Abstract
Our previous studies have characterized the inflammatory response of intratracheally instilled lipopolysaccharides (LPS) in F344/N rats. To better reflect the environmentally relevant form of LPS exposure, the present study evaluated the inflammatory response of F344/N rats exposed to LPS by inhalation. Rats were exposed by nose-only inhalation to aerosolized LPS at a median particle diameter of 1 μm and a dose range from 0.08 to 480 μg. Animals were sacrificed 72 h post exposure and the inflammatory cell counts and differentials, the cytokine/chemokine levels in the bronchoalveolar lavage fluid (BALF), and the changes in intraepithelial stored mucosubstances, mucous cells per mm basal lamina, and Bcl-2-positive mucous cells were quantified. We observed a dose-dependent increase reaching maximum values at the 75μg LPS dose for the numbers of neutrophils, macrophages and lymphocytes, for the levels of IL-6, IL-1α, IL-1β, TNFα, MCP-1 and GRO-KC. In addition, mucous cell metaplasia and the percentage of Bcl-2-positive mucous cells were increased with increasing deposited LPS dose. When rats were treated with the phosphodiesterase-4 (PDE4) inhibitor, rolipram (10 mg/kg), prior to exposure to aerosolized LPS neutrophil numbers in the BAL were reduced at 8 h but not at 24 or 72 h post LPS exposure. These results demonstrate that exposure to aerosolized LPS resulted in a more potent inflammatory response at lower doses and that inflammation was more uniformly distributed throughout the lung compared to inflammation caused by intratracheal LPS instillation. Therefore, this animal model will be useful for screening efficacy of anti-inflammatory drugs.
Keywords: pulmonary inflammation, airway epithelial hyperplasia, mucous cell metaplasia, apoptosis, phosphodiesterase inhibitor
INTRODUCTION
Lipopolysacharide (LPS) or endotoxin is a component of gram-negative bacteria cell wall and is commonly used to elicit an inflammatory response in the airways of laboratory animals. Humans are often exposed to LPS that is suspended in the air as part of house dust or as aerosols generated from contaminated water. Furthermore, occupational exposures to LPS are common for people in agricultural settings or in textile mills (Clapp et al., 1993), (Schwartz et al., 1995), (Schwartz, 1996). Macrophages and epithelial cells act as the first line of defense when healthy pulmonary airways are exposed to LPS. Activated macrophages release the cytokines IL-1β, TNFα, MCP-1 and MIP-1β (Chung, 2006) and epithelial cells secrete IL-8 and MCP-1 in vitro (Thorley et al., 2007). The release of these cytokines and chemokines is essential for the efficient recruitment of neutrophils from the pulmonary capillaries into the air spaces (Panina-Bordignon and D’Ambrosio, 2003). Once within the pulmonary airspace, neutrophils and other inflammatory cells release cytokines and chemokines, such as TNFα, IL-6 and IL-1β, which initiate epithelial cell proliferation and differentiation into mucous cells, a process termed mucus cell metaplasia (MCM) (Foster et al., 2003), (Tesfaigzi et al., 2004). Secreted mucus on airway epithelial surfaces traps foreign particles and allows particle transport out of the airways via the mucociliary function. Normally, MCM is reduced when the environmental insult is removed both by reducing expression of the mucin gene MUC5AC and by reducing the mucous cell numbers via programmed cell death (Harris et al., 2007). The reduction of MCM involves the downregulation of Bcl-2, an inhibitor of cell death (Harris et al., 2005).
Although the most common route of LPS exposure in the environment is by inhalation, this route of exposure is the least common mode utilized in laboratory studies. The majority of studies of LPS-induced pulmonary inflammation are conducted by either intravenous injection or instillation directly into nasal or tracheal passages. While studies conducted by intratracheal (IT) instillation can provide important information (Tesfaigzi et al., 2000), (Tesfaigzi et al., 2004), (Harris et al., 2005), we decided to characterize the inflammatory response of LPS delivered by inhalation as inhalation is the clinically relevant route for human subjects. We hypothesized that a more diffuse deposition (rather than bolus administration) of LPS in the respiratory tract by inhalation would better resemble inflammation in human disease and may be a useful model for screening anti-inflammatory drugs.
In the current study we describe a novel method of delivering aerosolized LPS to the lungs of male F344N rats at defined particle size distribution, and we characterized the inflammatory and epithelial cell response at 72 h post LPS inhalation. The dose of LPS over a wide range of concentrations was analyzed to better compare the effect of LPS when delivered as aerosol compared to LPS delivered by IT instillation (Harris et al., 2005), (Foster et al., 2003), (Tesfaigzi et al., 2000). After a detailed characterization of the inflammatory response, we tested the ability of the PDE4 inhibitor, rolipram, a known inhibitor of LPS-induced neutrophilic inflammation (Spond et al., 2001), to reduce pulmonary inflammation in the model of inhaled LPS in the rat. We found that LPS when delivered by inhalation increased inflammation and MCM at much lower concentrations and in a more linear fashion than when delivered by IT instillation and that this animal model is well-suited to screen for anti-inflammatory drugs.
MATERIALS AND METHODS
Animals
Male pathogen-free F344 rats (Charles River Laboratories, Wilmington, MA) aged 8–10 weeks were housed in pairs and provided food and water ad libitum. Rats were exposed to a 12:12 hour light:dark cycle and housed at 23°C with 20–40% humidity. Each rat was individually weighed and randomly assigned to each experimental group. All experiments were approved by the Lovelace Respiratory Research Institute Institutional Animal Care and Use Committee. Experiments were performed at the Institute in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Intratracheal Instillation
Intratracheal instillation was performed as previously described (Tesfaigzi et al., 2004). Briefly, rats designated for intratracheal instillation of LPS were anesthetized with 5% halothane in oxygen and intratracheally instilled with 1000 μg of LPS from Pseudomonas aeruginosa serotype 10, (Sigma, St. Louis, MO) in 500 μl deionized water.
