UNC5B induces apoptosis in the absence of its cognate ligand netrins and acts as a tumor suppressor. UNC5B is a direct transcriptional target of p53 upon UV stimulation. Here we show that Akt phosphorylates PIKE-A and regulates its association with UNC5B and inhibits UNC5B-provoked apoptosis in a p53-dependent manner.
Abstract
UNC5B acts as a tumor suppressor, and it induces apoptosis in the absence of its cognate ligand netrins. UNC5B is a direct transcriptional target of p53 upon UV stimulation. Here we show that Akt phosphorylates PIKE-A and regulates its association with UNC5B and inhibits UNC5B-provoked apoptosis in a p53-dependent manner. PIKE-A GTPase binds active Akt and stimulates its kinase activity in a guanine-nucleotide–dependent way. Akt feeds back and phosphorylates PIKE-A on Ser-472 and subsequently enhances its stimulatory effect on Akt kinase activity. Akt activity is significantly reduced in PIKE −/− Mouse Embryonic Fibroblast (MEF) cells as compared to wild-type cells. PIKE-A directly interacts with UNC5B, which is regulated by netrin-1–activated Akt. Overexpression of PIKE-A diminishes UNC5B expression through down-regulation of p53. Knocking down PIKE-A stabilizes p53, increases UNC5B, and escalates UV-triggered apoptosis. Depletion of Akt abrogates PIKE-A's inhibitory effect on both p53 and UNC5B. Hence our findings support the notion that Akt-phosphorylated PIKE-A inhibits UNC5B-elicited apoptosis and reduces its expression level through inactivation of p53.
INTRODUCTION
PI 3-kinase enhancer (PIKE) was originally identified as a brain-specific nuclear GTPase that binds PI 3-kinase and stimulates its lipid kinase activity (Ye et al., 2000). Nerve growth factor (NGF) activates PIKE by triggering PLC-γ1 nuclear translocation, which acts as a physiologic guanine nucleotide exchange factor for PIKE through its SH3 domain (Ye et al., 2002). To date, three forms of PIKE have been characterized: PIKE-S, PIKE-L, and PIKE-A. PIKE-S is the initially cloned shorter isoform. PIKE-L, a longer isoform of the CENTG1 gene, differs from PIKE-S by the addition of a 40-kDa C-terminal extension containing Arf-GAP and two ankyrin repeat domains. Whereas PIKE-S exclusively resides in the nucleus, PIKE-L occurs in both the nucleus and the cytoplasm (Chan and Ye, 2007). PIKE-L interacts with Homer 1, an mGluR I–binding adaptor protein. The Homer–PIKE-L complex couples PI 3-kinase to mGluR I and regulates a major action of group I mGluRs, prevention of neuronal apoptosis (Rong et al., 2003). Moreover, we recently showed that netrin-1 induces the interaction of UNC5B with the brain-specific GTPase PIKE-L. This interaction triggers the activation of PI 3-kinase signaling, prevents UNC5B's proapoptotic activity, and enhances neuronal survival (Tang et al., 2008). A third PIKE isoform, PIKE-A, was identified in human glioblastoma multiformes. Unlike the brain-specific PIKE-L and -S isoforms, PIKE-A distributes in various tissues. PIKE-A lacks the N-terminal proline-rich domain present in PIKE-L, which binds PI 3-kinase and PLC-γ1. Instead, PIKE-A specifically interacts with active Akt and up-regulates its activity in a GTP-dependent manner, mediating human cancer cell invasion and preventing apoptosis (Ahn et al., 2004a, 2004b). PIKE-A is overexpressed in numerous human cancers, escalating U87MG glioblastoma invasion and provoking NIH3T3 cell transformation. Our previous findings show that PIKE-A acts as a proto-oncogene, promoting cell transformation through Akt activation (Liu et al., 2007).
The three mammalian transmembrane receptors UNC5H1, UNC5H2, and UNC5H3 (also called UNC5A, UNC5B, and UNC5C when referring to humans) that belong to the UNC5H family of netrin-1 receptors were initially proposed as mediators of the chemorepulsive effect of netrin-1 on specific axons (Serafini et al., 1994, 1996). However, they were also shown to act as dependence receptors. Such receptors induce apoptosis when unbound to their ligands (Llambi et al., 2001, 2005). Mehlen and his colleagues showed that the expression of human UNC5A, B, and C is down-regulated in multiple cancers and they act as putative tumor suppressors (Thiebault et al., 2003).
The TP53 gene is mutated in approximately half of human cancers and encodes a transcription factor that is activated by various stresses, including hypoxia and DNA damage, and exerts its tumor-suppressive actions through a number of pathways mediated by its target genes (Arakawa, 2004). Intron 1 of UNC5B contains a p53-binding sequence, and genotoxic stress induces UNC5B expression in wild-type p53-expression cancer cells (Tanikawa et al., 2003), and netrin-1 inhibits p53-induced apoptosis when binding to UNC5B. The tumor-suppressor protein, p53, and the oncoprotein, Akt, are involved in a cross-talk that acts as the core of a cell's control machinery for switching between survival and death. This cross-talk is a combination of reciprocally antagonistic pathways emanating from p53 and Akt and also involves another oncogene, Mdm2 (Wee and Aguda, 2006). It is well accepted that Mdm2-mediated ubiquitination plays a crucial role in p53 regulation. In addition to proteasome-mediated degradation, ubiquitination of p53 by Mdm2 acts as a key signal for its nuclear export (Brooks et al., 2007). Akt has been shown to modulate the activity of p53 through its substrate MDM2 (Zhou et al., 2001; Ogawara et al., 2002). MDM2 is an E3 ubiquitin ligase that negatively regulates p53 transcriptional activity (Yin et al., 2002). Phosphorylation of MDM2 on Ser-166 and Ser-186 by Akt stimulates translocation of MDM2 to the nucleus, where it binds to p53 and targets p53 degradation by the proteosome (Zhou et al., 2001; Ogawara et al., 2002). In this report, we demonstrate that Akt phosphorylates PIKE-A and enhances its interaction with UNC5B and suppresses its proapoptotic activity. However, Akt-phosphorylated PIKE-A further escalates Akt kinase activity and promotes p53 degradation, leading to repression of UNC5B transcription and inhibition of apoptosis.
RESULTS
Akt phosphorylates PIKE-A on Ser-472
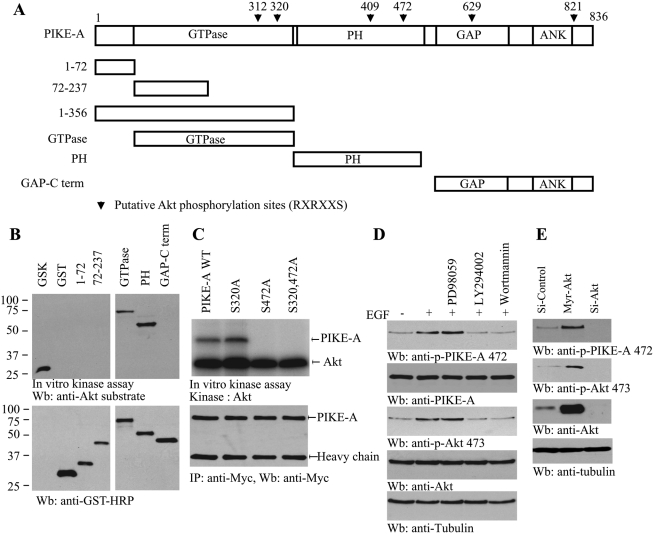
PIKE-A binds active Akt and enhances its kinase activity (Ahn et al., 2004b). In exploring the sequence of PIKE-A, we noticed that it contains numerous amino acids corresponding to a motif that is identified as a consensus Akt phosphorylation element present in various Akt substrates (Figure 1A). An in vitro Akt kinase assay revealed that both the GTPase domain (a.a. 73–356) and the PH domain (a.a. 357–555) were robustly phosphorylated by active Akt. By contrast, N-terminal domains (a.a. 1–72 and 72–237) were not phosphorylated (Figure 1B, top). Mutation with Ser-472A but not Ser-320A in PIKE-A completely abolished the phosphorylation of PIKE-A, suggesting that Ser-472 is the major residue that can be phosphorylated by Akt in vitro (Figure 1C, top). To explore whether endogenous PIKE-A can be phosphorylated by Akt in intact cells, we developed Ser-472 phosphorylation–specific antibody. We pretreated the cells with MEK1 inhibitor (PD98059) and PI3K inhibitors, Wortmannin and LY294002, respectively, followed by growth factor stimulation. Compared to control, epidermal growth factor (EGF) elicited potent PIKE-A phosphorylation, which was substantially blocked by PI3K inhibitors but not by the MEK1 inhibitor. The Akt activation status correlated with PIKE-A Ser-472 phosphorylation (Figure 1D, first and second panels). PIKE-A phosphorylation was markedly decreased when Akt1 was depleted by its siRNA compared to control siRNA. Overexpression of active plasma membrane myristoylated Akt (myr-Akt) substantially enhanced PIKE-A phosphorylation, underscoring that Akt is the physiological upstream kinase for PIKE-A (Figure 1E). Collectively, these data support that PIKE-A is a physiological substrate of Akt.
