Abstract
Background
The Leishmania protozoan parasites cause devastating human diseases. Leishmania have been considered to replicate clonally, without genetic exchange. However, an accumulation of evidence indicates that there are inter-specific and intra-specific hybrids among natural populations. The first and so far only experimental proof of genetic exchange was obtained in 2009 when double drug resistant Leishmania major hybrids were produced by co-infecting sand flies with two strains carrying different drug resistance markers. However, the location and timing of hybridisation events in sand flies has not been described.
Methodology/Principal Findings
Here we have co-infected Phlebotomus perniciosus and Lutzomyia longipalpis with transgenic promastigotes of Leishmania donovani strains carrying hygromycin or neomycin resistance genes and red or green fluorescent markers. Fed females were dissected at different times post bloodmeal (PBM) and examined by fluorescent microscopy or fluorescent activated cell sorting (FACS) followed by confocal microscopy. In mixed infections strains LEM3804 and Gebre-1 reached the cardia and stomodeal valves more rapidly than strains LEM4265 and LV9. Hybrids unequivocally expressing both red and green fluorescence were seen in single flies of both vectors tested, co-infected with LEM4265 and Gebre-1. The hybrids were present as short (procyclic) promastigotes 2 days PBM in the semi-digested blood in the endoperitrophic space. Recovery of a clearly co-expressing hybrid was also achieved by FACS. However, hybrids could not sustain growth in vitro.
Conclusions/Significance
For the first time, we observed L. donovani hybrids in the sand fly vector, 2 days PBM and described the morphological stages involved. Fluorescence microscopy in combination with FACS allows visualisation and recovery of the progeny of experimental crosses but on this occasion the hybrids were not viable in vitro. Nevertheless, genetic exchange in L. donovani has profound epidemiological significance, because it facilitates the emergence and spread of new phenotypic traits.
Introduction
Protozoan parasites of the genus Leishmania (Kinetoplastida: Trypanosomatidae) cause the leishmaniases - severe human diseases that may be mutilating or fatal. The Leishmania donovani complex (subgenus Leishmania) comprising L. donovani and L. infantum (synonym L. chagasi) causes visceral leishmaniasis, which has been responsible for catastrophic epidemics among vulnerable populations of Sudan and the Indian subcontinent and for suburban outbreaks in Brazil. Other members of the genus Leishmania cause cutaneous leishmaniasis in both the Old World (L. major; L. tropica; L. aethiopica) and the New World (L. mexicana; L. amazonensis) or devastating mucocutaneous leishmaniasis (L. braziliensis in the New World).
The Leishmania life-cycle includes morphologically and physiologically distinct forms in the sand fly vector and in the vertebrate host [1], [2], [3], [4], [5]. Intracellular amastigotes taken up in the sand fly bloodmeal undergo transformation to short procyclic promastigotes with short flagellum. These forms divide and later transform to elongated nectomonads that escape through broken peritrophic matrix [6] and attach to the midgut epithelium to prevent expulsion during defecation of bloodmeal remnants. After defecation, late-stage infections comprise mainly short promastigotes (leptomonads [4]), which migrate anteriorly to the thoracic midgut, and highly motile metacyclic forms infective for the vertebrate host [1], [2], [3], [4], [5].
For many years Leishmania parasites have been considered to replicate asexually. No sexual dimorphism has been seen and chromosomes cannot be observed directly because they do not condense during cell division. Although this clonal hypothesis [7] did not entirely rule out the possibility of genetic recombination, such events were believed to be rare and insufficient to disrupt a prevailing clonal population structure. In the absence of genetic exchange it was proposed that recombination in large tandemly repeated gene families was one mechanism by which genetic diversity was sustained [7], [8], [9], [10]. Nevertheless, genetic exchange has been discovered in the related trypanosomatids Trypanosoma brucei and Trypanosoma cruzi, agents of African and American trypansomiasis, respectively. In both cases these discoveries were initially based on population genetic evidence, followed by formal experimental proof in the laboratory [11], [12], [13], and in both cases genetic exchange events have epidemiological relevance.
During the last two decades, evidence of genetic exchange among natural populations of Leishmania has repeatedly emerged in the form of strains that on molecular characterisation appear to be interspecific hybrids [14], [15], [16], [17], [18], [19] or intraspecific hybrids [20], [21]. Genetic exchange was finally demonstrated experimentally in 2009 [22]: 18 hybrid clones of L. major were recovered from a natural vector sand fly species (Phlebotomus duboscqi) co-infected with two transgenic parental strains. All of the clones inherited a full set of allelic markers from both parents but only one parental maxicircle haplotype. The nuclear genotypes were consistent with a heterozygous first generation progeny. However, 7 of the 18 clones were triploid by analysis of DNA content and the precise mechanism of genetic exchange remains to be determined. A natural progression of such research is to co-infect sand flies with transgenic Leishmania carrying two different markers that are fluorescent, in an attempt to visualise the recombination events microscopically [13].
