Abstract
In the last decade several experimental studies have demonstrated that particular patterns of synaptic activity can induce postsynaptic parallel fibre (PF) long-term potentiation (LTP). This form of plasticity can reverse postsynaptic PF long-term depression (PF-LTD), which has been traditionally considered the principal form of plasticity underlying cerebellar learning. Postsynaptic PF-LTP requires a transient increase of intracellular Ca2+ concentration and, in contrast to PF-LTD, is induced without concomitant climbing fibre (CF) activation. Thus, it has been postulated that the polarity of long-term synaptic plasticity is determined by the amplitude of the Ca2+ transient during the induction protocol, with PF-LTP induced by a smaller Ca2+ signals without concomitant CF activation. However, this hypothesis is contradicted by recent studies. A quantitative analysis of Ca2+ signals associated with induction of PF-LTP indicates that the bidirectional induction of long-term plasticity is regulated by more complex mechanisms. Here we review the state-of-the-art of research on postsynaptic PF-LTP and discuss the principal open questions on this topic.
Keywords: Animals, Humans, Long-Term Potentiation, physiology, Long-Term Synaptic Depression, physiology, Neurons, physiology, Purkinje Cells, physiology
Introduction
According to the classical theory, the activity-dependent weakening of erroneously activated cerebellar granule cell (CGN) to Purkinje neuron (PN) synapses is the most important form of synaptic plasticity in the cerebellum [1, 2]. This view gained importance with the discovery that pairing parallel fiber (PF) and climbing fiber (CF) activity could induce long-term depression (LTD) of PF inputs [3, 4]. An important characteristic of the depression of PF excitatory postsynaptic potentials (EPSPs) is the postsynaptic site of induction and expression. PF-LTD requires elevation of free intracellular calcium concentration ([Ca2+]i) [5] and activation of type-1 metabotropic glutamate receptors (mGluR1) [6–8], leading to a decrease in AMPA receptor efficacy [9] and internalization [10]. To reverse PF silencing, the necessity of a “reset” mechanism has been postulated [11–13].
In the last decade, the existence of postsynaptic forms of PF-LTP has been demonstrated [11, 12]. This form of plasticity is a potential reset mechanism because it shares the same site of expression of PF-LTD. It was first shown that postsynaptic PF-LTP could be induced by repetitive (1 Hz) PF stimulation without concomitant CF activation. The induction mechanism required elevation of [Ca2+]i, but smaller than that associated with the induction of CF-dependent PF-LTD [12]. This evidence suggested that the polarity of PF plasticity could be given by the size of the [Ca2+]i transient which is higher for LTD [12, 13]. The idea of different Ca2+ thresholds responsible for plasticity mechanisms of different polarity was proposed in 1982 [14] and termed “BCM rule”. According to the BCM rule, lower and higher Ca2+ transients are associated with the induction of LTD and LTP respectively. Here, we will use the term “inverse BCM rule” [14] to refer to a scenario where lower and higher Ca2+ transients are associated with the induction of LTP and LTD respectively.
Recently, it has been shown that local [Ca2+]i elevation associated with bursts of PF-EPSPs can be much larger than that associated with the CF-EPSP, but repetitive application of this protocol leads to postsynaptic PF-LTP [15]. This finding, together with the evidence that LTP could not be induced solely by local Ca2+ photo-release [16] suggested that bidirectional induction of plasticity cannot be explained only in terms of [Ca2+]i signal amplitude.
Here we review the most recent findings on PF-LTP induction mechanisms and discuss our current understanding.
Revisiting bidirectional induction of postsynaptic plasticity
PF-LTD is classically induced by pairing PF input (either single stimuli or bursts) with CF activity and CF activation has been substituted by postsynaptic depolarization [17, 18]. PF-LTD is also induced by strong PF stimulation without pairing with CF activity [19]. Although some molecular pathways leading to synaptic depression may be similar in different induction protocols, the expected time course and the spatial localization of triggering signals remain essentially diverse. The same argument applies to PF-LTP. Thus far, LTP was induced by three stimulation protocols, namely the “one-pulse induced PF-LTP”, the “burst induced PF-LTP” and “single-train induced PF-LTP” (see Fig.1).
Fig.1.