FIGURE 1:
Akt phosphorylates PIKE-A on Ser-472. (A) The diagram of human PIKE-A. PIKE-A possesses six putative Akt phosphorylation motifs (RXRXX(S/T)), as indicated (▾) with residue numbers. (B) In vitro Akt kinase assay. Purified recombinant GST-fusion proteins were incubated with active Akt at 30°C for 30 min. GTPase and PH domains were robustly phosphorylated, while other fragments were not. GSK was a positive control. (C) Ser-472 residue in PIKE-A is phosphorylated by Akt. Myc–PIKE-A wild-type, Ser-320A, and Ser-472A and Ser-320/Ser-472A were incubated with active Akt at 30°C for 30 min. Wild-type PIKE-A and Ser-320A but not Ser-472A mutant were strongly phosphorylated (top). Equal amounts of immunoprecipitated proteins were employed (bottom). (D) PI 3-kinase inhibitors block PIKE-A phosphorylation on Ser-472. Brain cells were pretreated by 10 μM LY294002, 100 nM wortmannin, or 10 μM PD98059 and then treated with growth factor for 20 min. EGF stimulated PIKE-A phosphorylation, which was diminished by PI 3-kinase but not ERK inhibitor pretreatment (top left). Akt phosphorylation was verified (left, second panel). (E) Akt is required for PIKE-A Ser-472 phosphorylation. Active Akt robustly phosphorylates endogenous PIKE-A and knocking down of Akt eliminates its phosphorylation (top). Verification of Akt overexpression and knockdown (third panel).
Akt phosphorylation is required for PIKE-A to associate with netrin receptor UNC5B
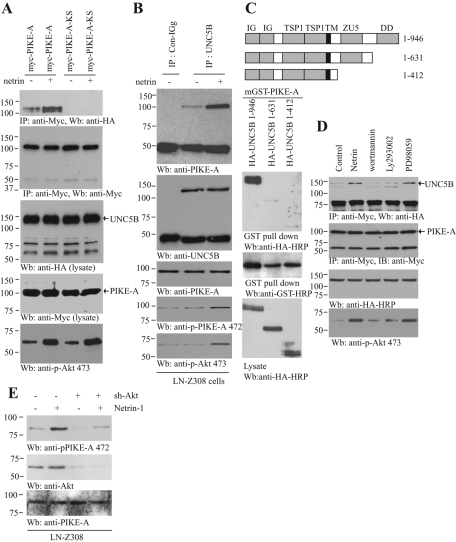
Our previous study shows that PIKE-L binds to UNC5B, for which PIKE-L's GTPase activity is indispensable, and netrin-1 treatment escalates the association (Tang et al., 2008). To examine whether netrin-1 modulates PIKE-A interaction with UNC5B, we cotransfected UNC5B into HEK293 cells with wild-type or dominant-negative PIKE-A-KS (K413AS414N). This mutant cripples PIKE-A's GTPase activity. It binds Akt but fails to activate it (Ye et al., 2000; Ahn et al., 2004a). Compared to the control, netrin-1 increased PIKE-A interaction with UNC5B; in contrast, PIKE-A-KS failed to bind UNC5B regardless of netrin-1 stimulation (Figure 2A), indicating that GTPase activity is essential for the association. Coimmunoprecipitation with UNC5B antibody revealed that endogenous PIKE-A selectively bound to UNC5B, and netrin-1 stimulated the association (Figure 2B, top), suggesting that PIKE-A specifically interacts with UNC5B in response to netrin-1. PIKE-A Ser-472 phosphorylation tightly coupled to Akt activation by netrin-1 (Figure 2B, bottom two panels). The binding assay demonstrated that truncation of the death domain in UNC5B completely abolished the association between PIKE-A and UNC5B (Figure 2C), supporting that the death domain in UNC5B is required for its binding to PIKE-A. To explore whether PIKE-A phosphorylation by Akt is required for its association with UNC5B, we transfected HEK293 cells with Myc-PIKE-A and HA-UNC5B and pretreated the transfected cells with PI3K inhibitors and MEK1 inhibitor. Netrin-provoked PIKE-A–UNC5B complex was substantially disrupted by PI3K inhibitors, whereas PD98059 had no effect (Figure 2D). PIKE-A is overexpressed in human glioblastoma cell line LN-Z308 (Ahn et al., 2004b). Netrin treatment enhanced PIKE-A phosphorylation on Ser-472, whereas depletion of Akt substantially blocked its phosphorylation (Figure 2E), underscoring that Akt is an endogenous upstream kinase responsible for phosphorylating PIKE-A on Ser-472. The death-associated protein kinase (DAPK) mediates UNC5B-induced apoptosis by interacting with the death domain (Llambi et al., 2005). To explore whether this downstream signaling is regulated by PIKE-A, we conducted a binding assay. PIKE-A enhanced the interaction between DAPK and UNC5B, and this interaction was further elevated by netrin-1 (Supplemental Figure 1, top). Interestingly, the Ser-308 phosphorylation on DAPK was slightly decreased in the presence of PIKE-A and was reduced more by netrin-1 treatment (Supplemental Figure 1, third panel). Therefore these data support that netrin-1 up-regulates the association between PIKE-A and UNC5B, for which Akt-mediated PIKE-A phosphorylation by netrin is required.
FIGURE 2:
Netrin-1–activated Akt mediates interaction between PIKE-A and UNC5B. (A) Netrin-1 increases the binding of PIKE-A to UNC5B. Wild-type and GTPase-dead (KS) PIKE-A were cotransfected into HEK293 cells with HA-UNC5B. The transfected cells were treated with netrin-1 for 30 min. PIKE-A was immunoprecipitated with anti-Myc antibody and analyzed with anti-HA antibody. Equal amounts of HA-UNC5B and Myc-PIKE-A were immunoprecipitated (second and third panels). (B) PIKE-A interaction with UNC5B in LN-Z308 cells. LN-Z308 cells were treated with netrin-1 for 30 min, endogenous UNC5B was immunoprecipitated with control IgG or anti-UNC5B antibody and the bound PIKE-A was detected using anti–PIKE-A antibody (top). Confirmation of Akt and PIKE-A phosphorylation status (fourth and fifth panels). (C) The UNC5B death domain is essential for the interaction between UNC5B and PIKE-A. The diagram of UNC5B domains is shown (top). Different UNC5B truncates were cotransfected into HEK293 cells with mGST–PIKE-A. PIKE-A was pulled down with glutathione beads and analyzed with anti-HA antibody. Truncation of the death domain in UNC5B or its whole intracellular domain abolished the interaction between PIKE-A and UNC5B (bottom). (D) PI 3-kinase signaling regulates the interaction between PIKE-A and UNC5B. HA-UNC5B and Myc–PIKE-A cotransfected HEK293 cells were pretreated with PD98059 (10 μM), wortmannin (100 nM), LY294002 (10 μM) for 30 min, before netrin-1 was introduced. Myc–PIKE-A was immunoprecipitated using Myc antibody, and the bound HA-UNC5B was detected using anti–HA-HRP antibody. Netrin-1 triggered the interaction between PIKE-A and UNC5B, but PI 3-kinase inhibitor markedly blocked it (top). Confirmation of Akt phosphorylation status (bottom). (E) Netrin-triggered PIKE-A phosphorylation is Akt dependent. LN-Z308 cells were infected with ad-shRNA-Akt for 36 h followed by 200 ng/ml netrin-1 treatment for 30 min. Immunoblotting was conducted with anti–phospho-PIKE-A Ser-472 (top), anti-Akt (middle), and anti–PIKE-A (bottom) antibodies.
