Abstract
The 5-nitroimidazole (NI) compound C17, with a side chain carrying a remote phenyl group in the 2-position of the imidazole ring, is at least 14-fold more active against the gut protozoan parasite Giardia lamblia than the 5-NI drug metronidazole (MTR), with a side chain in the 1-position of the imidazole ring, which is the primary drug for the treatment of giardiasis. Over 10 months, lines resistant to C17 were induced in vitro and were at least 12-fold more resistant to C17 than the parent strains. However, these lines had ID90 values (concentration of drug at which 10% of control parasite ATP levels are detected) for MTR of >200 μM, whilst lines induced to be highly resistant to MTR in vitro have maximum ID90 values around 100 μM (MTR-susceptible isolates typically have an ID90 of 5–12.8 μM). The mechanism of MTR activation in Giardia apparently involves reduction to toxic radicals by the activity of pyruvate:ferredoxin oxidoreductase (PFOR) and the electron acceptor ferredoxin. MTR-resistant Giardia have decreased PFOR activity, which is consistent with decreased activation of MTR in these lines, but C17-resistant lines have normal levels of PFOR. Therefore, an alternative mechanism of resistance in Giardia must account for these super-MTR-resistant cells.
Keywords: Pyruvate:ferredoxin oxidoreductase, Tinidazole, Ronidazole, 5-Nitroimidazole, Cross-resistance
1. Introduction
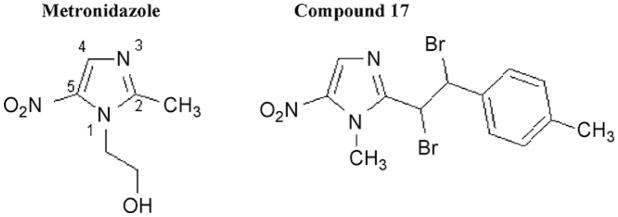
Metronidazole (MTR) (Fig. 1) belongs to the family of 5-nitroimidazole (NI) drugs that are the mainstay for treating infections caused by the clinically important anaerobic protozoa Giardia lamblia (synonymous with G. intestinalis and G. duodenalis), Trichomonas vaginalis, Entamoeba histolytica [1] and Blastocystis sp. (reviewed in [2]) and the anaerobic bacteria, particularly Helicobacter pylori, Clostridium difficile and Bacteroides fragilis [3]. MTR has a side chain in the 1-position of the imidazole ring, with the all important nitro group in the 5-position (Fig. 1). The nitro group is activated by low redox potential reactions in anaerobes, producing toxic nitro radicals that ultimately cause death of the anaerobic organism [4]. In G. lamblia, T. vaginalis and E. histolytica, this has been proposed to occur via reduction by the low electron potential couple, pyruvate:ferredoxin oxidoreductase (PFOR) and ferredoxin (FDOX) [5,6]. This proposal has recently been challenged by Leitsch et al. [7,8] who claim that in T. vaginalis and E. histolytica thioredoxin reductase (TrxR) has nitroreductase activity and that NADPH is the primary source of reducing power for MTR and tinidazole activation.
Fig. 1.
5-Nitroimidazole ring structure with side-chain position of metronidazole [2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanol] and compound 17 {2-[1,2-dibromo-2-(4-methylphenyl)ethyl]-1-methyl-5-nitro-1H-imidazole}.
1-Position-modified 5-NIs commonly in clinical use include tinidazole, recommended in cases of MTR treatment failures of T. vaginalis (reviewed in [9]), secnidazole and ornidazole, whilst ronidazole, a veterinary drug, and satranidazole are among the few commercially available 5-NIs with 2-position side chains. Some experimental 5-NI compounds with 2-position modifications (Fig. 1) demonstrate ca. 40-fold [10] to 80-fold (compound 29 in [10]) greater efficacy against G. lamblia than MTR, a 1-position 5-NI, and 5-NIs with 4-position side chains have also been reported to be more effective than MTR against T. vaginalis [11]. MTR treatment failures and clinical MTR resistance have been documented in cases of giardiasis [12]. In G. lamblia, the mechanism of MTR resistance has been proposed to involve downregulation of PFOR activity and the FDOX protein [13–15].
This study, as well as our previous reports [10,11,16,17], point towards the potential for developing a clinically safe, highly effective 5-NI that, similarly to MTR, is effective against a wide range of anaerobes but is far more potent and therefore likely to overcome MTR resistance. However, the limit of resistance that these parasites can develop must be tested—will new, more potent 5-NIs eventually induce more highly resistant organisms? In this study, we explore the potential of G. lamblia to develop resistance against one highly effective 2-position 5-NI compound.
2. Materials and methods
2.1. Isolates and culture
The following G. lamblia isolates were used: BRIS/83/HEPU/106 (106) and BRIS/87/HEPU/713 (713) [18]; the laboratory-induced MTR-resistant (MTRR) lines 713-M3 [19] and 106-2ID10 [20] derived from the above isolates, respectively; the laboratory-induced C17-resistant (C17R) line 106-17A; and MTRRC17R line 713-M3-C17 (see Section 2.3).
Giardia lamblia was cultured axenically in modified TYI-S-33 medium [21] as previously described [22]. MTRR Giardia lines 713-M3 and 106-2ID10 were maintained in the presence of 50 μM MTR and 10 μM MTR, respectively. Initially, 106-2ID10 was maintained in twice the 10% inhibitory dose of MTR for several years [20], but more recently we have increased the concentration to 10 μM MTR. C17R Giardia lines were established and maintained as described below (see Section 2.3).
