Abstract
The 5-Hydroxytryptamine3 (5-HT3) receptor is a member of the cys-loop family of ligand gated ion channels, of which the nicotinic acetylcholine receptor is the prototype. All other 5-HT receptors identified to date are metabotropic receptors. The 5-HT3 receptor is present in the central and peripheral nervous systems, as well as a number of non-nervous tissues. As an ion channel that is permeable to the cations, Na+, K+, and Ca2+, the 5-HT3 receptor mediates fast depolarizing responses in pre- and post-synaptic neurons. As such, 5-HT3 receptor antagonists that are used clinically block afferent and efferent synaptic transmission. The most well established physiological roles of the 5-HT3 receptor are to coordinate emesis and regulate gastrointestinal motility. Currently marketed 5-HT3 receptor antagonists are indicated for treatment of chemotherapy, radiation, and anesthesia-induced nausea and vomiting, as well as irritable bowel syndrome. Other therapeutic uses that have been explored include pain and drug addiction. The 5-HT3 receptor is one of a number of receptors that play a role in mediating nausea and vomiting, and as such, 5-HT3 receptor antagonists demonstrate greatest anti-emetic efficacy when administered in combination with other drug classes.
Keywords: 5-Hydroxytryptamine3, 5-HT, serotonin, antagonist, nausea and vomiting, irritable bowel syndrome
1. Introduction to the 5-HT3 receptor, a ligand-gated ion channel
The 5-Hydroxytryptamine3 (5-HT3) receptor (Derkach et al., 1989; Maricq et al., 1991) is a member of the cys-loop family of ligand-gated ion channels, which include the excitatory nicotinic acetylcholine receptor and the Zn2+ activated cation-selective channel, as well as the inhibitory g-amino butyric acid A (GABAA) and glycine receptors (Barnes et al., 2009). The 5-HT receptor primarily conducts the monovalent cations, Na+ and K+, and the divalent cation, Ca2+. At the resting membrane potential of most neurons in which 5-HT3 receptors are localized, the electrochemical gradient favors Na+ and Ca2+ entry. Thus, 5-HT3 receptors depolarize neurons and mediate fast, excitatory synaptic transmission. The highest number of 5-HT3 receptor binding sites in the central nervous system occurs in the area postrema and solitary tract nucleus (Gehlert et al., 1991; Tecott, 1993; Morales and Wang, 2002). In addition, large numbers of 5-HT3 receptors are found in the peripheral nervous system and gut (Tyers, 1990; Gershon, 1999; Glatzle et al., 2002). The most well-documented actions of 5-HT3 receptors are to alter gastrointestinal motility and to regulate the vomiting reflex (Sanger and Andrews, 2006), but they also appear to play a role in visceral pain and inflammation (Costall and Naylor, 2004). In addition, the 5-HT3 receptor has been implicated in altering the voluntary intake of ethanol in humans (Johnson et al., 1993; Johnson et al., 2002; Johnson, 2004) and rodents (Knapp and Pohorecky, 1992; Kostowski et al., 1993; Hodge et al., 2004).
1.1 Structure and function of the 5-HT3 receptor
To date, five subunits (A – E) of the 5-HT3 receptor have been cloned (Mariq et al., 1991; Davies et al., 1999; Dubin et al., 1999; Karnovsky et al., 2003; Niesler et al., 2003), but only the A and B subunits have been studied extensively. In common with other members of the cys-loop superfamily, the 5-HT3 receptor is an integral membrane protein that is pentameric in structure, with a central ion channel pore. Hydropathy plots of the deduced amino acid sequence of these receptor subunits suggest a putative long extracellular N-terminus, four transmembrane spanning domains (TM1-TM4), and a short C-terminus (Mariq et al., 1991). TM2 from each of the five subunits form the ion channel pore. A large intracellular loop between TM3 and TM4 is present, but is not required for expression of the homomeric A receptor in the plasma membrane or for its function (Jansen et al., 2008), based upon its substitution with a heptapeptide derived from the short intracellular loop between TM3 and TM4 in the prokaryotic homolog of the ion channel superfamily (Bocquet et al., 2007)>. The large intracellular loop of rodent A subunits is a site for alternative splicing (Isenberg et al., 1993; Hope et al., 1993; Lankiewicz et al., 1998). The large intracellular loops of the 5-HT3 receptor subunits also contain multiple consensus sites for protein kinase dependent phosphorylation (Mariq et al., 1991; Davies et al., 1999; Dubin et al., 1999; Karnovsky et al., 2003; Niesler et al., 2003).
The N-terminal domain contains the ligand recognition site for 5-HT (Eiselé et al., 1993). The binding of 5-HT occurs at the interface of two subunits. With the homomeric A subunit, there are five possible binding sites, but the binding of two molecules of agonist are necessary and sufficient to open the channel. Within these subunits are amino acid domains, so called “loops”, which participate in binding. The binding interfaces in the receptor are inequivalent, and Loops A–C are located in the principal binding component or face, whereas Loops D–F are located in the complementary component. Binding of an agonist causes a conformational change in the receptor which results in channel opening and ion flux (reviewed in Barnes et al., 2009). Residues important in determining cation selectivity of the mouse 5-HT3A receptor have been identified in the TM1-TM2 linker, as well as in two positions in TM2 (Gunthorpe and Lummis, 2001); a TM2 residue contributes to Ca2+ permeability (Livesay et al., 2008). The homomeric 5-HT3A receptor differs from the heteromeric 5-HT3A/B receptors in three key ways, lower single channel conductance, greater Ca2+ permeability, and slower kinetics of activation, deactivation, and desensitization (Davies et al., 1999; Hayrapetyan et al., 2005). Differences in desensitization kinetics may be observed in the representative tracings of mouse 5-HT3A and 5-HT3A/B receptors (Fig. 1). Chimeras of A/B or B/A subunits were utilized to identify the domain responsible for differences in biophysical properties. Three arginine residues in a domain of the large intracellular loop were determined to be important for both single channel conductance and Ca2+ permeability (Kelley et al., 2003; Livesay et al., 2008). This domain, present in each of the five subunits, is adjacent to TM4, is α helical, and is described as a membrane associated stretch that is a cytoplasmic extension of the conduction pathway.
Fig. 1.
Representative traces of inward currents evoked by 25 mM 5-HT in mouse 5-HT3A and 5-HT3A/B receptors. Patch clamp electrophysiological recordings were performed in HEK 293 cells transfected with mouse 5-HT3A or 5-HT3A plus 5-HT3B subunit cDNA's. Addition of the B subunit markedly enhanced desensitization relative to that in the homomeric receptor.
1.2 Subunit heterogeneity in and distribution of the 5-HT3 receptor
1.2.1 A subunits of the 5-HT3 receptor
To date, the 5-HT3A receptor has been cloned from brain or other nervous system derived tissues and colonic tissues from mouse (Maricq et al., 1989), rat (Isenberg, et al., 1995), human (Belelli et al., 1995; Miyake et al., 1995), guinea pig (Lankiewicz et al., 1998), ferret (Mochizuki et al., 2000), and dog (Jensen et al., 2006). All of these 5-HT3A subunits form functional homomeric receptors in heterologous expression systems, such as Xenopus laevis oocytes and HEK 293 cells. Among them, amino acid identities vary from ~ 80 – 90%, with rat and mouse receptors having the greatest identity. Where studied, the receptors have similar ion permeabilities and current-voltage relationships. Although the receptor orthologs have similar agonist potencies (including 5-HT) and affinities for numerous competitive antagonists, there are some differences in ligand recognition. For example, the human 5-HT3A receptor has ~ 1800 fold lower affinity for the competitive antagonist, curare, than the mouse receptor (Hope et al., 1999). In contrast, the agonist 2-methyl 5-HT has a greater efficacy at the human than mouse receptor (Werner et al., 1994; Miyake et al., 1995). Finally, 3-(2-hydroxy, 4-methoxybenzylidene)-anabaseine is a strong partial agonist at the mouse receptor and an apparent competitive antagonist at the human receptor (Zhang et al., 2006).
Distribution of the 5-HT3A receptor subunit, as measured by mRNA levels and immuncytochemistry, correlates well with radio-ligand binding studies conducted with high affinity competitive antagonists of the 5-HT3 receptor (Kilpatrick et al., 1987; Barnes et al., 1989; Laporte et al., 1992; Tecott et al., 1993; Morales et al, 1996; Morales and Bloom, 1997; Rosenberg et al., 1997). Expression of 5-HT3A receptor mRNA has been measured in the rat forebrain, hippocampus, amygdale, spinal tract of the trigeminal nerve, facial nerve, and the spinal cord dorsal horn (Tecott et al., 1993; Morales and Bloom, 1997). Morales and co-workers developed a polyclonal antibody against the A subunit and observed intensely immunoreactive neurons in rat forebrain, brainstem, and spinal cord (Morales et al., 1998). More intense staining in the forebrain was observed in layers II and III of the neocortex, anterior olfactory complex, hippocampus, and amygdala. Many of the neurons in the telencephalon that express that A subunit are inhibitory GABA interneurons (Morales and Bloom, 1997). Some intensely stained neurons were also found in the striatum, and less robustly stained neurons were found in the nucleus accumbens (Morales et al., 1998). The trigeminal motor and facial nuclei in the brainstem were intensely labeled, and staining was also present in the dorsal and ventral horns of the spinal cord (Morales et al., 1998). Trigeminal ganglia, nodose ganglia, and superior cervical ganglia express the A subunit, with the highest concentration found in the nodose ganglia (Morales and Wang, 2002). Green fluorescent protein expression, controlled by the 5-HT3A receptor promoter or the glutamic acid decarboxylase 67 promoter, has been used to demonstrate 5-HT3 receptor expression in vasoactive intestinal peptide and neuropeptide Y containing interneurons in the somatosensory cortex (Vucurovic et al., 2010) and in GABAergic neurons in the spinal superficial dorsal horn (Fukushima et al., 2009), respectively. Immunolabeling of 5-HT3A subunits in the gastrointestinal tract was observed in myenteric and submucosal neurons, interstitial cells of Cajal, and endocrine cells (Glatzle et al., 2002). Expression of the A subunit in virtually all tissues that express high affinity 5-HT3 receptor binding sites, as well as the requirement of the A subunit for function of heterologously expressed heteromeric 5-HT3 receptors as described below, underscores the dominance of the A subunit in the 5-HT3 receptor.