Generation of Exposure Atmosphere
All aerosol generation was conducted in a glove box connected to an exhaust system. Aerosolization was conducted by nebulization of LPS (Pseudomonas aeruginosa serotype 10 (Sigma, St. Louis, MO) that was diluted with deionized water. Solutions were nebulized with a mini-HEART™ (Critical Care Concepts, Atlanta, GA) nebulizer followed by a diffusion drier (custom flow-through drier with Dri-rite™ dessicant) that dried the aerosol (high humidity saturated sample filters and impactor substrates). Aerosolized LPS was delivered to a 24-port nose-only inhalation chamber (In-Tox Products, Inc., Albuquerque, NM) operated at approximately 30 L/min.
LPS concentration was determined by gravimetric analysis of filter samples collected during the exposure. Filter samples were collected approximately every 5 min from the time the first animal was introduced until the last animal was removed from the exposure plenum. Aerosols were collected (from the exposure plenum) on Type T60A20, 47-mm Pallflex membrane filters (Pall Gelman Sciences, Ann Arbor, MI) at a nominal volumetric flow rate of approximately 1 L/min. After collection the filters were removed from the filter holders, placed in Petri dishes and weighed.
Filters were extracted with water and assayed by the Limulus assay described below. Real-time analysis of aerosol concentration was conducted with a RAM-S nephelometer. The real-time data was utilized as a guide to adjust dilutions and maintain exposure concentrations.
Pulmonary dose of LPS was calculated using the following formula: Dose = (C × RMV × T × DF)/BW, where C is the average concentration of test article or LPS in the exposure atmosphere, T is exposure time and the deposition fraction (DF) is assumed to be 10% to target pulmonary deposition fraction (this is appropriate at the historical LPS particle size of ~1 μm). Respiratory minute volume (RMV; L/min) is calculated using the following allometric equation: RMV = 0.499BW0.809, where BW is average exposure day body weight in kg (Bide et al., 2000). Rats were exposed to filtered air or to LPS at 1 mg/m3 or 25 mg/m3. Doses of 0.08 and 0.8 μg were achieved by exposing for 5 and 50 min at 1 mg/m3. Doses of 5 and 75 μg were achieved by exposing to approximately 25 mg/m3 for approximately 20 and 180 min.
Rolipram Treatment
Two hours prior to LPS inhalation, rats were dosed by oral gavage with vehicle (0.4% methylcellulose) or 10 mg/kg rolipram. For this study, rats were exposed by a single nose-only inhalation to an aerosol concentration yielding an approximate 15 μg deposited pulmonary dose of LPS. Because of the change in the lot number, endotoxin activity for the dose-response study was 900,000 EU/mg and 3,000,000 EU/mg for the rolipram study.
Determination of Particle Size
Particle size distribution was determined with a 7-stage Mercer style impactor (In-Tox Products, Inc., Albuquerque, NM). The aerosol was withdrawn directly from the exposure chamber atmosphere. Particle size was approximately 1 μm with a geometric standard deviation of ~2.0.
LPS Inhalation Exposure, Necropsy, and Bronchoalveolar Lavage Fluid
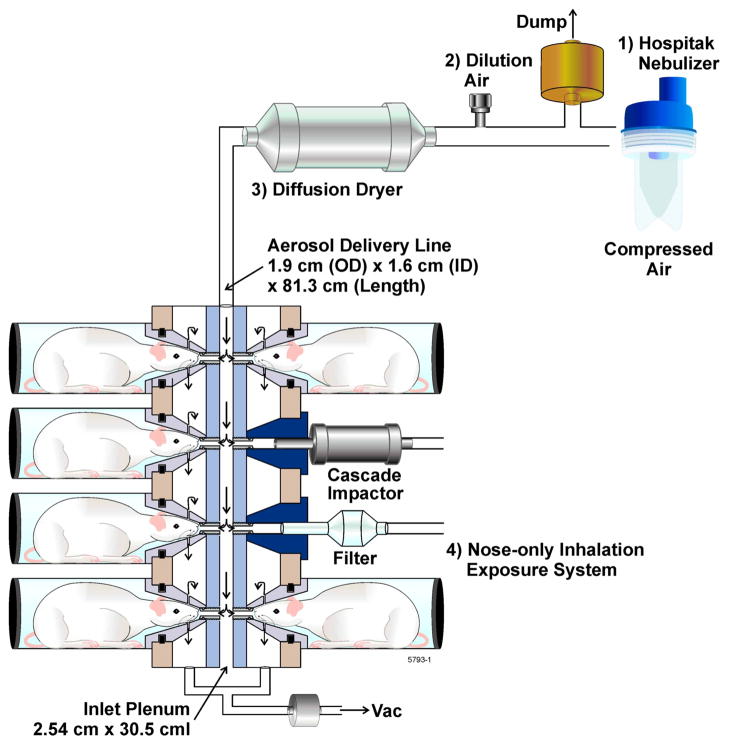
Rats were acclimated to the nose-only exposure tubes on two days preceding exposure (Fig. 1) by placing them individually in exposure tubes and waiting for 15 and 30 min sequentially. During LPS exposure, atmospheric oxygen content, temperature, and relative humidity were measured continuously to ensure that the environmental conditions were within acceptable limits. After exposure, animals were returned to shoe-box type cages and 72 h later euthanized by injecting them with pentobarbital followed by exsanguination through the renal artery. The lung vasculature was perfused via an injection of 10 ml cold pathogen-free saline through the heart. The trachea was cannulated with an 18 gauge blunt needle and the lungs were removed. For the LPS dose-response study, the lungs were lavaged three times using the same 5 ml of saline and inflated with 10% zinc formalin under a constant pressure of 25 cm H20 for 3 h. For the rolipram study, rats were euthanized at 8, 24 and 72 h post inhalation and the lungs were lavaged for BAL total cell counts and differentials. The whole lung was lavaged two times each using 5 ml of saline for lungs harvested at 8 and 24 h. The lungs harvested at 72 h, the right lungs were lavaged two times each with 3 ml of saline and the left lung was inflated with 10% zinc formalin under a constant pressure of 25 cm H2O for 3 h.