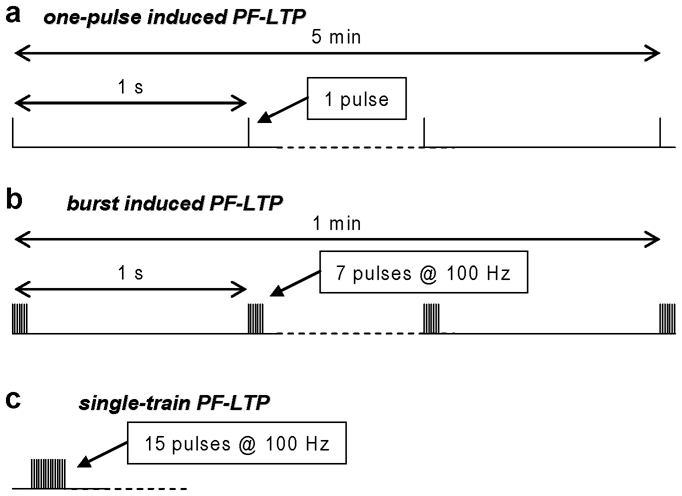
Protocols that induce postsynaptic PF-LTP. (a) One-pulse induced parallel PF-LTP: one PF stimulus repeated every second for five minutes. Characterised in brain slices of the rat at postnatal days 17–21 [11] and 18–27 [12]. (b) Burst induced parallel PF-LTP: A train of 7 PF stimuli at 100 Hz repeated every second for one minutes. Characterised in brain slices of the mouse at postnatal days 25-25, resistant to block of GABAA receptors, NMDA receptors and mGluR1 [14]. (c) Single-train induced parallel PF-LTP: One train of 15 PF stimuli at 100 Hz. Characterised in the mouse in vivo at postnatal months 5–8, abolished by blocking mGluR1 [19].
The first induction protocol to be characterized was the one-pulse induced PF-LTP (Fig. 1a), a repetition of a single pulse of PF stimulation at 1 Hz for 5 minutes [11, 12]. Pairing a PFEPSP with a CF-EPSP, with the CF-EPSP following the PF-EPSP, reversed LTP into LTD [12]. Both plasticity forms required transient [Ca2+]i elevation and 20 mM injection of the high affinity buffer BAPTA could lead to LTP induction with a CF pairing [12]. To interpret this result, it has been suggested that PF plasticity follows an inverse BCM rule compared to hippocampal synapses [13]. According to this view, LTP would be induced by a smaller [Ca2+]i signal compared to LTD and the additional Ca2+ would be provided by the CF depolarisation. Further support for this hypothesis came from a previous report showing that CF-EPSP was associated with a supra-linear Ca2+ signal and that for minimal PF activation this signal was mGluR1- dependent and limited to dendritic spines [20]. The inverse BCM rule hypothesis, however, did not take into account two factors that were reported in that study. First, the protocol used for pairing consisted of a burst of PF-EPSPs (typically 5–10 pulses at 100 Hz) and not in a single pulse. Second, by increasing the number of stimulated PF, a more prominent mGluR1- independent supra-linear Ca2+ signal was observed in a larger portion of the dendrite. It was later reported that the size of this supra-linear Ca2+ signal depended on the delay between the PF burst and the CF-EPSP, but this delay was different from that associated with mGluR1- dependent short-term synaptic depression [21] and PF-LTD [22]. Indeed, it has been shown that mGluR1-independent supra-linear Ca2+ summation is due to the local and transient saturation of the endogenous Ca2+ buffer and can elevate [Ca2+]i to micromolar concentrations, much higher compared to [Ca2+]i elevation associated with a CF-EPSP [15]. Repetition of a burst of 7 PFEPSPs at 1 Hz for one minute induced postsynaptic PF-LTP (burst induced PF-LTP, Fig 1b). The evidence that a [Ca2+]i signal larger than a CF-mediated [Ca2+]i signal induces LTP (and not LTD) led to a revision of the simple inverse BCM rule proposed before [12,13].
The general validity of the inversed BCM rule was also explored in a study where plasticity induction was tested by local Ca2+ photo-release. It was found that at high concentrations at spiny peri-dendritic regions could induce LTD, but no LTP was observed for smaller Ca2+ signals [16].
Both the one-pulse induced and the burst induced PF-LTP require repetitive application of the stimulating protocol. More recently, it was found that postsynaptic LTP can be also induced in vivo by a single train of 15 stimuli at 100 Hz (single-train induced PF-LTP, Fig. 1c) [23].
The interpretation of these experimental observations points to more complicated scenarios underlying the induction of postsynaptic PF-LTP, but also of PF-LTD. In the case of postsynaptic PF-LTD, the induction can occur with moderate PF stimulation and pairing with a CF-EPSP, or using a stronger PF stimulation intensity [15, 19] without concomitant CF stimulation. The burst of PF-EPSPs that induces PF-LTD is associated with a [Ca2+]i signal > 2 μM [15] and the evidence that Ca2+ photo-release can induce this form of plasticity [16] suggests that a large Ca2+ signal is sufficient to induce PF-LTD. In contrast, both postsynaptic PF-LTP and PF-LTD induced by weaker PF stimulation seem to require additional signalling associated with PF synaptic transmission. This signalling, that may involve well-defined spatio-temporal patterns of Ca2+ signals as well as Ca2+-independent biochemical pathways, may also be different for different protocols of plasticity induction. For instance, activation of mGluR1 was shown to be necessary for single-train induced PF-LTP [20] but not for burst induced PF-LTP [15].