1.2.2 B subunits of the 5-HT3 receptor
The human B subunit maps to human chromosome 11q23, the same location as the A subunit, and has ~ 41% amino acid identity with the A subunit (Davies et al., 1999; Dubin et al., 1999). There are three known splice variants of the B subunit, with differences in the translation initiation site; one of the variants lacks part of the N-terminus and is presumed to be non-functional (Tzvetkov et al., 2007). The B subunit does not assemble into receptors, but when co-expressed with the A subunit, forms functional heteromeric receptors (Davies et al., 1999; Dubin et al., 1999). In the absence of the A subunit, the B subunit is retained in the endoplasmic reticulum (Boyd et al, 2002, 2003). Rat and mouse (Hanna et al., 2000) B subunit cDNA's have also been cloned. Despite the fact that the 5-HT3A/B receptor differs in biophysical properties compared to the 5-HT3A receptor, as described above, pharmacological properties of drugs acting at the ligand binding domain demonstrate are very similar in homomeric and heteromeric receptors (Brady et al., 2001). Only drugs that are assumed to bind at sites other than the 5-HT recognition site, such as n-chain alcohols (Hayrapetyan et al., 2005; Stevens et al., 2005), volatile anesthetics (Stevens et al., 2005), and pictrotoxin (Das and Dillon, 2003), have differential potencies in the homomeric and heteromeric receptor complexes.
The 5-HT3B subunit mRNA is present in spleen, colon, small intestine, kidney, and brain (Davies et al., 1999; Dubin et al., 1999), but the distribution of the subunit protein in the CNS is in dispute. Morales and Wang (2002) described a virtual absence of the 5-HT3B receptor subunit in the rat CNS, except for the nerve terminals of the nodose ganglia that project to the dorsal vagal complex of the brainstem. In their studies, the 5-HT3B subunit is predominately expressed in peripheral neurons, but not all of them, raising the possibility of distinct populations of 5-HT3A and 5-HT3A/B receptors. In contrast, other investigators found 5-HT3B subunit staining in the hippocampus (Monk et al., 2001; Reeves and Lummis, 2006). Relatively large single channel conductances have been recorded in hippocampal 5-HT3 receptors (Jones and Suprenant, 1994), which are similar to that obtained with recombinant 5-HT3A/B receptors. It remains to be determined whether the B subunit or perhaps a C or E subunit combines with the A subunit to produce larger single channel conductances in native hippocampal receptors than that observed in heterologously expressed A homomers.
1.2.3 C, D, and E subunits of the 5-HT3 receptor
The C, D, and E subunits of the receptor were cloned most recently (Karnovsky et al., 2003; Niesler et al., 2003; Holbrook et al., 2009). These three genes map to an ~100 kb region on human chromosome 3q27 and may represent gene duplication events that happened after the divergence of the A and B subunits (Niesler et al., 2003). The C, D, and E subunits are expressed in numerous mammalian species (Holbrook et al., 2009), but are absent in mice and rats (Niesler et al., 2003; Karnovsky et al., 2003; Holbrook et al., 2009). Multiple sub-types within each subunit have been identified, including several presumed pseudogenes in C and E subunits that lack the signature N-terminal cys-loop present in all subunits of the superfamily and/or that lack signal peptides or have stop codons in the open reading frames (Karnovsky et al., 2003; Niesler et al., 2003; Holbrook et al., 2009). The expression and functionality of the C, D, and E subunits have been studied, and the expression of C, D, and E subunits as homomers is equivocal, with Holbrook et al., 2009 reporting that expression, but no function was observed in CHO cells. In contrast, Niesler et al., 2007 reported no expression of the homomers in HEK 293 cells. Both groups reported that upon co-expression of the A subunit with the C, D, or E subunits, the tagged subunits co-localized with the tagged A subunit, as measured by immunocytochemistry or immunoprecipitation. Any of the subunits co-expressed with the A subunit produced functional receptors, but there was no apparent difference in radio-ligand binding, current voltage-relationships, and kinetics of whole-cell currents of the A homomer versus the presumed heteromers. Therefore, it is unclear whether the C, D, and E receptors do form heteromeric receptors with the A subunit. Initial studies suggested that the D and E subunits had a very restricted localization to the colon or liver, but that the C subunit was present in brain, colon, intestine, lung, and kidney (Niesler et al., 2003). Holbrook et al, 2009 have performed extensive RT-PCR studies on the C, D, and E subunit variants, and have discovered a widespread distribution of the E subunit, in particular. Highest levels of C subunit mRNA was measured in lung, colon, and dorsal root ganglia and highest levels of D subunit transcripts were measured in the dorsal root ganglia, respectively.
1.3 Ligand-binding and initiation of channel gating in the 5-HT3 Receptor
1.3.1 Gating in the 5-HT3 receptor
Binding of an agonist causes a conformational change in the receptor which results in channel opening. In recent years, several advances have significantly impacted the cys-loop receptor field. The crystal structure of the molluscan acetylcholine binding protein (AChBP) (Brejc et al., 2001), a structural and functional homolog of the N-terminal domain of the nACh receptor, demonstrated that the ligand binding site is composed of loops from the principal face of one subunit and a series of β strands from the complementary face of an adjacent subunit. More recently, the extracellular domain of the α1 subunit of the nicotinic receptor was crystallized (Dellisanti et al., 2007). The crystal structures have provided the generalized three-dimensional configuration of the ligand binding domain, which has enabled investigators to model the N-terminal domains of all members of the cys-loop family. A second major advance occurred with electron microscopy studies of Torpedo nACh receptors at 4 angstrom resolution of the 1) TM domains (Miyazawa et al., 2003) and 2) the N-terminal domains, TM domains, and parts of the cytoplasmic loops (Unwin, 2005). Taken together, these findings have enabled investigators to begin mapping the ligand recognition site in the cys-loop family of receptors and to identify the intra-molecular movements in the receptors that couple ligand binding to channel gating.
1.3.2 Ligand binding in the 5-HT3 receptor
Agonist binding to the ligand binding domain is thought to initiate movement by bringing Loop C of the ligand binding domain from an uncapped and disengaged state to the capped and engaged position (Celie et al., 2004; Gao et al., 2005; Law et al., 2005; Unwin, 2005). Experimental evidence from several groups suggests that Loop 2, Loop 7 (cys-loop), Loop 9 (Loop F from the opposing face of the ligand binding domain), and Loop 10 interact with the TM2 – TM3 linker region and play key roles in transmitting agonist binding to channel opening (Bouzat et al., 2004; Chakrapani et al., 2004; Kash et al., 2003; Law et al., 2005; Reeves et al., 2005). This is accomplished through electrostatic and/or hydrophobic interactions, such that movement of the TM2–TM3 linker in turn moves TM2, the channel lining element, which results in channel opening. Lummis et al. (2005) have provided evidence that a proline in the TM2–TM3 linker of the 5-HT3A receptor undergoes cis - trans isomerization in the gating process. They propose that Trp183 in Loop B moves upon agonist binding and, in turn, moves the nearby Cys loop; movement of the Cys loop may provide the molecular switch to initiate isomerization and subsequent gating in members of the cys-loop family that contain proline at this key position. Lee and Sine (2005) have delineated another step in the pathway linking the binding of ligand to channel opening in Torpedo nACh receptors. They have identified an arginine in Loop 10, the domain between the ligand binding domain and TM1 that forms an electrostatic interaction with a glutamate in Loop 2; these residues are energetically coupled to another residue in Loop 2 and two residues in the TM2–TM3 linker previously identified as being critical in the gating process. A parallel pathway links the pre-TM1 domain and the TM2–TM3 linker via the cys-loop (Lee et al., 2009)
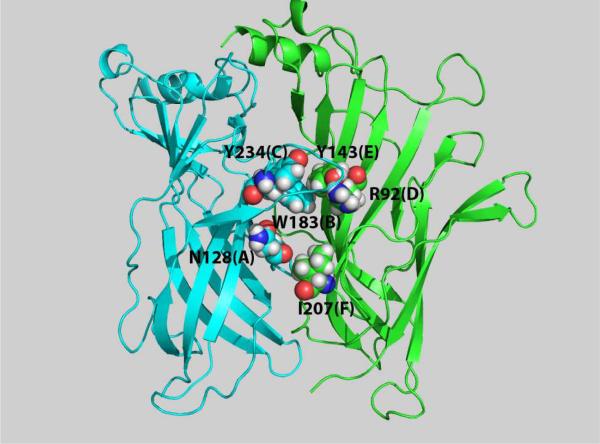
A number of ligand binding and electrophysiological approaches have been used to assess the roles of Loops A–F of the 5-HT3 receptor in ligand recognition. Homology models, using the AChBP and/or the N-terminal of the α1 subunit of the nicotinic receptor, have been constructed to orient ligands with their presumed contact points with amino acids in the agonist binding domain (Thompson et al., 2006; Zhang et al., 2007; Joshi et al., 2008; Nyce et al., 2010) and presented in Fig. 2. In this context, the three dimensional space of the ligand binding domain is being defined. Numerous tyrosine and tryptophan residues in Loops A–E have a major impact on binding and/or gating, and some differentially interact with agonists (m-chlorophenylbiguanide (m-CPBG)) and antagonists (granisetron and GR65630) (Yan et al., 1999; Spier and Lummis, 2000; Venkataraman et al., 2002; Price and Beene et al., 2004; Lummis, 2004; Yan and White, 2005). For example,W183 in Loop B forms a critical cation-pi interaction with the primary amine of 5-HT (Beene et al., 2002). Mutation of a series of residues in Loop A alters affinity of antagonists and/or agonists (Boess et al., 1997; Steward et al., 2000), and mutant-thermodynamic cycle analysis strongly suggests that 2' N of curare interacts directly with N128 (Yan et al., 2006). Substitution of E129 with unnatural amino acids strongly points to a hydrogen bond forming between this amino acid and the –OH group of 5-HT (Price et al., 2008). Mutagenesis of six Loop C residues has resulted in reduced affinity or elimination of [3H]-granisetron binding (Suryanarayanan et al., 2005; Thompson et al., 2005), reduced affinity of [3H]mCPBG and [3H]GR65630 (Schreiter et al., 2003) and altered relative efficacies of the partial agonist 2-Methyl 5-HT and/or 5-HT (Suryanarayanan et al., 2005). Loop C is highly variable between species and thus using interspecies chimeras, four groups identified Loop C as contributing to differential drug action at the in human, mouse, rat, and guinea pig 5-HT3A receptors (Lankiewicz et al., 1998; Hope et al., 1999; Mochizuki et al., 1999; Zhang et al., 2007). In Loop D, W90 strongly influences ligand binding (Spier and Lummis, 2000), whereas R92 likely interacts directly with the indazole ring of granisetron (Yan and White, 2005). In Loop E, mutation of G143 and Y143 to alanine completely eliminated granisetron binding (Venkataraman et al., 2002). Finally, the crystal structure of Loop F was poorly resolved in the AChBP, and thus, its structure in the ligand binding domain is uncertain. This loop has been studied the least. Thompson et al. (2006) investigated 21 mutations inclusive of and near Loop F and found that two regions displayed increases in Kd values for [3H]-granisetron. Two residues in Loop F of the human receptor, K195 and V202, account for the contribution of Loop F to the differential curare potency of human and mouse receptors (Zhang et al., 2007).