Figure 1.
Schematic of the LPS nose-only inhalation exposure system consisting of a Hospitak nebulizer, dilution air, and diffusion dryer. Samples for particle mass and size were obtained directly from the nose-ports.
BAL Cell Counts
BAL was centrifuged at 1000g at 4°C for 5 minutes. Supernatant was collected and aliquoted at −80°C for cytokine analyses. The cell pellet was diluted in 5 ml of media and BAL cell number was enumerated using a hematocytometer, cytospin slides were prepared with 50,000 cells, stained with Hematoxylin/Giesma, and cell differential quantified as described previously (Tesfaigzi et al., 1998).
Cytokine/chemokine detection in BALF
A standard multiplex assay kit (Lincoplex panel, Linco Research, Inc., St. Charles, MO) was used with the Luminex Flowmetrix system (Luminex, Austin, TX) to determine the levels of cytokines/chemokines (IL-1α, IL1-β, IL-6, IL-12, IL-18, TNF-α, IFN-γ, GMCSF, Gro/KC, MCP-1α) in BALF. The beads were incubated first with diluted standards or BALF overnight and then with a detector antibody cocktail for 60 min each at room temperature. After two washes in PBS supplemented with 0.02% Tween 20, 0.1% BSA, and 0.02% NaN3, the beads were incubated for 30 min with fluorescent dye-conjugated streptavidin. Cytokine levels were measured using a flow cytometer and were analyzed with Flowmetrix software (Luminex). Standard curves for each cytokine/chemokine were generated on a log-log plot for each assay, and the concentrations in each sample were calculated from the corresponding curve-fitting equations (Carson and Vignali, 1999). Cytokine/chemokine levels were measured from standard curves constructed from serial dilutions of the reference standard provided with the assay kit. The data were analyzed using a Luminex 100-plate reader.
Histopathology
The intrapulmonary airways of the left lung lobe from each animal were microdissected according to a modified version of a previously described procedure (Tesfaigzi et al., 2004). Dissection was performed under a high-resolution dissecting microscope (dual-viewing Wild M-8 stereomicroscope; Wild-Heerbrugg, Heerbrugg, Switzerland). Beginning at the lobar bronchus, the airways were split down the long axis of the axial pathway through the 11th airway generation. Lung sections at the level of the 5th (proximal) and 11th (distal) generation airways were embedded in paraffin, and 5 μm-thick sections were prepared for analysis of the axial airways.
Histochemical Staining and Analysis
Histochemical staining for Alcian Blue and periodic acid Schiff (AB-PAS) was carried out as previously described (Tesfaigzi et al., 2002). Mucous cell numbers per mm basal lamina (BL) were measured using the NIH Image analysis system (Bethesda, MD) by counting the number of mucous cells and dividing by the length of BL (Tesfaigzi et al., 2004). The volume of stored mucous substances was measured as previously described (Tesfaigzi et al., 2004). Briefly, the volume (μm3) of mucus per unit area (μm2) of basement membrane (Vs) was determined by quantifying the area of AB/PAS stained material within the epithelium using the following equation: Vs = (mucous area in mm2) × 1000)/(length of BL in mm × 1.27). In all cases, quantification and morphometry was carried out by a person unaware of slide identity.
Immunohistochemical Analysis
Endogenous peroxidase activity of the sample tissues was blocked with 2% hydrogen peroxide in methanol for 1 minute. Slides were re-hydrated in graded alcohol and deionized water, and then washed in Brij-35 in Dulbecco’s PBS (pH 7.4). Antigen retrieval was achieved by incubating the slides with Digest-all (Zymed Labs, San Francisco, CA) at a 1:3 dilution of trypsin:diluent at 32°C for 10 minutes. Following blocking in 100 mM Tris, pH 7.7, containing 550 mM NaCl, 10 mM KCl, 1% normal goat serum, and 2% BSA, slides were incubated for 48 h at room temperature with a 1:1000 dilution of Bcl-2 antibody (BD-Pharmingen, San Diego, CA). Bcl-2 immunoreaction was detected using a Vectastain ABC kit and the peroxidase substrate diaminobenzidine (Vector Laboratories, Burlingame, CA) according to the manufacturer’s directions. Mucus-containing cells were detected by staining with 0.05% AB for 10 minutes.
Statistical Analysis
Numerical data were expressed as mean group values ± SE. Data from experiments with various doses of LPS were analyzed by ANOVA. Tukey’s Multiple Comparison Test was used to determine differences between treatment groups. The criterion for significant difference was P < 0.05 in all studies.