PIKE-A binds to UNC5B and inhibits UNC5B-induced apoptosis
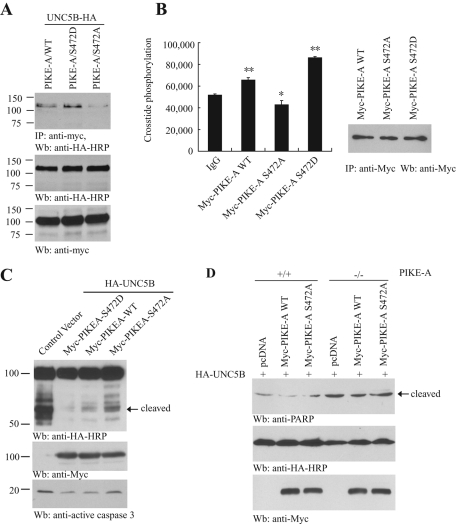
To test the notion that Akt phosphorylation is essential for PIKE-A to interact with UNC5B, we transfected HA-UNC5B into HEK293 cells with phosphorylation mimetic mutant PIKE-A Ser-472D and unphosphorylated mutant PIKE-A Ser-472A. Compared to wild-type PIKE-A, PIKE-A Ser-472D strongly bound to UNC5B, whereas Ser-472A barely interacted with UNC5B (Figure 3A). PIKE-A directly binds Akt and regulates its kinase activity (Ahn et al., 2004a). To explore if Ser-472 phosphorylation plays any role in mediating its stimulatory effect on Akt kinase activity, we immunoprecipitated various Myc-tagged PIKE proteins from transfected cells and monitored their effects on the purified Akt kinase in the presence of the substrate peptide Crosstide. Wild-type PIKE-A immunocomplex significantly increased Akt activity compared to control immunoglobulin G (IgG), and the stimulatory effect was further enhanced by phosphorylation mimetic mutant PIKE-A Ser-472D, indicating that Akt phosphorylation of PIKE-A feeds back and escalates Akt kinase activity. By contrast, the unphosphorylated PIKE-A Ser-472A mutant reduced Akt kinase activity (Figure 3B). In the absence of its cognate ligand netrins, UNC5B activates caspase-3 and yields the fragmented intracellular region that contains the death domain (Llambi et al., 2001, 2005). As expected, HA-UNC5B strongly displayed the cleaved band, which was predominantly blocked by wild-type PIKE or PIKE-A Ser-472D. In contrast, this inhibitory effect was evidently diminished in PIKE-A Ser-472A transfected cells (Figure 3C, top), fitting with its weak binding affinity to UNC5B. Accordingly, the caspase-3 activation pattern tightly correlated with UNC5B cleavage activity (Figure 3C, bottom). To further explore whether PIKE-A interaction with UNC5B is critical for suppressing its proapoptotic action, we employed wild-type and PIKE-A–null Mouse Embryonic Fibroblast (MEF) cells. Transfection of UNC5B provoked evident poly (ADP-ribose) polymerase (PARP) cleavage in PIKE-A −/− cells but not in wild-type MEF cells. Cotransfection of PIKE-A Ser-472A, which barely bound to UNC5B, failed to repress its proapoptotic effect. By contrast, wild-type PIKE-A decreased PARP cleavage in PIKE-A −/− MEF cells and almost completely suppressed PARP cleavage in wild-type MEF cells (Figure 3D). Hence these data support that Akt phosphorylation of PIKE-A up-regulates its association with UNC5B, which is critical for its inhibitory action on UNC5B-mediated apoptosis.
FIGURE 3:
Overexpressed PIKE-A decreases UNC5B-provoked apoptosis. (A) PIKE-A binding to UNC5B is Akt phosphorylation dependent. Myc-PIKE Ser-472A and Ser-472D were cotransfected into HEK 293 cells with HA-UNC5B, respectively. PIKE-A was immunoprecipitated by anti-Myc and was detected with HA-HRP antibody (top). The expression of HA-UNC5B and Myc–PIKE-A was verified (middle and bottom). (B) Phosphorylation of PIKE-A by Akt enhanced its stimulatory effect on Akt kinase activity. Myc-tagged wild-type PIKE-A and mutants were immunoprecipitated by anti-Myc antibody, and the immunocomplex was incubated with purified Akt protein, the Akt substrate crosstides, γ-32P- ATP, and cold ATP for 30 min at 30°C. A portion of the samples was loaded on filter papers and subjected to liquid scintillation counter analysis (left). Equal amounts of immunoprecipitated proteins were used (right). The data were represented as mean ± standard error of the mean (SEM) from three independent experiments (*p < 0.05, **p < 0.001, Student's t test). (C) PIKE-A binds UNC5B and suppresses its apoptotic cleavage. Control vector, Myc-PIKE wild type (WT), Ser-472A, and Ser-472D were cotransfected into HEK293 cells with HA-UNC5B, respectively. Immunoblotting was conducted with anti-HA (top), anti-Myc (middle), and anti-active caspase-3 antibodies (bottom). (D) PIKE-A inhibits apoptosis induced by UNC5B. Wild-type and PIKE-A −/− MEF cells were cotransfected with HA-UNC5B and control plasmid, Myc-PIKE-A WT, and Myc-PIKE-A Ser-472A. The expression of transfected PIKE-A and UNC5B was verified (middle and bottom). Overexpression of PIKE-A WT decreased PARP cleavage (top).