The fact that Leishmania can undergo genetic exchange is potentially of profound epidemiological significance. Hybrid offspring might show a strong selective advantage relative to the parental strains. Local abundance of L. braziliensis/L. peruviana hybrids was reported from Peru [23] and a putatively recombinant lineage of L. tropica has been widely disseminated [24]. Most importantly, hybrids characterised as L. major/L. infantum are transmissible by Phlebotomus papatasi, a principal vector of L. major that is normally refractory to L. infantum and L. donovani [13], [25], [26], [27].
The localisation and timing of Leishmania hybridisation events in the sand fly is as yet unresolved. Transgenic fluorescent trypanosomes have revealed that T. brucei undergoes recombination in the salivary glands of the tsetse fly vector [28]. Accordingly, here we have passaged several pairs of red and green transgenic Leishmania through appropriate sand fly vector species. We describe the behaviour of the pairs of L. donovani strains in competing infections in the sand fly and reveal the presence of unequivocally dual fluorescing hybrid organisms in single P. perniciosus and L. longipalpis co-infected with L. donovani strains LEM 4265 and Gebre-1. Furthermore, high resolution fluorescence microscopy allows provisional definition of the timing and localisation of hybridisation and the morphological stages that are involved.
Results
Experimental co-infections
Three pairs of GFP/RFP transfected L. donovani strains were used for experimental infections in two sand fly vector species, P. perniciosus and L. longipalpis: LEM3804 (RFP) and LV9 (GFP), LV9 (GFP) and Gebre-1 (RFP) and LEM4265 (GFP) and Gebre-1 (RFP) (Table 1). Engorged females were dissected at different time intervals PBM and used to study comparative distribution of co-infections, and for attempted recovery of hybrids in non-selective media and double-drug selective slopes, and for FACS analysis.
Table 1. Numbers of single and co-infected sand flies examined by fluorescence microscopy.
| L. donovani strains | Sand fly species | Day 2–3 PBM | Day 4 PBM | Day 7 PBM | Day 9–10 PBM | Total | ||||||||||||
| Dis | Inf | B | H | Dis | Inf | B | H | Dis | Inf | B | H | Dis | Inf | B | H | Dis | ||
| Co-infections | ||||||||||||||||||
| LEM3804/LV9 | L. longipalpis | 10 | 6 | 6 | 0 | - | - | - | - | 12 | 8 | 2 | 0 | 10 | 5 | 3 | 0 | |
| P. perniciosus | 10 | 10 | 10 | 0 | - | - | - | - | 12 | 6 | 5 | 0 | 15 | 10 | 8 | 0 | ||
| LV9/Gebre-1 | L. longipalpis | 12 | 12 | 12 | 0 | - | - | - | - | 12 | 12 | 4 | 0 | 11 | 7 | 2 | 0 | |
| P. perniciosus | 10 | 6 | 6 | 0 | - | - | - | - | 15 | 10 | 5 | 0 | 15 | 8 | 4 | 0 | ||
| LEM4265/Gebre-1 | L. longipalpis | 10 | 10 | 10 | 0 | - | - | - | - | 15 | 12 | 6 | 0 | 15 | 13 | 5 | 0 | |
| P. perniciosus | 70 | 56 | 56 | 1 | 40 | 34 | 27 | 0 | 41 | 33 | 21 | 0 | 15 | 10 | 4 | 0 | ||
| Total | L. longipalpis | 32 | 28 | 28 | 0 | - | - | - | - | 39 | 32 | 12 | 0 | 36 | 25 | 10 | 0 | |
| P. perniciosus | 90 | 72 | 72 | 1 | 40 | 34 | 27 | 0 | 68 | 49 | 31 | 0 | 45 | 28 | 16 | 0 | ||
| ∑ | 122 | 100 | 100 | 1 | 40 | 34 | 27 | 0 | 107 | 81 | 43 | 0 | 81 | 53 | 26 | 0 | 350 | |
| Single infections | ||||||||||||||||||
| LEM4265 | P. perniciosus | 30 | 21 | - | - | 30 | 23 | - | - | 38 | 23 | - | - | - | - | - | - | |
| Gebre-1 | P. perniciosus | 29 | 23 | - | - | 30 | 28 | - | - | 38 | 34 | - | - | - | - | - | - | |
| Total | P. perniciosus | 59 | 44 | - | - | 60 | 51 | - | - | 76 | 57 | - | - | - | - | - | - | 195 |
PBM, post bloodmeal;
Dis, number of dissected females;
Inf, number of females infected with at least one Leishmania strain;
B, number of females infected with both Leishmania strains;
H, number of females with hybrid promastigotes seen and photographed under fluorescence microscope;
-, not done.