RESULTS
Inflammatory Cells in BALF
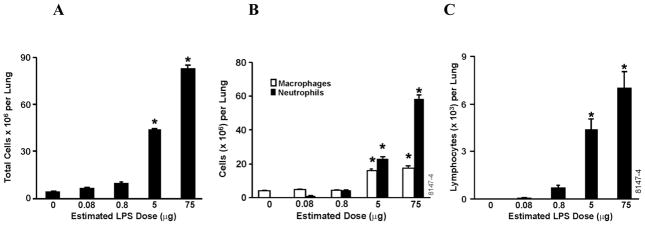
In the first exposure paradigm, we based our dosing on previously determined doses when LPS was administered by IT instillation (Foster et al., 2003). Rats were initially exposed to an estimated LPS dose deposited in the lungs that ranged from 50 to 480 μg. BAL was acquired from rat lungs 72 h post exposure and cell types and numbers were quantified. Total inflammatory cell numbers per lung were maximal at approximately 80×106 at 50 μg LPS dose and no further increases were observed at 90, 180, and 480 μg doses (data not shown). Therefore, for the subsequent studies, we used doses ranging from 0 to 75 μg deposited dose of LPS in the lung to better study the dose response in more detail. At these doses, the numbers of inflammatory cells recovered by lavage increased in a dose-dependent manner from 4 × 106 in rats exposed to filtered air to 10 × 106 at 0.8 and 80 × 106 at 75 μg dose (Figure 2A). These findings suggest that an approximate 100-fold increase in LPS dose from 0.8 to 75 μg increased the number of inflammatory cells by 10-fold.
Figure 2.
Inflammatory cell numbers recovered by BAL at 72 h following LPS inhalation. Lungs were lavaged three times using the same 5 ml of saline and BAL cells were analyzed by staining cytospins with Wright-Giemsa. (A) The total numbers of inflammatory cells increased in a dose-dependent manner. (B) The number of macrophages and neutrophils (C) and lymphocytes were quantified. Values are group means ± SE; (n = 6 rats per group). * = Significantly different from air-exposed- controls, P < 0.05.
The vast majority of the cells in the BAL of FA treated rats were macrophages (96%) and no significant increase was observed at the 0.08 and 0.8 μg LPS doses. However, macrophages were significantly increased at 5 μg LPS, with no further increase at the 75 μg dose (Figure 2B). The number of neutrophils was increased in a dose-dependent manner from 0.08 to 75 μg LPS doses (Figure 2B).
Lymphocytes were at virtually undetectable levels in the BAL of FA- and LPS-treated rats at 0.08 μg dose, but their numbers increased in a dose-dependent manner with 0.8, 5, and 75 μg deposited LPS doses reaching 7 × 103 at the 75 μg dose (Figure 2C).
Cytokines and Chemokines
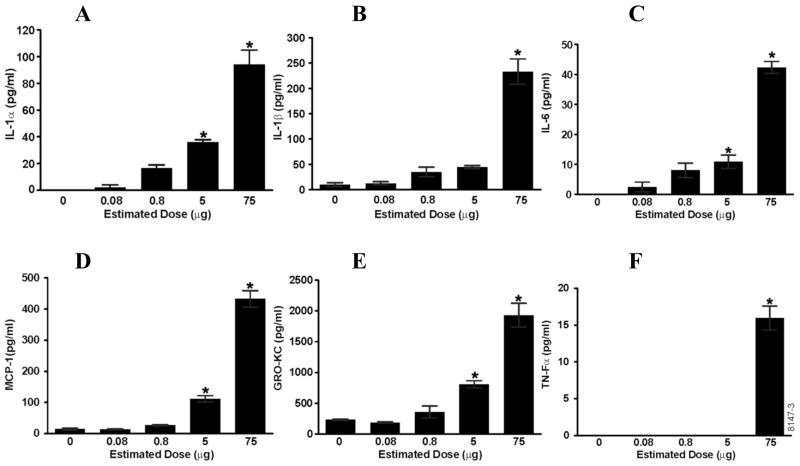
BAL supernatant was analyzed for proinflammatory cytokines and chemokines using a rat Luminex panel. The pro-inflammatory cytokines IL-1α, IL-1β, and IL-6 and the chemokines MCP-1 and KC were induced in a dose-dependent manner (Figure 3A, B, C, D, E). For each of these cytokines and chemokines, the maximum induction was achieved with the estimated lung deposited dose of 75 μg LPS. TNFα levels were undetectable at the 0 to 5 μg LPS doses and 15 pg/ml was detected at the 75 μg dose (Figure 3F). IL-12, IFN-γ, and GMCSF were not detected in any of the BAL samples.
Figure 3.
Proinflammatory cytokines and chemokines show a dose-dependent increase in response to aerosolized LPS treatment. BAL supernatant was analyzed using a rat cytokine/chemokine Luminex panel. (A) IL-1α, (B) IL-β, (C) IL-6, (D) MCP-1, and (E) GRO-KC showed dose-dependent increase and (F) TNF-α was only detectable at the highest dose of 75 μg LPS. Values are group means ± SE; (n = 6 rats per group). * = Significantly different from saline-instilled controls, P < 0.05.
Distribution of inflammation
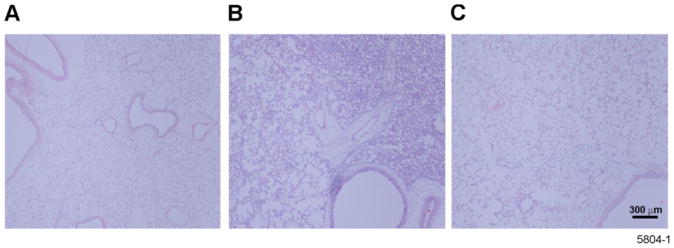
We have previously reported the number of inflammatory cells in the BAL of rats exposed to LPS by IT instillation (Foster et al., 2003). However, the overall distribution of the inflammation within the lung tissues was previously not described. Lung sections from rats treated with LPS via inhalation or IT instillation showed major differences in gross morphology. While control lungs from non-exposed rats showed no inflammation (Figure 4A), rat lungs exposed to LPS by IT instillation displayed focal areas of extensive inflammation while neighboring areas appeared less affected (Figure 4B). However, lungs from rats exposed to aerosolized LPS showed a more diffuse and less severe pattern of inflammation (Figure 4C).
