Abstract
RAS activation is implicated in physiologic and pathologic cardiac hypertrophy. Cross-talk between the Ras and calcineurin pathways, the latter also having been implicated in cardiac hypertrophy, has been suspected for pathologic hypertrophy. Our recent discovery that germ-line mutations in RAF1, which encodes a downstream RAS effector, cause Noonan and LEOPARD syndromes with a high prevalence of hypertrophic cardiomyopathy provided an opportunity to elaborate the role of RAF1 in cardiomyocyte biology. Here, we characterize the role of RAF1 signaling in cardiomyocyte hypertrophy with an aim of identifying potential therapeutic targets. We modeled hypertrophic cardiomyopathy by infecting neonatal and adult rat cardiomyocytes (NRCMs and ARCMs, respectively) with adenoviruses encoding wild-type RAF1 and three Noonan/LEOPARD syndrome-associated RAF1 mutants (S257L, D486N or L613V). These RAF1 proteins, except D486N, engendered cardiomyocyte hypertrophy. Surprisingly, these effects were independent and dependent of mitogen activated protein kinases in NRCMs and ARCMs, respectively. Inhibiting Mek1/2 in RAF1 overexpressing cells blocked hypertrophy in ARCMs but not in NRCMs. Further, we found that endogenous and heterologously expressed RAF1 complexed with calcineurin, and RAF1 mutants causing hypertrophy signaled via nuclear factor of activated T cells (Nfat) in both cell types. The involvement of calcineurin was also reflected by down regulation of Serca2a and dysregulation of calcium signaling in NRCMs. Furthermore, treatment with the calcineurin inhibitor cyclosporine blocked hypertrophy in NRCMs and ARCMs overexpressing RAF1. Thus, we have identified calcineurin as a novel interaction partner for RAF1 and established a mechanistic link and possible therapeutic target for pathological cardiomyocyte hypertrophy induced by mutant RAF1.
Keywords: RAF1, calcineurin, MEK1/2, ERK1/2, hypertrophic cardiomyopathy, Noonan/LEOPARD syndrome, cardiomyocyte hypertrophy
1. Introduction
Cardiac hypertrophy is elaborated via several intracellular signaling pathways including the calcineurin/nuclear factor of activated T cells (NFAT) and RAS/mitogen-activated protein kinase (MAPK) cascades [1]. Activation of RAS is implicated in cardiac hypertrophy, with cross-talk between the RAS and calcineurin pathways being suspected for pathologic hypertrophy but its basis being poorly understood [2]. Recently, RAF1 missense mutations with gain-of-function effects on RAF1’s kinase activity were found to cause Noonan and LEOPARD syndromes (NS and LS, respectively) with a high prevalence of hypertrophic cardiomyopathy (HCM) [3, 4]. The majority of RAF1 mutations causing NS and LS cluster around Ser259 and Ser621. Those HCM-associated mutations exhibited high kinase activites and increased Erk activation in Cos cells highlighting the significance of those two phosphorylation sites, particularly as it pertains to cardiac hypertrophy (eg. S257L and L613V). Mutations in the activation segment, which are infrequently associated with HCM (eg. D486N), showed impaired kinase and reduced Erk activation in Cos cells. Here, we elucidated the role of RAF1 in cardiac hypertrophy by expressing wild-type RAF1 and three NS/LS-associated RAF1 mutants representing the three mutation clusters, (S257L, D486N and L613V), in rat cardiomyocytes. Overexpression of the mutants from the Ser259 and Ser621 clusters (eg. S257L or L613V) induced hypertrophy that was independent and dependent of Erk1/2 activation in NRCMs and ARCMs, respectively. The activation segment mutant, D486N, failed to induce hypertrophy. By analyzing various hypertrophic signaling pathways to identify molecules that RAF1 may regulate in cardiomyocytes, we identified calcineurin (also known as protein phosphatase 2B) as a major interacting partner of RAF1 in cardiomyocytes in NRCMs and ARCMs. Strikingly, the wild type and the mutants (S257L or L613V) RAF1-calcineurin complexes regulated calcium signaling and initiated hypertrophy in NRCMs and ARCMs. However, wild-type RAF1 signals through Mef2 in NRCMs but not in ARCMs. L613V RAF1 signals through Nfat in both cell types. In contrast, D486N RAF1 failed to activate Nfat or Mef2. Blocking the Erk pathway using a dominant negative form of Mek1 blocked hypertrophy in ARCMs, but not in NRCMs, overexpressing RAF1. In contrast, inhibiting calcineurin with cyclosporine blocked the induction of cardiomyocyte hypertrophy in NRCMs and ARCMs. Thus, we have identified calcineurin as a novel interaction partner for RAF1 and established a novel interaction between two nodal pathways in hypertrophy that has therapeutic implications for pathological cardiomyocyte hypertrophy induced by RAF1 mutants.
2. Methods
All animal procedures were performed with the approval of IACUC at the Mount Sinai School of Medicine and in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals. The methods used in this study are detailed in the online data as supplement methods.
3. Results
3.1. Effect of a RAF1 mutant at the Ser621 cluster (Ad.RAF1L613V) in NRCMs
We infected NRCMs with recombinant adenoviruses encoding either wild-type or L613V RAF1. After 72 h, Ad.RAF1WT and Ad.RAF1L613V infections resulted in increased RAF1 protein levels compared to control cells infected with Ad.GFP. Of note, RAF1 levels in NRCMs expressing the L613V mutant were lower than in those expressing the wild-type protein, which was not due to a difference in infection efficiency or expression from that virus since GFP levels were comparable among all infected cells (Fig. 1a). Treatment with the proteasomal inhibitor, MG132, equalized RAF1 levels among infected NRCMs (Supplementary Fig. 1a). Since the stability of RAF1 is dependent upon the phosphorylation of Ser621 [6], which is near the mutation site, we considered the possibility that the L613V substitution might affect the phosphorylation status of that regulatory residue. Using phospho-specific antibodies, we found that Ser621 of L613V RAF1 was minimally phosphorylated, which was different from wild-type RAF1, while the phosphorylation status of Ser259, a residue that cooperatively regulates RAF1’s function with Ser621, was normally phosphorylated (Supplementary Fig. 1b and c). Collectively, these data suggest that the L613V mutation negatively affects the phosphorylation of Ser621 in cardiomyocytes, resulting in enhanced proteasomal degradation of this mutant RAF1 protein.
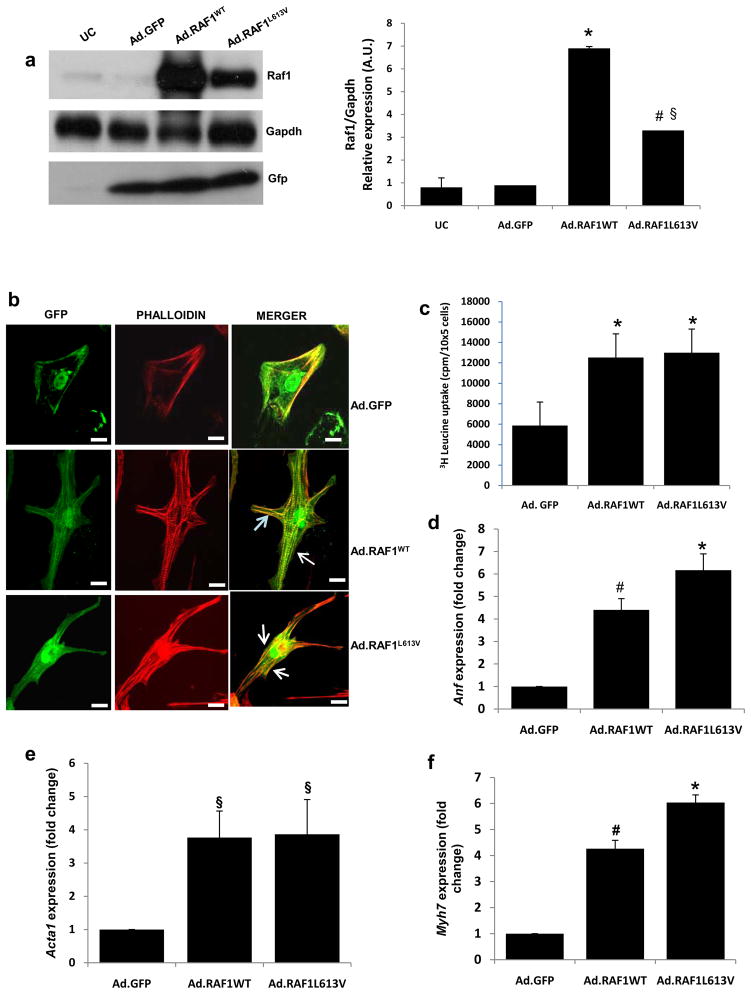
Figure 1. Overexpression of wild-type and L613V RAF1 proteins induces cardiomyocyte hypertrophy.