Infectivity rates and comparative distribution of co-infections
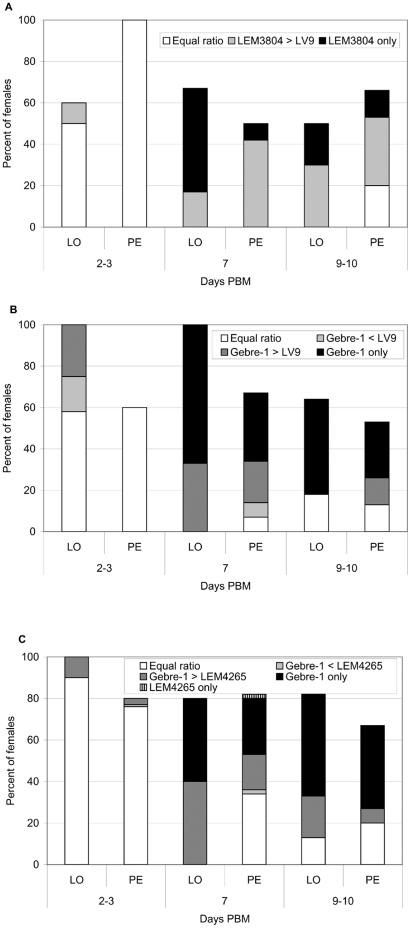
A total of 545 sand fly females were analysed microscopically (Table 1). By day 2–3 PBM the ratio of strains used for co-infections was mostly equal (Fig. 1). But in more established infections (day 7 and 9–10 PBM), LEM3804 prevailed over LV9 and Gebre-1 prevailed over both LV9 and LEM4265 in most specimens of both vector species (Fig. 1). LEM3804 and Gebre-1 infections also reached the cardia and colonized the stomodeal valve (SV) faster than LV9 and LEM4265 (Fig. 2). In these co-infection experiments sand fly females commonly became infected with LEM3804 or Gebre 1 alone, but single infections were absent for LV9 and rare for LEM4265 (Fig. 1).
Figure 1. Development of GFP/RFP transfected Leishmania strains in sand flies.
Infection rates and intensities of co-infections in three combinations of L. donovani strains in L. longipalpis (LO) and P. perniciosus (PE). A, LEM3804 plus LV9: in late stage infections (days 7 and 10) LEM3804 mostly over-grew LV9, in some females LV9 disappeared. B, Gebre-1 plus LV9: Gebre-1 grew better than LV9, in a high proportion of females LV9 was lost during late stage infections. C, Gebre-1 plus LEM4265: in late stage infections Gebre-1 mostly over-grew LEM4265, in a high proportion of females LEM4265 disappeared.
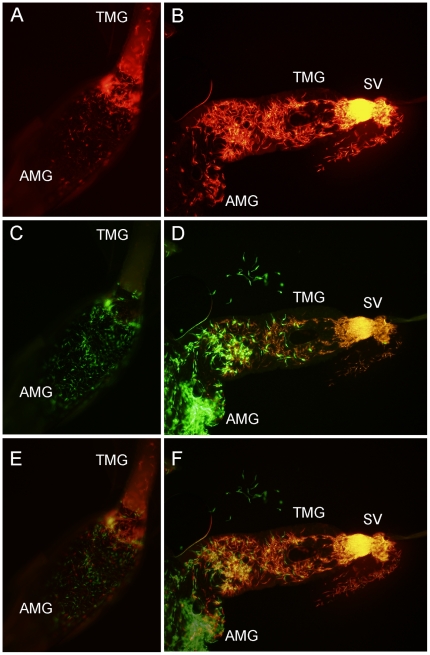
Figure 2. Different location of GFP/RFP transfected Leishmania strains in P. perniciosus.
Images from fluorescent microscopy (Olympus BX51). A, C, E, Early stage of infection (day 3 PBM), parasites of both strains have escaped from the peritrophic matrix to the ectoperitrophic space. LEM3804 parasite (red) starting migration from the abdominal midgut (AMG) into the thoracic part (TMG) while LV9 parasites (green) remained in the abdominal midgut. B, D, F, Late infections (day 7 PBM). Gebre-1 parasites (red) colonizing the stomodeal valve (SV) region while LEM4265 parasites (green) only reached the middle section of the thoracic midgut. A–B, images from red fluorescence, C–D images from green fluorescence and E–F, merged images (x100).
We also compared development of Gebre-1 and LEM4265 single infections with co-infections, in the same sand fly vector species, P. perniciosus. Parasite loads did not differ significantly between single infections and co-infections for either Gebre-1 (N = 191, P = 0.550, χ2 = 1.197, d.f. = 2) or for LEM4265 (N = 192, P = 0.257, χ2 = 4.041, d.f. = 3). Comparisons between single infections and co-infections were performed to determine if the slow colonization of the SV observed with LEM4265 strain was caused by competition with the faster developing Gebre-1 strain. Surprisingly, development of LEM4265 did not differ significantly between the females carrying single infections and co-infections (N = 136, P = 0.477, χ2 = 3.509, d.f. = 4). Parasites of LEM4265 consistently reached the cardia slowly and colonized the SV in a low percentage of females, if infected singly or co-infected with Gebre-1 parasites (Fig. 2).