Figure 4.
Lung tissue from aerosolized LPS-treated rats show more diffuse distribution of inflammatory cells than lungs from rats intratracheally instilled with LPS. Photomicrographs of H&E-stained tissue sections prepared from lung tissues at airway generation 5 in the left lung of rats exposed to (A) filtered air, (B) intratracheally instilled with LPS, or (C) to aerosolized LPS. Darkly stained tissue is indicative of inflammation. Note the even distribution of light staining tissue in the lungs exposed to aerosolized LPS compared to the localized dark stained tissue in the lungs of rats intratracheally instilled with LPS.
Mucous Cell Metaplasia
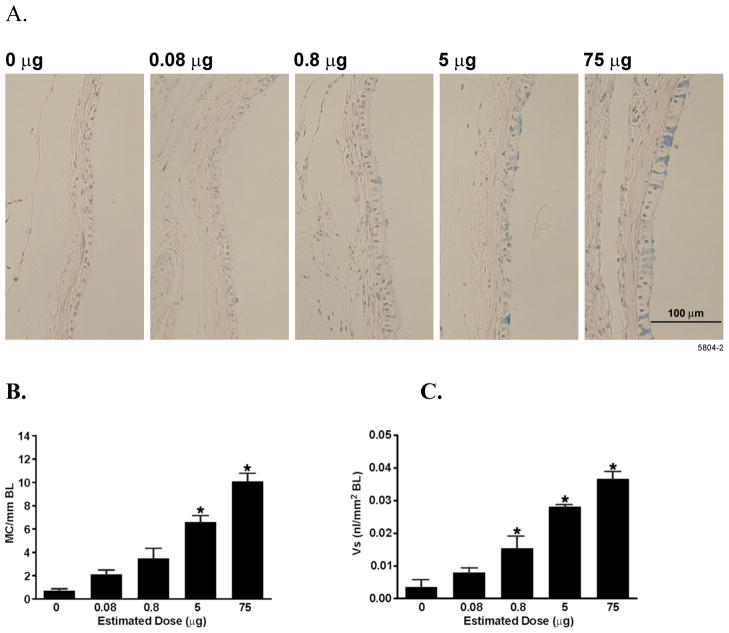
The left lungs of rats exposed to aerosolized LPS or FA were sectioned and processed for histology at airway generation 5, similar to the lungs that were analyzed previously after LPS instillation (Foster et al., 2003). Mucous cells were visible in airway epithelia with increasing LPS deposited dose (Figure 5A). Both the number of mucous cells per mm basal lamina (BL) and the volume of stored mucosubstances (Vs) showed a dose-dependent increase in response to LPS exposure (Figures 5B, C) with a 10-fold increase at the 75 μg dose.
Figure 5.
LPS inhalation induces a dose-dependent increase in MCM. Rats were exposed to increasing doses of aerosolized LPS or filtered air. Lungs were removed 72 h post exposure and analyzed for MCM following AB/PAS staining. (A) Representative photomicrograph of airway epithelia from rats exposed to increasing amounts of aerosolized LPS. (B) Mucus cell number per mm BL and (C) the amount of intraepithelial stored mucosubstances expressed as Vs. Values are group means ± SE; (n = 6 rats per group). * = Significantly different from saline-instilled controls, P < 0.05.
Bcl-2 expression in metaplastic mucous cells
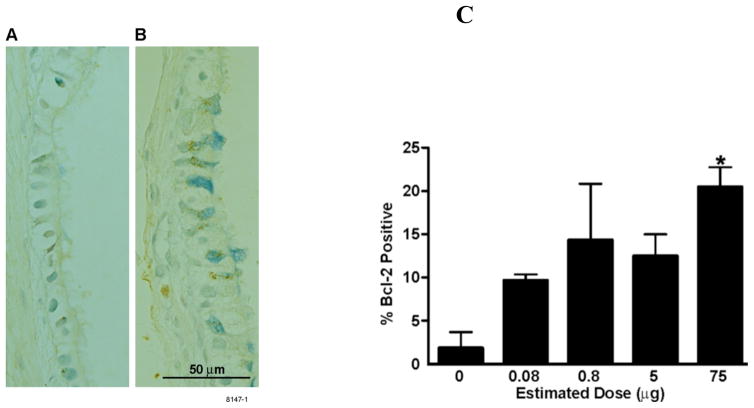
Expression and the role of Bcl-2 in metaplastic mucous cells has previously been studied extensively in rats intratracheally instilled with LPS (Tesfaigzi et al., 2004), (Foster et al., 2003), (Harris et al., 2005). Similarly, LPS exposure by inhalation caused Bcl-2 expression in metaplastic mucous cells (Figure 6A, B). While only 2–3% of airway mucous cells showed a positive immunoreaction for Bcl-2 in control rats, Bcl-2 positivity increased to 10% in rats treated with 0.08 μg LPS, and to 15–20% in rats treated with 0.8, 5 and 75 μg LPS, respectively (Figure 6C).
Figure 6.