PIKE-A blocks UNC5B-induced apoptosis through down-regulating p53
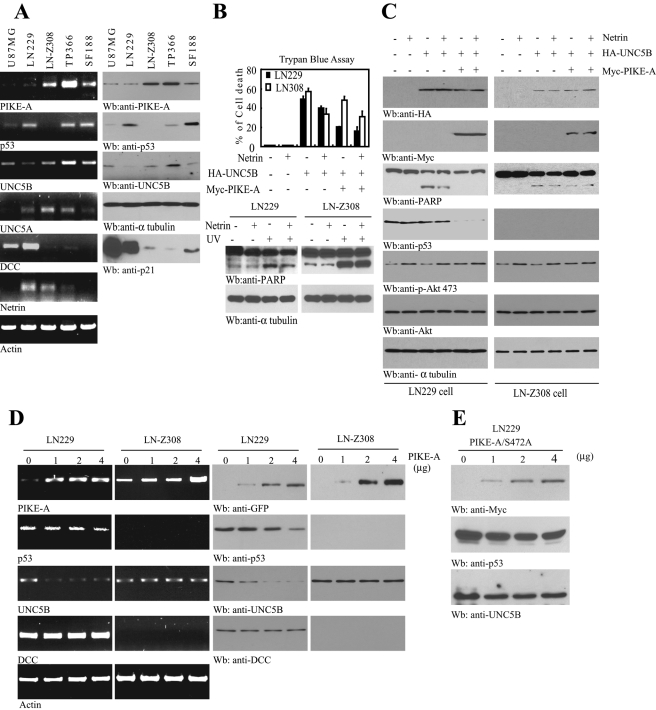
Netrin receptors UNC5 and DCC (deleted in colorectal cancer) are able to trigger apoptosis in the absence of netrin-1 (Llambi et al., 2001, 2005). To examine whether PIKE-A regulates UNC5B's apoptotic action in human cancer cells, we screened a panel of glioblastoma cell lines’ gene expression patterns including PIKE-A, p53, UNC5, DCC, netrin, etc. Reverse transcription PCR (RT-PCR) analysis revealed that netrin was strongly expressed in both LN229 and LN-Z308 cells. UNC5A and UNC5B were detectable in both cell lines, but LN-Z308 cells demonstrated more abundant UNC5B than LN229 cells. Interestingly, p53 was potently expressed in LN229, whereas it was negligible in LN-Z308 cells, indicating that other mechanisms but not p53 predominantly regulate UNC5B expression in LN-Z308 cells. However, DCC was markedly expressed in U87MG and LN229 cells, and its expression was not detectable in any other cells. Consistent with our previous report, PIKE-A was evidently overexpressed in LN-Z308 and TP366 (Figure 4A, top). A physiological target of p53—p21—was robustly expressed in some of these cells, including U87MG, LN229, and SF188, indicating that p53 in these cells is functional. We chose LN229 and LN-Z308 cells due to their different gene expression patterns. We transfected LN229 and LN-Z308 cells with HA-UNC5B in the presence or absence of PIKE-A and monitored cell death using trypan blue exclusion assay. Transfection of UNC5B provoked robust cell death in both cell lines, and netrin-1 treatment substantially decreased the apoptotic effect (Figure 4B, top, lanes 3 and 4), fitting with previous reports (Llambi et al., 2001, 2005). Interestingly, cotransfection of PIKE-A significantly diminished UNC5B-triggered cell death in LN229 cells, while the inhibitory effect by PIKE-A in LN-Z308 cells was modest (Figure 4B, top, lanes 3 and 5). Hence it is possible that p53 is required for PIKE-A to suppress UNC5B-mediated apoptosis, because p53 is null in LN-Z308 cells. As expected, netrin-1 treatment elicited notable reduction of cell death in both UNC5B and PIKE-A cotransfected cells (Figure 4B, top, lane 6). Consistently, UV-provoked PARP cleavage was reduced by netrin-1 in LN229 cells, and this inhibitory effect was not evident in LN-Z308 cells (Figure 4B, bottom panels). To monitor the molecular events in the transfected cells, we conducted immunoblotting analysis. PARP cleavage was demonstrable in UNC5B-transfected LN229 cells, which was strongly blocked by cotransfection of PIKE-A. Netrin treatment further elevated the inhibitory effect. Nonetheless, PARP cleavage was not substantially diminished in LN-Z308 cells regardless of netrin treatment or PIKE-A transfection or both (Figure 4C, third panel). This result fits with the trypan blue cell death assay. Strikingly, p53 expression was completely blocked in LN229 cells when PIKE-A was transfected. No p53 was detectable in LN-Z308 cells (Figure 4C, fourth panel). Hence overexpression of PIKE-A represses p53 expression and decreases UNC5B-stimulated cell death in LN229 cells. To explore whether p53 is implicated in the inhibitory action by PIKE-A to UNC5B-mediated apoptosis, we knocked down p53 in LN229 cells that were cotransfected with UNC5B and various PIKE-A plasmids. The depletion of p53 significantly blocked the inhibitory activity by PIKE-A on UNC5B-induced apoptosis (Supplemental Figure 2). This effect was similar to what occurred in p53-null LN-Z308 cells. Thus this finding indicates that PIKE-A blocks UNC5B-triggered apoptosis through p53.
FIGURE 4:
PIKE-A decreases UNC5B-provoked apoptosis and p53 stability. (A) The expression spectrum of various genes in various glioblastoma cell lines. Several indicated gene expressions were analyzed by RT-PCR and Western blotting. (B, C) PIKE-A suppresses UNC5B-provoked apoptosis in LN229 but not LN-Z308 cells. LN229 and LN-Z308 cells were exposed with UV (10 J/m2) followed by 24 h treatment with or without netrin-1(200 ng/ml). The cell lysates were subjected to immunoblotting analysis with anti-PARP (B, bottom). LN229 and LN-Z308 cells were transiently transfected with HA-UNC5B in the presence or absence of Myc-PIKE-A. Forty-eight hours after transfection, cells were treated with or without netrin-1, and cell death was measured by trypan blue exclusion assay. The expression of HA-UNC5B and Myc–PIKE-A was confirmed by Western blotting (C, top two panels). Tubulin was included as a loading control. PIKE-A overexpression repressed the cleavage of PARP and p53 protein levels (C, third and fourth panels). (D) PIKE-A regulates p53 protein stability. LN229 and LN-Z308 cells were transfected with the indicated amount of PIKE-A. Forty-eight hours after the transfection, isolated total RNA was examined by RT-PCR (left). The cell lysates were subjected to immunoblotting analysis with anti-p53, UNC5B, and DCC antibodies (right). (E) PIKE-A Ser-472A mutant does not alter p53 or UNC5B protein level in LN229 cells. LN229 cells were transfected with the indicated amount of PIKE-A Ser-472A for 48 h. The cell lysates were subjected to immunoblotting analysis with anti-p53, UNC5B, and Myc antibodies.
UNC5B is a direct transcriptional target of p53 (Tanikawa et al., 2003). To explore whether PIKE-A also antagonizes UNC5B through inhibiting p53, we conducted a titration assay. Gradually increasing PIKE-A levels progressively repressed p53 expression in LN229 cells. Accordingly, UNC5B was proportionally diminished, whereas DCC remained unchanged. By contrast, LN-Z308 cells lack the expression of p53, and overexpression of PIKE-A had no effect on UNC5B protein levels. Immunoblotting data were tightly correlated with RT-PCR results (Figure 4D). To further test this notion, we transfected PIKE-A Ser-472A that fails to bind UNC5B into LN229 cells. Overexpression of the PIKE-A mutant did not significantly alter p53 or UNC5B protein levels (Figure 4E). Because the PIKE-A mutant is unable to provoke Akt kinase activity, this experiment suggests that PIKE-A–provoked Akt kinase activity might be necessary for suppressing the p53 protein level and subsequently repressing UNC5B expression. Therefore our data support that PIKE-A down-regulates p53 and leads to a reduction of UNC5B expression.
PIKE-A is required for Akt activation and suppressing UV-provoked apoptosis
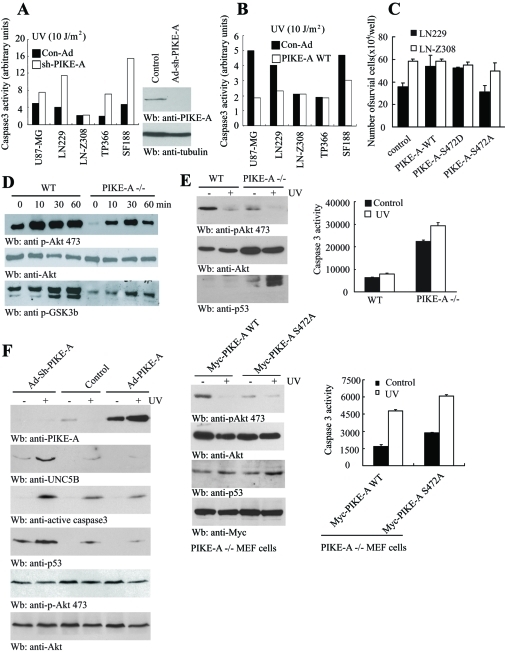
The transcription of UNC5B can be activated by various cellular stresses, including UV, in a p53-dependent manner (Tanikawa et al., 2003). To explore whether PIKE-A is required for protecting cells from UV-triggered apoptosis, we depleted PIKE-A in a variety of glioblastoma cells. Knocking down PIKE-A markedly increased caspase-3 activity in all tested cells except LN-Z308 cells (Figure 5A), suggesting that PIKE-A is required for the survival of all the tested cells but not LN-Z308 cells. Presumably LN-308 cells lack functional p53 for triggering UV-induced cell death. However, the overexpression of PIKE-A considerably protected U87MG, LN229, and SF188 cells from UV-provoked apoptosis, whereas no significant difference was detected in either LN-Z308 or TP366 cells (Figure 5B). This might be due to the high levels of overexpressed endogenous PIKE-A from genomic amplification in these two cell lines.