2.2. Drugs
MTR, ornidazole, ronidazole, furazolidone and albendazole were from Sigma (Australia), tinidazole was from AK Scientific Inc. (Mountain View, CA) and nitazoxanide was from Romark Laboratories, L.C. (Tampa, FL). The 2-position 5-NIs compound 17 (C17), compound 14 (C14) and compound 18 (C18) and the 4-position 5-NIs were synthesised as previously described [11,17]. All drugs were prepared as stock solutions ranging from 0.1 M to 0.001 M in dimethyl sulphoxide (DMSO) (Sigma) depending on drug solubility and assay working stocks were prepared in TYI-S-33 medium.
2.3. Development of C17-resistant lines
C17 resistance in G. lamblia isolate 106 was induced using a regimen of 1 h exposure to 10 μM or 20 μM C17 followed by constant and incremental increases in C17 drug treatment as outlined in Fig. 2. The resulting C17R line, 106-17A, is currently maintained in 10 μM C17. The MTRR line 713-M3 was treated with incremental increases in C17 as shown in Fig. 2. The resulting C17R line is maintained in 15 μM C17.
Fig. 2.
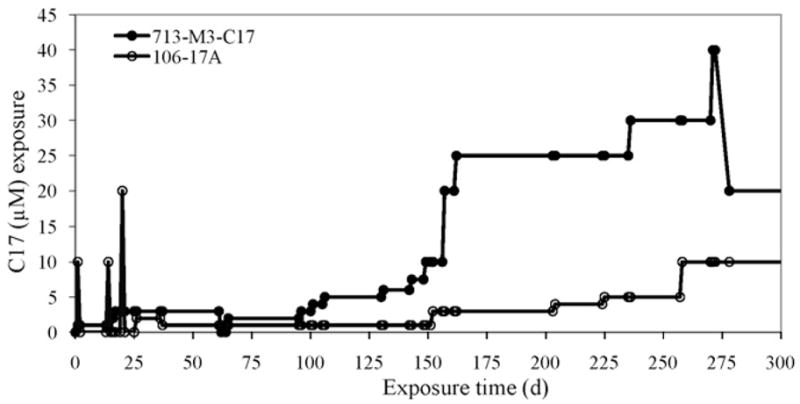
Time line for development of C17-resistant Giardia lines. Isolate 106 (°) was exposed to three 1 h spikes of C17 followed by recovery of parasites in drug-free medium over the first month. Intermittent drug treatment was then followed by incremental increases in C17 concentration in the medium, as shown on the y-axis, over a 9-month period. Parasites now grow relatively robustly in 10 μM C17. The metronidazole-resistant (MTRR) line 713-M3 (●) was exposed to incremental increases in C17 over a 9-month period. Levels reached at this stage were unsustainable and at 10 months the C17 concentration was reduced. At levels >20 μM C17 the drug precipitates. The parasites now grow well in 15 μM C17.
2.4. Drug susceptibility assays
Drug susceptibility assays were performed anaerobically as previously described [22] and modified as follows: drug and control DMSO working stocks were added to white-walled, flat, clear-bottomed, 96-well plates (Costar®) in 100 μL of media for each starting well before serial 1 in 2 dilutions down the plate into 50 μL of media. Each drug concentration was performed in triplicate. Trophozoites (2 × 104) were added in 50 μL of media to each well. Each plate was sealed in an incubation bag with an Anaerocult® IS (Merck, Darmstadt, Germany) sachet activated to produce anaerobic conditions (1% O2, 6.8% CO2, 16.5% H2) as per the manufacturer’s instructions. Plates were incubated at 37 °C for 48 h and minimal inhibitory concentrations (MICs) were determined as described previously [22] prior to ATP assays.
In each assay, ATP levels of viable drug-treated parasites compared with control-treated parasites were determined using the BacTiter-Glo™ Microbial Cell Viability Assay System (Promega, Madison, WI). Following the manufacturer’s instructions, 100 μL of BacTiter-G™ substrate/reagent mixture was added to each well at room temperature, generating a luminescent signal detected by a POLARStar Optima luminometer (BMG Labtech, Australia). Sample signal was compared with ATP (Sigma) standard signals resulting in determination of nM concentrations of ATP for each set of drug-treated replicates. Sample nM ATP concentrations were compared with ATP concentrations of control wells. ID90 values (concentration of drug at which 10% of control parasite ATP levels are detected) of drug for each assay were determined since these values match our previously reported MIC values [17,22]. The relative activity (RA) of each drug (compared with MTR), namely the ID90 for C17/ID90 for MTR, was determined for each MTR-susceptible (MTRS) isolate.
2.5. Assessment of pyruvate:ferredoxin oxidoreductase
2.5.1. Preparation of cell lysates
All chemicals involved in assessment of PFOR activity were from Sigma, unless otherwise stated. All solutions were flushed with nitrogen. Giardia lamblia isolates and lines 106, 106-2ID10 and 106-17A were grown to confluence. Dead cells and cellular debris were removed, cells were pelleted by centrifugation (290 × g, 5 min), washed in phosphate-buffered saline (PBS) and weighed. Cell pellets were either used immediately or flushed with nitrogen and stored at −80 °C. Cells were lysed with three cycles of freeze/thaw (dry ice/room temperature). To each gram of starting material, 5 mL of buffer [50 mM Tris–HCl (pH 7.5), 2 mM μ-mercaptoethanol] was added and the preparation was sonicated with a Branson Sonifier® for 2 × 20 s at 20% duty output. The resulting cell lysates were centrifuged (30 min, 40 000 × g, 4 °C) and the supernatant was used to determine PFOR presence and activity. Protein estimation was performed using the Bio-Rad Protein Assay with bovine serum albumin as standard.