Location and morphology of dual fluorescing hybrids found in situ
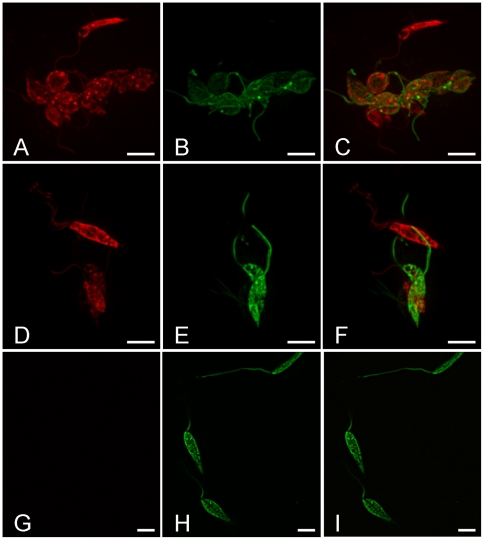
From 350 dissected sand flies that had been allowed to feed on a mixture of two parental GFP/RFP expressing strains, 196 were found to have successfully acquired infection with both strains (Table 1). Hybrid promastigotes clearly and unequivocally showing both red and green fluorescence were found in a single P. perniciosus female, co-infected with the LEM 4265 and Gebre-1 parental strains. The hybrid cells were present within the semi-digested bloodmeal in the endoperitrophic space 2 days PBM. Morphologically, hybrid parasites were rounded procyclic promastigotes with average body length 5.3 µm and body width 3.2 µm (n = 10, Fig. 3a–c) or short promastigotes with average body length 9.1 µm, body width 2.3 µm and flagellar length 12.1 µm (n = 2, Fig. 3d–f). All the hybrid cells showed a patched appearance of red and green fluorescence emitting sites (Fig. 3).
Figure 3. Hybrid promastigotes found in the sand fly midgut.
Images from the Olympus CellR 567 system showing dual fluorescence in Leishmania from a P. perniciosus female 2 days PBM (A–C). The female was infected with LEM 4265 (GFP transfected) and Gebre-1 (RFP transfected) parental strains. A, B, C, group of 10 hybrids together with four parasites showing red fluorescence only, D–F, group of two hybrids with two red parasites, G–I, GFP controls. A, D, G, images from red fluorescence, B, E, H, images from green fluorescence and C, F, I, merged images, scale bar: 5 µm.
FACS analysis
Numbers of P. perniciosus and L. longipalpis females co-infected with Gebre-1 (RFP) and LEM 4265 (GFP) examined by flow cytometry are given in Table 2. Although sorting was performed in two sequential steps (see Material and Methods), the resulting events showing both GFP and RFP fluorescence were checked visually by confocal microscopy to distinguish and exclude absolutely FACS sorted sand fly gut remnants with red or green autofluorescence and any sorted pairs of red and green cells from single dual fluorescing hybrid cells, clearly expressing both red and green fluorescence.
Table 2. Flow cytometry: numbers of sand flies co-infected with L. donovani (Gebre-1, RFP and LEM 4265, GFP) examined post bloodmeal (PBM).
| Sand fly species | Time PBM | |||||
| 1 day | 2 days | 3 days | 8–9 days* | 15–17 days* | Total | |
| P. perniciosus | 50 | 50 | 100 | 50 | 143 | 393 |
| L. longipalpis | 50 | 100 | 35 | 83 | 0 | 268 |
*In contrast to P. perniciosus, shorter longevity of L. longipalpis females does not allow later interval of dissections.
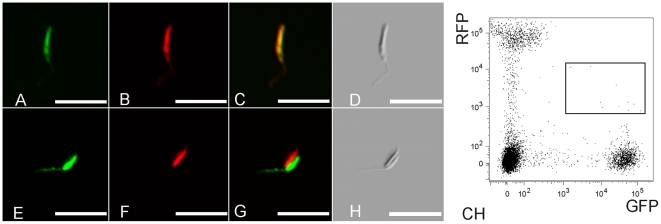
From 393 P. perniciosus females no putative hybrids were found. From 268 L. longipalpis, one Leishmania short promastigote, clearly showing colocalized GFP and RFP expression was sorted from the pool of 45 guts dissected at day 2 PBM. Fig. 4 shows clear co-expression of GFP and RFP in this parasite after recovery by FACS. No more hybrid cells co-expressing red and green fluorescence were observed in sand flies dissected after 2 days PBM or during later phases of the Leishmania infection. The frequency of hybridisation in L. longipalpis (based on phenotype) was estimated to be 4.8×10−5 or less.
Figure 4. Promastigotes sorted by flow cytometry 2 days PBM.
A–H, images from confocal microscopy (Olympus FV1000). A–D, hybrid promastigote, E–H, a doublet of red and green cells. A, E, green fluorescence, B, F, red fluorescence, C, G, RGB images, D, H, phase contrast, scale bar: 10 µm. C, H, Flow cytometry analysis detecting both GFP and RFP fluorescing parasites. A two day PBM sample containing many erythrocytes and non fluorescing particles. Rectangle encompasses the region sorted and screened for hybrids.