Neonatal rat cardiomyocytes were infected with adenoviruses encoding GFP alone (Ad.GFP), wild type RAF1 (Ad.RAF1WT) or a mutant RAF1 (Ad.RAF1L613V) and harvested after 72 hours. (a) Representative immunoblots with total lysates that were probed with anti-Raf1 (top panel), anti-Gapdh (middle panel) and anti-Gfp (lower panel) antibodies. Gapdh levels were used as loading control and Gfp levels were indicative of the efficiency of viral production. Uninfected cardiomyocytes (UC) were used as a negative control. Protein expression levels were normalized to respective Gapdh and expressed as relative expression. Data are mean values ± SD of three independent experiments §p <0.05 vs. Ad.GFP; *p <0.01 vs. Ad.GFP; #p <0.05 vs. WT (b) Cardiomyocytes infected with the viruses indicated at the right expressing GFP (left column) and stained with phalloidin to detect sarcomeric α-actinin(middle column) and merged (right column). Cells were imaged with confocal laser microscopy. Scale bar- 387×387nm. (c) [3H]-leucine incorporation in infected cardiomyocytes as an assessment of protein synthesis rates. *P <0.001 vs Ad.GFP. Data are mean values ± SD of three independent experiments. (d-f) Quantitative reverse transcription PCR analysis of fetal gene re-activation. Steady-state mRNA levels of (d) atrial natriuretic factor (Anf), (e) α-skeletal actin (Acta1) and (f) β-myosin heavy chain (Myh7) were performed. Expression levels were normalized to β-actin and expressed as fold change compared to level in the Ad.GFP cells. The mean fold induction ± SD of three independent experiments is shown. §p <0.05 vs. Ad.GFP; *p <0.001 vs. Ad.GFP; #p <0.01 vs. Ad.GFP.
3.2. RAF1 overexpression induces cardiomyocyte hypertrophy with cell elongation in NRCMs
To determine whether expression of the L613V RAF1 in NRCMs promoted cardiomyocyte hypertrophy, modeling the HCM observed in NS and LS patients, cell surface area, sarcomeric organization, protein synthesis rate and fetal gene expression were analyzed. Seventy-two hours after adenoviral infection, NRCMs overexpressing wild-type and L613V RAF1 had significantly increased average surface areas compared to Ad.GFP-infected NRCMs, attributable to dramatic increases in cell length (Table 1, Fig. 1b and Supplementary Fig. 1d and e). As generally observed with cardiomyocyte hypertrophy, NRCMs overexpressing wild-type RAF1 showed an increase in sarcomeric reorganization but those overexpressing the mutant RAF1 showed a disorganized and diffused pattern of sarcomeric architecture, which were not present in GFP-overexpressing cells (Fig. 1b and Supplementary Fig. 1d). We examined protein synthesis rates of NRCMs overexpressing wild-type and L613V RAF1 using the [3H]-leucine incorporation method and observed marked increases compared to GFP-expressing cells (Fig. 1c). We also found that the steady-state expression levels of atrial natriuretic factor (Anf), α-skeletal actin (Acta1) and β-myosin heavy chain (Myh7) were increased significantly in NRCMs overexpressing wild-type and L613V RAF1 compared to GFP-expressing cells (Fig. 1d-f), evidence of re-activation of the fetal gene program. Collectively, these data support the conclusion that overexpression of wild-type and L613V RAF1 promotes cardiomyocyte hypertrophy, but only L613V RAF1 causes sarcomeric disorganization.
Table 1.
Morphometric analysis of cardiomyocytes
| Cell treatment | Area (μm2) | Major axis (μm) | Minor axis (μm) | Major/minor |
|---|---|---|---|---|
| Ad.GFP | 2123 ± 632 | 62 ± 11 | 40 ± 10 | 1.42 ± 0.32 |
| Ad.RAF1 WT | 3540 ± 1050 | 136 ± 35 | 33 ± 13 | 4.70 ± 1.98* |
| Ad.RAF1L613V | 3821 ± 1000 | 140 ± 38 | 32 ± 13 | 4.98 ± 2.15* |
| Ad.GFP+ Ad.MEKDN | 1982 ± 870 | 65 ± 15.34 | 43 ± 17.62 | 1.00 ± 0.41 |
| Ad.RAF1 WT+ Ad.MEKDN | 3780 ± 1100 | 142 ± 37.02 | 35 ± 11.43 | 4.50 ± 2.00* |
| Ad.RAF1 L613V+ Ad.MEKDN | 3928 ± 1032 | 141 ± 40 | 34 ± 13.72 | 4.62 ± 2.15* |
| Ad.GFP+ Ad.ASKDN | 1962 ± 670 | 54 ± 15 | 44 ± 13.56 | 1.00 ± 0.21 |
| Ad.RAF1 WT+ Ad.ASKDN | 3092 ± 890 | 142 ± 37.73 | 37 ± 9.2 | 4.60 ± 2.05* |
| Ad.RAF1 L613V+ Ad.ASKDN | 3789 ± 980 | 136 ± 43.98 | 34 ± 13 | 4.90 ± 4.15* |
| Ad.GFP+ Ad.VIVIT | 1937 ± 423 | 51 ± 02 | 41 ± 11 | 1.20 ± 0.01 |
| Ad.RAF1 WT+ Ad.VIVIT | 3370 ± 992 | 132 ± 41 | 34 ± 11.2 | 4.59 ± 1.88* |
| Ad.RAF1 L613V+ Ad.VIVIT | 2003 ± 312 | 59 ± 13 | 39 ± 15 | 1.35 ± 0.12 |
| Ad.GFP+ Ad.MEF2DN | 1999 ± 356 | 49 ± 3 | 40 ± 8 | 1.01 ± 0 |
