Harita et al., 2008, 2009). Expression vectors for Fyn (a gift from T. Tezuka, Tokyo University) (Tezuka et al., 1999), mouse TRPC6-HA (a gift from C. Hisatsune, RIKEN Brain Science Institute, Japan) (Hisatsune et al., 2004), and human Flag-PLC-γ1 (a gift from P. G. Suh, Pohang University, Korea) (Bae et al., 2000) were described. SH2 mutants of PLC-γ1 were made according to the literature (Bae et al., 1998; Plattner et al., 2003). Mammalian expression plasmids encoding mouse TRPC6 mutants, Y31F, Y50F, Y85F, Y107F, Y206F, Y208F, Y284F, Y895F, P112Q, N143S, S270T, K874X, R895C, E897K and deletion (98–104) mutant, and a nephrin-Flag deletion mutant (1216–27) were prepared using standard PCR methods. DNA sequencing was performed to validate all the constructs. Oligonucleotides used in PCR are described in the Supplementary Material.
Cell-surface biotinylation
Surface expression of TRPC6 was assayed according to Winn et al. (2005). Briefly, HEK293T cells or cultured podocytes transfected with indicated plasmids were surface biotinylated for 30 min at 4ºC with 2 mg/ml Sulfo-NHS-SS-Biotin (Pierce). The biotinylated proteins were recovered with streptavidin beads (Pierce) and were analyzed by Western blot analyses.
Immunoprecipitation
Cells were lysed with lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Nonidet P-40 [NP40], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], 50 mM NaF, 10 μg/ml antipain, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM sodium vanadate) for 15 min on ice. Lysates were clarified by centrifugation and incubated with beads conjugated with M2 α-Flag antibody or with 3F10 α-HA antibody for 1 h at 4ºC. Beads were washed three times with Tris-buffered saline–1% NP40, and bound proteins were eluted with 100 mM glycine–HCl (pH 2.6) and analyzed by Western blotting.
Bacterial fusion protein expression
GST-tagged nephrin cytoplasmic region (nephrin-CD: amino acids 1102–1252) was previously described (Harita et al., 2009). GST-nephrin-CD deletion mutants (1102–1211, 1102–1215, 1102–1227) were prepared using standard PCR methods. Bacterial pellets were resuspended and sonicated in a solution containing 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% NP40, 2 mM dithiothreitol, 1 mM PMSF, 10 μg/ml antipain, 10 μg/ml leupeptin, and insoluble material was removed by centrifugation. GST fusion protein was purified on a glutathione–Sepharose column (GE Healthcare, Buckinghamshire, UK) and eluted with free glutathione. Oligonucleotides used to make GST-nephrin-CD deletion mutants are listed in the Supplementary Material.
Pull-down assay
GST-nephrin-CD immobilized on glutathione-Sepharose beads was phosphorylated with Fyn as described previously (Harita et al., 2008, 2009). HA-TRPC6 expressing HEK293T whole-cell lysates were incubated with phosphorylated GST-nephrin-CD or nonphosphorylated GST-nephrin-CD immobilized on beads at 4ºC overnight. Beads were washed extensively with wash buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% NP40). Nephrin and bound proteins were eluted with free glutathione and analyzed by Western blotting and Coomassie Brilliant Blue staining. For pull-down analysis using peptides, peptides were fixed on SulfoLink Coupling Gel (Pierce).
Determination of proteins using mass spectrometry
Proteins of interest were excised from silver-stained gel and digested with 1 pmol of Achromobacter protease I in 40 μl of digestion buffer (10 mM Tris, pH 8.5) at 37ºC overnight. Peptides were purified and subjected to peptide mass fingerprinting using liquid chromatograph-mass/mass spectrometry (LC-MS/MS) (Q-STAR Elite; Applied Biosystems, Carlsbad, CA). Peptide ions were analyzed with ProteinPilot (version 3.0; Applied Biosystems) software.
Isolation of rat kidney glomeruli
The glomeruli were isolated from the rat renal cortexes using a graded sieving technique and were saved for protein extraction. All experiments were carried out according to the guidelines set by the Animal Center of the Institute of Medical Science, the University of Tokyo. Wistar rats were purchased from Charles River Laboratories Japan (Atsugi, Japan).