Recovery of hybrids
Initially, when both selective drugs were incorporated into the overlay, 25 isolates were recovered from 121 dissected females. However, when the selective antibiotics were incorporated into both components of the cultivation medium, the blood agar and the overlay, no further isolates were recovered from 89 experimental co-infections. Nor did the 25 positive isolates sustain growth in this highly selective medium. Furthermore, none of the 25 isolates contained both neomycin and hygromycin drug resistance markers after PCR amplification. In addition, 157 isolation attempts from the sand fly co-infections were made into non-selective medium. Of these 70 produced viable cultures but growth was not sustained for more than 1 or 2 sub-cultures in double drug selective medium. Thus, although putative hybrids were clearly seen in sand flies and a single putative hybrid was recovered by FACS, viable hybrids could not be grown in vitro.
Discussion
Leishmania have, controversially, been considered to be propagated clonally. However, accumulating evidence from genotyping of natural populations of both Old World and New World Leishmania has revealed the presence of putative hybrids between several combinations of described species and between strains within species. Finally, in 2009, the landmark experiments of Akopyants et al. [22] proved that two strains of L. major carrying different drug resistance markers could undergo genetic exchange, and that this process occurred in the sand fly vector, with an approximate frequency of 2.5×10−5.
We have described a quite extensive series of experiments passaging three pairs of transgenic drug resistant GFP or RFP labelled L. donovani strains through their natural sand fly vectors P. perniciosus and L. longipalpis and dissecting at intervals PBM. Gut contents were examined by fluorescence microscopy and by FACS analysis and also subjected to double drug selections in an attempt to recover viable hybrids.
In experiments aimed at examining L. donovani behaviour in P. perniciosus and L. longipalpis we obtained 196 successful co-infections from 350 sand fly females given bloodmeals containing pairs of L. donovani strains (Table 1). Multiple hybrids were seen in a single P. perniciosus co-infected with the strains LEM4265 and Gebre 1, checked in situ. Retrieval of a single cell unequivocally expressing both fluorescent reporters was achieved also by FACS analysis from the pool of L. longipalpis co-infected with the same combination of parental strains. This demonstrated the immense value of FACS for detecting and sorting hybrid progeny.
In these co-infections, the strains involved did not appear to behave competitively. Strains displayed the same broad level of distribution of infection in single and co-infected flies. We did not recover sustained growth of viable hybrid progeny in non selective media or through rigorous double drug selection. However, the importance of this work is that the fluorescence did allow us to visualise the distribution and course of the infection in the sand flies with great clarity, to see hybrid organisms displaying both red and green fluorescence and to make several key, novel observations. This demonstrates that the L. donovani complex almost certainly has the capacity for genetic exchange within its natural sand fly vector species, and confirms the validity of this experimental approach.
Furthermore, we made observations indicating that hybridisation events probably occur in the early phase of bloodmeal digestion, 2 days PBM, when all parasites are located in the endoperitrophic space within the bloodmeal surrounded by peritrophic matrix. We obtained spectacular high resolution images of the promastigote morphological stages involved, as illustrate in Figures 3 and 4. However, hybrids were not seen in long term sand fly infections and proved to be non-viable in the culture conditions used here and as a result we were unable to characterise hybrid progeny in detail by molecular methods.
These data from microscopic examinations and FACS experiments suggest that similarly low frequency of sexual events occurred in both tested sand fly species. The low frequency of hybrid occurrence has several possible explanations. The strains used had been maintained in the laboratory for prolonged periods. Some genotyping data were available but genetic compatibilities are not known. Furthermore, sand flies were infected with promastigotes derived from 4 day old cultures, not with amastigotes within macrophages, and bloodmeals were artificially comprised. However, in a previous study L. major hybrids [22] were achieved using the same mode of sand fly infection with strains maintained long-term in culture, yielding 12 positive double drug resistant isolates from only 102 dissected sand flies.
Our findings suggest that there may be a species-specific difference among Leishmania in the capacity for sexual reproduction. Rarity of genetic exchange in our experiments may reflect natural situations where there is minimal chance of hybrid clones arising and being more viable than parental genotypes. However, hybridisation events when they occur may fail to yield progeny that are viable in the long term or progeny that have a selective advantage and allow the hybrids to out-compete parental populations. Genetic exchange may infrequently break a prevailing pattern of clonal population structure [7].
Nevertheless, the success of hybridization and its epidemiological importance in Leishmania is unquestionable from genetic analysis of natural populations, and in particular from experimental demonstration of the vigour and transmissibility of hybrids described as L. major/L. infantum [25]. Future experimental crosses with transgenic Leishmania should a) be based on putative parental and hybrid strains recently isolated in endemic regions from sympatric populations b) probably employ strains predetermined to have similar growth rates and intensities in sand flies c) incorporate sequential as well as mixed infections d) include genetically similar strains to allow that genetic exchange might frequently involve closely related populations. We have observed that hybrid formation can occur between L. donovani strains in the natural vector sand fly species, and that the cell types involved are short (procyclic) promastigotes as early as 2 days PBM. We have also shown that fluorescence microscopy with FACS analysis and sorting of individual cells provides a strong combined approach for visualization and recovery of hybrid progeny.