2.5.2. Pyruvate:ferredoxin oxidoreductase activity gel assays
Crude cell lysate containing 40 μg of protein was diluted in sample buffer [60 mM Tris–HCl (pH 6.8), 10% glycerol, 0.001% bromophenol blue, 10% α-monothioglycerol] and loaded onto a 6% polyacrylamide gel with a 5% non-sodium dodecyl sulphate stacking gel. Non-denaturing polyacrylamide gel electrophoresis (PAGE) [13] was performed for 4 h at 85 V at 4 °C in running buffer (25 mM Tris, 192 mM glycine) containing 800 μL/L β-mercaptoethanol. Following electrophoresis, the gel was rinsed (1 × 5 min) in wash buffer [50 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2, 200 μM thiamine pyrophosphate (TPP)] containing 0.4 μL/mL β-mercaptoethanol and transferred to an anaerobic chamber (Forma-Scientific). Gel slices were prepared and washed for 15 min in pre-reduced wash buffer without β-mercaptoethanol prior to incubation in pre-reduced assay buffer [50 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2, 200 μM TPP, 200 μM coenzyme A, 10 μg/mL nitrotetrazolium blue chloride, 10 mM pyruvate] for ca. 10 min. Enzymatic activity was detected by the formation of an insoluble blue/purple formazan precipitate in the gel matrix. The reaction was stopped with several changes of Milli-Q water.
2.5.3. Pyruvate:ferredoxin oxidoreductase Western blotting
Giardia extracts were subjected to denaturing PAGE and transferred to a nitrocellulose membrane (Micron Separations Inc.) [14]. The membrane was blocked with 5% non-fat milk powder in PBS containing 0.1% Tween-20 (PBS-T) and incubated overnight at 4 °C in anti-PFOR antibody (raised in mice against isolate 106 PFOR, purified as described by Townson et al. [13] and validated against pure PFOR; data not shown) diluted 1:1000 in 5% non-fat milk in PBS-T. After washing with PBS-T, the membrane was incubated for 1 h at room temperature in goat anti-mouse–horseradish peroxidase (HRP) conjugate (Zymed) (1:10 000) and StrepTactin–HRP conjugate (Bio-Rad) (1:10 000) in 5% non-fat milk in PBS-T.
3. Results
3.1. Drug susceptibility of Giardia parent lines
Consistent with previous reports [10,17,23], we have shown that 5-NIs [C14, C17 and C18 (see [17] and Fig. 1) and commercially available ronidazole] modified in the 2-position of the imidazole ring display greater activity against G. lamblia than the more traditional 1-position 5-NIs (MTR, tinidazole and ornidazole), with MTR consistently displaying the lowest activity (Table 1). The RA of C17 compared with MTR was 17.5 and 14.3 for G. lamblia MTRS isolates 106 and 713, respectively (Table 1). C17 appears to be more effective than C14 and C18, which had RA values of 7.9–12.7 against the MTRS G. lamblia isolates (Table 1). Ronidazole was more effective than the 1-position 5-NIs but was not as effective as C14, C17 and C18, which carry aromatic rings in their side chains (Table 1). Both tinidazole and ornidazole had similar activities against the MTRS isolates, ranging from 3.3- to 4.4-fold better than MTR (Table 1). The 4-position 5-NI compounds described in Crozet et al. [11] were tested against isolates of Giardia, but none were more active than MTR (data not shown). Furazolidone and nitazoxanide, which are also compounds with a 5-nitro group relative to a 2-position side chain, are 1.1–1.5 and 3.0–7.3 times more effective, respectively, than MTR against lines 106 and 713 (Table 1).
Table 1.
Efficacy of 5-nitro group drugs against metronidazole-susceptible isolates and drug-resistant lines of Giardia lamblia Nitroimidazole.
| 5-nitro group drug | ID90 (μM) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 106 |
713 |
|||||||
| MTRS | RA | MTRRa | C17Rb, c | MTRS | RA | MTRRa | MTRRC17Rb,d | |
| 2-position | ||||||||
| C17 | 0.4–0.63(3) | 17.5 | 1.23–2.23(2) | 1.3–8.6(4) | 0.35–0.6(3) | 14.3 | 1.33–2.25(3) | 1.68–7.5(3) |
| C14 | 1.00 | 9.5 | 3.8 | 1.9 | 0.68–0.98(2) | 7.9 | 1.68 | 2.76 |
| C18 | 1.10 | 8.6 | 5.8 | 3.2 | 0.52 | 12.7 | 2.65 | 11 |
| Ronidazole | 1.60 | 5.9 | 9 | 40 | 1.40 | 4.7 | 16.5 | 25 |
| Furazolidone | 6.20 | 1.5 | 7.6 | 11–19(2) | 7.35 | 1.1 | 14.5–19.7(2) | 15.7–20.7(2) |
| Nitazoxanide | 1.3 | 7.3 | 6 | 17 | 2.2 | 3.0 | 10 | 14 |
| 1-position | ||||||||
| Tinidazole | 2.30 | 4.1 | 20 | >200 | 2.00 | 3.3 | 46.3 | >200 |
| Ornidazole | 2.40 | 3.9 | 18 | 198 | 1.50 | 4.4 | 41.7 | >200 |
| Metronidazole | 6.2–12.8(4) | 1.0 | 90 | 174–186(2) | 5–7.5(4) | 1.0 | 97 | >200 |
ID90, concentration of drug at which 10% of control parasite ATP levels are detected; MTRS, metronidazole-susceptible; RA, relative activity (drug activity relative to MTR for MTRS isolates calculated using average ID90 values); MTRR, metronidazole-resistant; C17R, C17-resistant lines.
(n) Indicates number of times experiment was done if more than once, with average values quoted in the text.