Measurement of [Ca2+]i
HEK293T cells or cultured podocytes grown on coverslips were loaded with 4 μM Fura-2/AM (Dojindo, Kumamoto, Japan) and bathed in HEPES-buffered saline (HBS; 115 mM NaCl, 5.4 mM KCl, 20 mM HEPES, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose, pH 7.4) for 30 min before Ca2+ measurements. The cells were successively perfused at room temperature with Ca2+-free HBS (115 mM NaCl, 5.4 mM KCl, 20 mM HEPES, 2 mM MgCl2, 10 mM glucose, 0.05 mM EGTA, pH 7.4) and HBS. The fluorescence of Fura-2–loaded cells was monitored under an Olympus IX70 microscope with a 20× objective lens (N.A. 0.75). Fluorescence of Fura-2 was excited alternately by 340- and 380-nm wavelength light every 10 s, and images were recorded with a cooled CCD camera (ORCA-ER; Hamamatsu Photonics, Hamamatsu, Japan). The time course of ratio of fluorescence intensity (F340/F380) was calculated from all the cells (400∼500) in each view field and averaged. Image acquisition and data analysis were performed with custom-made TI Workbench software written by T. I on a Macintosh computer (Bannai et al., 2004).
Peptide delivery
A nephrin peptide (amino acids 1216–1227, CWPEVQCEDPRGI) or a control peptide (nephrin C terminus; amino acids 1241–1252, CSSLPFELRGHLV) was delivered into HEK293T cells using Transport Protein Delivery Reagent (Takara, Tokyo, Japan). Cultured podocytes were incubated with 3 μM fluorescein isothiocyanate (FITC)-conjugated 11R-nephrin peptide (1216–1227, RRRRRRRRRRRGGGWPEVQCEDPRGI) or FITC-conjugated 11R-control peptide (nephrin C terminus; amino acids 1241–1252, RRRRRRRRRRRGGGSSLPFELRGHLV) for 24 h. The cells were washed three times with phosphate-buffered saline, and the fluorescence of FITC was monitored under an Olympus IX71 microscope with a 60× objective lens (N.A. 0.7).
Statistics
Values are presented as means ± SEM. Comparison between two groups of [Ca2+]i was assessed with the Mann–Whitney U test. The unpaired Student's t test was used to compare the band intensities in Western blot analyses. Values of p < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank H. Kosako for valuable comments on the manuscript. We also thank T. Tezuka (Tokyo University, Japan), C. Hisatsune (RIKEN, Japan), and J. K. Kim and P. G. Suh (Pohang University, Korea) for plasmids. This study was partially supported by the Molecular Nephrology Forum and the Japan Foundation for Pediatric Research. This study was also supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS) Fellows from the JSPS (to K.S.), a Grant-in-Aid for Young Scientists (B) (20790719, 22790991) (to H.Y.), and a Grant-in-Aid for Scientific Research (B) (22390204) (to H.Y., H.S., and I.T.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations used:
- CD
cytoplasmic domain
- EGF
epidermal growth factor
- FITC
fluorescein isothiocyanate
- FSGS
1focal segmental glomerulosclerosis
- GST
glutathione S-transferase
- HA
hemagglutinin
- HBS
HEPES-buffered saline
- LC-MS/MS
liquid chromatograph-mass/mass spectrometry
- NP40
Nonidet P-40
- PH
pleckstrin homology
- PLC
phospholipase C
- PMSF
phenylmethylsulfonyl fluoride
- PTK
protein tyrosine kinase
- SH2
Src homology 2
- TRPC
transient receptor potential canonical
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-12-0929) on April 6, 2011.
REFERENCES
- Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SS, Perry DK, Oh YS, Choi JH, Galadari SH, Ghayur T, Ryu SH, Hannun YA, Suh PG. Proteolytic cleavage of phospholipase C-gamma1 during apoptosis in Molt-4 cells. FASEB J. 2000;14:1083–1092. doi: 10.1096/fasebj.14.9.1083. [DOI] [PubMed] [Google Scholar]
- Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- Bannai H, Inoue T, Nakayama T, Hattori M, Mikoshiba K. Kinesin dependent, rapid, bi-directional transport of ER sub-compartment in dendrites of hippocampal neurons. J Cell Sci. 2004;117:163–175. doi: 10.1242/jcs.00854. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, et al. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- Caraveo G, van Rossum DB, Patterson RL, Snyder SH, Desiderio S. Action of TFII-I outside the nucleus as an inhibitor of agonist-induced calcium entry. Science. 2006;314:122–125. doi: 10.1126/science.1127815. [DOI] [PubMed] [Google Scholar]
- Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem. 2004;279:7241–7246. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Rost BR, Gudermann T. The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Pflugers Arch. 2005;451:72–80. doi: 10.1007/s00424-005-1460-0. [DOI] [PubMed] [Google Scholar]
- Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, Hattori S. Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J Biol Chem. 2008;283:9177–9186. doi: 10.1074/jbc.M707247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, Ohsawa I, Ohta S, Hattori S. Phosphorylation of nephrin triggers Ca2+ signaling by recruitment and activation of phospholipase C-gamma 1. J Biol Chem. 2009;284:8951–8962. doi: 10.1074/jbc.M806851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem. 2004;279:18887–18894. doi: 10.1074/jbc.M311274200. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Jones N, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- Kawasaki BT, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc Natl Acad Sci USA. 2006;103:335–340. doi: 10.1073/pnas.0508030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Patterson RL. The integrative function of TRPC channels. Front Biosci. 2009;14:45–58. doi: 10.2741/3230. [DOI] [PubMed] [Google Scholar]
- Krall P, et al. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One. 2010;5:e12859. doi: 10.1371/journal.pone.0012859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahdenpera J, Kilpelainen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int. 2003;64:404–413. doi: 10.1046/j.1523-1755.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- Lepage PK, Lussier MP, Barajas-Martinez H, Bousquet SM, Blanchard AP, Francoeur N, Dumaine R, Boulay G. Identification of two domains involved in the assembly of transient receptor potential canonical channels. J Biol Chem. 2006;281:30356–30364. doi: 10.1074/jbc.M603930200. [DOI] [PubMed] [Google Scholar]
- Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- Mio K, Ogura T, Kiyonaka S, Hiroaki Y, Tanimura Y, Fujiyoshi Y, Mori Y, Sato C. The TRPC3 channel has a large internal chamber surrounded by signal sensing antennas. J Mol Biol. 2007;367:373–383. doi: 10.1016/j.jmb.2006.12.043. [DOI] [PubMed] [Google Scholar]
- Moller CC, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T, Peters J. TRP channels in disease. Sci STKE. 2005;2005:re8. doi: 10.1126/stke.2952005re8. [DOI] [PubMed] [Google Scholar]
- Nishida M, Watanabe K, Sato Y, Nakaya M, Kitajima N, Ide T, Inoue R, Kurose H. Phosphorylation of TRPC6 channels at Thr69 is required for anti-hypertrophic effects of phosphodiesterase 5 inhibition. J Biol Chem. 2010;285:13244–13253. doi: 10.1074/jbc.M109.074104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell AF, Scott JL, Van Helden DF. Epidermal growth factor induces tyrosine phosphorylation, membrane insertion, and activation of transient receptor potential channel 4. J Biol Chem. 2005;280:37974–37987. doi: 10.1074/jbc.M503646200. [DOI] [PubMed] [Google Scholar]
- Okada T, et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–468. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Ford DL, Hurt KJ, Bae SS, Suh PG, Kurosaki T, Snyder SH, Gill DL. Phospholipase C-gamma is required for agonist-induced Ca2+ entry. Cell. 2002;111:529–541. doi: 10.1016/s0092-8674(02)01045-0. [DOI] [PubMed] [Google Scholar]
- Plattner R, Irvin BJ, Guo S, Blackburn K, Kazlauskas A, Abraham RT, York JD, Pendergast AM. A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-gamma1. Nat Cell Biol. 2003;5:309–319. doi: 10.1038/ncb949. [DOI] [PubMed] [Google Scholar]
- Poulin B, Sekiya F, Rhee SG. Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-gamma1. Proc Natl Acad Sci USA. 2005;102:4276–4281. doi: 10.1073/pnas.0409590102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol. 2009;20:2534–2545. doi: 10.1681/ASN.2009010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L. beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci USA. 2006;103:14110–14115. doi: 10.1073/pnas.0602587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol. 2009;296:C558–C569. doi: 10.1152/ajpcell.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Lemonnier L, Vazquez G, Bird GS, Putney JW., Jr Dissociation of regulated trafficking of TRPC3 channels to the plasma membrane from their activation by phospholipase C. J Biol Chem. 2006;281:11712–11720. doi: 10.1074/jbc.M510541200. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-d-aspartate receptor subunit NR2A. Proc Natl Acad Sci USA. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, Schiffer M. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J Biol Chem. 2010;285:25285–25295. doi: 10.1074/jbc.M109.087239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, St JBird G, McKay RR, Birnbaumer L, Putney JW., Jr Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem. 2003;278:16244–16252. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278:20716–20723. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J, et al. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest. 2004;114:1475–1483. doi: 10.1172/JCI22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MP, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- Woudenberg-Vrenken TE, Bindels RJ, Hoenderop JG. The role of transient receptor potential channels in kidney disease. Nat Rev Nephrol. 2009;5:441–449. doi: 10.1038/nrneph.2009.100. [DOI] [PubMed] [Google Scholar]
- Zachos NC, van Rossum DB, Li X, Caraveo G, Sarker R, Cha B, Mohan S, Desiderio S, Patterson RL, Donowitz M. Phospholipase C-gamma binds directly to the Na+/H+ exchanger 3 and is required for calcium regulation of exchange activity. J Biol Chem. 2009;284:19437–19444. doi: 10.1074/jbc.M109.006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.