Fig. 2.
Molecular model of the mouse 5-HT3A receptor N-terminal domain in the unliganded/antagonist bound conformation. The model was created using MODELLER (www.saliab.org) with antagonist-bound forms of the AChBP as the templates (Nyce et al., 2010). Residues that are important in ligand recognition in each of the six loops of the ligand binding domain of the receptor are indicated: Loop A: N128 (Yan et al., 2006), Loop B: W183 (Thompson et al., 2005), Loop C: Y234 (Thompson et al., 2005), Loop D: R92 (Yan and White, 2005), Loop E: Y143 (Beene et al., 2004), and Loop F: I207 (Zhang et al., 2007).
2. Therapeutic uses of 5-HT3 receptor antagonists
2.1 Available 5-HT3 receptor antagonists for gastrointestinal disorders
The 5-HT3 receptor antagonists currently available for therapeutic used in the U.S. are ondansetron (Zofran), granisetron (Kytril), dolasetron (Anzemet), palonosetron (Aloxi), and alosetron (Lotronex), Table 1. Tropisetron and ramosetron are marketed outside the U.S. Ondansetron, granisetron, dolanestron, and palonsetron are used for chemotherapy-induced and post-operative nausea and vomiting (PONV), whereas alosetron is available for irritable bowel syndrome (Aapro, 2005; Fayyaz and Lackner, 2008; Walstab et al., 2010). Ondansestron, granisetron, dolasetron, and alosetron are competitive antagonists of the 5-HT3 receptor, with structural similarity to 5-HT, and with carbazole, indazole, indole, and imidazole main-chains, respectively. In contrast, palonosetron is a non-competitive antagonist of the 5-HT3 receptor, with a fused tricyclic ring and a quinuclidine side chain (Rojas et al., 2008).
Table 1.
5-HT3 Receptor Antagonists Available in the United States
| Name | Chemical Class | Mechanism of Action | Therapeutic Use | Side Effects |
|---|---|---|---|---|
| Ondansetron (Zofran™) | Carbazole | Competitive Antagonist | CTNV & PONV* | headache, dizziness, & constipation |
| Granisetron (Kytril™) | Indazole | Competitive Antagonist | CTNV & PONV | headache, dizziness, & constipation |
| Dolasetron (Anzemet™) | Indole | Competitive Antagonist | CTNV & PONV | headache, dizziness, & constipation |
| Alosetron (Lotronex™) | Imidazole | Competitive Antagonist | IBS**, Diarrhea Predominant-restricted use by the FDA | headache, dizziness, & constipation risk of severe constipation, ischemic colitis |
| Palonosetron (Aloxi™) | Isoquinoline | Non-competitive Antagonist | CTNV & PONV | headache, dizziness, & constipation |
CTNV & PONV: Chemotherapy-Induced Nausea and Vomiting and Post-Operative Nausea and Vomiting,
Irritable Bowel Syndrome
2.2 Treatment of nausea and vomiting with 5-HT3 receptor antagonists
2.2.1 Physiology of nausea and vomiting
Vomiting is coordinated by neurons in the medulla oblongata. This complex process requires stepwise gastric fundus relaxation, intense contraction of abdominal, intercostal, and diaphragmatic muscles, closing of the glottis, and relaxation of the upper esophageal sphincter prior to the expulsion of gastric contents. These physiological responses are the result of the activation of numerous efferent neurons whose nuclei are spread throughout the medulla oblongata. This region of the brainstem that controls the motor outflow is called the vomiting center or more recently, the central pattern generator (Hornby, 2001). Nausea and vomiting may be initiated by a number of factors. Noxious chemicals or toxins may act directly on the gastric mucosa to stimulate abdominal vagal afferents, which project to the dorsal vagal complex that contains the nucleus of the solitary tract, the dorsal motor nucleus of the vagal nerve, and the area postrema. Circulating noxious chemicals act directly at the chemoreceptor trigger zone (CTZ) of the area postrema. The CTZ is located on the floor of the fourth ventricle, an area of the CNS not bound by the blood-brain-barrier. Motion sickness and other vestibular disturbances stimulate nausea and vomiting. Changes in intracranial pressure, trauma to the brain, or lesions in or near the vomiting center can evoke vomiting, as can extreme emotion, bad odors, or hormonal changes associated with pregnancy. Thus, inputs from the meninges and higher brain centers, e.g., the cortex, thalamus, and hypothalamus, are thought to innervate the vomiting center. Finally, gastrointestinal dysfunction, such as gastroparesis, can produce nausea and vomiting (Hornby, 2001; Costall and Naylor, 2004; Sanger and Andrews, 2006).
2.2.2 Discovery of antiemetic properties of 5-HT3 receptor antagonists
The development of 5-HT3 receptor antagonists and the recognition of the 5-HT3 receptor's role in nausea and vomiting preceded the receptor's identification as a ligand-gated ion channel. In the 1950's, two laboratories identified the 5-HT3 receptor through its contraction of guinea pig ileum. It was initially given the name “5-Hydroxytryptamine M” receptor because of morphine's ability to block the contractile response (Rocha et al., 1953; Gaddum, 1953; Costall and Naylor, 2004). Twenty-five years later, Fozard and co-workers (Fozard and Mobarok, 1978) identified a 5-HT-M receptor in isolated rabbit heart and began investigating it pharmacologically. Their observation that the dopamine receptor antagonist, metoclopramide, blocked the action of 5-HT at the M receptor led to the development of early M-selective agents, such as MDL 72222. The observation by Florczyk et al., 1982 that high dose metoclopramide relieved cisplatin-induced vomiting in ferrets led two groups of investigators to demonstrate that MDL 72222 is very effective in reducing cisplatin-induced vomiting in ferrets (Costall et al., 1986; Miner and Sanger, 1986).
2.2.3 Possible mechanisms of 5-HT3 receptors in mediating nausea and vomiting
The mechanism(s) through which 5-HT3 receptor activation initiates nausea and vomiting may involve both a peripheral and central component. In the gut, enterochromaffin cells release 5-HT in response to gastric irritation or cellular damage. Released 5-HT binds to 5-HT3 receptors on vagal afferents in the gastric mucosa, which project to the dorsal vagal complex of the vomiting center (Andrews et al., 1990; Hillsley and Grundy, 1998). 5-HT3 receptors are on the nerve terminals of the projecting vagal afferents to the dorsal vagal complex. Post-synaptic 5-HT3 receptors may also be present in the dorsal vagal complex (Glaum et al, 1992). It is important to point out that other neurotransmitter systems are important in initiating and coordinating nausea and vomiting. Besides the 5-HT3 receptor, three other major classes of neurotransmitters receptors have been identified as targets for antiemetic drugs: the dopamine D2 receptor, muscarinic M1 cholinergic receptor, and histamine H1 receptor (Scuderi, 2003). Antiemetic drugs have also been developed that antagonize the cannabinoid receptor and neurokinin1 (NK1) receptor, which is activated by Substance P. That so many drugs are marketed highlights the fact that no single drug class can successfully block nausea and vomiting produced by any possible cause. Accordingly, 5-HT3 receptor antagonists are limited to the treatment of nausea and vomiting associated with chemotherapy or radiation treatment and surgery, since they have little or no efficacy in treating other causes of emesis (Scuderi, 2003, Sanger and Andrews, 2006).
2.2.4 Efficacy of 5-HT3 receptor antagonists in chemotherapy and radiation induced nausea and vomiting
The introduction of 5-HT3 receptor antagonists into clinical use revolutionized the treatment of nausea and vomiting in cancer patients receiving chemo- or radiation therapy (Aapro, 1991). At the time of ondansestron's release, dopamine D2 receptor antagonists, anti-histamines, and anti-muscarinics were the primary antiemetic pharmacotherapies. None of these three classes was as efficacious in decreasing emesis associated with cancer treatment as ondansetron proved to be. The relative risk for developing nausea and vomiting with chemotherapy ranges from 30 – 90% and is dependent upon the chemotherapeutic agent used. Relative risk for nausea and vomiting with radiation therapy is ~ 40% (Kovac, 2003). All currently marketed 5-HT3 receptor antagonists have good efficacy in reducing acute emesis associated with chemo- and radiation therapy, and in fact are generally well tolerated with limited side effects. Most common side effects are headache, dizziness, constipation, diarrhea, fever, malaise, and transient increases in serum transaminases (Hesketh, 2000). Cardiovascular effects, primarily changes in ECG recordings, have been reported and are dose related; these are considered to be clinically insignificant, as they are small in magnitude and are transient (Jantunen et al., 1996; Keefe, 2002). However, 5-HT3 receptor antagonists must be used judiciously in patients who have long QT syndrome.