The electrophysiological induction protocol is the first step of one or more sequences of events leading to a change in the efficacy or in the number of synaptic receptors. In this dynamic process, a measurable fundamental molecule is Ca2+. Thus the first aspect that will be carefully analysed is the spatial distribution and the time-course of Ca2+ signals associated with PF- and CF-EPSPs during different induction protocols. Ca2+ signals are fundamental variables in the biochemical cascades activated by the induction mechanisms, where other proteins are involved. For many different molecules, although a role in synaptic plasticity has been demonstrated, the direct association with a particular pathway was limited by the available pharmacological or genetic tools. Some controversial issues arising from molecular analysis are discussed below. Finally, upstream to the molecular pathways, induction protocols are produced by artificial electrical stimulation at a given site. Several studies have shown that induction protocols in brain slices can produce different results according to the position of the stimulating electrode and to the orientation of the slice. These discrepancies may be due to the architecture of the presynaptic fibres as well as to the localization of synaptic contacts and the use of spatially well defined stimulation may help to isolate specific signalling pathways. In addition, this information can shed light on the functional organization of the cerebellar circuitry. Thus, this is the third aspect addressed in this review.
Potential Ca2+ signals involved in postsynaptic PF-LTP induction
Although the size of [Ca2+]i transients cannot be directly correlated with the polarity of longterm synaptic plasticity, the spatial distribution and the time-course of Ca2+ signals can be a determinant for bidirectional induction of plasticity. Indeed, the contribution of Ca2+ in a particular pathway may in general depend on its co-localization with a particular Ca2+-binding protein (localization of Ca2+ signal) as well as on the kinetics of the Ca2+-protein interaction.
The contribution of Ca2+ entry via AMPA receptors is negligible in mature PNs [24] and there is no evidence of dendritic Ca2+ entry via NMDA receptors although there is recent evidence that these receptors are still expressed in PNs in mature mice [25]. Thus, following excitatory postsynaptic activity, [Ca2+]i elevation is due to Ca2+ entry via voltage-gated Ca2+ channels (VGCCs) activated by dendritic depolarisation, or by secondary pathways triggered by mGluR1 activation in the case of PF activity (Fig. 2a). Activation of VGCCs following dendritic depolarisation triggers local dendritic Ca2+ spikes [26, 27]. A single dendritic Ca2+ spike can be elicited either by a CF-EPSP, in the bulk of the dendritic arbour, or by one or more PF-EPSPs locally [15]. The fast [Ca2+]i elevation associated with a calcium spike has a peak of ~100–200 nM and a duration of few milliseconds as measured using low-affinity dyes [15, 28] and depicted in Fig. 2b. When several calcium spikes occur during a high-frequency burst, the transient saturation of the endogenous calcium buffer can elevate [Ca2+]i to higher concentrations (see Fig. 2c), depending on the number and the architecture of the PFs activated. This phenomenon can be observed only in association with PF-EPSP bursts, or with a CF-EPSP following a PFEPSP burst [15]. The time-course of the [Ca2+]i elevation following a PF-EPSP burst depends on the timing of calcium spikes. The different spatial distribution of calcium spikes elicited either by CF or PF stimulation can play an important role in the bidirectional induction of plasticity and this hypothesis should be further investigated.
Fig.2.