| Ad.RAF1 WT+ Ad.MEF2DN | 1890 ± 567 | 49 ± 02 | 39 ± 6.87 | 0.99 ± 0.2 |
| Ad.RAF1 L613V+ Ad.MEF2DN | 3800 ± 890 | 141 ± 33 | 29 ± 12 | 4.99 ± 1.68* |
Values are means of SD of three independent experiments;
P≤ 0.01 versus GFP cells
3.3. RAF1 overexpression induces cardiomyocyte hypertrophy independent of Mapk activation in NRCMs
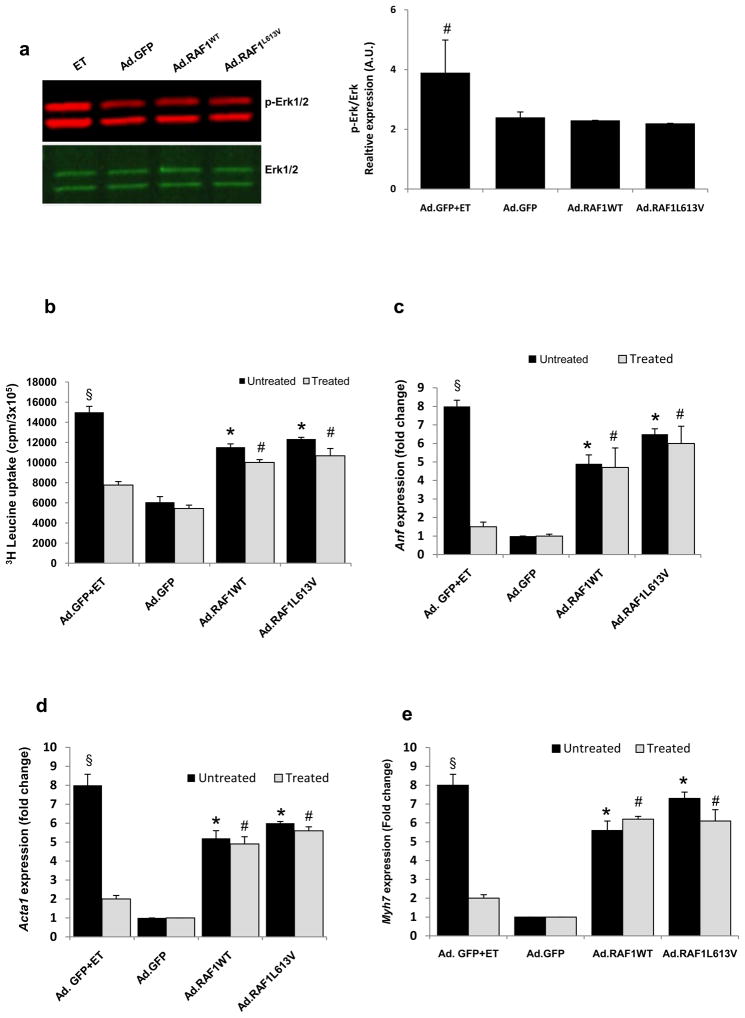
Raf1 phosphorylates Mek1/2, which then activate Erk1/2. To test our hypothesis that Erk activation was modulating the hypertrophy in cardiomyocytes overexpressing RAF1 proteins, we assessed the phosphorylation status of Erk1/2. Surprisingly, we observed that the ratio of phospho Erk1/2 to total Erk1/2 was unchanged in NRCMs overexpressing wild-type and L613V RAF1 compared to GFP-expressing cells respectively (Fig. 2a). Since this result was unexpected, we considered the possibility that RAS signaling was being desensitized as cardiomyocyte hypertrophy progressed. To test this, we expressed a dominant-negative form of Mek1 along with the RAF1 proteins to block signaling to Erk1/2 and found no abrogation of the cardiomyocyte hypertrophy (Table 1 and Fig. 2b–e). Since overexpressed RAF1 proteins were able to induce cardiomyocyte hypertrophy independently of Erk1/2 activation in NRCMs, we explored whether activation of other Mapks was mediating this effect. Activation of Jnk (c-Jun N-terminal kinase) and p38 Mapk were similar and decreased, respectively, in NRCMs overexpressing wild-type and L613V RAF1 as compared to GFP-expressing cardiomyocytes (Supplementary Fig. 2 and 3). We then examined the status of Erk5 because its activation has been associated with cardiomyocyte elongation [7], but found that it was also unchanged in RAF1-expressing NRCMs (Supplementary Fig. 4a). Thus, we concluded that the overexpressed RAF1 proteins were able to signal independently of Mapk to engender NRCM hypertrophy.
Figure 2. Wild-type and L613V RAF1 does not cause cardiomyocyte hypertrophy via the mitogen-activated protein kinases (Mapk), Erk1/2.
(a) Representative immunoblot analysis of phospho-Erk1/2 in neonatal rat cardiomyocytes infected with Ad.GFP, Ad.RAF1WT and Ad.RAF1L613V. A Mapk agonist, endothelin-1 (ET), that induces cardiac hypertrophy via Erk1/2, served as the positive control. Protein expression levels were normalized to total Erk and expressed as relative expression. Data are mean values ± SD of three independent experiments. #p <0.05 vs. Ad.GFP (b-e) To show that signaling via Erk1/2 was not necessary for RAF1-induced cardiomyocyte hypertrophy, a dominant-negative form of Mek1 was co-expressed using Ad.MEKDN. Co-infected cardiomyocytes were analysed 72 hours post infection for (b) protein synthesis rates and steady-state expression levels of (c) Anf, (d) Acta1 and (e) Myh7. Data are mean values ± SD of three independent experiments. *p <0.01 vs. untreated Ad.GFP; #p <0.01 vs. treated Ad.GFP; §p <0.001 vs. untreated Ad.GFP.
3.4. Search for the mechanism underlying RAF1-Induced hypertrophy in NRCMs
Next, we assessed the status of several phosphoproteins participating in signaling pathways previously implicated in cardiac hypertrophy. We observed that the levels of phospho-Stat3 (Signal transducers and activators of transcription), -Akt (Protein kinase B), -Gsk3β (Glycogen synthase kinase), and –Acc (Acetyl-CoA carboxylase) (Supplementary Fig. 4b, 5a-b & 6) were not altered in NRCMs expressing wild-type or L613V RAF1 compared to GFP-expressing cells. These results suggested that signaling through the Jak-Stat, Akt-Gsk3β, and Ampk-Acc pathways was not engendering the RAF1-induced cardiomyocyte hypertrophy.
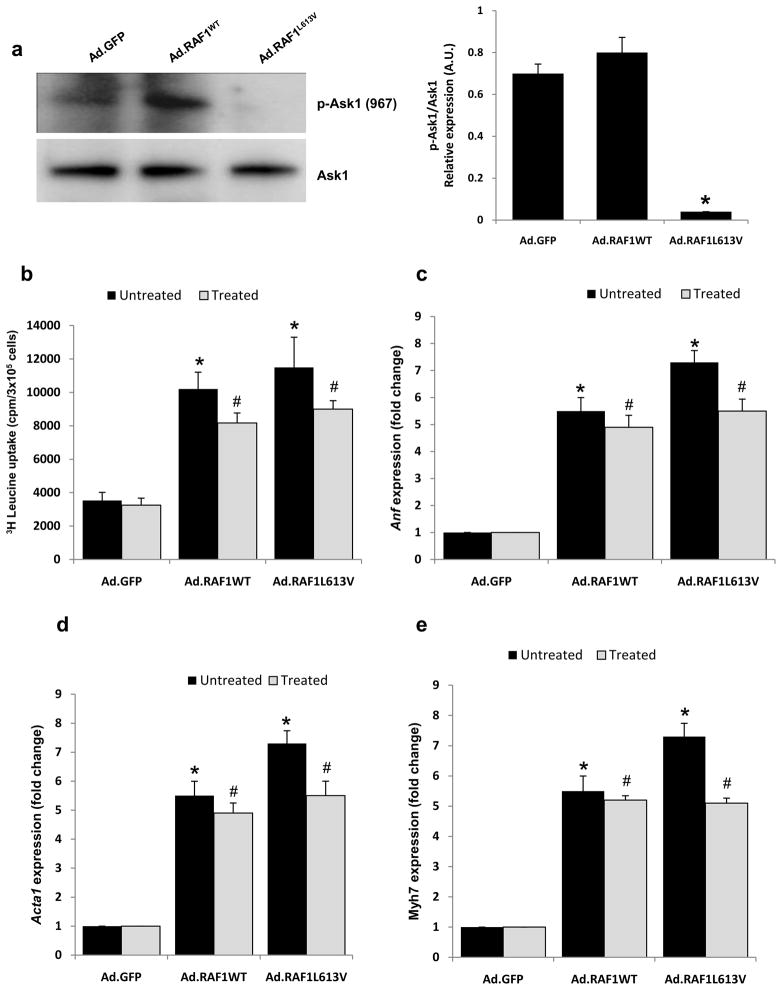
Ask1 (Apoptosis signal-regulating kinase 1) is known to interact directly with Raf1 and has roles in cardiomyocyte cell death and, possibly, hypertrophy [8–10]. Calcineurin dephosphorylates Ask1 at Ser967, resulting in a loss of 14-3-3 binding and Ask1 activation [9]. We observed that NRCMs overexpressing L613V RAF1 had notably lower levels of pAsk1 (Ser967) compared to those overexpressing wild-type RAF1 or GFP (Fig. 3a) suggesting that Ask1 was activated by the mutant. However, co-expression of a dominant-negative form of Ask1 with RAF1 L613V failed to suppress cardiomyocyte hypertrophy (Table 1, Fig. 3b-e). Therefore, we concluded that Ask1 activation was not mediating this phenomenon.