Early on, it was observed that ondansetron's therapeutic effect was limited to the acute phase of nausea and vomiting and had little effect on the delayed phase occurring > 24 hours after treatment (Scuderi, 2003; Sanger and Andrews, 2006). The combination of a 5-HT3 receptor antagonist and a corticosteroid, such as dexamethasone or methylprednisolone, increases the antiemetic efficacy of both drugs in the early and delayed phases. The drug regimen includes intravenous therapy prior to and during the cancer treatment, followed by oral administration of dexamethasone for two to four days. The mechanism by which corticosteroids exert their antiemetic effect is unknown. The NK1 receptor antagonist, aprepitant, is effective in the delayed phase of nausea and vomiting and is currently used in combination with a 5-HT3 receptor antagonist and a corticosteroid for treatment of delayed nausea and vomiting for highly emetogenic chemotherapy (Kris et al., 2006).
Despite the efficacy of 5-HT3 receptor antagonists of drugs as a whole, some patients respond to this class of drugs better than others. In addition, some patients are resistant to the antiemetic effects of ondansetron, but are successfully treated with granisetron (Kovac, 2003; de Wit et al., 2005). With regard to patients who do not respond well to the class of drugs, two hypotheses have been put forward. First, ondansetron is primarily metabolized by CYP2D6, and patients with allelic variants of the enzyme that cause ultra-rapid metabolism may have reduced efficacy (Kaiser et al., 2002). Second, there are allelic variants of 5-HT3 receptor subunits that have been identified that may play a role (Niesler, 2008). The 5-HT3B receptor has several polymorphisms, one of which is a deletion in the promoter that has been associated with reduced efficacy of ondansetron and tropisetron in moderate to highly emetogenic chemotherapy (Tremblay et al., 2003). Polymorphisms in the 5-HT3C subunit may have predictive value in determining the relative risk for nausea and vomiting in moderately emotogenic chemotherapy (Fasching et al., 2008). The intriguing finding that some patients do not respond to ondansetron, but are treated successfully with granisetron may, in part, depend on the properties of the drug and genetic variability in the patient population (de Wit et al., 2005). Ondansetron has a shorter duration of action and a lower affinity and selectivity in comparison with granisetron. Thus, patients who are rapid metabolizers, or who have the short serotonin transporter polymorphism resulting in reduced activity and expression (Lesch et al., 1996) and increased lifetime of 5-HT in the gut, might be predicted to have a poorer outcome with ondansetron.
2.2.5 Treatment of post-operative nausea and vomiting with 5-HT3 receptor antagonists
Post-operative nausea and vomiting have been reported to range from 12 – 40% and even higher in patients who are at greater risk. Patients at greater risk are those who have a previous history of PONV or motion sickness, who are female, and who are non-smokers. Risk is also associated with specific anesthetics drugs, such as nitrous oxide and volatile anesthetics, as well as opiates. Type and length of surgery also play a factor (Gan, 2007). Although the mechanism(s) through which PONV occurs has not been elucidated, it may be due to anesthetic-induced release of 5-HT in the gut and activation of 5-HT3 receptors locally; a direct action on the vomiting center may also occur. Interestingly, volatile anesthetics stimulate 5-HT3 receptor mediated currents (Machu and Harris, 1994), but propofol has been reported to have no effect on (Machu and Harris, 1994) or to inhibit (Rusch et al., 2007) 5-HT3 receptor function. Propofol is an intravenous general anesthetic that produces far less nausea and vomiting than volatile anesthetics. Similar to chemotherapy induced nausea and vomiting, 5-HT3 receptor antagonists appear to most effective in the prophylaxis of nausea and vomiting and in the treatment of the early phase of PONV. Accordingly, the efficacy of 5-HT3 receptor antagonists in the early and late phases of PONV is enhanced when given in combination with dexamethasone or in a triple combination, with the addition of aprepitant (Scuderi, 2003; Yang and Scott, 2009).
2.3 Treatment of irritable bowel syndrome with 5-HT3 receptor antagonists and 5-HT4 receptor partial agonists
2.3.1 Irritable bowel syndrome: features of disease and prevalence
Irritable bowel syndrome (IBS) is a disorder that is characterized by periods of intense abdominal symptoms, followed by periods in which there are no symptoms. Severe intestinal cramping or pain, in the absence of any organic disease, is the hallmark of IBS. It may be accompanied by diarrhea, constipation, or diarrhea that alternates with constipation. The Rome guidelines, developed and refined over a period of years (Drossman, 1999; Drossman and Dumitrascu, 2006), define criteria by which IBS is diagnosed. In a period of 12 months, the patient must experience a minimum of 12 weeks of abdominal pain or discomfort, with two out of three of the following features. Abdominal distress is 1) relieved by defecation, 2) is associated with a change in stool frequency, or 3) is associated with change in appearance of stool. The sub-type of IBS, constipation or diarrhea predominant, mixed, alternating, or unspecified, is based upon stool consistency. IBS is one of the most common disorders that gastroenterologists treat, with an incidence of 10 – 20 % worldwide (Fayyaz and Lackner, 2008). It has ~ a two-fold greater incidence in females than in males (Sandler, 1990). The frequency and severity of IBS symptoms and even its onset may be influenced by the patient's medical history, such as emotional stress or psychiatric disorder. However, the presence of a psychosocial disorder is not a requirement for diagnosis of IBS (Drossman and Dumitrascu, 2006).
2.3.2 Irritable bowel syndrome: possible mechanisms and involvement of the 5-HT3 and 5-HT4 receptors
Gastrointestinal function is controlled by intrinsic and extrinsic factors, including endocrine and paracrine mediators, as well as the sympathetic, parasympathetic, and enteric nervous systems. Nerve plexuses in the enteric nervous system form reflex arcs that coordinate activity in the GI tract. The enteric nervous system can function independently of the sympathetic and parasympathetic nervous systems, but is normally modulated by their input (Furness et al., 1998; Goyal, 2000). IBS has features of both altered motility and enhanced visceral sensitivity (Drossman, 1999). Given the large number of hormones and neurotransmitters involved in GI motility and in transmitting sensory information in the gut, including pain, it is not surprising that IBS has been treated with so many different classes of drugs, including anti-cholinergics, tricyclic antidepressants, anti-diarrheal drugs or laxatives, fiber, pro-biotics, peppermint oil, non-absorbable antibiotics, selective C-2 chloride channel activators, 5-HT selective re-uptake inhibitors, 5-HT4 receptor agonists, and 5-HT3 receptor antagonists (Mertz, 2003; Brandt et al., 2009).
The mechanisms underlying irritable bowel syndrome are poorly understood. They are likely multi-factorial and may differ depending upon the sub-type of IBS that the patient displays (Brandt et al, 2009). The therapeutic efficacy that some anti-cholinergics (anti-spasmodic agents) demonstrate in a sub-set of patients lent early support to the idea that autonomic dysregulation may contribute to the syndrome. Tricyclic antidepressants have efficacy at doses that are anti-muscarinic, but that are not sufficient to produce anti-depressant activity. Tricyclic antidepressants also have analgesic and neuromodulatory effects. In general, most studies suggest that there is an increase in sympathetic versus parasympathetic activity in IBS patients. In these studies, cardiovascular function, e.g., heart rate variability, blood pressure, and fingertip blood flow, were the primary measures (Manabe et al., 2009). Aggarwal et al. (1994) compared the autonomic function in the IBS sub-groups and found vagal dysfunction and increased sympathetic tone in constipation predominant and diarrhea predominant IBS, respectively.
Serotonin has multiple roles in regulation of GI function (Fayyaz and Lackner, 2008; Sikander et al., 2009). It is released by enterchromaffin cells and binds to multiple 5-HT receptor subtypes to directly stimulate excitatory (acetylcholine) or inhibitory (nitric oxide) motor neurons that regulate smooth muscle tone. Serotonin also binds to 5-HT1p receptors on intrinsic primary afferent neurons (IPANs), which are the primary sensors and regulators of the enteric nervous system. IPANs secrete acetylcholine and calcitonin gene related peptide, which stimulate interneurons that control motor or sensory output. 5-HT3 receptors play a relatively minor role in enhancing GI motility via activation of IPANs and other neurons. A greater role of 5-HT3 receptors is to transmit sensory information to the central nervous system, including pain via spinal afferents and non-painful sensory information, such as the sensation of bloating, via parasympathetic afferents (Gershon, 2004). Thus, the 5-HT3 receptor antagonist, alosetron, has been marketed to reduce the visceral sensation associated with IBS that is diarrhea predominant. Indeed, antagonism of 5-HT3 receptors on sensory nerve endings or on pre-synpatic nerve terminals of Substance P containing neurons may play a role in reducing pain associated with fibromyalgia and other chronic pain disorders (Faerber et al., 2007). The 5-HT4 receptor is present on distal terminals of IPANs and its stimulation by 5-HT or a partial agonist, such as tegasarod, augments 5-HT1p mediated release of acetylcholine and calcitonin gene related peptide (Gershon, 2004). As such, tegasarod has been marketed for IBS that is constipation predominant.
Several meta-analyses have supported the efficacy of alostetron, tegaserod, and the investigational 5-HT3 receptor antagonist, cilansetron, in the treatment of IBS (Jones et al., 2002; Lesbros-Pantoflickova et al., 2004; Evans et al., 2007; Rahimi et al., 2008; Ford et al., 2009). However, alosetron produced severe constipation and ischemic colitis in two subsets of patients, respectively. It was removed from the market and later re-introduced with tightly controlled regulation by the FDA. Tegasarod produced cerbrovascular or cardiovascular ischemia in a small subset of patients and was also removed from the market. It is now only available for emergency use as an investigational drug (Ford et al., 2009).