Expression of Bcl-2 in mucous cells treated with LPS. (A) Representative photomicrograph of axial airways at generation 5 in the left lung lobes of rats treated with filtered air (0 μg) (A) or 75μg aerosolized LPS (B). Following immunostaining with Bcl-2 antibody, Bcl-2 was detected by immunohistochemistry and diaminobenzidine (DAB) and appears dark brown. Mucous cells were then stained with Alcian Blue. (C) The percentage of Bcl-2 positive mucous cells at airway generations 5 and 11 in the left lung were quantified in rats treated with filtered air or various doses of aerosolized LPS. Values are group means ± SE; (n = 6 rats per group). * = Significantly different from saline-instilled controls, P < 0.05.
Effect of rolipram treatment on inflammatory cells and MCM
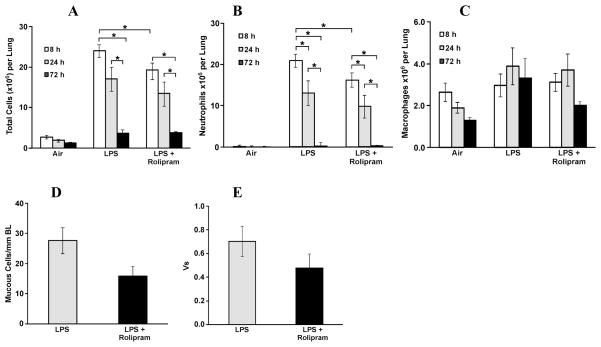
To test whether this animal model can be used for screening of anti-inflammatory drugs we treated rats by oral gavage with vehicle or rolipram 2 h prior to LPS aerosol exposure. BAL was acquired from lungs of rats at 8, 24 and 72 h post exposure to LPS and inflammatory cell types and numbers were quantified. Total cells (Figure 7A) and neutrophils (Figure 7B) were at a maximum at 8 h and decreased over 24 and 72 h post LPS exposure. While rolipram treatment showed a trend toward decreasing total inflammatory cells and neutrophils at all time points we observed a significant reduction for total cells and neutrophils only at 8 h (Figures 7A–B). Conversely, macrophages were at a minimum at 8 h post exposure to LPS and rolipram treatment showed a trend in attenuating the increase at 72 h (Figure 7C). In addition, treatment of rats with rolipram resulted in a trend toward reduced MCM as measured by both the number of mucous cells per mm basal lamina (Figure 7D) and the volume of stored mucosubstances (Vs) at 72 h post LPS exposure (Figure 7E).
Figure 7.
Effects of rolipram on BAL inflammatory cells and mucous cell metaplasia in airway epithelium. Rats were gavaged with vehicle or 10 mg/kg body weight rolipram 2 h prior to exposure to LPS aerosol (15 μg deposited pulmonary dose). BAL was performed at 8, 24 and 72 h following LPS inhalation and the numbers of (A) total cells, (B) neutrophils, and (C) macrophages were quantified. For the harvest at 8 and 24 h, the whole lung was lavaged two times each using 5 ml of saline. For the harvest at 72 h, the right lungs were lavaged two times each with 3 ml of saline and the left lung was inflated with 10% zinc formalin under a constant pressure for 3 h. (D) mucous cell number per mm BL and (E) the amount of intraepithelial stored mucosubstances expressed as Vs were quantified. Values are group means ± SE; (n = 6 rats per group). * = Significantly different, P < 0.05.
DISCUSSION
The present study demonstrates the efficacy of aerosolized LPS exposure for inducing inflammation that is more evenly distributed throughout the lung compared to LPS delivered by IT instillation. Dose-dependent increases in inflammatory cell numbers and cytokine/chemokine levels were found in the BALF at significantly lower doses when LPS was delivered by aerosol than by IT instillation. Furthermore, aerosolized LPS-induced MCM increased more linearly with increasing LPS doses as compared to the less linear dose-response curve achieved via instillation (Foster et al., 2003).
Our initial exposures that spanned 0.08 – 480 μg deposited LPS dose showed that total number of inflammatory cells in the lavage reached maximum at ~50 μg LPS dose. Dose-dependent increase in total cell infiltration was observed between 0.08 to 75 μg of deposited LPS dose. A 10-fold lower dose of aerosolized LPS was sufficient to cause a 10-fold increase in inflammatory cell numbers as was observed for LPS that was delivered by IT instillation (Foster et al., 2003). In addition, a previous study quantified the number of inflammatory cells 10 h post IT instillation of 1000 μg LPS and found approximately the same number of cells (data not shown) as was observed at 8 h post LPS aerosol exposure at 15 μg deposited dose. Therefore, extent of inflammation as measured by total cells retrieved by lavage at these doses resembled those observed at much larger doses (50– 1000 μg) when LPS was instilled (Foster et al., 2003). The deposition of instilled LPS throughout the lung is likely to be uneven thereby causing massive inflammation in regions where a high LPS bolus was delivered with some areas of the lungs remaining unaffected. LPS inhalation resulted in a 4-fold increase in neutrophil infiltration compared to instilled LPS and the number of macrophages retrieved by lavage reached maximum at approximately 20×106 cells per lung for both routes of LPS delivery. However, macrophage numbers were decreased when 1000 μg LPS was instilled compared to the lower 500 μg dose, while the macrophage numbers remained constant from 5 to 75 μg aerosolized LPS. Furthermore, while lymphocytes were undetectable in the BALF of LPS-instilled rats, LPS inhalation resulted in a dose-dependent increase in lymphocyte infiltration reaching ~7 × 103 cells per lung. We postulate these relative increases in neutrophil and lymphocyte infiltration are due to the widespread and evenly distributed inflammation caused by LPS inhalation that allows increased recruitment from the vasculature.