MTRR lines 106-2ID10 and 713-M3.
C17R lines 106-17A and 713-M3-C17.
Developing C17R line was cultured in the presence of 2–10 μM C17. Assays were performed from 208 days to 676 days following first exposure of line to C17.
Developing C17R line cultured in the presence of 2–15 μM C17. Assays were performed from 42 days to 474 days following first exposure of line to C17.
Previously, we reported MICs as a measure of drug susceptibility [17,22] and here we report ID90 values since these two values correlate well in 5-NI inhibition assays. Typically, the MIC for C17 against the MTRS isolate 713 is 0.6 μM, similar to the ID90 concentration of 0.35–0.6 μM (Table 1). As expected, the ID50 concentration for C17 against MTRS isolates is ca. 0.23 μM. However, this is not the case with albendazole assays where ATP levels curiously remain above the 10% of parasite control ATP levels for more than 3 days (thus preventing ID90 value determination; data not shown). Therefore, albendazole inhibition is reported as MIC.
3.2. Development of resistance in Giardia against C17
C17 was chosen for this study because of its efficacy and ease of synthesis [10,17]. We attempted to grow four isolates (106, WB1B, 713 and 1279) [18,19] and six drug-resistant lines (MTRR 106-2ID10, WB1B-M3 and 713-M3; quinacrine-resistant WB1B-Q and WB1B-M3-Q; and albendazole-resistant WB1B-M3-Alb) [19,20,24,25] in the presence of C17, commencing at 0.5 μM and with incremental increases up to 6 μM. All parasites except line 713-M3 succumbed. The highest concentration reached prior to its demise by one other isolate (713) was 5 μM. However, 713-M3 adapted to C17, after 10 months being able to grow in 20 μM C17 (Fig. 2) and producing the MTRRC17R line 713-M3-C17 that is maintained in 15 μM C17. 713-M3-C17 has a generation time[26] of 28.3 h, being 1.6 times longer than 713-M3 and 4.1 times longer than the parent 713 isolate that has a generation time of 6.9 h (Table 2). With removal of drug pressure from routine culture of 713-M3-C17-ND for up to 40 days, the generation time reverts to 15.1 h, similar to the 17.4 h generation time of the MTRR line 713-M3 (Table 2).
Table 2.
Giardia lines 106 and 713 generation times (g) and susceptibility to metronidazole (MTR) and compound C17 following removal of drug from routine culture of the C17-resistant Giardia lines.
| Giardia line | Days in culture without druga |
||||
|---|---|---|---|---|---|
| 40 g (h) b | 18 MTR ID90 (μM) | 46 | 18 C17 ID90 (μM) | 46 | |
| 106 | 9 | – | – | – | – |
| 106-2ID10 | 14.7 | – | – | – | – |
| 106-17Ac | 21.1 | >200 | >200 | >6.25 | >6.25 |
| 106-17A-NDd | 21.1 | 182 | 147 | >6.25 | >6.25 |
| 713 | 6.9 | – | – | – | – |
| 713-M3 | 17.4 | – | – | – | – |
| 713-M3-C17e | 28.3 | >200 | 200 | >6.25 | >6.25 |
| 713-M3-C17-NDf | 15.1 | 189 | 92 | >6.25 | 3.1 |
ID90, concentration of drug at which 10% of control parasite ATP levels are detected.
Applies to 106-17A-ND (no drug) and 713-M3-C17-ND (no drug) lines only.
Generation time (g) in hours calculated using the formula [26]: g = T2 − T1/log2 (n2/n1).
C17-resistant line was cultured in the presence of 10 μM C17. Assays were performed at 704 days and 732 days following first exposure of line to C17.
C17-resistant line was cultured in the presence of 10 μM C17 prior to removal of drug from culture at 686 days after first exposure of line to C17 to produce line 106-17A-ND.
C17-resistant line was cultured in the presence of 7.5–10 μM C17. Assays were performed at 503 days and 531 days following first exposure of line to C17.
C17-resistant line was cultured in the presence of 7.5 μM C17 prior to removal of drug from culture at 485 days after first exposure of line to C17 to produce line 713-M3-C17-ND.
Using an alternative method, we exposed isolate 106, over 1 month, to three spikes of 10–20 μM C17 for 1 h periods, followed by recovery in drug-free medium for several days and then exposure to sequential culture in 1–10 μM C17 (Fig. 2). Four lines survived this treatment, with the first two lines succumbing to 5 μM C17 by 214 days and 235 days, respectively, of C17 treatment (data not shown). The third line succumbed by 238 days of C17 treatment, only reaching survival in 2–4 μM C17 (data not shown). However, during the 9-month treatment period, a fourth line survived resulting in the C17R line (Fig. 2), designated 106-17A that is maintained in 10 μM C17. 106-17A has a generation time of 21.1 h, 2.3 times greater than the parent isolate 106 that has a generation time of 9 h (Table 2), whilst the MTRR line 106-2ID10 has a generation time of 14.7 h. On removal of C17 drug pressure from culture 106-17A-ND for up to 40 days, the generation time remained constant at 21.1 h (Table 2).