3. Investigational uses of 5-HT3 receptor antagonists
3.1 5-HT3 receptor antagonists and ethanol and other drugs of abuse
The function of the 5-HT3A (Lovinger and White, 1991; Machu and Harris, 1994; Jenkins et al., 1996), but not the 5-HT3A/B (Hayrapetyan et al., 2005) receptor, is enhanced by pharmacologically relevant concentrations of ethanol. In common with positive allosteric modulation by other drugs, ethanol only enhances 5-HT3 receptor mediated currents at low gating concentrations of agonist. Ethanol does not alter the affinity of the 5-HT3 receptor for either partial agonists (Lovinger et al., 2000) or competitive antagonists (Hellevuo et al., 1991). The use of single channel measurements to determine the mechanisms of ethanol's effects on the 5-HT3A receptor is not feasible, given the low conductance (~ 0.5 pS) of the homomer (Kelley et al., 2003). Likewise, the larger conducting 5-HT3A/B receptor is relatively insensitive to ethanol (Hayrapetyan et al., 2005). Therefore, measurements of kinetics of whole cell currents with rapid application methods have been performed to identify mechanisms through which alcohols enhance current (Zhou et al., 1998). Ethanol likely enhances 5-HT3 receptor mediated currents by stabilization of the open channel state through at least three ways. Ethanol and other alcohols increase the activation rate, i.e., increase the rate of opening the receptors. Ethanol and other alcohols reduce the intrinsic desensitization rate of the receptor. Finally, deactivation rate, or unbinding of 5-HT is slowed.
Both neurochemical and behavioral data support the idea that 5-HT3 receptors play a role in alcohol intake. Dopaminergic neurons in the ventral tegmental area that project to the nucleus accumbens, amygdala, and the pre-frontal cortex are part of the “reward pathway”, i.e., the areas of the brain that are stimulated when drugs of abuse, such as alcohol, are ingested or taken through other routes of administration (Dworkin and Smith, 1987). 5-HT3 receptors are located on nerve terminals of the dopaminergic neurons in the nucleus accumbens and amygdala (Morales et al., 1998). Ethanol enhances dopamine release, and 5-HT3 receptor antagonists have been demonstrated to reduce the evoked release, and either inhibit or have no effect on basal release (Carboni et al., 1989; Wozniak, et al., 1990, Campbell et al., 1996). Likewise, in the absence of alcohol, 5-HT stimulated dopamine release was blocked by selective 5-HT3 receptor antagonists (Campbell et al, 1996). Thus, ethanol-induced activation of the 5-HT3 receptor may, in part, provide a cellular basis for enhanced dopamine release by ethanol.
Studies in both rodents and humans have demonstrated the effectiveness of 5-HT3 receptor antagonists in reducing alcohol intake. However, there are inconsistencies in rodent studies regarding 5-HT3 receptor antagonist efficacy and access to alcohol. Some studies demonstrated that 5-HT3 receptor antagonists were only effective in reducing alcohol intake under limited access conditions (Dyr and Kostowski, 1995; Tompkins et al., 1995), whereas another study showed that these drugs reduced intake under 24 hour alcohol access (Knapp and Pohorecky, 1992). The differences in experimental findings may be explained by strain or line of rat or the antagonist itself, some of which are not that selective for the 5-HT3 receptor (Engleman et al., 2008). In a 5-HT3A receptor knock-out study, the presence of the 5-HT3A receptor was required for the selective 5-HT3A receptor antagonist, LY-278-584, to reduce alcohol consumption, underscoring the importance of this receptor in alcohol consumption (Hodge et al., 2004). Transgenic mice overexpressing the 5-HT3A receptor drank less alcohol than wild-type mice (Engel et al., 1998), but these mice had increased sensitivity to the low dose effects of alcohol (Engel and Allan, 1999). Decreased sensitivity to ethanol in humans has been shown to correlate with a higher alcohol consumption and greater risk for alcoholism (Schuckit, 1994). Thus, increased sensitivity to alcohol may result in reduced consumption. In humans, ondansetron reduces alcohol intake and the pleasurable effects of alcohol (Johnson et al., 1993; Swift et al., 1996). Johnson and co-workers' studies suggest that ondansetron has its greatest efficacy in early onset alcoholics (Johnson, 2000; Johnson et al., 2002), that is, those that are at greatest risk of severe abuse of alcohol, because of genetic vulnerability and possible serotonergic hypo-function accompanied by an up-regulation of 5-HT3 receptors.
There is less than compelling evidence that 5-HT3 receptor antagonists alter the use of other drugs of abuse. In rats, 5-HT3 receptor antagonists had no effect on heroin (Higgins et al., 1994), nicotine (Corrigall and Coen, 1994), or cocaine (Walsh and Cunningham, 1997; Engleman et al., 2008) self-administration. In contrast, morphine self-administration was reduced by ondansetron or tropisetron (Hui et al., 1993). In humans, ondansetron did not alter methamphetamine use, withdrawal, or craving (Johnson et al., 2008).
4. Conculsions and future directions for 5-HT3 receptor antagonists
The most beneficial therapeutic use of 5-HT3 receptor antagonists to date is for nausea and vomiting. Ondansetron revolutionized the treatment of radiation and chemotherapy-induced nausea and vomiting. The use of alosetron in diarrhea predominant irritable bowel syndrome has been hampered by the life-threatening side effect of ischemic colitis in a very small number of patients. It is likely that treatment for IBS could be improved by development of 5-HT3 receptor antagonists with less propensity for producing severe constipation and colitis. Further classification of IBS subtypes and development of biomarkers for the subtypes may also play a future role in predicting patients that could most likely benefit from 5-HT3 receptor antagonist therapy. The presence of 5-HT3 receptors on sensory nerve endings and on the nerve terminals of neurons that release pain mediators, such as Substance P, has generated interest in the use of 5-HT3 receptor antagonists in diseases that cause chronic pain, such as fibromyalgia and peripheral neuropathies. The use of 5-HT3 receptor antagonists in the treatment of alcoholism has been explored, but they are likely to benefit, at best, only a subpopulation of patients with the disease. The disease states or conditions in which 5-HT3 receptor antagonists have been shown to be beneficial are complex and are likely mediated through multiple pathways. Therefore, therapies employing 5-HT3 receptor antagonists in combination with agents from other drug classes may be a common theme in therapeutics, as is already the case in the treatment of nausea and vomiting. Pharmacogenomics, whether through the classification of a patient's polymorphisms of 5-HT3 receptor subunits or his polymorphisms of cytochrome p450 enzymes responsible for metabolism of 5-HT3 receptor anatogonists, are expected to help predict the patient population that will benefit most from treatment with this class of drugs.
Acknowledgements
I thank Dr. Michael M. White at the Dept. of Biochemistry, Drexel University College of Medicine, Philadelphia, PA, for generating the molecular model of the mouse 5-HT3A receptor presented in Fig. 2.
Abbreviations
- ACh
acetylcholine
- 5-HT
5-Hydroxytryptamine
- GABA
γ-amino butyric acid
- IBS
irritable bowel syndrome
- IPANs
intrinsic primary afferent neurons
- PONV
post-operative nausea and vomiting
- TM
transmembrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5 References
- Aapro M. 5-HT3 receptor antagonists: an overview of their present status and future potential in cancer-therapy-induced emesis. Drugs. 1991;42:551–568. doi: 10.2165/00003495-199142040-00002. [DOI] [PubMed] [Google Scholar]
- Aapro M. 5-HT3 receptor antagonists in the management of nausea and vomiting in cancer and cancer treatment. Oncol. 2005;69:97–109. doi: 10.1159/000087979. [DOI] [PubMed] [Google Scholar]
- Aggarawal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlatewith specific autonomic nervous abnormalities. Gastroenterol. 1994;106:945–950. doi: 10.1016/0016-5085(94)90753-6. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Hales TG, Lummis SCR, Peters JA. The 5-HT3 receptor- the relationship between structure and function. Neuropharmacol. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Ironside JW, Naylor RJ. Identification and characterization of 5-hydroxytryptamine3 recognition sites in human brain tissue. J Neurochem. 1989;53:1787–1793. doi: 10.1111/j.1471-4159.1989.tb09244.x. [DOI] [PubMed] [Google Scholar]
- Beene DL, Price KL, Lester HA, Dougherty DA, Lummis SCR. Tyrosine residues that control binding and gating in the 5-Hydroxytryptamine3 receptor revealed by unnatural amino acid synthesis. J Neurosci. 2004;24:9097–9104. doi: 10.1523/JNEUROSCI.2429-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beene DL, Brandt GS, Zhong W, Zacharias NM, Lester HA, Dougherty DA. Cation-pi interactions in ligand recognition by serotonergic (5-HT3A) and nicotinic acetylcholine receptors: the anomalous binding properties of nicotine. Biochem. 2002;41:10262–10269. doi: 10.1021/bi020266d. [DOI] [PubMed] [Google Scholar]
- Belelli D, Balcarek JM, Hope AG, Peters JA, Lambert JJ, Blackburn TP. Cloning and functional expression of a human 5-Hydroxytryptamine type 3As receptor subunit. Mol Pharmacol. 1995;48:1054–1062. [PubMed] [Google Scholar]
- Bocquet N, de Carvalho LP, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux J-P, Corringer P-J. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- Boess FG, Steward LJ, Steele JA, Liu D, Reid J, Glencourse TA, Martin IL. Analysis of the ligand binding site of the 5-HT3 receptor using site directed mutagenesis: importance of glutamine 106. Neuropharmacol. 1997;36:637–347. doi: 10.1016/s0028-3908(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Bouzat C, Gumllar F, Spitzmaul G, Wang H-L, Rayes D, Hansen SB, Taylor P, Sine SM. Coupling of agonist binding to channel gating in an Ach-binding protein linked to an ion channel. Nature. 2004;430:896–900. doi: 10.1038/nature02753. [DOI] [PubMed] [Google Scholar]