The number of total inflammatory cells and neutrophils peaked at 8 h post LPS exposure and declined by 24 h and near baseline by 72 h. This resolution is consistent with previous studies demonstrating that LPS-induced pulmonary neutrophilia in rats increases at 2 h, peaked by 16 h, and declined thereafter but remained elevated for up to 48 h (Spond et al., 2001), (Harris et al., 2007). Similarly, the observed increase in macrophages at 8 and 72 h compared to the unexposed group is consistent with previous reports (Harris et al., 2007).
As expected, the pro-inflammatory cytokines IL-1α, IL-1β, IL-6, MCP-1, and GRO-KC were increased in the lavage fluid with increasing LPS dose. The chemokine MCP-1 activates and recruits macrophages into the pulmonary airways (Murugan and Peck, 2009). LPS inhalation initiates a dose-dependent increase of MCP-1 in BALF which correlates with the dose dependant increase in macrophage infiltration into the lungs. The chemokine KC is an important regulator of neutrophil recruitment and activation during LPS induced pulmonary inflammation (Frevert et al., 1995). As expected, we observed a correlation between the LPS-induced increase in the numbers of BALF neutrophils and increasing amounts of KC. TNF-α was detected at the highest dose only, likely because this early response cytokine at lower LPS doses was reduced as part of the resolution process of the inflammatory response (Harris et al., 2007). Because IL-1β stimulates mucin production in vitro (Koo et al., 2002), we anticipated that the LPS dose that achieved the highest IL-1β levels in the lavage would be associated with maximum MCM.
Both the mucous cell number per mm BL and Vs increased with increasing deposited dose of aerosolized LPS, each displaying a 3-fold increase at 0.8 μg LPS and a 10-fold increase at the highest dose of 75 μg over FA controls. As expected, this increase correlated with the inflammatory response as measured by the inflammatory cell numbers and cytokine/chemokine levels in BALF. Similarly, IT instillation of LPS also increased mucous cells per mm BL and Vs in a dose-dependent manner; however at the highest instilled LPS dose of 1000 μg, these parameters were increased only 2- and 5-fold compared to saline-instilled controls (Foster et al., 2003). These findings suggest that the mechanical injury caused by saline instillation may already have caused increase in MCM and thereby somewhat minimizing the effect of LPS on MCM.
The observation that the number of mucous cells per mm BL and the Vs were increasing proportionally with increasing LPS dose suggests that the number of mucus-producing cells were increasing rather than the stored mucosubstances per cell. However, in rats instilled with LPS, Vs continued to increase with higher LPS doses while the number of mucous cells per mm BL remained essentially unchanged over 50 – 1000 μg doses (Foster et al., 2003) suggesting that instilled LPS caused increased storage of mucins in cells rather than increasing the number of mucus-producing cells.
The maximum number of mucous cells per mm BL was approximately 10 for exposure by inhalation but 40 for exposure by instillation (Foster et al., 2003). This significant difference may again be explained by the differences in LPS deposition. Because the quantification for all rats was performed at airway generation 5 of the rat’s left lung it is possible that the 1000 μg of instilled LPS is preferentially deposited directly onto this area of the lung tissue causing maximum local inflammation. However, due to the distribution of the relatively low dose of aerosolized LPS throughout the lung, extent of local inflammation at airway generation 5 would be expected to be significantly lower than when 1000 μg LPS was IT instilled causing significantly higher local inflammation and MCM.
Despite the differences in extent of MCM at airway generation 5 the percentage of Bcl-2-positive mucous cells was 10% at 0.08 μg and ~20% at 75 μg of aerosolized LPS. In rats instilled with LPS, the percentage of Bcl-2-positive mucous cells did not increase appreciably over controls at doses below 250 μg LPS but increased 5-fold at 500 and 1000 μg to ~20% (Foster et al., 2003). These results are consistent with previous LPS studies in which maximum Bcl-2 positivity is reached at approximately 20% (Tesfaigzi et al., 2004).
Rolipram significantly attenuated the total cell and neutrophil response at 8 h post LPS exposure with a decrease also noted at 24 h. These findings are consistent with a previous report showing that rolipram inhibits neutrophil recruitment at 4 h post LPS treatment in rats (Escofier et al., 1999). These observations suggest that rolipram while effective in reducing early neutrophilic inflammation does not affect the resolution of inflammation. However, while statistically not significant in our present study, rolipram showed a role in suppressing the increase in macrophages post LPS exposure and showed a trend toward reducing MCM at 72 h post LPS treatment. It is important to note the half-life of rolipram in rats is less than 15 minutes (Krause and Kuhne, 1988) and this may explain the lack of significance in the ability of rolipram to inhibit the LPS-induced inflammatory response at later times. The inflammatory cell number at 72 h in the dose-response study (Fig. 2) and the rolipram treatment study (Fig. 7) show discrepancies because different lots of LPS were used for exposures and different volumes of saline were used for BAL preparation. However, four subsequent studies since then have shown that the inflammatory response is highly reproducible when the same lot of LPS and lavage procedures are used (data not shown).
Collectively, these results support the efficacy of this aerosolized LPS delivery system for initiation of pulmonary inflammation and MCM in rats. A single dose of aerosolized LPS was sufficient to initiate an inflammatory response as measured by cell infiltration, cytokine/chemokine release, and an increase in MCM. LPS inhalation resulted in a more linear dose response at much lower doses compared to the doses required for initiating inflammation by IT instillation. Overall, LPS-induced pulmonary inflammation was attenuated by the PDE4 inhibitor rolipram. Furthermore, assessment of tissue histopathology and MCM revealed that LPS causes a much more evenly distributed inflammatory response when aerosolized than when intratracheally instilled. We conclude that delivery of aerosolized LPS requires a smaller dose and results in a more evenly distributed pulmonary inflammatory response in rats suggesting this route of delivery should be preferred when designing studies for screening of effective drugs to better approximate the human condition.