3.3. Drug susceptibility of metronidazole- and C17-resistant Giardia lines
Giardia lamblia drug-resistant lines were exposed to test drugs under anaerobic conditions and ATP concentrations were measured to determine growth inhibition or drug resistance of the parasites. The ID90 of C17 against the C17R line 106-17A ranged from 1.3 μM to 8.6 μM [assays performed from 208 days following exposure to C17 (1.3 μM) to the present time (8.6 μM)], with the average ca. 8-fold higher than against the parent 106 isolate (average ID90 of 0.54 μM for 106, increased to average ID90 of 4.2 μM for 106-17A) (Table 1; Fig. 3). This level of resistance was maintained by 106-17A when cultured in the absence of C17 drug pressure for up to 46 days, producing an ID90 of >6.25 μM (Table 2) and demonstrating no short-term (over 40 days) loss in C17 resistance. Against the MTRR line (106-2ID10), the ID90 for C17 ranged between 1.23 μM and 2.23 μM (Table 1; Fig. 3), with the average (1.7 μM) being ca. 3-fold higher than for the parent isolate 106 (average 0.54 μM). Similarly, increased C17 average ID90 values of 1.9 μM and 4.1 μM for the 713 MTRR line and 713 MTRRC17R line were seen compared with 0.46 μM for the MTRS parent isolate (Table 1 for ID90 ranges; Fig. 3). C17 resistance was maintained by 713-M3-C17 cultured without C17 drug pressure, with an ID90 for C17 of >6.25 μM after 18 days without drug and of 3.1 μM after 46 days without drug. C14 was more effective than C18 against MTRR and C17R lines, with ID90 values ranging from 1.68 μM to 3.8 μM for C14 compared with 2.65 μM to 11 μM for C18 (Table 1). Without a ring structure in the 2-position side chain, ronidazole was clearly less effective than C14, C17 and C18 against the MTRR and C17R lines (Table 1; Fig. 3).
Fig. 3.
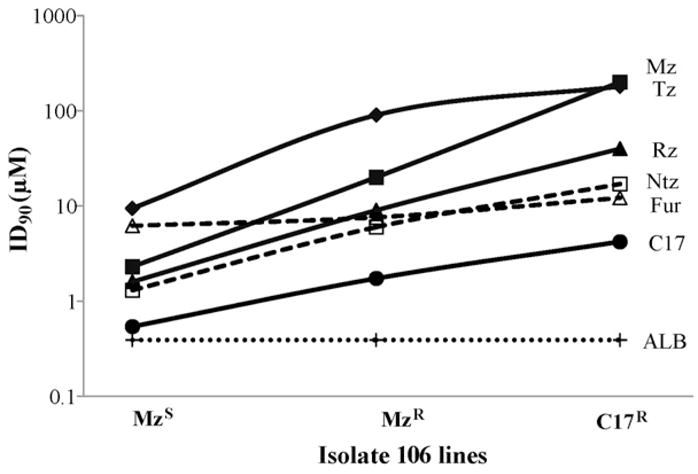
Increase in resistance to the 5-nitroimidazole (NI) drugs by the Giardia metronidazole-resistant (MTRR) and C17-resistant (C17R) 106 lines compared with the metronidazole-susceptible (MTRS) parent isolate. ID90 values (concentration of drug at which 10% of control parasite ATP levels are detected) are expressed as μM on a log scale (y-axis) for: the 1-position 5-NIs metronidazole (MTR) and tinidazole (Tz); the 2-position 5-NIs C17 and ronidazole (Rz); the 5-nitrofuran furazolidone (Fur); and the 5-nitrothiazole nitazoxanide (Ntz). For comparison, the minimal inhibitory concentration of 0.39 μM of the benzimidazole albendazole (ALB) for both 106 lines and the parent isolate is included.
Furazolidone and nitazoxanide had ID90 values against MTRS isolates of 6.2–7.35 μM and 1.3–2.2 μM, respectively (Table 1). However, against the MTRR (713-M3) and C17R (106-17A and 713-M3-C17) lines, ID90 values for furazolidone (11–20.7 μM) and nitazoxanide (10–17 μM) were higher, indicating cross-resistance to the 5-NI drugs (Table 1; Fig. 3). Albendazole, a benzimidazole antigiardial drug with no nitro group, was equally effective against all MTRS, MTRR and C17R lines, with a maximum MIC of 0.39 μM (Fig. 3) (see Section 3.1).
Tinidazole, ornidazole and MTR were less effective than all of the 2-position 5-NI drugs against the MTRR lines, with MTR having an ID90 of 90 μM and 97 μM against 106-2ID10 and 713-M3, respectively (Table 1). Tinidazole and ornidazole were at least two-fold more effective against 106-2ID10 than 713-M3. However, the ID90 values for all of the 1-position 5-NIs against the C17R lines were ca. 200 μM (Table 1; Fig. 3). Of note, line 106-17A was not exposed to MTR or any other 1-position 5-NI at any stage prior to drug susceptibility assessment. MTR resistance to the C17R lines remained high even when cultured without drug pressure for up to 18 days and 46 days, with ID90 values of 182 μM and 147 μM, respectively, for 106-17A, and 189 μM and 92 μM, respectively, for 713-M3-C17 (Table 2). These results demonstrate the highest level of MTR resistance among any Giardia isolates or lines so far reported.
3.4. Pyruvate:ferredoxin oxidoreductase in drug-resistant lines
We and others have shown a decrease in both PFOR and FDOX protein and mRNA in MTRR cells [13–15]. Our data were obtained primarily using the line 106-2ID10 in comparison with its parent isolate 106 and the conclusions were that the PFOR/FDOX couple activates MTR to its toxic state and that these decreased levels of proteins allow the cell to become MTRR. We repeated activity assays and indeed showed that 106-2ID10 has decreased PFOR activity in comparison with its MTRS parent isolate (Fig. 4A). Decreased levels of PFOR in 106-2ID10 were confirmed by Western blot data using an antibody raised against purified PFOR (Fig. 4B). However, the derived C17R line 106-17A did not show any decrease in PFOR activity or concentration but is clearly highly MTRR (Fig. 4).
Fig. 4.