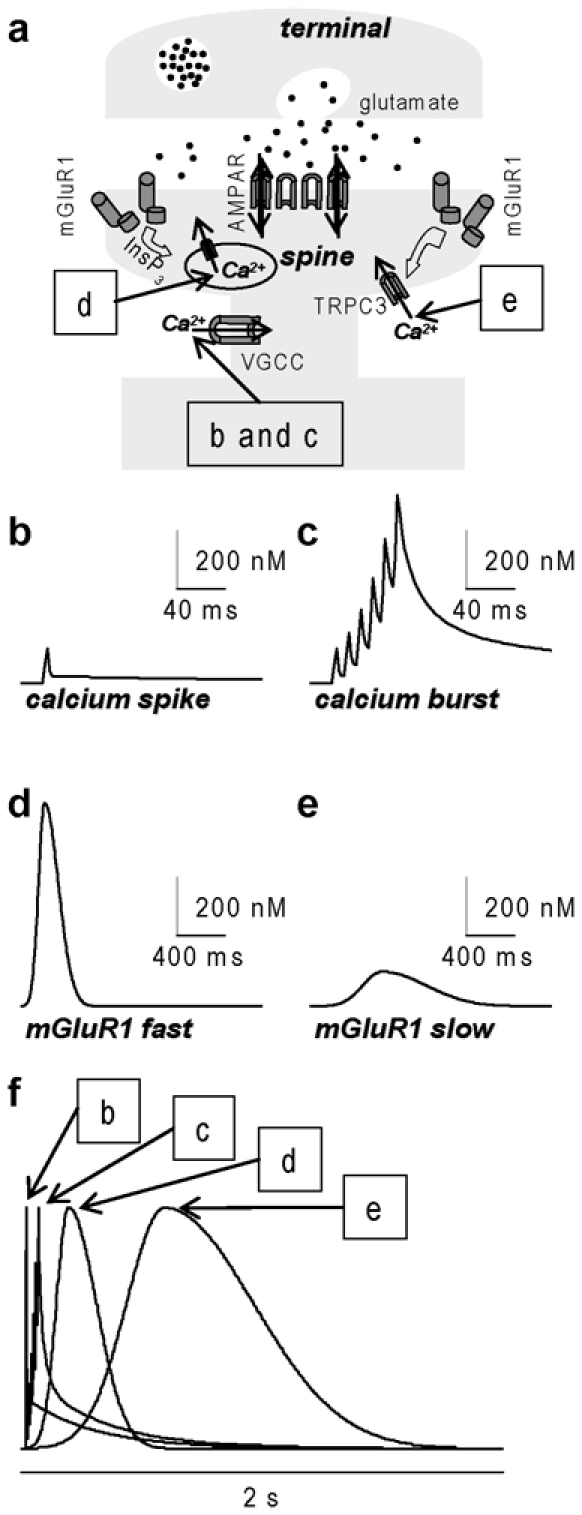
Schematic of four [Ca2+]i signals associated with excitatory synaptic transmission. (a) Associated with glutamate release from presynaptic terminals and depolarisation due to AMPA receptor (AMPAR) activation, [Ca2+]i can elevate via Ca2+ entry through VGCCs (signals b and c), via Ca2+ release from stores triggered by mGluR1 activation and InsP3 (signal d) and via Ca2+ entry through TRPC3 triggered by mGluR1 activation (signal e). (b) [Ca2+]i signal associated with one calcium spike: peak ~100–200 nM, duration ~10 ms; estimate from [14]. (c) [Ca2+]i signal associated with a burst of 6 calcium spikes at 100 Hz (calcium bursts): peak 0.5-2 μM, duration ~80 ms; estimate from [14]. (d) [Ca2+]i signal mediated by mGluR1 and Ca2+ release from stores (fast mGluR1): peak: ~1 μM, delay from mGluR1 activation ~50 ms, duration ~100 ms; estimate from [26]. (e) [Ca2+]i signal mediated by mGluR1 and slow Ca2+ influx (slow mGluR1): peak: ~100–200 nM, time-to-peak from mGluR1 activation ~0.5-1s, duration ~1s; estimate from [26, 30]. (f) The four [Ca2+]i signals normalised in amplitude and superimposed.
Activation of mGluR1 is associated with PF-EPSPs. Currently, there is evidence for two Ca2+ signals mediated by mGluR1 activation [29]. The faster signal is Ca2+ release from stores via InsP3 receptors. Evidence from several laboratories indicates that Ca2+ release from stores can be localised in dendritic spines [29–31]. In rats, this signal has well-defined kinetics, as depicted in Fig. 2d, and it peaks ~100 ms after mGluR1 activation [29]. It is primed by Ca2+ influx [29] and facilitated by a concomitant CF-EPSP [32]. A slower signal is Ca2+ influx via nonselective cation conductance [33]. A recent study suggested that this conductance is the TRPC3 channel, widely expressed in PNs [34]. This signal is slower and smaller in size compared to Ca2+ release from stores [29] as depicted in Fig. 2e.
Fig. 2f shows the four [Ca2+]i transients reported in Fig. 2b–e normalised and superimposed. The time course of different [Ca2+]i transients can play a role in synaptic plasticity by selective activation of proteins with different kinetics. Both Ca2+ influx associated with Ca2+ spikes and Ca2+ release from stores can in principle occur in dendritic spines, in the bulk of the dendrite or in both and a different spatial distribution can result in the activation of different molecular pathways. Finally, the interplay of [Ca2+]i transients with well-defined kinetics can play a crucial role in the timing of signals associated with different patterns of stimulation that induce long-term synaptic plasticity.