Acknowledgments
These studies were supported by grants from the National Institutes of Health (HL68111 and ES015482).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest related to the studies described in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bide RW, Armour SJ, Yee E. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol. 2000;20:273–290. doi: 10.1002/1099-1263(200007/08)20:4<273::aid-jat657>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Carson RT, Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- Chung KF. Cytokines as targets in chronic obstructive pulmonary disease. Current drug targets. 2006;7:675–681. doi: 10.2174/138945006777435263. [DOI] [PubMed] [Google Scholar]
- Clapp W, Thorne P, Frees K, Zhang X, Lux C, Schwartz D. The effects of inhalation of grain dust extract and endotoxin on upper and lower airways. Chest. 1993;104:825–830. doi: 10.1378/chest.104.3.825. [DOI] [PubMed] [Google Scholar]
- Escofier N, Boichot E, Germain N, Silva PM, Martins MA, Lagente V. Effects of interleukin-10 and modulators of cyclic AMP formation on endotoxin-induced inflammation in rat lung. Fundamental & clinical pharmacology. 1999;13:96–101. doi: 10.1111/j.1472-8206.1999.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Foster JE, Gott K, Schuyler MR, Kozak W, Tesfaigzi Y. LPS-induced neutrophilic inflammation and Bcl-2 expression in metaplastic mucous cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L405–414. doi: 10.1152/ajplung.00249.2002. [DOI] [PubMed] [Google Scholar]
- Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–344. [PubMed] [Google Scholar]
- Harris JF, Aden J, Lyons R, Tesfaigzi Y. Resolution of LPS-induced airway inflammation and goblet cell hyperplasia is independent of IL-18. Respir Res. 2007;8:24–36. doi: 10.1186/1465-9921-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JF, Fischer MJ, Hotchkiss JA, Monia BP, Randell SH, Harkema JR, Tesfaigzi Y. Bcl-2 sustains increased mucous and epithelial cell numbers in metaplastic airway epithelium. Am J Respir Crit Care Med. 2005;171:764–772. doi: 10.1164/rccm.200408-1108OC. [DOI] [PubMed] [Google Scholar]
- Koo JS, Kim YD, Jetten AM, Belloni P, Nettesheim P. Overexpression of mucin genes induced by interleukin-1 beta, tumor necrosis factor-alpha, lipopolysaccharide, and neutrophil elastase is inhibited by a retinoic acid receptor alpha antagonist. Experimental lung research. 2002;28:315–332. doi: 10.1080/01902140252964393. [DOI] [PubMed] [Google Scholar]
- Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica; the fate of foreign compounds in biological systems. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- Murugan V, Peck MJ. Signal transduction pathways linking the activation of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease. Experimental lung research. 2009;35:439–485. doi: 10.1080/01902140902759290. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, D’Ambrosio D. Chemokines and their receptors in asthma and chronic obstructive pulmonary disease. Current opinion in pulmonary medicine. 2003;9:104–110. doi: 10.1097/00063198-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Schwartz D. Grain dust, endotoxin, and airflow obstruction. Chest. 1996;109:57S–63S. doi: 10.1378/chest.109.3_supplement.57s. [DOI] [PubMed] [Google Scholar]
- Schwartz D, Thorne P, Yagla S, Burmeister L, Olenchock S, Watt J, Quinn T. The role of endotoxin in grain dust-induced lung disease. Am J Respir Crit Care Med. 1995;152:603–608. doi: 10.1164/ajrccm.152.2.7633714. [DOI] [PubMed] [Google Scholar]
- Spond J, Chapman R, Fine J, Jones H, Kreutner W, Kung TT, Minnicozzi M. Comparison of PDE 4 inhibitors, rolipram and SB 207499 (ariflo), in a rat model of pulmonary neutrophilia. Pulmonary pharmacology & therapeutics. 2001;14:157–164. doi: 10.1006/pupt.2001.0291. [DOI] [PubMed] [Google Scholar]
- Tesfaigzi J, Wood MB, Johnson NF, Nikula KJ. Apoptosis is a major pathway responsible for the resolution of endotoxin-induced type II cell hyperplasia in the rat. Int J Exp Pathol. 1998;79:303–312. doi: 10.1046/j.1365-2613.1998.720402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaigzi Y, Fischer MJ, Martin AJ, Seagrave J. Bcl-2 in LPS- and allergen-induced hyperplastic mucous cells in airway epithelia of Brown Norway rats. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1210–L1217. doi: 10.1152/ajplung.2000.279.6.L1210. [DOI] [PubMed] [Google Scholar]
- Tesfaigzi Y, Harris JF, Hotchkiss JA, Harkema JR. DNA synthesis and Bcl-2 expression during the development of mucous cell metaplasia in airway epithelium of rats exposed to LPS. Am J Physiol Lung Cell Mol Physiol. 2004;286:L268–274. doi: 10.1152/ajplung.00172.2003. [DOI] [PubMed] [Google Scholar]
- Tesfaigzi Y, Singh SP, Foster JE, Kubatko J, Barr EB, Fine PM, McDonald JD, Hahn FF, Mauderly JL. Health Effects of Subchronic Exposure to Low Levels of Wood Smoke in Rats. Toxicol Sci. 2002;65:115–125. doi: 10.1093/toxsci/65.1.115. [DOI] [PubMed] [Google Scholar]
- Thorley AJ, Ford PA, Giembycz MA, Goldstraw P, Young A, Tetley TD. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages. J Immunol. 2007;178:463–473. doi: 10.4049/jimmunol.178.1.463. [DOI] [PubMed] [Google Scholar]