- Boyd GW, Doward AI, Kirkness EW, Millar NS, Connolly CN. Cell surface expression of 5-Hydroxytryptamine type 3 receptors is controlled by an endoplasmic reticulum retention signal. J Biol Chem. 2003;278:27681–27687. doi: 10.1074/jbc.M304938200. [DOI] [PubMed] [Google Scholar]
- Boyd GW, Low P, Dunlop JI, Robertson LA, Vardy A, Lambert JJ, Peters JA, Connolly CN. Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: the role of oligomerization and chaperone proteins. Mol Cell Neurosci. 2002;21:38–50. doi: 10.1006/mcne.2002.1160. [DOI] [PubMed] [Google Scholar]
- Brady CA, Stanford IM, Ali I, Lin L, Williams JM, Dubin AE, Hope AG, Barnes NM. Pharmacological comparison of human homomeric 5-HT3A receptors versus heteromeric 5-HT3A/3B receptors. Neuropharmacol. 2001;41:282–284. doi: 10.1016/s0028-3908(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EMM, Moayyedi P. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(S1):S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–76. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Frau R, Di Chiarra G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur J Pharmacol. 1989;164:515–519. doi: 10.1016/0014-2999(89)90259-8. [DOI] [PubMed] [Google Scholar]
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AchBP crystal structures. Neuron. 2004;41:841–842. doi: 10.1016/s0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Chakrapani S, Bailey TD, Auerbach A. Gating dynamics of the acetylcholine receptor. J Gen Physiol. 2004;123:341–356. doi: 10.1085/jgp.200309004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine self-administration and locomotor activity are not modified by the 5-HT3 antagonists ICS 205–930 and MDL 72222. Pharmacol Biochem Behav. 1994;49:67–71. doi: 10.1016/0091-3057(94)90457-x. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:27–37. doi: 10.2174/1568007043482624. [DOI] [PubMed] [Google Scholar]
- Costall B, Domeney AM, Naylor RJ, Tattersall FD. 5-Hydroxytryptamine M-receptor antagonism to prevent cisplatin-induced emesis. Neuropharmacol. 1986;25:959–961. doi: 10.1016/0028-3908(86)90030-4. [DOI] [PubMed] [Google Scholar]
- Das P, Dillon GH. The 5-HT3B subunit confers reduced sensitivity to picrotoxin when co-expressed with the 5-HT3A receptor. Mol Brain Res. 2003;119:207–212. doi: 10.1016/j.molbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- de Witt R, Aapro M, Blower P. Is there a pharmacological basis for the differences in 5-HT3-receptor antagonist efficacy in refractory patients? Cancer Chemother Pharmacol. 2005;56:231–238. doi: 10.1007/s00280-005-1033-0. [DOI] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR α-1bound to α-bungarotoxin at 1.94Å resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Drossman DA. The functional gastrointestinal disorders and the Rome II process. GUT. 1999;45(Suppl II):II1–II15. doi: 10.1136/gut.45.2008.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- Dubin AE, Huvar R, D'Andreas MR, Pyati J, Zhu JY, Koy KC, Wilson SJ, Galindo JE, Glass CA, Lin L, Jackson MR, Lovenberg TW, Erlander MG. The pharmacological and functional characteristics of the serotonin 5-HT(3A) receptor are specifically modified by a 5-HT(3B) receptor subunit. J Biol Chem. 1999;274:30799–30810. doi: 10.1074/jbc.274.43.30799. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Smith JE. Neurobiological aspects of drug-seeking behaviors. In: Thompson T, Drews PB, Barrett JE, editors. Advances in behavioral pharmacology. Vol. 6. Erlbaum & Assoc.; Hillsdale, N.J.: 1987. pp. 1–44. [Google Scholar]
- Dyr W, Kostowski W. Evidence that the amygdala is involved in the inhibitory effects of 5-HT3 receptor antagonists on alcohol drinking in rats. Alcohol. 1995;12:387–391. doi: 10.1016/0741-8329(95)00023-k. [DOI] [PubMed] [Google Scholar]
- Eisele¢ JL, Bertrand S, Galzi JL, Deillers-Thiery A, Changeux J-P, Bertrand D. Chimeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature. 1993;336:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- Engel SR, Allan AM. 5-HT3 receptor over-expression enhances ethanol sensitivity in mice. Psychopharmacol. 1999;144:411–415. doi: 10.1007/s002130051025. [DOI] [PubMed] [Google Scholar]
- Engel SR, Lyons CR, Allan AM. 5-HT3 receptor over-expression decreases ethanol self administration in transgenic mice. Psychopharmacol. 1998;140:243–248. doi: 10.1007/s002130050763. [DOI] [PubMed] [Google Scholar]
- Englemann EA, Rodd ZA, Bell RL, Murphy JM. The role of 5-HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS & Neurolog Disord – Drug Targets. 2008;7:454–467. doi: 10.2174/187152708786927886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev. 2007;17:CD00396. doi: 10.1002/14651858.CD003960.pub3. [DOI] [PubMed] [Google Scholar]
- Faerber L, Dreschler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research- evolving concepts in management of pain and inflammation. E J Pharmacol. 2007;560:1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Fasching PA, Kollmannsberger B, Strissel PL, Niesler B, Engel J, Kreis H, Lux MP, Weihbrecht S, Lausen B, Bani MR, Beckmann MW, Strick R. Polymorphisms in the novel serotonin subunit gene HTR3C show different risks for acute chemotherapy-induced vomiting after anthracycline chemotherapy. J Cancer Res Clin Oncol. 134:1079–1086. doi: 10.1007/s00432-008-0387-1. [DOI] [PubMed] [Google Scholar]
- Fayyaz M, Lackner JM. Serotonin receptor modulators in the treatment of irritable bowel syndrome. Ther Clin Risk Manag. 2008;4:41–48. doi: 10.2147/tcrm.s140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florczyk AP, Schurig JE, Bradner WT. Cisplatin-induced emesis in the ferret. A new animal model. Cancer Treat Rep. 1982;66:187–189. [PubMed] [Google Scholar]
- Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831–1843. doi: 10.1038/ajg.2009.223. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Mobarok AA. Blockade of neuronal tryptamine receptors by metoclopramide. Eur J Pharmacol. 1978;49:109–112. doi: 10.1016/0014-2999(78)90228-5. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Ohtsubo T, Tsuda M, Yanagawa Y, Hori Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in spinal superficial dorsal horn. J Neurophysiol. 2009;102:1459–1471. doi: 10.1152/jn.91160.2008. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Gaddum JH. Tryptamine receptors. J Physiol. 1953;119:363–368. doi: 10.1113/jphysiol.1953.sp004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan TJ. Mechanisms underlying postoperative nausea and vomiting and neurotransmitter receptor antagonist-based pharmacotherapy. CNS Drugs. 2007;21:813–833. doi: 10.2165/00023210-200721100-00003. [DOI] [PubMed] [Google Scholar]
- Gao E, Bren N, Burghardt TP, Hansen S, Henchman RH, Taylor P, McCammon JA, Sine SM. Agonist-mediated conformational changes in acetylcholine-binding protein revealed by simulation and intrinsic tryptophan fluorescence. J Biol Chem. 2005;280:8443–8451. doi: 10.1074/jbc.M412389200. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Roles played by 5-hydroxytryptamine in the physiology of the bowel (review) Ailment Pharmacol Ther. 1999;13(suppl 2):15–30. [PubMed] [Google Scholar]
- Gershon MD. Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol & Ther. 2004;20(S7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Wong DT, Robertson DW. Localization of 5-HT3 receptors in the rat brain using [3H]-LY278584. Brain Res. 1991;553:149–154. doi: 10.1016/0006-8993(91)90242-n. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Raybold HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterol. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Brookes PA, Spyer KM, Miller RJ. 5-Hydroxytryptamine-3 receptors modulate synaptic activity in the rat nucleus tractus solitarius in vitro. Brain Res. 1992;589:62–68. doi: 10.1016/0006-8993(92)91162-8. [DOI] [PubMed] [Google Scholar]
- Goyal RK. Targets of the enteric motor neurones: smooth muscle cells. Gut. 2000;47(Suppl IV):IV38–IV39. doi: 10.1136/gut.47.suppl_4.iv38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Lummis SC. Conversion of the ion selectivity of the 5-HT(3a) receptor from cationic to anionic reveals a conserved feature of the ligand-gated ion channel superfamily. J Biol Chem. 2001;276:10977–10983. [PubMed] [Google Scholar]
- Hanna MC, Davies PA, Hales TG, Kirkness EF. Evidence for expression of heteromeric serotonin 5-HT(3) receptors in rodents. J Neurochem. 2000;75:240–247. doi: 10.1046/j.1471-4159.2000.0750240.x. [DOI] [PubMed] [Google Scholar]
- Hayrapetyan V, Jenschke M, Dillon GH, Machu TK. Co-expression of the 5-HT3B subunit with the 5-HT3A receptor reduces alcohol sensitivity. Mol Brain Res. 2005;142:146–150. doi: 10.1016/j.molbrainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Hellevuo K, Hoffman PL, Tabakoff B. Ethanol fails to modify [3H]GR65630 binding to 5-HT3 receptors in NCB-20 cells and in rat cerebral membranes. Alcoholism Clin Exp Res. 1991;15:775–778. doi: 10.1111/j.1530-0277.1991.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Hesketh P. Comparative review of 5-HT3 receptor antagonists in the treatment of actue chemotherapy-induced nausea and vomiting. Cancer Invest. 2000;18:163–173. doi: 10.3109/07357900009038248. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Wang Y, Corrigall WA, Sellers EM. Influence of 5-HT3 receptor antagonists and the indirect 5-HT agonist, dexfenfluramine, on heroin self-administration in rats. Psychopharmacol. 1994;114:611–619. doi: 10.1007/BF02244992. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Grundy D. Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol (London) 1998;509.3:717–727. doi: 10.1111/j.1469-7793.1998.717bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Kelley SP, Bratt AM, Iller K, Schroeder JP, Besheer J. 5-HT(3A) receptor subunit is required for 5-HT3 antagonist-induced reductions in alcohol drinking. Neuropsychopharmacol. 2004;29:1807–1813. doi: 10.1038/sj.npp.1300498. [DOI] [PubMed] [Google Scholar]