In conclusion, it must be pointed out that in induction mechanisms produced by repetitive stimulation not only the [Ca2+]i transient associated with one individual stimulation, but also its evolution during the repetition as well as the change in the basal [Ca2+]i must be taken into account.
Molecular pathways associated with repetitive stimulations
It is well established that changes in the phosphorylation state of AMPA receptor subunits regulate the efficacy of AMPA receptors to glutamate binding as well as their insertion/internalization balance (for a review see [35]). Thus, research on molecular cascades leading to different forms of postsynaptic cerebellar plasticity focused on pathways that could produce changes in kinase/phosphatase activation. Several types of kinase inhibitors affect LTD (see for a review [17]). However, the same kinase/phosphatase may act at different stages of a molecular cascade or within different concomitant pathways. This multiple action of a single enzyme complicates the analysis and generates discrepancies in the interpretation of results. A typical example is the old controversy on the role of protein kinase C (PKC). At postsynaptic PNs, PKC can phosphorylate the ser880 site of GluR2 leading to its internalization [36], but also interacts with mitogen-activated protein kinase involved in LTD [37]. Presynaptically, PKC regulates the granule cell synthesis of nitric oxide (NO) [38] which is involved both in LTP [11] and in LTD [39] induction. Thus, unspecific block of PKC prevents CF-dependent LTD in cultures [40], but this form of plasticity is surprisingly still intact in mice which are deficient of PKC-γ, exclusively expressed in PNs [41].
In a recent investigation, it was reported that inhibition of several types of phosphatases prevents postsynaptic LTP, suggesting that bidirectional plasticity may be finally controlled by the balance of kinase/phosphatase activation [42]. Interestingly, in another report, LTP was prevented by the block N-ethylmaleimide-sensitive factor, suggesting that the binding of this molecule to GluR2 and not GluR2 dephosphorylation may be responsible for LTP [43].
In summary, it is important to remark that mechanisms underlying different induction protocols are likely to be associated with different molecular pathways, but the interpretation of results from pharmacological and genetic explorations is often limited by the fact that the same molecule may be involved in more steps of a putative molecular pathway underlying synaptic plasticity. In addition, the situation is further complicated by the involvement of the same molecule in pathways occurring in parallel during the application of a particular induction protocol.
Geometric constraints of postsynaptic LTP and LTD induction
In brain slices, EPSPs (and related induction protocols) are evoked by a stimulating electrode positioned at a particular site of the slice cut with a particular orientation. Whereas initial studies on cerebellar plasticity did not take into account this factor, more recently it was found that an important aspect of postsynaptic PF-LTP (or PF-LTD) induction is its critical dependence on the spatial organisation of the synapses with respect to dendritic geometry which reflects the architecture of the afferents. PF synapses, formed along the parallel axons of the molecular layer, are ~90% of the contacts between granule cells and PNs, whereas the remaining 10%, called ascending fibers (AFs), are formed in the ascending tracts of the granule cell axons [44]. In experiments performed in coronal slices of the rat, where activation of PF and of AF synapses can be separated (Fig. 3a), both one-pulse induced parallel PF-LTP and PFLTD have been shown to be inducible only by PF stimulation and not by AF stimulation [45, 46].
Fig.3.
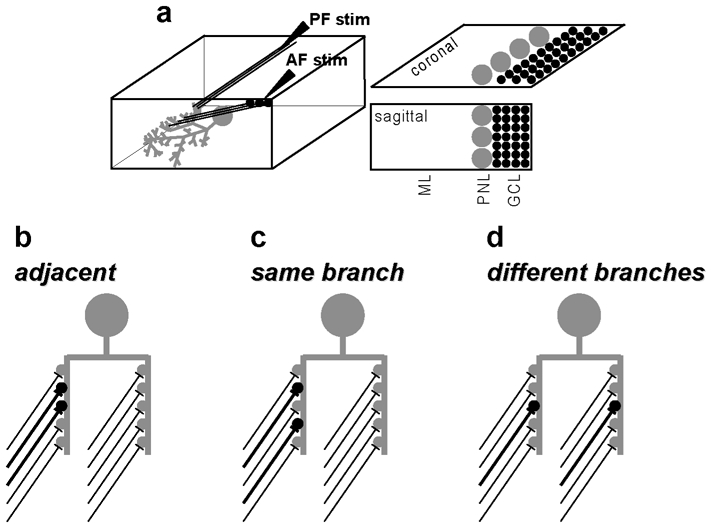
Possible spatial arrangement of two simultaneously active CGC-PN synapses. (a) Schematic of a sagittal/coronal section of the cerebellum with Molecular Layer (ML), PN Layer (PNL) and Granule Cell layer (GCL); PF stimulation: stimulation in the ML; AF stimulation: stimulation in the GCL behind the PN. (b) Adjacent: afferents contacting two adjacent spines in the same dendritic branch allowing for chemical crosstalk between the two synapses. (c) Same branch: afferents contacting non-adjacent spines but in the same dendritic branch allowing for local depolarisation of the dendrite. (d) Different branches: afferents contacting two different dendritic branches.