Materials and Methods
Leishmania strains
Four Leishmania donovani strains were used in experimental crosses: LEM3804 (MCAN/SD/99/LEM3804), LV9 (MHOM/ET/67/L82/LV9), Gebre-1 (MHOM/ET/72/GEBRE1) and LEM4265 (MHOM/SD/2001/AHSAF2). Strains originate from Sudan (LEM 3804, LEM 4265) or Ethiopia (LV9, GEBRE1) and could be distinguished, in our laboratory, by SNP differences in four housekeeping genes. Preliminary experiments confirmed that all these strains were able to develop in sand flies and establish heavy late-stage infections with colonization of the stomodeal valve. All the strains were maintained as stabilates and at 23°C either in liquid alpha-MEM medium (minimum essential media, Invitrogen) supplemented with 20% heat inactivated FCS or in blood agar base over-laid with alpha-MEM. G418 and hygromycin were added to final concentrations of 150 µg/ml when double drug selection was applied, as described below.
Preparation of transgenic fluorescent Leishmania strains
Parental lines (LEM3804, LV9, Gebre-1, LEM4265) of transgenic fluorescent Leishmania were produced using the LEXSY expression system (Jena Bioscience) and the expression vectors pF4X1.4neo and pF4X1.4hyg incorporating eGFP and RFP, respectively, as fluorescent markers. This vector contains optimised untranslated regions flanking the target gene insertion site and splicing signals for post-transcriptional mRNA processing. Insertion of eGFP and RFP was verified by vector cleavage using restriction enzymes (NcoI and NotI) to confirm replacement of the 0.7kb stuffer fragment with the appropriate fluorescent protein cassette. The expression vectors were initially constructed in E. coli and introduced into Leishmania by electroporation using d = 4 mm cuvettes and two pulses at 1500V, 25uF [29]. Expression of the target genes in Leishmania followed integration of the expression cassette into the chromosomal 18S rRNA locus (SSU). Integration was confirmed by diagnostic PCR amplification from genomic DNA, with one primer hybridising within the expression cassette and one to a SSU sequence not present in the plasmid. Expression of eGFP and RFP, which is dependent on successful integration, was confirmed by fluorescence microscopy and transgenic Leishmania clones showing stable fluorescence were selected for the crossing experiments. Transgenic cell lines retained vigorous growth in vitro.
Experimental infection of sand flies
Separate colonies of P. perniciosus and L. longipalpis, proven vectors of L. infantum, part of the L. donovani complex, were maintained at 26°C, on a 14-h light/10-h dark photoperiod [30] and fed on a 50% sucrose solution. Sand fly females were infected by feeding through a chick-skin membrane on heat inactivated rabbit blood (purchased from Bioveta, Ivanovice na Hane, Czech Rep.) containing a mixture of two parental strains at 106 promastigotes/ml, derived from four-day old α-MEM or blood agar cultures. Engorged sand flies were maintained in the same conditions as the colony and dissected at various time intervals PBM (Table 1) either for microscopic observation or for isolation of Leishmania.
Recovery of live Leishmania from sand flies
To recover live Leishmania, sand flies were dissected in sterile saline and gut contents were inoculated into blood agar slopes overlaid with alpha-MEM medium supplemented with 20% heat inactivated FCS, fluorocytosine (100 ug/ml) and gentamicin (100 ug/ml). Selective antibiotics (150 ug/ml hygromycin, 150 ug/ml G418) were applied either in the liquid overlay or in both the overlay and agar base of the primary cultures. However, some primary cultures were inoculated without the addition of selective antibiotics, and double drug selection was only applied when recovered Leishmania were sub-cultured. Cultures were examined weekly by microscopy for the presence of Leishmania and if positive the promastigotes were cryopreserved in liquid nitrogen for further characterisation of the recovered organisms.
Dissection of sand flies and microscopy
Dissected sand fly guts were fixed in situ on the slide with 1% paraformaldehyde and examined by microscopy. Levels of Leishmania infection were graded as reported previously [31] as light (<100 parasites/gut), moderate (100–1000 parasites/gut) and heavy (>1000 parasites/gut). The course of infection and location of Leishmania infection, including individual parasites, in the sand fly digestive tract were determined and recorded photographically with an Olympus BX51 fluorescent microscope and Olympus camera. Putative hybrids expressing both red and green fluorescence were detected by merging images from red and green fluorescence filters using Adobe Photoshop 7.0.1. In addition, guts were also very carefully examined with an Olympus CellR system which provides multi-fluorescence imaging and allows direct scrutiny of hybrid parasites emitting both green and red fluorescence. Putative hybrids sorted by flow cytometer were checked and single cells scrutinised and photographed with confocal fluorescence microscopy on an Olympus FV1000.
Preparation of Leishmania clones
Leishmania recovered in selective media were cloned by plating on solid media using the methodology described by [32]. Ten clonal colonies were selected from each plate and transferred to non selective media (alpha-MEM supplemented with 20% heat inactivated FCS). Following DNA extraction (Qiagen) the presence of the expression cassette, containing resistance and fluorescent markers, was confirmed by PCR amplification (30 cycles of 30 sec at 94°C, 1 min at 53°C, and 2 min at 72°C), with the primers A3804 (CCGATGGCTGTGTAGAAGTACTCG) and F3002 (CTGCAGGTTCACCTACAGCTAC). Integration of the expression cassette into the ssu locus resulted in a characteristic 1.8 kbp fragment.