Figure 3. ASK1 is not involved in RAF1-induced cardiomyocyte hypertrophy.
(a) Representative immunoblots analysis of phospho-Ask1 (Ser967) and total Ask1 in neonatal rat cardiomyocytes overexpressing GFP, wild-type RAF1 and L613V RAF1. Protein expression levels were normalized to total Erk and expressed as relative expression. Data are mean values ± SD of three independent experiments. *p <0.01 vs. Ad.GFP. Neonatal rat cardiomyocytes were infected with Ad.GFP, Ad.RAF1WT and Ad.RAF1L613V along with Ad.ASK1DN (treated). Seventy-two hours post infection, the cardiomyocytes were analyzed for protein synthesis rates (b) and steady-state mRNA levels of Anf (c), Acta1 (d) and Myh7 (e). *p <0.01 vs. untreated Ad.GFP; #p <0.01 vs. treated Ad.GFP. Mean values ± SD of three independent experiments are shown.
3.5. Wild-type and L613V RAF1 signal through calcineurin to induce cardiomyocyte hypertrophy
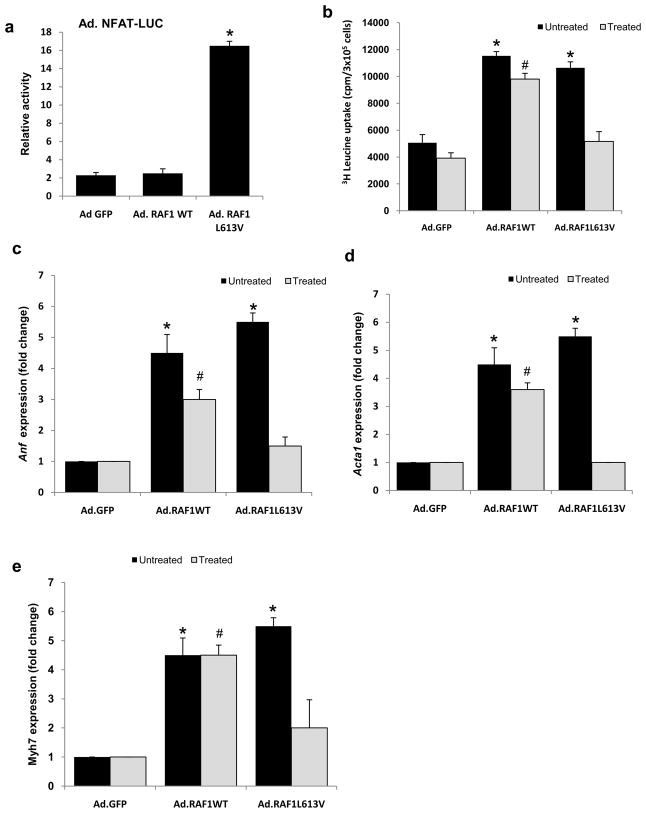
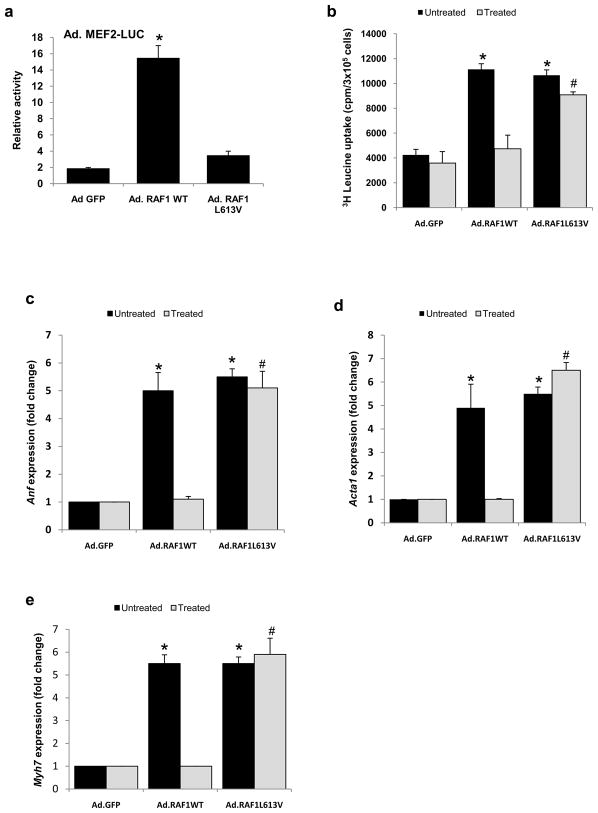
Next, we explored a possible role of the calcineurin-Nfat pathway in RAF1-mediated cardiomyocyte hypertrophy since signaling through the Nfat transcription factors has been associated with pathologic cardiac hypertrophy. This seemed particularly plausible since p38 Mapk, which can inhibit signaling via Nfat [11, 12], showed reduced activity in NRCMs overexpressing wild-type and L613V RAF proteins (Supplementary Fig. 2). Using a luciferase reporter with nine multimerized Nfat binding sites, we observed that the Nfat activity in NRCMs expressing the L613V RAF1 was increased approximately 16 fold compared to cells infected with Ad.GFP or Ad.RAF1WT (Fig. 4a). To determine whether the increased transcriptional activity of Nfat was necessary for RAF1 L613V-induced cardiomyocyte hypertrophy, we co-infected cells with an adenovirus expressing VIVIT (Ad.VIVIT), a peptide that selectively blocks calcineurin’s Nfat binding site without affecting its phosphatase activity. VIVIT expression nearly completely abrogated cardiomyocyte hypertrophy for NRCMs expressing L613V RAF1 but not for those expressing wild-type RAF1 and had minimal effect on cardiomyocytes expressing only GFP (Table 1 and Fig. 4b-e).
Figure 4. L613V RAF1 but not wild-type RAF1 causes cardiomyocyte hypertrophy via Nfat.
(a) Nfat activity was determined in neonatal rat cardiomyocytes infected with Ad.GFP, Ad.RAF1WT and Ad.RAF1L613V along with Ad.NFAT-LUC. Luciferase activity shown as relative light units (RLU) represent mean values ± SD of three independent experiments. *P <0.001 vs Ad.GFP. Neonatal rat cardiomyocytes were co-infected with Ad.GFP, Ad.RAF1WT and Ad.RAF1L613V along with Ad.VIVIT (treated) that blocks NFAT specifically. Seventy-two hours hours post infection, cardiomyocytes were analyzed for protein synthesis rates (b) and steady-state mRNA levels of Anf (c), Acta1 (d) and Myh7 (e). *p <0.01 vs. untreated Ad.GFP; #p <0.01 vs. treated Ad.GFP. Mean values ± SD of three independent experiments are shown.