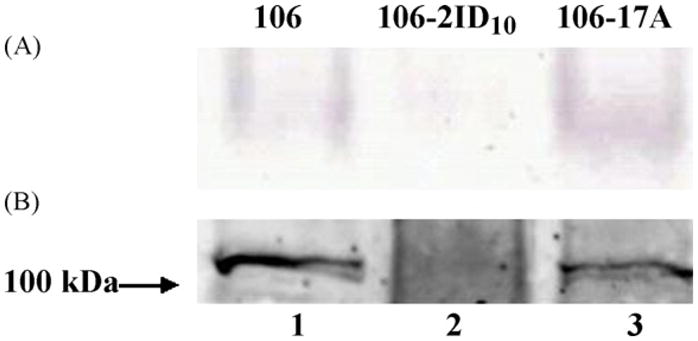
(A) Pyruvate:ferredoxin oxidoreductase (PFOR) activity gel and (B) Western blot of Giardia lamblia 106 isolate and drug-resistant lines106-2ID10 and 106-17A. 40 μg of cell lysate of isolate 106 (lane 1), of the metronidazole-resistant (MTRR) line 106-2ID10 (lane 2) and the C17-resistant (C17R) line 106-17A (lane 3) was loaded in each lane. (A) PFOR was separated in a non-denaturing polyacrylamide gel and its activity was determined by the precipitate of insoluble blue nitrotetrazolium blue chloride in the gel following reduction by PFOR. (B) PFOR, a homodimer of 138 kDa subunits [13], was identified after separation by denaturing polyacrylamide gel electrophoresis (PAGE) with polyclonal mouse anti-PFOR antiserum. The markers were Precision Plus Protein™ WesternC™ Standards (Bio-Rad).
4. Discussion
The 2-position 5-NI compound C17 is extremely effective against G. lamblia in vitro, with ID90 values 14.3–17.7-fold more effective than MTR. These ID90 values are consistent with our previous reports using different assay readouts [10,17]. Also consistent with C17 being highly effective was the difficulty in developing C17R G. lamblia lines. Many unsuccessful attempts were made to grow a range of isolates and lines in low levels of C17, including quinacrine- and albendazole-resistant lines that clearly have different mechanisms of resistance to the 5-NI-resistant lines [24,25]. However, after ca. 9 months of drug concentration spikes and incremental increases, we produced a line, 106-17A, that now grows in 10 μM C17. This line not surprisingly has an eight-fold higher ID90 for C17 than the parent isolate 106 from which it was derived. What is surprising is the >200 μM ID90 of MTR against the C17R line (which has never been exposed to MTR prior to the assay). The ID90 against the syngeneic MTRR line is less than half this value.
The basis of resistance to C17 in 106-17A is not the same as resistance to MTR in 106-2ID10. In the latter, resistance appears to be a result of downregulation of PFOR activity, as shown here and previously [13]. Importantly, Dan et al. [27] have also shown increased MTR resistance when PFOR was knocked down. In contrast, the highly MTRR 106-17A has normal PFOR activity (perhaps even higher than its parent isolate) and protein levels as determined in activity gels and by Western blotting, respectively. Therefore, in this C17R line, the unusually high levels of MTR cross-resistance do not correlate with decreased PFOR levels and thus a new mechanism of MTR resistance must apply. Argüello-García et al. [28] reported that their MTRR lines produced after extensive exposure to MTR did not have downregulated PFOR and they suggest that induction of MTR resistance is multifactorial, highlighting the molecular variability and variation in Giardia.
A second line, 713-M3-C17, developed to be C17R was already MTRR (713-M3). However, the ID90 of this line against MTR was greater than two-fold higher than its MTRR parent line and it too has normal PFOR activity (data not shown). In addition, 106-17A and 713-M3-C17 are 1.6–4-fold more resistant to ronidazole and 4–10-fold more resistant to tinidazole and ornidazole than the MTRR lines 106-2ID10 and 713-M3. 106-17A and 713-M3-C17 are more resistant to MTR than any MTRR G. lamblia line or isolate previously reported. The C17R lines and, to a lesser extent, the MTRR lines demonstrate cross-resistance (assuming all of the 5-NIs have similar mechanisms of resistance) against the 5-nitrofuran furazolidone and the 5-nitrothiazole nitazoxanide. These data suggest a common mechanism of resistance in Giardia against the 5-NIs and nitazoxanide [15,29] and between 5-NIs and furazolidone, which we have previously reported is activated by the enzyme NADH oxidase [30]. Albendazole, which targets the cytoskeleton of Giardia, is equally effective (as measured by MIC) against all the lines and isolates described here, indicating a mechanism of resistance in the C17R lines specific for the 5-NI drugs. Our albendazole data are a working example of how a single assay system (e.g. measurement of ATP) cannot be applied in all cases. Drug efficacy against susceptible and resistant populations is also commonly measured by its lethality (minimum lethal concentration) [31].
When C17 was removed from the medium of the C17R lines, susceptibility to C17 and MTR and growth rate did not return to parent levels even after several weeks, indicating generalised and possibly permanent changes in the parasites. Identification of these changes awaits mechanistic and genomic studies.
Not all 2-position 5-NIs are effective antiprotozoal agents [16,17], but having a ring structure in the 2-position side chain appears to favour increased efficacy over MTR against G. lamblia. This is a generalisation from our previous studies [10,17] and these data with C17, C14 and C18 support this. However, ronidazole, with no ring structure in its side chain, is clearly more effective than the 1-position 5-NIs shown here and previously [23].
In addition to the 1- and 2-position 5-NIs, two 4-position 5-NIs have been reported to be more effective than MTR against T. vaginalis [11]. However, using the compounds described in Crozet et al. [11] we have not been able to show any improved efficacy over MTR of any 4-position 5-NIs against G. lamblia (data not shown). This result suggests different mechanisms of activation of the 5-NIs in different species.