FIGURE 5:
PIKE-A regulates DNA damage-mediated apoptosis in glioblastoma cells. (A) Depletion of PIKE-A makes glioblastoma cells vulnerable to UV-triggered apoptosis. Activation of caspase-3 was quantified in glioblastoma cells infected with control and sh-PIKE-A adenovirus. Control and sh-PIKE-A adenovirus were used to infect different glioblastoma cells. Forty-eight hours after infection, the cells were exposed to 10 J/m2 of UV. Quantitative apoptotic assay in various cell lines is shown. Immunoblotting analysis of PIKE-A knockdown in LN-Z308 cells (right). (B) PIKE-A overexpression represses UV-triggered apoptosis. PIKE-A infected U87-MG, LN229, and SF188 cells revealed the decreased caspase-3 activity, but LN-Z308 and TP366 cells remained the same compared to control. (C) Akt phosphorylation of PIKE-A is required for its prosurvival activity in LN229 cells. LN229 and LN-Z308 glioblastoma cells were transfected with control and various PIKE constructs, followed by UV stimulation. Apoptosis was analyzed after 24 h. (D) PIKE-A is required for FBS-stimulated Akt activation. Wild-type and PIKE-A −/− MEF cells were treated with 10% FBS at various time points. The cell lysates were analyzed with anti–p-Akt 473, anti-Akt, and anti–p-GSK3 antibodies, respectively. (E) Wild-type but not PIKE-A Ser-472A mutant prevents PIKE-null MEF cells from UV-induced apoptosis. Top, wild-type and PIKE-A–null MEF cells were stimulated with UV. After 24 h, the cells were analyzed by immunoblotting with various indicated antibodies and caspase-3 activity was quantified by Caspase-Glo 3/7 assay. Bottom, PIKE-A–null MEF cells were transfected with Myc-PIKE-A WT or Ser-472A followed by UV stimulation. Akt activity and p53 levels were analyzed (bottom left). Caspase-3 activity was quantified by Caspase-Glo 3/7 assay (bottom right). (F) Knocking down of PIKE-A up-regulates p53 and UNC5B, enhancing apoptosis. TP366 cells were infected with control adenovirus or adenovirus expressing shRNA or wild-type PIKE-A. Over 24 h, the infected cells were exposed with UV. After 24 h, the cell lysates were analyzed with various antibodies as indicated.
Our previous study shows that PIKE-A promotes cell survival through activating Akt (Ahn et al., 2004a). To assess whether PIKE-A phosphorylation by Akt plays any role in mediating its survival action, we transfected both LN229 and LN-Z308 cells with various PIKE-A constructs, and we treated the cells with UV. Compared to the control, the overexpression of wild-type PIKE-A and the phosphorylation mimetic Ser-472D mutant elevated cell survival in LN229 cells. However, transfection of PIKE-A Ser-472A even reduced cell survival compared to the control. Nonetheless, in LN-Z308 cells, UV-provoked cell death was not significantly altered regardless of transfection of any form of PIKE-A (Figure 5C), which might be due to highly overexpressed endogenous PIKE-A in LN-Z308 cells or a lack of p53. Therefore our findings support that Akt phosphorylation of PIKE-A regulates its prosurvival action.
To examine the role of PIKE-A in activating Akt and how it promotes survival against apoptosis through p53 and UNC5B, we first determined the effect of PIKE-A in regulating Akt activity in wild-type and PIKE-A–null MEF cells. Fetal bovine serum (FBS, 10%) stimulates a time-dependent Akt activation, whereas Akt activity was dramatically reduced in PIKE-A–null MEF cells (Figure 5D, top). Subsequently the phosphorylation of GSK3β, a physiological substrate of Akt, was also decreased in PIKE −/− cells (Figure 5D, bottom). Hence PIKE-A is required for the full activation of Akt. Notably, UV treatment strongly decreased Akt activity in wild-type MEF cells and it completely eliminated Akt activity in PIKE-null cells. As expected, p53 was substantially up-regulated in PIKE-A −/− cells upon UV stimulation (Figure 5E, top left panels). Consequently, UV stimulation provoked much stronger apoptosis in PIKE-A–null cells than in wild-type MEF cells (Figure 5E, top right). Transfection of wild-type PIKE-A back into PIKE-A −/− MEF cells elicited much stronger Akt activation than the PIKE-A Ser-472A mutant cells, which were greatly diminished by UV treatment. Accordingly, the p53 level was up-regulated upon UV treatment (Figure 5E, bottom left panels). Consistently, the apoptosis activity was tightly coupled to p53 levels in PIKE-A–null cells (Figure 5E, bottom right panel). To further assess whether PIKE-A is necessary for suppressing p53/UNC5B-triggered apoptosis, we knocked down PIKE-A in TP366 cells using adenovirus expressing its specific shRNA, followed by UV treatment. Compared to control cells, knockdown of PIKE-A elevated UNC5B, and UV treatment further increased UNC5B levels. Accordingly, caspase-3 was more strongly activated than in control cells. However, overexpression of PIKE-A greatly repressed UV-induced UNC5B and caspase-3 activation (Figure 5F, top three panels). As expected, p53 levels were notably up-regulated when PIKE-A was depleted. It was substantially blocked by PIKE-A overexpression. As predicted, Akt activity in control cells and PIKE-A overexpressed cells was higher than in PIKE-A knockdown cells (Figure 5F, fifth and sixth panels).