FACS analysis
At different time intervals PBM (Table 2), guts of infected sand flies were dissected into a small volume of the cultivation medium (35–50 guts in 15 µl), filtered using a 30 µm filter (Partec) into PBS buffer and analyzed with a LSRII flow cytometer (BD Biosciences). Numbers of GFP positive events (excitation with the 488 nm laser, emission detection at 530/30 nm), RFP positive events (excitation with the 561 nm laser, emission detection at 585/15 nm) and putative hybrid cells, i.e. events simultaneously emitting at both GFP and RFP wavelengths, were determined. The cytometer was calibrated using both positive controls (GFP transfected Leishmania line, RFP transfected Leishmania line) and a negative control (wild type Leishmania strain).
Putative hybrid cells were sorted with FACSVantage SE cell sorter (BD Biosciences) with the laser emitting at 488 nm wavelengths and detection of emission at 530/30 nm (GFP positive events) and 585/42 nm (RFP positive events). Sorting was performed in two steps: in the first phase all GFP positive events were separated, secondly a further sorting was performed on the GFP separated parasites. Events showing double positive emission were collected into a glycerol drop containing 1% formaldehyde and immediately checked visually on the Olympus FV1000, for single cells co-expressing red and green fluorescence (See Results, FACS analysis, above)..
Ethics Statement
This study does not include experiments on animals or humans.
Acknowledgments
We would like to thank Ondrej Sebesta, Zdenek Cimburek and Jan Svoboda for skilled assistance during fluorescent microscopy and FACS assays.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work was financed by Wellcome Trust and JS, VS and PV were also funded by Ministry of Education (projects LC06009, MSM0021620828). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bates PA, Rogers ME. New insights into the developmental biology and transmission mechanisms of Leishmania. Curr Mol Med. 2004;4:601–609. doi: 10.2174/1566524043360285. [DOI] [PubMed] [Google Scholar]
- 2.Cihakova J, Volf P. Development of different Leishmania major strains in the vector sandflies Phlebotomus papatasi and P. duboscqi. Ann Trop Med Parasitol. 1997;91:267–279. doi: 10.1080/00034989761120. [DOI] [PubMed] [Google Scholar]
- 3.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Rogers ME, Chance ML, Bates PA. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology. 2002;124:495–507. doi: 10.1017/s0031182002001439. [DOI] [PubMed] [Google Scholar]
- 5.Walters LL. Leishmania differentiation in natural and unnatural sand fly hosts. J Eukaryot Microbiol. 1993;40:196–206. doi: 10.1111/j.1550-7408.1993.tb04904.x. [DOI] [PubMed] [Google Scholar]
- 6.Sadlova J, Volf P. Peritrophic matrix of Phlebotomus duboscqi and its kinetics during Leishmania major development. Cell Tissue Res. 2009;337:313–325. doi: 10.1007/s00441-009-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tibayrenc M, Ayala FJ. The clonal theory of parasitic protozoa: 12 years on. Trends Parasitol. 2002;18:405–410. doi: 10.1016/s1471-4922(02)02357-7. [DOI] [PubMed] [Google Scholar]
- 8.Tibayrenc M, Kjellberg F, Ayala FJ. A clonal theory of parasitic protozoa: the population structures of Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium, Trichomonas, and Trypanosoma and their medical and taxonomical consequences. Proc Natl Acad Sci U S A. 1990;87:2414–2418. doi: 10.1073/pnas.87.7.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tibayrenc M, Kjellberg F, Ayala FJ. The clonal theory of parasitic protozoa - a taxonomic proposal applicable to other clonal organisms. Bioscience. 1991;41:767–774. [Google Scholar]
- 10.Victoir K, Dujardin JC. How to succeed in parasitic life without sex? Asking Leishmania. Trends Parasitol. 2002;18:81–85. doi: 10.1016/s1471-4922(01)02199-7. [DOI] [PubMed] [Google Scholar]
- 11.Gaunt MW, Yeo M, Frame IA, Stothard JR, Carrasco HJ, et al. Mechanism of genetic exchange in American trypanosomes. Nature. 2003;421:936–939. doi: 10.1038/nature01438. [DOI] [PubMed] [Google Scholar]
- 12.Gibson W, Bailey M. Genetic exchange in Trypanosoma brucei: evidence for meiosis from analysis of a cross between drug-resistant transformants. Mol Biochem Parasitol. 1994;64:241–252. doi: 10.1016/0166-6851(94)00017-4. [DOI] [PubMed] [Google Scholar]
- 13.Miles MA, Yeo M, Mauricio IL. Leishmania exploit sex. Science. 2009;324:187–189. doi: 10.1126/science.1172789. [DOI] [PubMed] [Google Scholar]