We have previously demonstrated that purified Giardia PFOR and FDOX can reduce MTR in vitro [5,13]. This presumably translates to the same pathway of MTR activation in vivo and we assume this would apply to activation of all 5-NI compounds, although this has not been demonstrated as yet. A similar proposed mechanism of action in Trichomonas and Entamoeba, however, has been queried by Leitsch et al. [7,8] who claim that reduction via TrxR is the major pathway for MTR and tinidazole activation in these organisms and that 5-NI resistance also involves thioredoxin pathways. Rasoloson et al. [32] believe that hydrogenosomal malic enzyme is involved in MTR reduction and, like PFOR, is downregulated in MTRR trichomonads. Pal et al. [33] also raise alternative mechanisms of MTR activity in Giardia, Trichomonas and Entamoeba by oxygen-insensitive nitroreductases and nim gene products, known to be involved in MTR activation and resistance in bacteria, but the action of these has not yet been implicated in wild-type parasites.
The ability of Giardia to develop resistance against a highly effective drug such as C17 is not likely to affect its clinical potential, since C17 remains lethal to C17R cells at concentrations of drug (ca. 4 μM) lower than the concentration of MTR necessary to kill MTRS isolates (ca. 6–10 μM). In addition, the slow rate of resistance developed in vitro against C17 is highly unlikely to be seen clinically. C14 and C18 are also effective against C17R lines, with ID90 values of ≤11 μM. These drugs and others [10] are more likely to be developed clinically since the C17 Br moiety appears to be toxic [10].
This work demonstrates the presence of alternative mechanisms of 5-NI resistance in Giardia that may also compromise other 5-NI drugs. We earlier suggested that more than one mechanism of MTR resistance in Giardia was probable [13]. We have also argued that the development of isogenic lines of drug-selected resistant cells by diverse selection protocols will provide a base for analysing clinical isolates and determining the subsets of possible evolving resistance mechanisms on a controlled background of possibilities that can then be dissected to their validity and importance [34]. Newly discovered mechanisms can then be placed in context.
Furthermore, the development of new drugs is extremely important in this dissection and discovery approach and will be so in the future when we further analyse the pharmacodynamics and pharmacokinetics of drug handling and delivery of new analogues (of which we already have more potent 5-NIs than C17 [10]) and the possibility of drug resistance development.
Acknowledgments
The authors thank Marc S. Ayers from Romark Laboratories, L.C. (Tampa, FL) for the nitazoxanide.
Funding: This work was supported by the National Health and Medical Research Council of Australia, the Australian Research Council and by U01 Cooperative Research Agreement AI75527 from the US National Institutes of Health (NIH). This study was facilitated by the commissioning of synthesis of C17 by NIH from Southern Research Institute (USA).
Footnotes
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–64. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan KSW. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev. 2008;21:639–65. doi: 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Land KM, Johnson PJ. Molecular basis of metronidazole resistance in pathogenic bacteria and protozoa. Drug Resist Updat. 1999;2:289–94. doi: 10.1054/drup.1999.0104. [DOI] [PubMed] [Google Scholar]
- 4.Edwards DI. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 5.Townson SM, Hanson GR, Upcroft JA, Upcroft P. A purified ferredoxin from Giardia duodenalis. Eur J Biochem. 1994;220:439–46. doi: 10.1111/j.1432-1033.1994.tb18641.x. [DOI] [PubMed] [Google Scholar]
- 6.Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983;93:165–71. [PubMed] [Google Scholar]
- 7.Leitsch D, Kolarich D, Wilson IBH, Altmann F, Duchêne M. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol. 2007;5:1820–34. doi: 10.1371/journal.pbio.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitsch D, Kolarich D, Binder M, Stadlmann J, Altmann F, Duchêne M. Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol Microbiol. 2009;72:518–36. doi: 10.1111/j.1365-2958.2009.06675.x. [DOI] [PubMed] [Google Scholar]
- 9.Dunne RL, Dunn LA, Upcroft P, O’Donoghue PJ, Upcroft JA. Drug resistance in the sexually transmitted protozoan Trichomonas vaginalis. Cell Res. 2003;13:239–49. doi: 10.1038/sj.cr.7290169. [DOI] [PubMed] [Google Scholar]
- 10.Valdez CA, Tripp JC, Miyamoto Y, Kalisiak J, Hruz P, Andersen YS, et al. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J Med Chem. 2009;52:4038–53. doi: 10.1021/jm900356n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crozet MD, Botta C, Gasquet M, Curti C, Rémusat V, Hutter S, et al. Lowering of 5-nitroimidazole’s mutagenicity: towards optimal antiparasitic pharmacophore. Eur J Med Chem. 2009;44:653–9. doi: 10.1016/j.ejmech.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Lemée V, Zaharia I, Nevez G, Rabodonirina M, Brasseur P, Ballet JJ, et al. Metronidazole and albendazole susceptibility of 11 clinical isolates of Giardia duodenalis from France. J Antimicrob Chemother. 2000;46:819–21. doi: 10.1093/jac/46.5.819. [DOI] [PubMed] [Google Scholar]