Since the cardiomyocyte hypertrophy engendered by overexpression of wild-type RAF1 could not be attributed to signaling through Nfat, we considered the possibility that Mef2, another major downstream effector of calcineurin, was relevant [13]. Using a luciferase reporter with eight multimerized Mef2 binding sites, we observed that the Mef2 activity in NRCMs expressing wild-type RAF1 was increased approximately 15 fold compared to cells that were expressing GFP or L613V RAF1 (Fig. 5a). To determine whether the increased transcriptional activity of Mef2 was necessary for cardiomyocyte hypertrophy induced by wild-type RAF1, we co-infected cells with an adenovirus expressing dominant-negative Mef2. Dominant-negative Mef2 nearly completely abrogated cardiomyocyte hypertrophy for NRCMs expressing wild-type RAF1 but not for those expressing L613V RAF1 and had minimal effect on cells expressing only GFP (Table 1 and Fig. 5b-e).
Figure 5. Wild-type RAF1 but not L613V RAF1 causes cardiomyocyte hypertrophy via Mef2.
(a) Mef2 activity was determined in neonatal rat cardiomyocytes infected with Ad.GFP, Ad.RAF1WT and Ad.RAF1L613V along with Ad.MEF2-LUC. Luciferase activity shown as relative light units (RLU) represent mean values ± SD of three independent experiments. *P <0.001 vs Ad.GFP. Neonatal rat cardiomyocytes were co-infected with Ad.GFP, Ad.RAF1WT and Ad.RAF1L613V along with Ad.MEF2DN (treated). Seventy-two hours post infection, cardiomyocytes were analyzed for protein synthesis rates (b) and steady-state mRNA levels of Anf (c), Acta1 (d) and Myh7 (e). *p <0.01 vs. untreated Ad.GFP; #p <0.01 vs. treated Ad.GFP. Mean values ± SD of three independent experiments are shown.
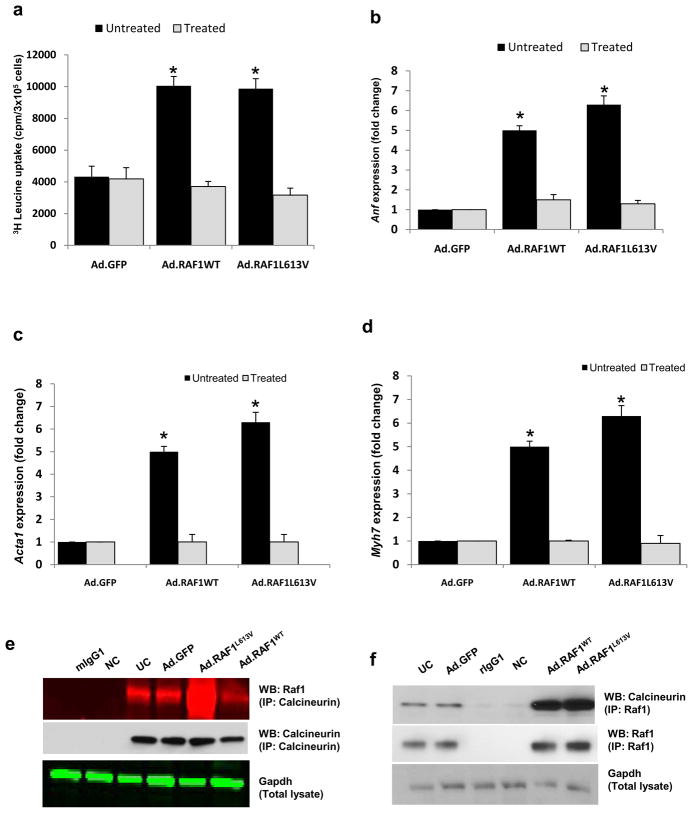
The foregoing results support the idea that wild-type and L613V RAF1 induce cardiac hypertrophy by signaling through calcineurin, albeit through different effectors (Mef2 and Nfat, respectively). To provide evidence that this signaling required calcineurin, we used a pharmacologic approach. Treatment with the calcineurin-inhibitor, cyclosporine A, eliminated cardiomyocyte hypertrophy for NRCMs expressing wild-type or L613V RAF1 as assessed by cell area, protein synthesis rate and re-expression of fetal markers (Table1, Fig. 6a-d).
Figure 6. Calcineurin inhibition blocks the hypertrophy induced by wild-type and L613V RAF1 proteins.
Neonatal rat cardiomyocytes were infected with Ad.GFP, Ad.RAF1WT and Ad.RAF1L613V and treated with cyclosporine after 48 hours. Seventy-two hours post infection, cardiomyocytes were analyzed for protein synthesis rates (a) and steady-state mRNA levels of Anf (b), Acta1 (c) and Myh7 (d). *P <0.01 vs untreated Ad.GFP. Mean values ± SD of three independent experiments are shown. Raf1 complexes with calcineurin (e) Immunoblots with the indicated antibodies after immunoprecipitation with anti-calcineurin antibody, rabbit pre-immune IgG (rIgG1) or Gapdh as negative control (NC) and reprobed for calcineurin (e) Immunoblots with the indicated antibodies after immunoprecipitation with anti-RAF1 antibody, mouse pre-immune IgG (mIgG1) or Gapdh as negative control (NC) and reprobed for Raf1 (f).
3.6. RAF1 directly interacts with calcineurin
Since wild-type and L613V RAF1 proteins were signaling through calcineurin, we considered the possibility that there was a physical interaction between the RAF1 and calcineurin. Using co-immunoprecipitation, we detected RAF1 and calcineurin immunologically in immunoprecipitates from cells overexpressing RAF1 (Fig. 6e & f). More importantly, we detected Raf1-calcineurin complexes in uninfected NRCMs, documenting that this interaction was not an artifact of RAF1 overexpression. Taken together, these data suggest that the interaction of Raf1 and calcineurin is likely to mediate physiological and pathological signaling in cardiomyocytes.