PIKE-A decreases the protein levels of p53 and UNC5B through stimulating Akt activity
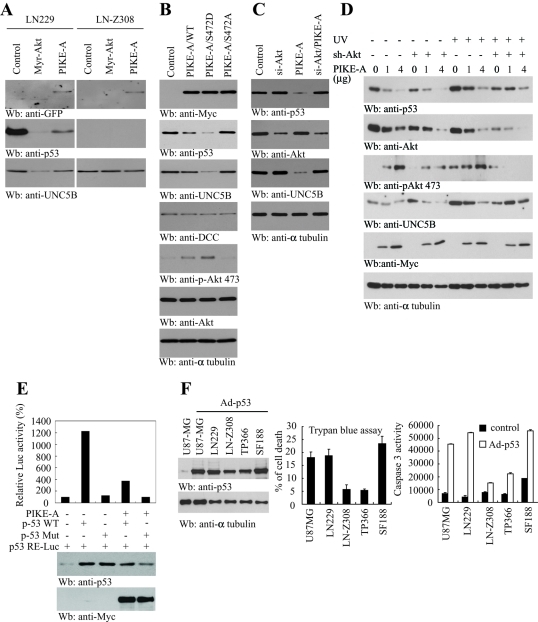
UNC5B is a direct transcriptional target for the tumor suppressor p53 and mediates p53 proapoptotic activity (Tanikawa et al., 2003). Akt down-regulates the p53 protein level through phosphorylating MDM2, an E3 ubiquitin ligase for p53 (Zhou et al., 2001). To examine whether PIKE-A represses the UNC5B protein level through the Akt-p53 pathway, we transfected LN229 and LN-Z308 cells with active Akt or GFP-PIKE-A. Overexpression of plasma membrane–associated myristoylated Akt (Myr-Akt) completely eliminated p53 expression and transfection of PIKE-A markedly diminished the p53 level. Consequently, UNC5B protein levels were substantially decreased in Myr-Akt and PIKE-A overexpressed cells, coupling to p53 expression levels in LN229 cells. By contrast, UNC5B remained unchanged in p53-deficient LN-Z308 cells regardless of Akt or PIKE-A overexpression (Figure 6A). Transfection of wild-type PIKE-A increased Akt activation, which was further enhanced by Akt-phosphorylation mimetic mutant PIKE-A Ser-472D. As expected, Akt activation was not detectable in Ser-472A-transfected LN229 cells (Figure 6B, fifth panel). Both p53 and UNC5B protein levels tightly and inversely fitted with Akt activation by PIKE-A transfection. As a control, the DCC protein level remained unchanged (Figure 6B). To further investigate whether PIKE-A down-regulating p53 or UNC5B is mediated through Akt, we depleted Akt1 with its specific siRNA in the presence or absence of PIKE overexpression. Knocking down endogenous Akt slightly increased both p53 and UNC5B protein levels. By contrast, overexpressing PIKE-A markedly diminished both p53 and UNC5B protein levels (Figure 6C, lanes 2 and 3). As expected, knocking down Akt in PIKE-A–transfected cells considerably increased the p53 level and blunted PIKE-A's inhibitory action on UNC5B protein level (Figure 6C, lane 4), suggesting that PIKE-A inactivates p53 or UNC5B through activating Akt. To explore, under stimulation conditions, whether PIKE-A decreases the protein levels of p53 and UNC5B via Akt, we knocked down Akt in LN229 cells that were transfected with various amounts of Myc–PIKE-A and treated the transfected cells with or without UV. Without UV stimulation, we found that p53 and UNC5B proteins were reduced by PIKE-A in a dose-dependent manner. Depletion of Akt increased UNC5B levels, though p53 levels were not significantly elevated (Figure 6D, first and fourth panels, lanes 1–6). Interestingly, UV stimulation substantially increased p53 levels. The extent of p53 reduction by PIKE-A overexpression was evidently reduced when Akt was depleted. We made an observation similar to that for UNC5B protein levels as compared to control cells and Akt knockdown cells when PIKE-A was overexpressed (Figure 6D, first and fourth panels, lanes 7–12). Akt activation and its total levels were verified (Figure 6D, second and third panels). Hence PIKE-A decreases p53 and UNC5B protein levels via activation of Akt.
FIGURE 6:
PIKE-A regulates Akt-mediated p53 stability and transcriptional activity. (A) Active Akt and PIKE-A regulate p53 protein level. LN229 and LN-Z308 cells were transfected with the indicated plasmid and monitored by Western blotting. p53 and UNC5B protein levels decreased in active Akt or PIKE-A transfected LN229 cells (left). No p53 expressed in LN-Z308 cells and UNC5B protein levels remained unchanged (right). (B) Akt phosphomimetic PIKE-A Ser-472D decreases p53 protein levels. Various PIKE-A mutants were transfected into LN229 cells and incubated for 48 h. The lysates were examined by Western blotting using indicated antibodies. PIKE-A wild-type and Ser-472D mutant decreased p53 and UNC5B expression (second and third panels). Akt was highly activated in PIKE-A Ser-472D transfected cells (fifth panel). (C) PIKE-A diminishes p53 protein level through Akt. LN229 cells were transfected with PIKE-A, si-Akt, and si-Akt/PIKE-A. Forty-eight hours after transfection, the lysates were assessed by Western blotting using indicated antibodies. The p53 level increased in Si-Akt transfected cells, whereas its level decreased when PIKE-A was transfected. The p53 level returned when Akt was depleted in PIKE-A transfected cell (top). (D) PIKE-A decreases the protein level of p53 and UNC5B via activation of Akt. LN229 cells were transfected with indicated amount of PIKE-A and were infected with ad-control or ad-sh-Akt followed by 10 J/m2 UV treatment. Cells were lysed 24 h after UV treatment and the lysates were subjected to immunoblotting analysis with indicated antibodies. (E) The p53 transcriptional activities assay. Mutant and wild-type p53 were cotransfected with luciferase-conjugated p53-sensitive construct in the presence or absence of PIKE-A. Wild-type p53 strongly elevated p53 RE-Luc promoter activity, which can be blocked by PIKE-A cotransfection (top). Verification of p53 and PIKE-A protein levels (middle and bottom). (F) Overexpression of p53 induces apoptosis in glioblastoma cells with lower levels of PIKE-A. A panel of glioblastoma cells was infected with control adenovirus or adenovirus expressing p53. After 24 h, cell lysates were analyzed with immunoblotting with anti-p53 antibody (left). The apoptosis was assessed by trypan blue exclusion assay and caspase-3 enzyme-linked immunosorbent assay (middle and right).
To further examine whether PIKE-A down-regulates UNC5B expression through diminishing p53, we cotransfected PIKE-A with wild-type and mutated p53 in the presence of p53 responsive reporter construct. When the individual reporter construct was transiently cotransfected with empty pCMV, pCMV-p53, or pCMV-p53mt135 vector into LN229 cells, we found that transfection of pCMV-p53 but not pCMV-p53mt135 increased the luciferase activity of the reporter plasmid, demonstrating that the reporter plasmid is p53 sensitive. Cotransfection of PIKE-A greatly diminished the p53 protein level and p53-mediated transcriptional activity (Figure 6E), which supports that PIKE-A blocks p53 transcriptional activity through decreasing its protein levels. To explore whether overexpressing p53 in glioblastoma cells that also express PIKE-A and UNC5B would override the prosurvival function of PIKE-A, we infected various human brain tumor cell lines with adenovirus expressing p53. Overexpression of p53 strongly augmented much more robust apoptosis in U87MG, LN229, and SF188 cells than LN-Z308 and TP366 cells that expressed high PIKE-A levels than other human cancer cells (Figure 6F). Thus higher levels of PIKE-A antagonize p53-induced apoptosis. Together, these data support that Akt-phosphorylated PIKE-A highly activates Akt, leading to down-regulation of p53 and UNC5B, for which Akt is indispensable.
DISCUSSION
In the current study, we show that PIKE-A is a physiological substrate of Akt. Consequently, Akt phosphorylation of PIKE-A enhances its stimulatory effect on Akt kinase activity. PIKE-A binds UNC5B, which is enhanced by netrin-1. The association between PIKE-A and UNC5B is tightly regulated by Akt-mediated phosphorylation of PIKE-A, because the blockade of PI3K/Akt signaling disrupts the association between UNC5B and PIKE-A. Moreover, the Akt phosphorylation mimetic PIKE-A Ser-472D mutant robustly binds UNC5B, whereas unphosphorylated PIKE-A Ser-472A barely binds UNC5B. Accordingly, cotransfection of PIKE-A evidently blocks UNC5B-provoked apoptosis in a p53-dependent manner. Therefore PIKE-A inhibits UNC5B's apoptotic action, presumably through both direct interaction with the dependence receptor and down-regulation of its transcription level. Employing siRNA to knock down Akt, we show that PIKE-A suppresses p53 and UNC5B expression through activating Akt. Thus this study establishes the model that PIKE-A prevents UNC5B's apoptotic effect through both protein–protein interaction and transcriptional repression mechanisms.