- 13.Townson SM, Upcroft JA, Upcroft P. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol Biochem Parasitol. 1996;79:183–93. doi: 10.1016/0166-6851(96)02661-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu SM, Brown DM, O’Donoghue P, Upcroft P, Upcroft JA. Ferredoxin involvement in metronidazole resistance of Giardia duodenalis. Mol Biochem Parasitol. 2000;108:137–40. doi: 10.1016/s0166-6851(00)00194-8. [DOI] [PubMed] [Google Scholar]
- 15.Müller J, Sterk M, Hemphill A, Müller N. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. J Antimicrob Chemother. 2007;60:280–7. doi: 10.1093/jac/dkm205. [DOI] [PubMed] [Google Scholar]
- 16.Upcroft JA, Campbell RW, Benakli K, Upcroft P, Vanelle P. Efficacy of new 5-nitroimidazoles against metronidazole-susceptible and -resistant Giardia, Trichomonas, and Entamoeba spp. Antimicrob Agents Chemother. 1999;43:73–6. doi: 10.1128/aac.43.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upcroft JA, Dunn LD, Wright JM, Benakli K, Upcroft P, Vanelle P. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob Agents Chemother. 2006;50:344–7. doi: 10.1128/AAC.50.1.344-347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upcroft JA, Boreham PFL, Campbell RW, Shepherd RW, Upcroft P. Biological and genetic analysis of a longitudinal collection of Giardia samples derived from humans. Acta Trop. 1995;60:35–46. doi: 10.1016/0001-706x(95)00100-s. [DOI] [PubMed] [Google Scholar]
- 19.Townson SM, Laqua H, Upcroft P, Boreham PFL, Upcroft JA. Induction of metronidazole and furazolidone resistance in Giardia. Trans R Soc Trop Med Hyg. 1992;86:521–2. doi: 10.1016/0035-9203(92)90095-t. [DOI] [PubMed] [Google Scholar]
- 20.Boreham PFL, Phillips RE, Shepherd RW. Altered uptake of metronidazole in vitro by stocks of Giardia intestinalis with different drug sensitivities. Trans R Soc Trop Med Hyg. 1988;82:104–6. doi: 10.1016/0035-9203(88)90278-7. [DOI] [PubMed] [Google Scholar]
- 21.Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329–41. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upcroft JA, Upcroft P. Drug susceptibility testing of anaerobic protozoa. Antimicrob Agents Chemother. 2001;45:1810–4. doi: 10.1128/AAC.45.6.1810-1814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boreham PFL, Phillips RE, Shepherd RW. A comparison of the in-vitro activity of some 5-nitroimidazoles and other compounds against Giardia intestinalis. J Antimicrob Chemother. 1985;16:589–95. doi: 10.1093/jac/16.5.589. [DOI] [PubMed] [Google Scholar]
- 24.Upcroft JA, Campbell RW, Upcroft P. Quinacrine-resistant Giardia duodenalis. Parasitology. 1996;112:309–13. doi: 10.1017/s0031182000065823. [DOI] [PubMed] [Google Scholar]
- 25.Upcroft J, Mitchell R, Chen N, Upcroft P. Albendazole resistance in Giardia is correlated with cytoskeletal changes but not with a mutation at amino acid 200 in β-tubulin. Microb Drug Resist. 1996;2:303–8. doi: 10.1089/mdr.1996.2.303. [DOI] [PubMed] [Google Scholar]
- 26.Boreham PFL, Phillips RE, Shepherd RW. The sensitivity of Giardia intestinalis to drugs in vitro. J Antimicrob Chemother. 1984;14:449–61. doi: 10.1093/jac/14.5.449. [DOI] [PubMed] [Google Scholar]
- 27.Dan M, Wang AL, Wang CC. Inhibition of pyruvate–ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol Microbiol. 2000;36:447–56. doi: 10.1046/j.1365-2958.2000.01863.x. [DOI] [PubMed] [Google Scholar]
- 28.Argüello-García R, Cruz-Soto M, Romero-Montoya L, Ortega-Pierres G. In vitro resistance to 5-nitroimidazoles and benzimidazoles in Giardia duodenalis: variability and variation in gene expression. Infect Genet Evol. 2009;9:1057–64. doi: 10.1016/j.meegid.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Wright JM, Dunn LA, Upcroft P, Upcroft JA. Efficacy of antigiardial drugs. Expert Opin Drug Saf. 2003;2:529–41. doi: 10.1517/14740338.2.6.529. [DOI] [PubMed] [Google Scholar]
- 30.Brown DM, Upcroft JA, Upcroft P. A H2 O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. Eur J Biochem. 1996;241:155–61. doi: 10.1111/j.1432-1033.1996.0155t.x. [DOI] [PubMed] [Google Scholar]
- 31.Argüello-García R, Cruz-Soto M, Romero-Montoya L, Ortega-Pierres G. Variability and variation in drug susceptibility among Giardia duodenalis isolates and clones exposed to 5-nitroimidazoles and benzimidazoles in vitro. J Antimicrob Chemother. 2004;54:711–21. doi: 10.1093/jac/dkh388. [DOI] [PubMed] [Google Scholar]
- 32.Rasoloson D, Vanácová S, Tomková E, Rázga J, Hrdý I, Tachezy J, et al. Mechanisms of in vitro development of resistance to metronidazole in Trichomonas vaginalis. Microbiology. 2002;148:2467–77. doi: 10.1099/00221287-148-8-2467. [DOI] [PubMed] [Google Scholar]
- 33.Pal D, Banerjee S, Cui J, Schwartz A, Ghosh SK, Samuelson J. Giardia, Entamoeba, and Trichomonas enzymes activate metronidazole (nitroreductases) and inactivate metronidazole (nitroimidazole reductases) Antimicrob Agents Chemother. 2009;53:458–64. doi: 10.1128/AAC.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upcroft P. Drug resistance in Giardia: clinical versus laboratory isolates. Drug Resist Updat. 1998;1:166–8. doi: 10.1016/s1368-7646(98)80035-6. [DOI] [PubMed] [Google Scholar]