- Holbrook JD, Gill CH, Zebda N, Spencer JP, Leyland R, Rance KH, Trinh H, Balmer G, Kelly FM, Yusaf SP, Coutenay N, Luck J, Rhodes A, Modha S, Moore SE, Sanger GJ, Gunthorpe MJ. Characterization of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: evolution, distribution and function. J Neurochem. 2009;108:384–396. doi: 10.1111/j.1471-4159.2008.05775.x. [DOI] [PubMed] [Google Scholar]
- Hope AG, Belelli D, Mair ID, Lambert JJ, Peters JA. Molecular Determinants of (+)-Tubocurarine Binding at Recombinant 5-Hydroxytryptamine3A Receptor Subunits. MolPharmacol. 1999;55:1037–1043. doi: 10.1124/mol.55.6.1037. [DOI] [PubMed] [Google Scholar]
- Hope AG, Downie DL, Sutherland L, Lambert JJ, Peters JA, Burchell B. Cloning and functional expression of an apparent splice variant of the murine 5-HT3 receptor A subunit. Eur J Pharmacol. 1993;245:187–192. doi: 10.1016/0922-4106(93)90128-v. [DOI] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111:106S–112S. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological, and functional diversity of the 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hui S-CG, Sevilla EL, Ogle CW. 5-HT3 antagonists reduce morphine self-administration in rats. Br J Pharmacol. 1993;110:1341–1346. doi: 10.1111/j.1476-5381.1993.tb13966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg KE, Ukhun IA, Holstad SG, Jafir S, Uchida U, Zorumski CF, Yang J. Partial cDNA cloning and NGF regulation of a rat 5-HT3 receptor subunit. Neuroreport. 1993;5:121–124. doi: 10.1097/00001756-199311180-00006. [DOI] [PubMed] [Google Scholar]
- Jansen M, Bali M, Akabas, M.H. Molecular design of Cys-loop ligand-gated ion channels: functional 5-HT3 and GABA ρ1 receptors lacking the large cytoplasmic M3M4 loop. J Gen Physiol. 2008;131:137–146. doi: 10.1085/jgp.200709896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantunen IT, Kataja VV, Muhonen TT, Parviainen T. Effects of granisetron with doxorubicin or epirubicin on ECG intervals. Cancer Chemother Pharmacol. 1996;37:502–504. doi: 10.1007/s002800050420. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Franks NP, Lieb WR. Actions of general anesthetics on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br J Pharmacol. 1996;117:1507–1515. doi: 10.1111/j.1476-5381.1996.tb15314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TN, Nielsen J, Frederiksen K, Ebert B. Molecular cloning and pharmacological characterization of serotonin 5-HT3A receptor subtype in dog. Eur J Pharmacol. 2006;538:23–31. doi: 10.1016/j.ejphar.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Jones BW, Moore DJ, Robinson SM, Song F. A systematic review of tegaserod for treatment of irritable bowel syndrome. J Clin Pharmacol. 2002;27:343–352. doi: 10.1046/j.1365-2710.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- Jones KA, Suprenant A. Single channel properties of the 5-HT3 subtype of serotonin receptor in primary cultures of rodent hippocampus. Neurosci Lett. 1994;174:133–136. doi: 10.1016/0304-3940(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Role of the serotonergic system in the neurobiology of alcoholism: Implications for treatment. CNS Drugs. 2004;18:1105–1118. doi: 10.2165/00023210-200418150-00005. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Campling GM, Griffiths P, Cohen PJ. Attenuation of some alcohol-induced mood changes and the desire to drink by 5-HT3 receptor blockade: a preliminary study in healthy male volunteers. Psychopharmacol. 1993;112:142–144. doi: 10.1007/BF02247375. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Zanca NA, Velasquez M. Ondansetron reduces the craving of biologically predisposed alcoholics. Psychopharmacol. 2002;160:408–413. doi: 10.1007/s00213-002-1002-9. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Elkashef AM, Smith EV, Kahn R, Vocci F, Li SH, Bloch DA. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of methamphetamine dependence. Int J Neuropsychopharmacol. 2008;11:1–14. doi: 10.1017/S1461145707007778. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Javors MA, DiClemente C, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963–971. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- Joshi PR, Suryanarayanan A, Hazai E, Schulte MK, Maksay G, Bikadi Z. Interactions of granisetron with an agonist-free 5-HT3A receptor model. Biochem. 2006;45:1099–1105. doi: 10.1021/bi051676f. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Sezen O, Papies A, Bauer S, Schelenz C, Tremblay PB, Possinger K, Roots I, Brockmoller J. Patient-tailored antiemetic treatment with 5-Hydroxytryptamine type 3 receptor antagonists according to P-450 2D5 genotypes. J Clin Oncol. 2002;20:2805–2811. doi: 10.1200/JCO.2002.09.064. [DOI] [PubMed] [Google Scholar]
- Karnovsky AM, Gotow LF, McKinley DD, Piechan JL, Ruble CL, Mills CJ, Schellin KAB, Slightom JL, Fitzgerald LR, Benjamin CW, Roberds SL. A cluster of novel serotonin receptor 3-like genes on human chromosome 3. GENE. 2003;319:137–148. doi: 10.1016/s0378-1119(03)00803-5. [DOI] [PubMed] [Google Scholar]
- Kash TL, Jenkins A, Kelley JC, Trudell JR, Harrison NL. Coupling of agonist binding to channel gating in the GABA(A) receptor. Nature. 2003;421:272–275. doi: 10.1038/nature01280. [DOI] [PubMed] [Google Scholar]
- Keefe DL. The cardiotoxic potential of the 5-HT3 receptor antagonist anti-emetics: Is there cause for concern? Oncologist. 2002;7:65–72. doi: 10.1634/theoncologist.7-1-65. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Dunlop JI, Kirkness EF, Lambert JJ, Peters JA. A cytoplasmic region determines single channel conductance in 5-HT3 receptors. Nature. 2003;424:321–324. doi: 10.1038/nature01788. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of the 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Pohorecky LA. Zacopride, a 5-HT3 receptor antagonist, reduces voluntary ethanol consumption in rats. Pharmacol Biochem and Behav. 1992;41:847–850. doi: 10.1016/0091-3057(92)90237-a. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Dyr W, Krzascik P. The abilities of 5-HT3 receptor antagonist ICS 205–930 to inhibit alcohol preference and withdrawal seizures in rats. Alcohol. 1993;10:369–373. doi: 10.1016/0741-8329(93)90022-g. [DOI] [PubMed] [Google Scholar]
- Kovac AL. Benefits and risks of newer treatments for chemotherapy-induced and postoperative nausea and vomiting. Drug Safety. 2003;26:227–259. doi: 10.2165/00002018-200326040-00003. [DOI] [PubMed] [Google Scholar]
- Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24:2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- Lankiewicz S, Lobitz N, Wetzel CHR, Rupprecht R, Gisselmann G, Hatt H. Molecular cloning, functional expression, and pharmacological characterization of 5-hydroxytryptamine3 receptor cDNA and its splice variants from guinea pig. Mol Pharmacol. 1998;53:202–212. doi: 10.1124/mol.53.2.202. [DOI] [PubMed] [Google Scholar]
- Laporte AM, Koscielniak T, Ponchant M, Verge D, Hamon M, Gozlan H. Quantitative autoradiographic mapping of 5-HT3 receptors in the rat CNS using [125I]iodozacapride and [3H]zacopride as ligands. Synapse. 1992;10:271–281. doi: 10.1002/syn.890100402. [DOI] [PubMed] [Google Scholar]
- Law RJ, Henchman RH, McCammon JA. A gating mechanism proposed from a simulation of a human a7 nicotinic acetylcholine receptor. Proc Natl Acad Sci (USA) 2005;102:6813–6818. doi: 10.1073/pnas.0407739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438:167–168. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- Lee WY, Free CR, Sine SM. Binding to gating transduction in nicotinic receptors: cys-loop energetically couples to pre-M1 and M2-M3 regions. J Neurosci. 2009;29:3189–3199. doi: 10.1523/JNEUROSCI.6185-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesbros-Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum AL. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:1253–1269. doi: 10.1111/j.1365-2036.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Mueller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Sci. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Livesay MR, Cooper MA, Deeb TZ, Carland JE, Kozuska J, Hales TG, Lambert JJ, Peters JA. Structural determinants of Ca2+ permeability and conduction in the human 5-Hydroxytryptamine3A receptor. J Biol Chem. 2008;283:19301–19313. doi: 10.1074/jbc.M802406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G. Ethanol potentiation of 5-Hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- Lovinger DM, Sung KW, Zhou Q. Ethanol and trichloroethanol alter gating of 5-HT3 receptor-channels in NCB-20 neuroblastoma cells. Neuropharmacol. 2000;39:561–570. doi: 10.1016/s0028-3908(99)00164-1. [DOI] [PubMed] [Google Scholar]
- Lummis SCR, Beene DL, Lee LW, Lester HA, Broadhurst RW, Dougherty DA. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature. 2005;438:248–252. doi: 10.1038/nature04130. [DOI] [PubMed] [Google Scholar]
- Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-Hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther. 1994;271:898–905. [PubMed] [Google Scholar]
- Manabe N, Tanaka T, Hata J, Kusunoki H, Haruma K. Pathophysiology underlying irritable bowel syndrome – from the viewpoint of dysfunction of autonomic nervous system activity. J Sm Musc Res. 2009;45:15–23. doi: 10.1540/jsmr.45.15. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5-HT3 receptor, a serotonin-gated ion channel. Sci. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Mertz HR. Drug therapy: irritable bowel syndrome. N Engl J Med. 2003;349:2136–2146. doi: 10.1056/NEJMra035579. [DOI] [PubMed] [Google Scholar]
- Miner WD, Sanger GJ. Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism. Br J Pharmacol. 1986;88:497–499. doi: 10.1111/j.1476-5381.1986.tb10228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Molecular cloning of human 5-Hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol. 1995;48:407–416. [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanisms of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Miyake A, Furuichi K. Identification of a domain affecting agonist potency of meta-chlorophenylbiguanide in 5-HT3 receptors. Eur J Pharmacol. 1999;369:125–132. doi: 10.1016/s0014-2999(99)00058-8. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Watanabe T, Miyake A, Saito M, Furuichi K. Cloning, expression, and characterization of ferret 5-HT3 receptor subunit. Eur J Pharmacol. 2000;399:97–106. doi: 10.1016/s0014-2999(00)00371-x. [DOI] [PubMed] [Google Scholar]