The postsynaptic PF-LTD induced by pairing the one-pulse protocol with CF stimulation is mGluR1-dependent and a critical correlation was found between glutamate spillover and PFLTD induction [47]. Thus, in another study, it was suggested that the difference between PF and AF susceptibility reported before [46] was actually due to the spatial arrangement of the afferents [48]. According to this view, PF stimulation results in the activation of afferents contacting postsynaptic Purkinje neurons at adjacent dendritic spines and in the local glutamate accumulation necessary for spillover and chemical crosstalk among adjacent spines.
Because the one-pulse induced LTD was shown to be different for the PF and the AF pathways, the same type of analysis in coronal slices was performed for the burst-induced PFLTP [15]. In contrast to what reported for one-pulse induced plasticity, LTP was found both at the PF and at the AF pathways. This induction protocol did not require mGluR1-mediated Ca2+ signals [15] associated with glutamate spillover [47], but required Ca2+ influx associated with Ca2+ spikes that could be elicited both by the activation of the PF and the AF pathways [15].
To explore more in details the differences in synaptic plasticity, regardless of whether synaptic contacts originate from PF or AF pathways, we summarize in Fig.3a which hypothetical geometric scenarios of CGC-PN synapses can occur under different constraints. In the first case, depicted in Fig. 3b, adjacent CGC axons are stimulated. This configuration can occur by stimulating in the vicinity of the PN dendrite in sagittal slices or in the molecular layer in coronal slices. Thus, CGC-PN synapses can target adjacent spines allowing for glutamate accumulation, transient uptake saturation and glutamate spillover onto adjacent spines [47, 48]. In the second case, depicted in Fig. 3c, axons that activate spines belonging to the same dendritic branch (but not adjacent) are excited. This configuration may be what happens when AF are stimulated in coronal slices, by position the stimulating electrode in the granule cell layer below the PN. Under this condition, synaptic activation may be insufficient for chemical crosstalk but may still allow localised depolarisation and excitation. Finally, sparse spines belonging to different dendritic branches may be activated (see Fig. 3d). This configuration can hypothetically occur by using more stimulating electrodes.
The evidence that different forms of LTP (and LTD) are remarkably dependent on the physical arrangement of the afferents implies an important functional role of the spatial organization of the CGC-PN synapses. The possibility of synaptic crosstalk, either by activation of adjacent synapses via glutamate spillover, or by the local summation of depolarisation leading to dendritic calcium spikes, may underlie the translation of the spatial organisation into a functional organisation of PF afferents.
Summary and future directions
It is proven that both postsynaptic PF-LTP and postsynaptic PF-LTD require [Ca2+]i elevation, but the hypothesis that the polarity of postsynaptic PF long-term synaptic plasticity is determined by Ca2+ signals of different amplitude appears unlikely. More realistically, [Ca2+]i elevation following PF and/or CF activation may occur at different spatial and temporal scales triggering local biochemical pathways and activating sensors with different kinetics. In addition, Ca2+-dependent pathways may interact with Ca2+-independent pathways as suggested by the evidence that Ca2+ photo-release does not mimic the induction of long-term synaptic plasticity observed by synaptic stimulation [16]. In these experiments, PF-LTP was not observed even when [Ca2+]i was within the range normally associated with LTP. This leaves two options: either the Ca2+ signal did not properly mimic the physiological signal or the synaptically evoked Ca2+ increase is accompanied by an as-of-yet undetermined signal. The first option might be explored by optimizing the spatio-temporal characteristics of the photo-released Ca2+. For the second option, some of the candidates of necessary signals that are not present by Ca2+ photo-release are presynaptic release of NO or postsynaptic binding of glutamate to the AMPA receptors and subsequent membrane depolarization.