3.7. RAF1 impairs calcium signaling by down-regulating sarco/endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a)
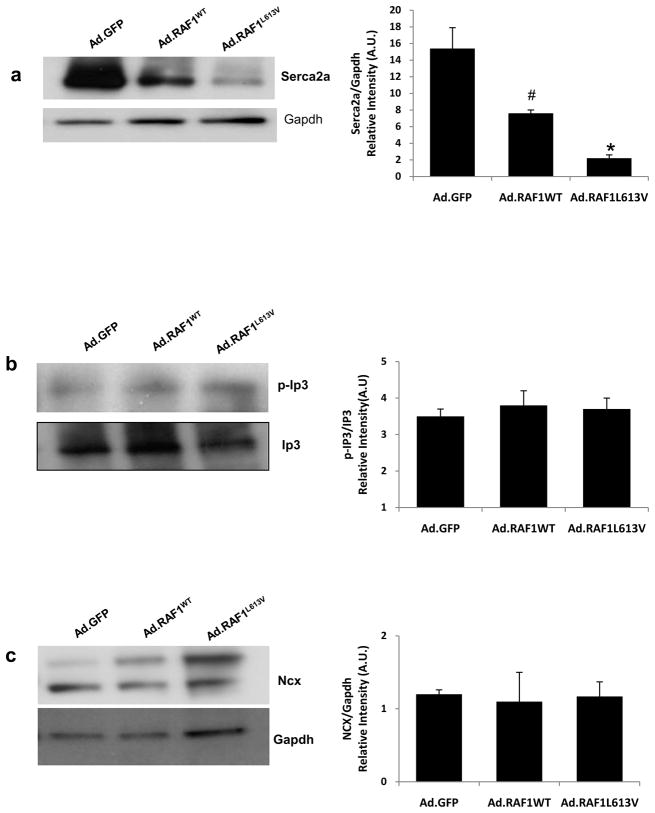
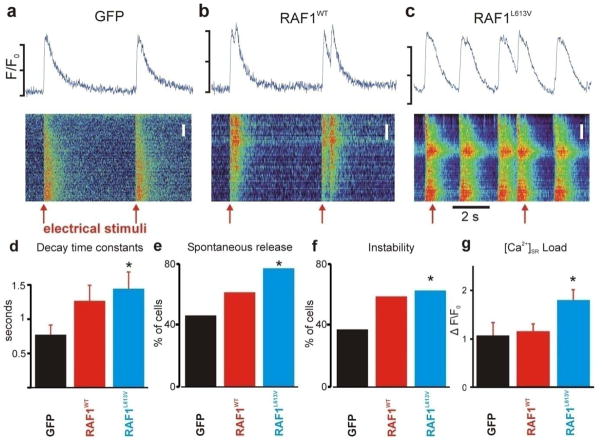
Calcineurin/Nfat signaling has been implicated as a critical transducer that uniquely links changes in intracellular calcium handling in cardiac hypertrophy [14, 15]. Thus, the observed interaction also pointed to the possibility that RAF1 might target components of calcium regulatory proteins through calcineurin and regulate calcium activity. We measured the total expression levels of several proteins (Serca2a, sodium-calcium exchanger (Ncx1) and inositol 1, 4, 5-triphosphate (Ip3)) that are known to play vital roles in calcium signaling in conjunction with calcineurin. We found that Serca2a was significantly down regulated in NRCMs overexpressing wild-type and L613V RAF1 but that the other proteins were unchanged (Fig. 7a–c). To evaluate whether altered signaling through the RAF1-calcineurin complex perturbed intracellular Ca2+ handling, intracellular cytoplasmic Ca2+ transients were measured. With slow pacing at 0.2 Hz, Ca2+ transients in cardiomyocytes expressing GFP followed electrical stimuli with a 1:1 correspondence, whereas the RAF1-overexpressing cells exhibited spontaneous Ca2+ transients that were unassociated with electrical stimuli, indicative of unstable Ca2+ handling (Fig. 8a-c). To quantify the degree of Ca2+ handling dysfunction, we computed three measures as described in Methods and Supplementary Methods. During 0.2-Hz pacing, the wild-type and L613V RAF1-overexpressing cardiomyocytes exhibited slower average Ca2+ transient rates of decay (Fig. 8d) and greater percentages of cells exhibiting spontaneous Ca2+ transients (Fig. 8e) compared to control cardiomyocytes expressing GFP. Pacing at 2 Hz induced irregular Ca2+ transients to a greater extent in the wild-type and L613V RAF1-overexpressing cardiomyocytes than in control cells (Fig. 8f). Further, the sarcoplasmic reticulum (SR) calcium load in the L613V RAF1-overexpressing cardiomyocytes was increased compared to wild type and GFP (Fig. 8g). Thus, the present data establish a potential link between signaling from RAS through RAF1 for pathologic hypertrophy and calcium dysregulation, which could be potentially arrhythmogenic.
Figure 7. Serca2a is downregulated, but other calcium regulatory proteins are unchanged, in cardiomyocytes expressing RAF1.
Representative immunoblots with total lysates from neonatal rat cardiomyocytes expressing GFP, wild-type RAF1 or L613V RAF1 that were probed with anti-Serca2,-Gapdh, p-Ip3, -Ip3, -Ncx, and -Gapdh antibodies in panels from top to bottom (a-c). Protein expression levels were normalized to indicated antibodies and expressed as Relative Expression. Data are mean values ± SD of three independent experiments. *p <0.01 and #p <0.01 vs. Ad.GFP.
Figure 8. Intracellular calcium handling is dysregulated in neonatal rat cardiomyocytes expressing wild-type and L613V RAF1.
(a-c) Intracellular Ca2+ transients from the three groups, as indicated, expressed as space-time images (bottom) and average intracellular fluorescence (units of F/F0, top). Image scaling and Ca2+ transient scale bars are different in the three cases because of different baseline Ca2+ transient amplitudes. Top scale bars indicate F/F0 ranges of 1.0–1.5 (left), 1.0–2.0 (middle), and 1.0–2.0 (right). Red arrows indicate timing of electrical stimuli, here delivered every 5 seconds. Spatial scale bars indicate 2 μm in all cases. (d) Ca2+ transient decay time constants, calculated from the following numbers of cells: n=42 (GFP), n=39 (WT RAF1) and n=36 (L613V RAF1). *P<0.05 vs. GFP by two-tailed Student’s t-test. (e) Percentage of cells in each group exhibiting spontaneous Ca2+ release when paced at 0.2 Hz. *P<0.05 vs. GFP by two-tailed Fisher’s exact test. (f) Percentage of cells in each group showing unstable beat-to-beat Ca2+ release when paced at 2 Hz. *P<0.05 vs. GFP by two-tailed Fisher’s exact test. (g) SR Ca2+ load, quantified as peak ΔF/F0 in response to 20 mM caffeine. Bars calculated from n=30 cells from each group. *P<0.05 vs. GFP by two-tailed Student’s t-test. See Supplementary Methods for details of calculation of these metrics.
3.8. Effects of RAF1 mutations at the Ser259 cluster and activating segment
The L613V mutation is representative of the HCM-associated mutations that cluster at the C-terminus near Ser621. To understand the effects of mutations at the Ser259 cluster and the activating segment, we infected NRCMs with representative recombinant adenoviruses encoding either S257L or D486N RAF1 and performed key experiments including assessments of the phosphorylation status of RAF1, Erk1/2 activation, cellular hypertrophy, Nfat and Mef2 signaling, and RAF1-calcineurin complexing as well as determining the effect of treating with cyclosporine to rescue the hypertrophic phenotype. As anticipated, we found that S257L RAF1 negatively affected the phosphorylation of Ser259 but phosphorylation of that residue was normal in the D486N protein (Supplementary Fig. 7). The levels of pErk1/2 activation were unchanged in NRCMs overexpressing the S257L or D486N RAF1 proteins (Supplementary Fig. 8). Overexpression of the S257L RAF1 induced cardiac hypertrophy similarly to L613V protein whereas the kinase-dead mutant, D486N, failed to induce hypertrophy as assessed by protein synthesis rate and fetal gene expression (Supplementary Fig. 9a-c). Further, transcription activity from Nfat, but not Mef2, was increased in cardiomyocytes overexpressing S257L RAF1 whereas overexpression of D486N protein failed to induce Nfat or Mef2 activity (Supplementary Fig. 10a and b). As with endogenous Raf1 and overexpressed wild-type and L613V RAF1 proteins, S257L RAF1 complexed with calcineurin; that interaction was abrogated for overexpressed D486N RAF1 (Supplementary Fig. 11). Finally, we were able to rescue the hypertrophic phenotype engendered by S257L RAF1 overexpression using cyclosporine (Supplementary Fig. 9). Collectively, these results suggest that RAF1 mutations at the Ser259 and Ser621 clusters in NRCMs mediate cardiomyocyte hypertrophy through a comparable mechanism dependant upon signaling through calcineurin but not Erk1/2 and also explain the negative association of HCM with RAF1 mutations residing in the activation loop.