- Monk SA, Desai K, Brady CA, Williams JM, Lin L, Princivalle A, Hope AG, Barnes NM. Generation of a selective 5-HT3B subunit-recognising polyclonal antibody; identification of immunoreactive cells in rat hippocampus. Neuropharmacol. 2001;41:1013–1016. doi: 10.1016/s0028-3908(01)00153-8. [DOI] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5-HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- Morales M, Battenberg E, de Lecea L, Sanna PP, Bloom FE. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Mol Brain Res. 1996;36:251–260. doi: 10.1016/0169-328x(96)88406-3. [DOI] [PubMed] [Google Scholar]
- Morales M, Wang S-D. Differential composition of 5-Hyrdoxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. GENE. 2003;319:101–111. doi: 10.1016/s0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- Niesler B, Kapeller J, Hammer C, Rappold G. Serotonin type3 genes: HTR3A, B, C, D, E. Pharmacogenom. 2008;9:501–504. doi: 10.2217/14622416.9.5.501. [DOI] [PubMed] [Google Scholar]
- Niesler B, Walstab J, Combrink S, Möller D, Kapeller J, Rietdorf J, Bönisch H, Göthert M, Rappold G, Brüss M. Characterization of the Novel Human Serotonin Subunits 5-HT3C, 5-HT3D, and 5-HT3E. Mol Pharmacol. 2007;72:8–17. doi: 10.1124/mol.106.032144. [DOI] [PubMed] [Google Scholar]
- Nyce HL, Stober ST, Abrams CF, White MM. Mapping spatial relationships between residues in the ligand-binding domain of the 5-HT3 receptor using a molecular ruler. Biophys J. 2010;98:1847–1855. doi: 10.1016/j.bpj.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KL, Lummis SCR. The role of tyrosine residues in the extracellular domain of the 5-Hydroxytryptamine3 receptor. J Biol Chem. 2004;279:23294–23301. doi: 10.1074/jbc.M314075200. [DOI] [PubMed] [Google Scholar]
- Price KL, Bower KS, Thompson AJ, Lester HA, Dougherty DA, Lummis SC. A hydrogen bond in Loop A is critical for the binding and function of the 5-HT3 receptor. Biochem. 2008;47:6370–6377. doi: 10.1021/bi800222n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled 12 week trials. Clin Ther. 2008;30:884–901. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Reeves DC, Lummis SC. Detection of human and rodent 5-HT3B receptor subunits by anti-peptide polyclonal antibodies. BMC Neurosci. 2006;7:27. doi: 10.1186/1471-2202-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves DC, Jansen M, Bali M, Lemster T, Akabas MH. A role for the b1–b2 loop in the gating of 5-HT3 receptors. J Neurosci. 2005;25:9358–9366. doi: 10.1523/JNEUROSCI.1045-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha E, Silva M, Valle JR, Picarelli ZP. A pharmacological analysis of the mode of action of serotonin (5-hydroxytryptamine) upon the guinea pig ileum. Br J Pharmacol. 1953;8:378–388. doi: 10.1111/j.1476-5381.1953.tb01333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, Rubenstein E, Sebastiani S, Cantoreggi S, Snyder SH, Slusher B. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008;107:469–478. doi: 10.1213/ane.0b013e318172fa74. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Pie B, Cooper E. Developing neonatal rat sympathetic and sensory neurons differ in their regulation of 5-HT3 receptor expression. J Neurosci. 1997;17:6629–6638. doi: 10.1523/JNEUROSCI.17-17-06629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch D, Braun HA, Wulf H, Schuster A, Raines DE. Inhibition of 5-HT(3A) and 5-HT(3AB) receptors by etomidate, propofol, and pentobarbital. Eur J Pharmacol. 2007;273:60–64. doi: 10.1016/j.ejphar.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler RS. Epidemiology of irritable bowel syndrome in the United States. Gastroenterol. 1990;99:409–415. doi: 10.1016/0016-5085(90)91023-y. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Andrews PLR. Treatment of nausea and vomiting: Gaps in our knowledge. Auto Neurosci: Basic & Clin. 2006;129:3–16. doi: 10.1016/j.autneu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Schreiter C, Hovius R, Costioli M, Pick H, Kellenberger S, Schild L, Vogel H. Characterization of the ligand binding site of the 5-HT3 receptor. J Biol Chem. 2003;278:22709–22715. doi: 10.1074/jbc.M301801200. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psych. 1994;151:184–191. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Scuderi PE. Pharmacology of antiemetics. Int Anesthesiol Clin. 2003;41:41–66. doi: 10.1097/00004311-200341040-00006. [DOI] [PubMed] [Google Scholar]
- Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Spier AD, Lummis SC. The role of tryptophan residues in the 5-Hydroxytryptamine3 receptor ligand binding domain. J Biol Chem. 2000;275:5620–5625. doi: 10.1074/jbc.275.8.5620. [DOI] [PubMed] [Google Scholar]
- Stevens R, Rusch D, Solt K, Raines DE, Davies PA. Modulation of human 5-Hydroxytryptamine3AB receptors by volatile anesthetics and n-alcohols. J Pharmacol Exp Ther. 2005;314:338–345. doi: 10.1124/jpet.105.085076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward LJ, Boess FG, Steele JA, Liu D, Wong N, Martin IL. Importance of phenylalanine 107 in agonist recognition by the 5-hydroxytryptamine(3A) receptor. Mol Pharmacol. 2000;57:1249–1255. [PubMed] [Google Scholar]
- Suryanarayanan A, Joshi PR, Bikadi Z, Muthalagi M, Kulkarni TR, Gaines C, Schulte MK. The Loop C region of the murine 5-HT3A receptor contributes to the differential actions of 5-Hydroxytryptamine and m-Chlorophenylbiguanide. Biochem. 2005;44:9140–9149. doi: 10.1021/bi050661e. [DOI] [PubMed] [Google Scholar]
- Swift RM, Davidson D, Whelihan W, Kuznetsov O. Ondansetron alters human intoxication. Biol Psych. 1996;40:514–521. doi: 10.1016/0006-3223(95)00432-7. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci USA. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Padgett CL, Lummis SC. Mutagenesis and molecular modeling reveal the importance of the 5-HT3 receptor F-loop. J Biol Chem. 2006;281:16576–16582. doi: 10.1074/jbc.M601265200. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Price KL, Reeves DC, Chan SL, Chau P-L, Lummis SCR. Locating an antagonist in the 5-HT3 receptor binding site using modeling and radioligand binding. J Biol Chem. 2005;280:20476–20482. doi: 10.1074/jbc.M413610200. [DOI] [PubMed] [Google Scholar]
- Tompkins DM, Le AD, Sellers EM. Effect of the 5-HT3 antagonist ondansetron on voluntary ethanol intake in rats and mice maintained on a limited access procedure. Psyhcopharmacol. 1995;117:479–485. doi: 10.1007/BF02246222. [DOI] [PubMed] [Google Scholar]
- Tremblay P-B, Kaiser R, Sezer O, Rosler N, Schelenz C, Possinger K, Roots I, Brockmoller J. Variations in the 5-Hydroxytryptamine 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J Clin Oncol. 2003;21:2147–2155. doi: 10.1200/JCO.2003.05.164. [DOI] [PubMed] [Google Scholar]
- Tyers MB. 5-HT3 receptors. Ann NY Acad Sci. 1990;600:194–200. doi: 10.1111/j.1749-6632.1990.tb16882.x. [DOI] [PubMed] [Google Scholar]
- Tzvetkov M, Meineke V, Oetjen E, Hirsch-Ernst K, Brockmoller J. Tissue-specific alternative promoters of the serotonin receptor gene HTR3B in human brain and instestine. GENE. 2007;386:52–62. doi: 10.1016/j.gene.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4 angstrom resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Venkataraman P, Venkatachalan SP, Joshi PR, Muthalagi M, Schulte MK. Identification of critical residues in loop E in the 5-HT3ASR binding site. BMC Biochem. 2002;3:15. doi: 10.1186/1471-2091-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis T. Serotonin3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cer Cor. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing, and subjective effects of cocaine. Psychopharmacol. 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- Walstab J, Rappold G, Niesler B. 5-HT3 receptors: role in disease and target of drugs. Pharm & Ther. 2010;128:146–169. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Werner P, Kawashima E, Reid J, Hussy N, Lundstrom K, Buell G, Humbert Y, Jones KA. Organization of the mouse 5-HT3 receptor gene and functional expression of two splice variants. Mol Brain Res. 1994;26:233–241. doi: 10.1016/0169-328x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Wozniak KM, Pert A, Linnoila M. Antagonism of 5-HT3 receptors attenutates the effects of ethanol on extracellular dopamine. Eur J Pharmacol. 1990;187:287–289. doi: 10.1016/0014-2999(90)90015-x. [DOI] [PubMed] [Google Scholar]
- Yan D, White MM. Spatial orientation of the antagonist granisetron in the ligand-binding domain of the 5-HT3 receptor. Mol Pharmacol. 2005;68:365–371. doi: 10.1124/mol.105.011957. [DOI] [PubMed] [Google Scholar]
- Yan D, Meyer JK, White MM. Mapping residues in the ligand-binding domain of the 5-HT3 receptor onto d-tubocurarine structure. Mol Pharmacol. 2006;70:571–578. doi: 10.1124/mol.106.024075. [DOI] [PubMed] [Google Scholar]
- Yan D, Schulte MK, Bloom KE, White MM. Structural features of the ligand-binding domain of the serotonin 5-HT3 receptor. J Biol Chem. 1999;274:5537–5541. doi: 10.1074/jbc.274.9.5537. [DOI] [PubMed] [Google Scholar]
- Yang LPH, Scott LJ. Palonosetron: In the prevention of nausea and vomiting. Drugs. 2009;69:2257–2278. doi: 10.2165/11200980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Zhang R, White NA, Soti FS, Kem WR, Machu TK. N-terminal domains in mouse and human 5-Hydroxytryptamine3A receptors confer partial agonist and antagonist properties to benzylidene analogs of anabaseine. J Pharmacol Exp Ther. 2006;317:1276–1284. doi: 10.1124/jpet.106.101485. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wen X, Militante J, Hester B, Rhubottom HE, Sun H, Leidenheimer NJ, Yan D, White MM, Machu TK. The role of loop F residues in determining differential d-tubocurarine potencies in mouse and human 5-hydroxytryptamine3A receptors. Biochem. 2007;46:1194–1204. doi: 10.1021/bi0616100. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Verdoorn TA, Lovinger DM. Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol. 1998;507.2:335–352. doi: 10.1111/j.1469-7793.1998.335bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]