The mechanisms and conditions necessary for the induction of postsynaptic PF-LTP are a cornerstone in the relationship between cerebellar activity and memory formation. Our current understanding on this topic is a mosaic of information with several apparent discrepancies. Part of these discrepancies arise from the fact that postsynaptic PF-LTP has been characterised under different experimental conditions, i.e. different induction stimulation protocols (see Fig. 1) but also different species and different stages of development. For instance, whereas in vitro studies in rats [11, 12] and mice [15] have been carried out in 3–5 weeks old animals, in vivo LTP has been characterised in adult mice [23]. Interestingly, one-pulse induced PF-LTP, as well as PF-LTD induced by CF pairing, have been observed also in a non-mammalian nervous system [49]. Other discrepancies originate from the incomplete interpretation of non-quantitative data on Ca2+ signalling, missing information on the spatio-temporal distribution of [Ca2+]i transients associated with the different induction protocols. Advanced optical techniques are available, allowing Ca2+ measurements at the level of single spines [50, 51]. In particular, using two-photon microscopy, it is possible to measure [Ca2+]i transients from individual spines using low affinity dyes [20] that produce minimal perturbation of the physiological [Ca2+]i dynamics. The use of two-photon voltage measurements, recently available [52], may provide additional information on the spatial distribution of local excitation that appears essential for long-term plasticity induction. A final aspect that may produce controversial results is the spatial arrangement of CGC-PN synapses, which mirrors the highly ordered functional architecture of the cerebellar circuitries. The use of stimulating electrodes can bias the conditions for postsynaptic PF-LTP induction by artificially selecting pathways of stimulation and these pathways may be very different in different experimental conditions. This aspect must be taken into account especially when performing experiments on sagittal cerebellar slices, which are ideal for imaging studies, but preclude the possibility to control the arrangements of the stimulated presynaptic afferents.
Acknowledgments
This work was supported by the University of Basel and by the SNSF grant 3100A0_122000 (M.C.). We thank prof. Josef Kapfhammer for valuable comments on the manuscript.
References
- 1.Marr D. A theory of cerebellar cortex. J Physiol (Lond ) 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus JS. A theory of cerebellar function. Math Biosci. 1971;28:167–171. [Google Scholar]
- 3.Ito M, Sakurai M, Tongroach P. Climbing fiber induced depression of both mossy fiber responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol (Lond) 1982;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karachot L, Kado RT, Ito M. Stimulus parameters for induction of long-term depression in in vitro rat Purkinje cells. Neurosci Res. 1995;21:161–168. doi: 10.1016/0168-0102(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 5.Konnerth A, Dreessen J, Augustine GJ. Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 1992;89:7051– 7055. doi: 10.1073/pnas.89.15.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, et al. Deficient cerebellar longterm depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 7.Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 8.Hartell NA. Induction of cerebellar long-term depression requires activation of glutamate metabotropic receptors. NeuroReport. 1994;5:913–916. doi: 10.1097/00001756-199404000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Hemart N, Daniel H, Jaillard D, Crepel F. Properties of glutamate receptors are modi. ed during long-term depression in rat cerebellar Purkinje cells. Neurosci. 1994;19:213–221. doi: 10.1016/0168-0102(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci U S A. 2002;99:8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;18:691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canepari M, Vogt KE. Dendritic Spike Saturation of Endogenous Calcium Buffer and Induction of Postsynaptic Cerebellar LTP. PLoS ONE. 2008;3:e4011. doi: 10.1371/journal.pone.0004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka K, Khiroug L, Santamaria F, Doi T, Ogasawara H, Ellis-Davies GC, Kawato M, Augustine GJ. Ca2+ requirements for cerebellar long-term synaptic depression: role for a postsynaptic leaky integrator. Neuron. 2007;54:787–800. doi: 10.1016/j.neuron.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Ito M. Cerebellar Long-Term Depression: Characterization, Signal Transduction, and Functional Roles. Physiol rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 18.Hartell NA. Parallel fiber plasticity. Cerebellum. 2002;1:3–18. doi: 10.1080/147342202753203041. [DOI] [PubMed] [Google Scholar]
- 19.Hartell NA. Strong activation of parallel fibers produces localized calcium transients and a form of LTD that spreads to distant synapses. Neuron. 1996;16:601–610. doi: 10.1016/s0896-6273(00)80079-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang SS, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 21.Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2006;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Chen G, Gao W, Ebner T. Long-term potentiation of the responses to parallel fiber stimulation in mouse cerebellar cortex in vivo. Neuroscience. 2009;162:713–722. doi: 10.1016/j.neuroscience.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuruma A, Inoue T, Mikoshiba K. Dynamics of Ca2+ and Na+ in the dendrites of mouse cerebellar Purkinje cells evoked by parallel fibre stimulation. Eur J Neurosci. 2003;18:2677–2689. doi: 10.1111/j.1460-9568.2003.02977.x. [DOI] [PubMed] [Google Scholar]