3.9. Effects of RAF1 mutant (L613V) on adult cardiomyocytes (ARCMs)
To understand the effects of the RAF1 mutations in ARCMs, we infected those cells with representative recombinant adenoviruses encoding either WT or L613V RAF1 and performed key experiments including assessments of the cell surface area, phosphorylation status of RAF1, Erk1/2 activation, cellular hypertrophy, Nfat and Mef2 signaling, and RAF1-calcineurin complexing as well as determining the effect of treating with cyclosporine to rescue the hypertrophic phenotype. Cell surface areas were increased in the ARCMs overexpressing WT and L613V compared to GFP (Supplementary Fig. 12 a and b). As observed with NRCMs, the overexpression of L613V was low compared to WT (Supplementary Fig. 13a) and phosphorylation was reduced at S621 but unchanged at S259 (Supplementary Fig. 13b). In contrast to the findings with NRCMs, overexpression of L613V RAF1 increased the levels of pErk1/2 in ARCMs (Supplementary Fig. 14). Overexpression of the WT and L613V RAF1 induced cardiac hypertrophy as assessed by fetal gene expression (Supplementary Fig. 15a, b and c). Further, transcription activity from Nfat was increased in cardiomyocytes overexpressing L613V RAF1 whereas overexpression of WT failed to induce Nfat or Mef2 activity (Supplementary Fig. 16a). WT and L613V RAF1 interact with calcineurin in ARCMs as had been observed in NRCMs (Supplementary Fig. 16b). Finally, we were able to rescue the hypertrophic phenotype engendered by RAF1 overexpression by co-expressing a dominant negative form of MEK or by treating with cyclosporine (Supplementary Fig. 15 and 17 a, b and c). Collectively, these results suggest that the hypertrophic effects of RAF1 in ARCMs depend upon signaling through Mek/Erk and calcineurin.
4. Discussion
Collectively, our results provide important, molecular and clinically-relevant insights into the signaling pathways leading to cardiac hypertrophy. Cardiac hypertrophy is stimulated by biomechanical and neurohumoral mechanisms. Neurohumoral stimuli include ligands such as growth factors, hormones and cytokines that utilize G-protein-coupled receptors, gp-130-linked receptors and receptors kinases, particularly receptor tyrosine kinases (RTKs). Activation of RAS, which is a well-described result of RTK stimulation, occupies a critical role in cardiac hypertrophy as first established with in vitro studies with cardiomyocytes and in vivo studies with transgenic mice expressing constitutively active Hras (G12V) [16, 17]. Transgenic mice with cardiac-specific expression of Hras G12V developed HCM with myofibrillary disarray and Anf re-expression, phenocopying human HCM observed in individuals with sarcomeric gene defects as well in NS.
The canonical signaling pathway downstream from RAS is RAF–MEK-ERK. Numerous studies have examined the role of this pathway in cardiac hypertrophy with interesting, if complex, results. Overexpression of activated Mek1 in the mouse heart resulted in concentric hypertrophy with Erk1/2 activation and re-activation of Anf expression but did not cause cardiac fibrosis, diastolic dysfunction or lethality, consistent with physiologic rather than pathologic hypertrophy [18]. Pharmacologic inhibition of Raf and Mek in cultured cardiomyocytes blocked endothelin-1 and phenylephrine-induced hypertrophy and Mek inhibition prevented L-NAME-induced hypertrophy in rats [19, 20]. Transgenic expression of a dominant-negative Raf1 in mouse heart resulted in a reduced hypertrophic response to pressure overload with diminished Erk1/2 activation and no re-activation of Anf expression [21]. In contrast, genetic reduction of Erk activation per se (Erk1−/− and Erk2+/− mice as well as Dusp6 transgenic mice) did not abrogate the hypertrophic response to pressure overload [22]. Recently, Erk1/2 has also been shown to be a critical regulator of cell growth and size in cardiac hypertrophy. Eccentric (elongated) hypertrophy does not involve Erk1/2 whereas concentric (width wise) hypertrophy requires Erk1/2 [23]. Our data are consistent with these findings as overexpression of RAF1 proteins induced elongated growth without Erk1/2 activation in NRCMs but concentric growth with Erk1/2 activation in ARCMs. Taken as a whole, it appears that signaling from RAS to ERK1/2 plays some role in cardiac hypertrophy but the involvement of other pathways is suggested.
Our results provide new insights into how RTK activation signaling through RAS induces hypertrophy. We showed that overexpression of wild-type and gain-of-function RAF1 in ARCMs caused cardiomyocyte hypertrophy by activating the Erk1/2 and calcineurin pathways whereas similar RAF1 overexpression in NRCMs only activated calcineurin signaling. Activation of multiple pathways either in parallel or independently by RAS-MAPK members including RAS, SOS1 and MEK are widely observed. For example, overexpression of Hras G12V in NRCMs resulted in increased Jnk and Nfat activity [24]. Also, activated Mek expression in NRCMs and in transgenic mice increased Erk1/2 [23] and Nfat activity in a calcineurin-independent manner [7]. Interestingly, cardiac tissue from Sos1 mouse model also showed upregulation of multiple pathways including Erk1/2, Rac and Stat3 [25]. Our observations that RAF1 overexpression induced hypertrophy via multiple pathways (Erk1/2 and calcineurin) depending on the developmental stage of heart, and that RAF1 and calcineurin interact directly outlines a crucial role for these pathways in the pathogenesis of cardiomyopathy in NS. Following the seminal discovery of the role of signaling via calcineurin-NFAT in pathologic hypertrophy [26], interactions between that pathway and RAS signaling have been contemplated. Based on our present data, we suggest that the level of cross-talk between the RAS and calcineurin pathways might be crucial at the RAF1 level. However, RAF1 regulation is highly intricate, depending upon various factors including the type of cardiomyocte, localization, time, dose, type of stimulus and feedback mechanisms. Further adding to the complexity, NRCMs and ACMs have overlapping and significant physiological and functional differences as reviewed [27]. Thus, it is possible that in vitro overexpression may result in complex results. Clearly, additional studies using a Raf1 mouse model at various cardiac developmental stages addressing the differences in the activation of Erk1/2 and/or calcineurin-Nfat pathways will shed light on this particular hypertrophic signaling pathway.
Clinical observations concerning inherited RAS pathway disorders correlate well with the experimental data about the roles of specific proteins in that pathway vis à vis cardiac hypertrophy. To date, germline MEK1 and MEK2 mutations have been identified in 28 individuals with cardio-facio-cutaneous syndrome, of whom 8 (29%) had HCM [28–31]. Data about HCM severity or its outcome were not provided save the single case described by Dentici et al., in which the HCM was described as mild [31]; no arrhythmia has been associated with a MEK mutation. Of note, all MEK mutants that have been characterized biochemically demonstrate increased kinase activity with some being constitutively active [31, 32]. The prevalence of HCM among patients with Noonan syndrome caused by RAF1 defects is considerably higher- 27/35 (77%) if all mutations are included and 26/29 (90%) for gain-of-function mutations clustering near Ser259 and Ser621 [3, 4, 32, 33]. Moreover, the severity of the HCM appears to be greater with several instances of sudden death and one of atrial ectopic tachycardia [34]. Of interest, HRAS Gly12 and Gly13 missense mutations underlying Costello syndrome also engender HCM and atrial arrhythmias (19 and 17 of 40 patients for prevalence rates of 47% and 42%, respectively) [35]. The commonest allele, constituting 82% of cases, is G12S, which has substantially less GTPase activity than the G12V allele that caused remarkable hypertrophy in NRCMs and transgenic mice. Thus, germline gain-of-function RAF1 and MEK1/2 mutations in humans are experiments of nature that provide evidence that RAF1 occupies a more important role in pathologic cardiac hypertrophy than do MEK1/2. These clinical observations are consistent with our current findings showing that RAF1 can signal through multiple pathways depending on the developmental stage of the heart to induce pathologic hypertrophy, even bypassing the Erk1/2
Finally, calcium handling in cardiomyocytes with increased Ras signaling has previously been shown to be altered. In transgenic mice expressing Hras G12V in the myocardium, Serca2a protein levels were modestly depressed [36]. These findings are consistent with our observations with NRCMs overexpressing gain-of-function RAF1, in which Serca2a was also reduced and there was decreased decay of calcium transients with spontaneous calcium transients. These abnormalities in calcium handling may provide the rationale for the atrial arrhythmias observed in Costello syndrome and RAF1-associated Noonan syndrome as well as the sudden death risk associated with the latter.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Children’s Cardiomyopathy Foundation (B.D.G.), the National Institutes of Health (HL071207 to B.D.G.; R01HL078731, R01HL080498, R01HL083156, R01HL093183, R01HL088434 and P20HL100396 to R.J.H.; HL076659 to D.L.; HL076230 to E.A.S.), the Transatlantic Leducq Foundation (R.J.H.), the ERA-Net for research programmes on rare diseases 2009 (M.T.) and Telethon-Italy GGP10020 (M.T.)