The tumor supressor gene TP53 encodes a transcription factor that exerts its physiological functions by binding to a specific sequence within its target gene and activating its transcription (Vogelstein et al., 2000; Vousden and Woude, 2000). The p53 family includes two other members, p63 and p73. Each of them uses multiple promoters and alternative splicing to generate an array of isoforms, including full-length isoforms with transactivation (TA) domain homologues to that of full-length p53, and N-terminal truncated (ΔN) isoforms. Whereas the full-length TA isoforms of p63 and p73 can activate downstream target genes and induce apoptosis, ΔN isoforms that lack the TA domain can function as dominant inhibitors of the full length of p53, p63, and p73, inhibiting transactivation of target genes and induction of apoptosis (Muller et al., 2006). Interestingly, Gespach and his colleagues recently showed that netrin-1induced apoptosis in human cervical tumor cells and p53-deficient HEK293 cells through up-regulation of Tap73α by preventing its ubiquitination and degradation. Hence the transcriptionally active Tap73α is implicated in apoptosis induced by netrin-1 in a p53-independent and DCC/ubiquitin-proteasome–dependent manner (Roperch et al., 2008). Recently UNC5B was shown to be a direct target gene for p53 that mediates p53-dependent apoptosis (Tanikawa et al., 2003). UNC5B is also known to be a dependence receptor that regulates apoptosis either positively or negatively depending on its interaction with netrin-1 (Llambi et al., 2001). It remains unknown whether p63 or p73 is implicated in regulating UNC5B transcription. Conceivably, netrin-1 has a dual role in provoking apoptosis or cell survival in human cancer cells, depending on the genetic context, including p53 family members, netrin receptors, expression profiles, etc. UNC5B-induced apoptosis appears independent of the mitochondrial and death-receptor pathways. This might therefore represent a third pathway for p53-dependent apoptosis (Arakawa, 2004). Our recently study demonstrates that PIKE-L selectively binds to the death domain of UNC5B through PIKE's GTPase domain, preventing the apoptotic actions of UNC5B. This interaction is mediated by Fyn phosphorylation of PIKE-L upon netrin treatment. Thus, PIKE-L acts as a downstream survival effector for netrin-1 by blocking UNC5B in the nervous system (Tang et al., 2008). In the current study, we establish that PIKE-A acts as a physiological substrate of Akt and find that Ser-472 phosphorylation on PIKE-A by Akt is critical for its affinity to UNC5B. In addition to direct association with the UNC5B receptor and blockade of its apoptotic action, PIKE-A also mediates UNC5B transcription through down-regulating p53 by activating Akt. Hence PIKE-A exerts its inhibitory effect on UNC5B through at least two different molecular mechanisms.
Our previous study shows that PIKE-A binds Akt and enhances it kinase activity (Ahn et al., 2004a). Here we show that PIKE-A is also a downstream target of Akt (Figure 1). Akt phosphorylation of PIKE-A substantially increases its stimulatory effect on Akt kinase activity (Figure 3). However, knocking out PIKE-A notably diminishes Akt activation (Figure 5). Thus a positive feedback loop exists between PIKE-A and Akt. They mutually regulate each other through direct protein–protein interaction. However, Liu and his colleagues recently reported that PIKE-A was phosphorylated by Akt on the Ser-692 site, which is different from the Ser-472 residue that we identified here. Unfortunately, they did not verify the phosphorylation residue by preparing a phosphospecific antibody. In addition, the Ser-692A mutation failed to completely abolish PIKE-A phosphorylation by Akt (Cai et al., 2009). Obviously, Ser-692 is not the major Akt phosphorylation site on PIKE-A. Recently we showed that PIKE-A acts as a proto-oncogene, promoting cell transformation through Akt activation (Liu et al., 2007). These findings are confirmed by a recent report showing that PIKE-A is overexpressed in human prostate cancers, and PIKE-A enhances proliferation, foci formation, and tumor progression in vivo (Cai et al., 2009).
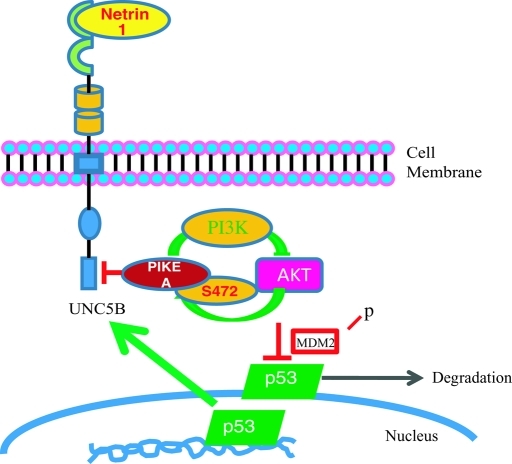
Netrin-1, an axon navigation cue, plays a crucial role during colorectal tumorigenesis by regulating apoptosis. The netrin-1 receptors, UNC5H, are initially proposed as mediators of the chemorepulsive effect of netrin-1 on specific axons. The netrin-1 receptors DCC and UNC5H belong to the family of dependence receptors that share the ability to induce apoptosis in the absence of their ligands. Both inactivation of UNC5C and overexpression of netrin-1 in the gastrointestinal tract are associated with intestinal tumor progression (Mazelin et al., 2004; Bernet et al., 2007), supporting that loss of netrin-1 dependence receptors might be a causal factor for tumor progression (Grady, 2007). It has been reported that the expression of human UNC5A, UNC5B, or UNC5C is down-regulated in multiple human cancers. The loss or reduction of expression may be a crucial mechanism for tumorigenicity because the expression of UNC5 receptors inhibits tumor cell anchorage-independent growth and invasion. Furthermore, these hallmarks of malignant transformation can be restored by netrin-1 addition or apoptosis inhibition. Hence UNC5 receptors have been proposed to act as tumor suppressors that inhibit tumor extension outside the region of netrin-1 availability by inducing apoptosis (Thiebault et al., 2003). Interestingly, PIKE-A only selectively binds to UNC5B but not to UNC5A or UNC5C (Supplemental Figure 3A). However, it also displays a srtonger binding affinity to UNC5B than PIKE-S and PIKE-L in the absence of netrin-1 (Supplemental Figure 3B). UNC5B contains a death domain in its intracellular region, and deletion of the death domain of UNC5B completely abrogates UNC5B-induced apoptosis, implying that the death domain is essential for this process (Llambi et al., 2001). It has been proposed that DAPK might mediate UNC5B-induced apoptosis by interacting with the death domain. The cleavage of UNC5B by caspases seems to be indispensable for UNC5B-induced apoptosis (Llambi et al., 2005). Most recently, we provided evidence that the PI3K–Akt pathway is involved in signaling in netrin-1–regulated antiapoptosis in neurons (Tang et al., 2008). In glioblastomas, netrin-1 might provoke Akt activation, which, in turn, phosphorylates PIKE-A and escalates its interaction with UNC5B, preventing its apoptotic cleavage and blocking its proapoptotic action. Moreover, Akt-phosphorylated PIKE-A further elevates Akt kinase activity and leads to p53 degradation through the Akt–MDM2 pathway, culminating in down-regulation of UNC5B transcription (Figure 7). Taken together, our data support that PIKE-A exerts its prosurvival functions by directly interacting with UNC5B, suppressing its proapoptotic action, and down-regulating p53 by enhancing Akt activation.
FIGURE 7:
A schematic diagram of a model of how PIKE-A suppresses UNC5B's proapoptotic action. In response to UNC5B ligand netrin-1 treatment, PI3K/Akt signaling is activated, which phosphorylates PIKE-A on Ser-472 residue. This phosphorylation enhances PIKE-A association with UNC5B, blocking UNC5B apoptotic fragmentation and inhibiting programmed cell death. Moreover, phosphorylated PIKE-A further enhances Akt kinase activity that triggers p53 degradation, leading to suppression of UNC5B transcription and apoptosis.
MATERIALS AND METHODS
Cells and reagents
HEK293, U87-MG, LN229, LN-Z308, TP366, and SF188 cells were maintained in DMEM including 10% FBS and 100 units penicillin-streptomycin. All cells were maintained at 37°C with 5% CO2 atmosphere in a humidified incubator. EGF and NGF were from Roche (Indianapolis, IN). Myc antibody was from Calbiochem (San Diego, CA). Wortmannin, LY294002, PD98059, and GST-HRP were from Sigma (St. Louis, MO). Anti–phospho-Akt Ser-473, anti-p53, and anti-His were from Cell Signaling (Danvers, MA). Si-TP53 and Akt (sc5298) antibody were from Santa Cruz (Santa Cruz, CA). Active Akt protein and Akt substrate crosstides were from Upstate Biotechnology (Waltham, MA). All the chemicals not included above were from Sigma.