- 14.Banuls AL, Jonquieres R, Guerrini F, Le Pont F, Barrera C, et al. Genetic analysis of Leishmania parasites in Ecuador: are Leishmania (Viannia) panamensis and Leishmania (V.) guyanensis distinct taxa? Am J Trop Med Hyg. 1999;61:838–845. doi: 10.4269/ajtmh.1999.61.838. [DOI] [PubMed] [Google Scholar]
- 15.Belli AA, Miles MA, Kelly JM. A putative Leishmania panamensis/Leishmania braziliensis hybrid is a causative agent of human cutaneous leishmaniasis in Nicaragua. Parasitology. 1994;109(Pt 4):435–442. doi: 10.1017/s0031182000080689. [DOI] [PubMed] [Google Scholar]
- 16.Delgado O, Cupolillo E, Bonfante-Garrido R, Silva S, Belfort E, et al. Cutaneous leishmaniasis in Venezuela caused by infection with a new hybrid between Leishmania (Viannia) braziliensis and L. (V.) guyanensis. Mem Inst Oswaldo Cruz. 1997;92:581–582. doi: 10.1590/s0074-02761997000500002. [DOI] [PubMed] [Google Scholar]
- 17.Dujardin JC, Banuls AL, Llanos-Cuentas A, Alvarez E, DeDoncker S, et al. Putative Leishmania hybrids in the Eastern Andean valley of Huanuco, Peru. Acta Trop. 1995;59:293–307. doi: 10.1016/0001-706x(95)00094-u. [DOI] [PubMed] [Google Scholar]
- 18.Kelly JM, Law JM, Chapman CJ, Van Eys GJ, Evans DA. Evidence of genetic recombination in Leishmania. Mol Biochem Parasitol. 1991;46:253–263. doi: 10.1016/0166-6851(91)90049-c. [DOI] [PubMed] [Google Scholar]
- 19.Ravel C, Cortes S, Pratlong F, Morio F, Dedet JP, et al. First report of genetic hybrids between two very divergent Leishmania species: Leishmania infantum and Leishmania major. Int J Parasitol. 2006;36:1383–1388. doi: 10.1016/j.ijpara.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Chargui N, Amro A, Haouas N, Schonian G, Babba H, et al. Population structure of Tunisian Leishmania infantum and evidence for the existence of hybrids and gene flow between genetically different populations. Int J Parasitol. 2009;39:801–811. doi: 10.1016/j.ijpara.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Rougeron V, De Meeus T, Hide M, Waleckx E, Bermudez H, et al. Extreme inbreeding in Leishmania braziliensis. Proc Natl Acad Sci U S A. 2009;106:10224–10229. doi: 10.1073/pnas.0904420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akopyants NS, Kimblin N, Secundino N, Patrick R, Peters N, et al. Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science. 2009;324:265–268. doi: 10.1126/science.1169464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolder D, Roncal N, Davies CR, Llanos-Cuentas A, Miles MA. Multiple hybrid genotypes of Leishmania (Viannia) in a focus of mucocutaneous Leishmaniasis. Am J Trop Med Hyg. 2007;76:573–578. [PubMed] [Google Scholar]
- 24.Schwenkenbecher JM, Wirth T, Schnur LF, Jaffe CL, Schallig H, et al. Microsatellite analysis reveals genetic structure of Leishmania tropica. Int J Parasitol. 2006;36:237–246. doi: 10.1016/j.ijpara.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Volf P, Benkova I, Myskova J, Sadlova J, Campino L, et al. Increased transmission potential of Leishmania major/Leishmania infantum hybrids. Int J Parasitol. 2007;37:589–593. doi: 10.1016/j.ijpara.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volf P, Myskova J. Sand flies and Leishmania: specific versus permissive vectors. Trends Parasitol. 2007;23:91–92. doi: 10.1016/j.pt.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volf P, Sadlova J. Sex in Leishmania. Science. 2009;324:1644. doi: 10.1126/science.324_1644b. [DOI] [PubMed] [Google Scholar]
- 28.Gibson W, Peacock L, Ferris V, Williams K, Bailey M. The use of yellow fluorescent hybrids to indicate mating in Trypanosoma brucei. Parasit Vectors. 2008;1:4. doi: 10.1186/1756-3305-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson KA, Beverley SM. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2003;128:217–228. doi: 10.1016/s0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 30.Benkova I, Volf P. Effect of temperature on metabolism of Phlebotomus papatasi (Diptera: Psychodidae). J Med Entomol. 2007;44:150–154. doi: 10.1603/0022-2585(2007)44[150:eotomo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Myskova J, Votypka J, Volf P. Leishmania in sand flies: comparison of quantitative polymerase chain reaction with other techniques to determine the intensity of infection. J Med Entomol. 2008;45:133–138. doi: 10.1603/0022-2585(2008)45[133:lisfco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Yeo M, Lewis MD, Carrasco HJ, Acosta N, Llewellyn M, et al. Resolution of multiclonal infections of Trypanosoma cruzi from naturally infected triatomine bugs and from experimentally infected mice by direct plating on a sensitive solid medium. Int J Parasitol. 2007;37:111–120. doi: 10.1016/j.ijpara.2006.08.002. [DOI] [PubMed] [Google Scholar]