- 25.Renzi M, Farrant M, Cull-Candy SG. Climbing-fibre activation of NMDA receptors in Purkinje cells of adult mice. J Physiol. 2007;585:91–101. doi: 10.1113/jphysiol.2007.141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol (Lond) 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rancz EA, Häusser M. Dendritic calcium spikes are tunable triggers of cannabinoid release and short-term synaptic plasticity in cerebellar Purkinje neurons. J Neurosci. 2006;26:5428–5437. doi: 10.1523/JNEUROSCI.5284-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt H, Stiefel KM, Racay P, Schwaller B, Eilers J. Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k. J Physiol. 2003;551:13–32. doi: 10.1113/jphysiol.2002.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canepari M, Ogden D. Kinetic, pharmacological and activity-dependent separation of two Ca2+ signalling pathways mediated by type 1 metabotropic glutamate receptors in rat Purkinje neurones. J Physiol (Lond) 2006;573:65–82. doi: 10.1113/jphysiol.2005.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 31.Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- 32.Sarkisov DV, Wang SS. Order-dependent coincidence detection in cerebellar Purkinje neurons at the inositol trisphosphate receptor. J Neurosci. 2008;28:133–142. doi: 10.1523/JNEUROSCI.1729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canepari M, Auger C, Ogden D. Ca2+ ion permeability and single-channel properties of the metabotropic slow EPSC of rat Purkinje neurons. J Neurosci. 2004;24:3563–3573. doi: 10.1523/JNEUROSCI.5374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roche KW, Tingley WG, Huganir RL. Glutamate receptor phosphorylation and synaptic plasticity. Curr Opin Neurobiol. 1994;4:383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 36.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka K, Augustine GJ. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron. 2008;59:608–620. doi: 10.1016/j.neuron.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada D. Protein kinase C modulates calcium sensitivity of nitric oxide synthase in cerebellar slices. J Neurochem. 1995;64:1298–1304. doi: 10.1046/j.1471-4159.1995.64031298.x. [DOI] [PubMed] [Google Scholar]
- 39.Daniel H, Hemart N, Jaillard D, Crepel F. Long-term depression requires nitric oxide and guanosine 39,59 cyclic monophosphate production in rat cerebellar Purkinje cells. Eur J Neurosci. 1993;5:1079–1082. doi: 10.1111/j.1460-9568.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 40.Linden DJ, Connor JA. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science. 1991;254:1656–1659. doi: 10.1126/science.1721243. [DOI] [PubMed] [Google Scholar]
- 41.Abellovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC-mutant mice. Cell. 1993;75:1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- 42.Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kakegawa W, Yuzaki M. A mechanism underlying AMPA receptor trafficking during cerebellar long-term potentiation. Proc Natl Acad Sci U S A. 2005;102:17846–17851. doi: 10.1073/pnas.0508910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gundappa-Sulur G, De Schutter E, Bower JM. Ascending granule cell axon: an important component of cerebellar cortical circuitry. J Comp Neurol. 1999;408:580–596. doi: 10.1002/(sici)1096-9861(19990614)408:4<580::aid-cne11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 45.Sims RE, Hartell NA. Differences in transmission properties and susceptibility to long-term depression reveal functional specialization of ascending axon and parallel fiber synapses to Purkinje cells. J Neurosci. 2005;25:3246–3257. doi: 10.1523/JNEUROSCI.0073-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sims RE, Hartell NA. Differential susceptibility to synaptic plasticity reveals a functional specialization of ascending axon and parallel fiber synapses to cerebellar Purkinje cells. J Neurosci. 2006;26:5153–5159. doi: 10.1523/JNEUROSCI.4121-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:776–781. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcaggi P, Attwell D. Short- and long-term depression of rat cerebellar parallel fibre synaptic transmission mediated by synaptic crosstalk. J Physiol. 2007;578:545–550. doi: 10.1113/jphysiol.2006.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han VZ, Zhang Y, Bell CC, Hansel C. Synaptic plasticity and calcium signaling in Purkinje cells of the central cerebellar lobes of mormyrid fish. J Neurosci. 2007;27:13499–13512. doi: 10.1523/JNEUROSCI.2613-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denk W, Yuste R, Svoboda K, Tank DW. Imaging calcium dynamics in dendritic spines. Curr Opin Neurobiol. 1996;6:372–378. doi: 10.1016/s0959-4388(96)80122-x. [DOI] [PubMed] [Google Scholar]
- 51.Sabatini BL, Maravall M, Svoboda K. Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol. 2001:349–356. doi: 10.1016/s0959-4388(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 52.Fisher JA, Barchi JR, Welle CG, Kim GH, Kosterin P, Obaid AL, et al. Two-photon excitation of potentiometric probes enables optical recording of action potentials from mammalian nerve terminals in situ. J Neurophysiol. 2008;99:1545–1553. doi: 10.1152/jn.00929.2007. [DOI] [PubMed] [Google Scholar]