Footnotes
Disclosures
Drs. Gelb and Tartaglia have a patent pending for genetic testing for RAF1 mutations causing Noonan and LEOPARD syndromes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63(3):467–75. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39(8):1007–12. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 4.Razzaque MA, Nishizawa T, Komoike Y, Yagi H, Furutani M, Amo R, et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39(8):1013–17. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- 5.Kim M, Oh JK, Sakata S, Liang I, Park W, Hajjar RJ, Lebeche D. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45(2):270–80. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noble C, Mercer K, Hussain J, Carragher L, Giblett S, Hayward R, et al. CRAF autophosphorylation of serine 621 is required to prevent its proteasome-mediated degradation. Mol Cell. 2008;31(6):862–72. doi: 10.1016/j.molcel.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20(11):2757–67. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Q, Sargent MA, York AJ, Molkentin JD. ASK1 regulates cardiomyocyte death but not hypertrophy in transgenic mice. Circ Res. 2009;105(11):1110–17. doi: 10.1161/CIRCRESAHA.109.200741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol. 2006;26(10):3785–97. doi: 10.1128/MCB.26.10.3785-3797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Fujii K, Zhang L, Roberts T, Fu H. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc Natl Acad Sci U S A. 2001;98(14):7783–88. doi: 10.1073/pnas.141224398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanna B, Bueno OF, Dai YS, Wilkins BJ, Molkentin JD. Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol Cell Biol. 2005;25(3):865–78. doi: 10.1128/MCB.25.3.865-878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111(10):1475–86. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, et al. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 2001;20(22):6414–23. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colella M, Pozzan T. Cardiac cell hypertrophy in vitro: role of calcineurin/NFAT as Ca2+ signal integrators. Ann N Y Acad Sci. 2008;1123:64–68. doi: 10.1196/annals.1420.008. [DOI] [PubMed] [Google Scholar]
- 15.Colella M, Grisan F, Robert V, Turner JD, Thomas AP, Pozzan T. Ca2+ oscillation frequency decoding in cardiac cell hypertrophy: role of calcineurin/NFAT as Ca2+ signal integrators. Proc Natl Acad Sci U S A. 2008;105(8):2859–64. doi: 10.1073/pnas.0712316105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH. The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of Rho kinase. J Biol Chem. 1998;273(13):7725–30. doi: 10.1074/jbc.273.13.7725. [DOI] [PubMed] [Google Scholar]
- 17.Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270(39):23173–78. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 18.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19(23):6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Proud CG. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor agonists in adult cardiomyocytes. Circ Res. 2002;91(9):821–29. doi: 10.1161/01.res.0000041029.97988.e9. [DOI] [PubMed] [Google Scholar]
- 20.Sanada S, Node K, Minamino T, Takashima S, Ogai A, Asanuma H, et al. Long-acting Ca2+ blockers prevent myocardial remodeling induced by chronic NO inhibition in rats. Hypertension. 2003;41(4):963–67. doi: 10.1161/01.HYP.0000062881.36813.7A. [DOI] [PubMed] [Google Scholar]
- 21.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110(6):718–23. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 22.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104(35):14074–79. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108(2):176–83. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichida M, Finkel T. Ras regulates NFAT3 activity in cardiac myocytes. J Biol Chem. 2001;276(5):3524–30. doi: 10.1074/jbc.M004275200. [DOI] [PubMed] [Google Scholar]
- 25.Chen PC, Wakimoto H, Conner D, Araki T, Yuan T, Roberts A, et al. Activation of multiple signaling pathways causes developmental defects in mice with a Noonan syndrome–associated Sos1 mutation. J Clin Invest. 2010;120(12):4353–65. doi: 10.1172/JCI43910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molkentin JD, Robbins J. With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure. J Mol Cell Cardiol. 46(2):130–6. doi: 10.1016/j.yjmcc.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nava C, Hanna N, Michot C, Pereira S, Pouvreau N, Niihori T, et al. Cardio-facio-cutaneous and Noonan syndromes due to mutations in the RAS/MAPK signalling pathway: genotype-phenotype relationships and overlap with Costello syndrome. J Med Genet. 2007;44(12):763–71. doi: 10.1136/jmg.2007.050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armour CM, Allanson JE. Further delineation of cardio-facio-cutaneous syndrome: clinical features of 38 individuals with proven mutations. J Med Genet. 2008;45(4):249–54. doi: 10.1136/jmg.2007.054460. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311(5765):1287–90. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- 31.Dentici ML, Sarkozy A, Pantaleoni F, Carta C, Lepri F, Ferese R, et al. Spectrum of MEK1 and MEK2 gene mutations in cardio-facio-cutaneous syndrome and genotype-phenotype correlations. Eur J Hum Genet. 2009;17(6):733–40. doi: 10.1038/ejhg.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Viciana P, Rauen KA. Biochemical characterization of novel germline BRAF and MEK mutations in cardio-facio-cutaneous syndrome. Methods Enzymol. 2008;438:277–89. doi: 10.1016/S0076-6879(07)38019-1. [DOI] [PubMed] [Google Scholar]
- 33.Ko JM, Kim JM, Kim GH, Yoo HW. PTPN11, SOS1, KRAS, and RAF1 gene analysis, and genotype-phenotype correlation in Korean patients with Noonan syndrome. J Hum Genet. 2008;53(11–12):999–1006. doi: 10.1007/s10038-008-0343-6. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Aoki Y, Niihori T, Cavé H, Verloes A, Okamoto N, et al. Molecular and clinical analysis of RAF1 in Noonan syndrome and related disorders: dephosphorylation of serine 259 as the essential mechanism for mutant activation. Hum Mutat. 2010 Mar;31(3):284–94. doi: 10.1002/humu.21187. [DOI] [PubMed] [Google Scholar]
- 35.Gripp KW, Lin AE, Stabley DL, Nicholson L, Scott CI, Jr, Doyle D, et al. HRAS mutation analysis in Costello syndrome: genotype and phenotype correlation. Am J Med Genet A. 2006;140(1):1–7. doi: 10.1002/ajmg.a.31047. [DOI] [PubMed] [Google Scholar]
- 36.Zheng M, Dilly K, Dos Santos Cruz J, Li M, Gu Y, Ursitti JA, et al. Sarcoplasmic reticulum calcium defect in Ras-induced hypertrophic cardiomyopathy heart. Am J Physiol Heart Circ Physiol. 2004;286(1):H424–33. doi: 10.1152/ajpheart.00110.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.