Caspase-3 activity and cell death analysis
Caspase-3 activity was measured by means of the CaspACE assay system fluorometric kit or Caspase-Glo 3/7 Assay (Promega, Madison, WI). Cells were initially seeded at a density of 4.5 × 105 in six-well plates and infected with control, sh-PIKE-A, PIKE-A wild-type adenoviruses. After 48 h infection, cells were UV irradiated at 10 J/m2 using UV cross-linker, and caspase-3 activity was measured according to the manufacturer's instructions. HA-UNC5B and/or Myc–PIKE-A–transfected LN229 and LN-Z308 cell death were examined using trypan blue.
RT-PCR
Total RNA was isolated from various cell lines using the TRIzol reagent (Invitrogen Carlsbad, CA). Two micrograms of RNA was transcribed into cDNA by Superscript III (Invitrogen) in 20 μl, and 2 μl was subjected to RT-PCR. RT-PCR was examined using specific primers.
In vitro kinase assay
Purified GST fusion proteins or immunoprecipitated Myc tagged proteins were incubated with active Akt with or without crosstides in 20 μl of kinase reaction buffer (20 mM Tris, pH 7.5, with 10 mM MgCl2) containing 25 μM ATP and 2.5 μCi of γ-[32P]ATP for 20 min at 30°C. Reactions were terminated by adding 7 μl of Laemmli's sample buffer. A portion of the sample (15 μl) was separated on SDS-polyacrylamide gel and autoradiographed, analyzed by a PhosphorImage analyzer, or subjected to liquid scintillation assay.
Luciferase reporter assay
The information about the plasmids used in this study, including a p53-luc reporter plasmid, a pCH110 plasmid encoding β-galactosidase, pCMV-p53, and pCMV-p53mt135 expression vectors, the purification and transfection of these plasmids, and the luciferase activity assay, were described in detail previously (Liu et al., 2004). Luciferase and β-galactosidase assays were performed using the luciferase and β-galactosidase enzyme assay systems (Promega), respectively. Luciferase activity was normalized with β-galactosidase activity.
Coimmunoprecipitation and in vitro binding assay
A 10-cm plate of transfected HEK293 cells was washed once in phosphate-buffered saline, lysed in 1 ml of lysis buffer (50 mM Tris, pH 7.4, 40 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1.5 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate, 10 mM sodium β-glycerophosphate, protease inhibitor cocktail), and centrifuged for 10 min at 16,000 × g at 4°C. The supernatant was transferred to a fresh tube and mixed with a variety of antibody. After SDS-PAGE, the samples were transferred to a nitrocellulose membrane. Western blotting analysis was performed with a variety of antibodies.
Supplementary Material
Acknowledgments
This work is supported by a grant from NIH (RO1, CA127119; NS-045627) to K. Ye.
Abbreviations used:
- DAPK
death-associated protein kinase
- DCC
deleted in colorectal cancer
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- IgG
immunoglobulin G
- MEF
Mouse Embryonic Fibroblast
- NGF
nerve growth factor
- PARP
poly (ADP-ribose) polymerase
- PIKE
PI 3-kinase enhancer
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-11-0923) on April 1, 2011.
REFERENCES
- Ahn JY, Hu Y, Kroll TG, Allard P, Ye K. PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc Natl Acad Sci USA. 2004a;101:6993–6998. doi: 10.1073/pnas.0400921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JY, Rong R, Kroll TG, Van Meir EG, Snyder SH, Ye K. PIKE (phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J Biol Chem. 2004b;279:16441–16451. doi: 10.1074/jbc.M312175200. [DOI] [PubMed] [Google Scholar]
- Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4:978–987. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- Bernet A, Mazelin L, Coissieux MM, Gadot N, Ackerman SL, Scoazec JY, Mehlen P. Inactivation of the UNC5C netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology. 2007;133:1840–1848. doi: 10.1053/j.gastro.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CL, Li M, Gu W. Mechanistic studies of MDM2-mediated ubiquitination in p53 regulation. J Biol Chem. 2007;282:22804–22815. doi: 10.1074/jbc.M700961200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Wang J, Li R, Ayala G, Ittmann M, Liu M. GGAP2/PIKE-A directly activates both the Akt and nuclear factor-kappaB pathways and promotes prostate cancer progression. Cancer Res. 2009;69:819–827. doi: 10.1158/0008-5472.CAN-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CB, Ye K. PIKE GTPase are phosphoinositide-3-kinase enhancers, suppressing programmed cell death. J Cell Mol Med. 2007;11:39–53. doi: 10.1111/j.1582-4934.2007.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady WM. Making the case for DCC and UNC5C as tumor-suppressor genes in the colon. Gastroenterology. 2007;133:2045–2049. doi: 10.1053/j.gastro.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu Y, Hao C, Rempel SA, Ye K. PIKE-A is a proto-oncogene promoting cell growth, transformation and invasion. Oncogene. 2007;26:4918–4927. doi: 10.1038/sj.onc.1210290. [DOI] [PubMed] [Google Scholar]
- Liu X, Yue P, Khuri FR, Sun SY. p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res. 2004;64:5078–5083. doi: 10.1158/0008-5472.CAN-04-1195. [DOI] [PubMed] [Google Scholar]
- Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 2001;20:2715–2722. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Lourenco FC, Gozuacik D, Guix C, Pays L, Del Rio G, Kimchi A, Mehlen P. The dependence receptor UNC5H2 mediates apoptosis through DAP-kinase. EMBO J. 2005;24:1192–1201. doi: 10.1038/sj.emboj.7600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, Bredesen DE, Scoazec JY, Mehlen P. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- Muller M, Schleithoff ES, Stremmel W, Melino G, Krammer PH, Schilling T. One, two, three–p53, p63, p73 and chemosensitivity. Drug Resist Updat. 2006;9:288–306. doi: 10.1016/j.drup.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances MDM2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Roperch JP, El Ouadrani K, Hendrix A, Emami S, De Wever O, Melino G, Gespach C. Netrin-1 induces apoptosis in human cervical tumor cells via the TAp73alpha tumor suppressor. Cancer Res. 2008;68:8231–8239. doi: 10.1158/0008-5472.CAN-08-1483. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Tang X, et al. Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol. 2008;10:698–706. doi: 10.1038/ncb1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa C, Matsuda K, Fukuda S, Nakamura Y, Arakawa H. p53RDL1 regulates p53-dependent apoptosis. Nat Cell Biol. 2003;5:216–223. doi: 10.1038/ncb943. [DOI] [PubMed] [Google Scholar]
- Thiebault K, Mazelin L, Pays L, Llambi F, Joly MO, Scoazec JY, Saurin JC, Romeo G, Mehlen P. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci USA. 2003;100:4173–4178. doi: 10.1073/pnas.0738063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Woude GF. The ins and outs of p53. Nat Cell Biol. 2000;2:E178–180. doi: 10.1038/35036427. [DOI] [PubMed] [Google Scholar]
- Wee KB, Aguda BD. Akt versus p53 in a network of oncogenes and tumor suppressor genes regulating cell survival and death. Biophys J. 2006;91:857–865. doi: 10.1529/biophysj.105.077693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, Snyder SH. Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature. 2002;415:541–544. doi: 10.1038/415541a. [DOI] [PubMed] [Google Scholar]
- Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, Blackshaw S, Ferris CD, Snyder SH. PIKE. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell. 2000;103:919–930. doi: 10.1016/s0092-8674(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Yin Y, Stephen CW, Luciani MG, Fahraeus R. p53 stability and activity is regulated by MDM2-mediated induction of alternative p53 translation products. Nat Cell Biol. 2002;4:462–467. doi: 10.1038/ncb801. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.