Abstract
Viral infections are often linked to altered drug metabolism in patients; however, the underlying molecular mechanisms remain unclear. Here, we describe a mechanism by which activation of anti-viral responses by the synthetic double stranded RNA ligand, polyinosinic-polycytidylic acid (polyI:C), leads to decreased acetaminophen (APAP) metabolism and hepatotoxicity. PolyI:C administration downregulates expression of retinoic X receptor-α (RXRα) as well as its heterodimeric partner pregnane X receptor (PXR) in mice. This downregulation results in suppression of downstream cytochrome P450 enzymes involved in conversion of APAP to its toxic metabolite. While the effects of polyI:C on drug metabolism are often attributed to interferon production, we report that polyI:C can decrease APAP metabolism in the absence of the type I interferon receptor. Furthermore, we demonstrate that polyI:C can attenuate APAP metabolism through both its membrane-bound receptor, Toll-like receptor 3 (TLR3), as well as cytoplasmic receptors.
Conclusion
This is the first study to illustrate that in vivo administration of polyI:C affects drug metabolism independent of type I Interferon production or in the absence of TLR3 through crosstalk between nuclear receptors and anti-viral responses.
Altered states of drug metabolism were first noticed by physicians over 30 years ago when virally infected patients would exhibit new or pronounced adverse reactions to pharmacologic agents (1). Such perturbations in drug metabolism have been linked to the effects of infections or inflammatory stimuli on altering the activities and expression of various hepatic cytochrome P450 (CYP) enzymes (2,3). CYPs are responsible for pharmacological activation or inactivation of many drugs as well as their clearance from the circulation (4). In light of the considerable progress in our understanding of host anti-viral innate immune responses and pathways during the last decade, it is surprising that little recent research has been conducted on the mechanisms by which CYP enzymes are modulated during viral infections.
Hepatic CYPs have been shown to be affected differently in response to various inflammatory stimuli (4). This selectivity is important clinically and implies the existence of multiple mechanisms for CYP regulation. We previously demonstrated a novel mechanism by which viral infection leads to transcriptional downregulation of nuclear hormone receptor Retinoid X Receptor α (RXR α) and its downstream CYP enzymes required for Aspirin (ASA) metabolism (5). Our results provided an explanation for how ASA consumption can cause Reye’s syndrome, a condition where children with viral infections develop hepatotoxicity and neurological side effects (6). Given these adverse effects of ASA in patients with viral infections, acetaminophen (APAP) is often the first line of therapy to manage pain in children (7). In this study, we evaluate the effects of cross-talk between nuclear hormone receptors and anti-viral pathways on metabolism and toxicity of APAP.
APAP-induced toxicity is the leading cause of acute hepatic failure in the United States and many other developed countries worldwide (8). APAP is metabolized by CYP family members CYP1A2, CYP2E1, and CYP3A11 (murine homologue of human CYP3A4) into N-acetyl-p-benzoquinone-imine (NAPQI) in mice (9,10). NAPQI is a highly reactive intermediate that is normally conjugated to glutathione by glutathione S-transferase enzymes in order to become more excretable (11). Accumulation of NAPQI causes cell death and toxicity through covalent binding to cysteine groups on proteins and formation of APAP-protein adducts. The generation of these APAP-protein adducts has been correlated with hepatotoxicity through oxidation of NAPQI-conjugated proteins (12).
Among the CYP enzymes involved in NAPQI generation, CYP3A isoforms and CYP1A2, are subject to regulation by nuclear hormone receptors (13). Nuclear hormone receptors are transcription factors that are activated by a wide range of molecules, such as lipids, cholesterols, bile acids, and xenobiotics. RXRα is an important nuclear hormone receptor and acts as a heterodimer with other nuclear hormone receptors such as Pregnane X Receptor (PXR) and Constitutive Androstane Receptor (CAR) (14). RXR/PXR heterodimer is an important regulator of CYP3A isoforms, however the involvement of this complex in transcriptional regulation of CYP1A2 is not well-established. CYP1A2 is mainly regulated by aryl hydrocarbon receptor; however, PXR-deficient mice and hepatocyte RXRα-deficient mice express lower hepatic mRNA levels of CYP1A2 and CYP3A11 compared to wild-type mice, particularly after APAP administration (15–17). Consequently, these knockout mice are resistant to APAP-induced hepatotoxicity (15,17). Thus, any changes in the expression of these nuclear hormone receptors in response to activation of anti-viral pathways could potentially alter APAP-induced toxicity through modulation of NAPQI generation.
Since viral infections can lead to significant induction of type I Interferons (IFN), many groups have used IFN or IFN inducing agents to study the impact of activation of anti-viral responses on drug metabolism (18). One such agent is polyinosinic-polycytidylic acid (polyI:C), a viral double stranded RNA mimetic, which has been shown to impair drug metabolism (19). Although the effects of polyI:C on drug metabolism have been ascribed to its ability to induce IFN, there has not been a conclusive study supporting this hypothesis. PolyI:C does induce other cytokines such as Tumor Necrosis Factor α (TNFα) and Interleukin-1 (IL-1) that could affect activity or expression of CYPs. IFNs as well as TNFα and IL-1 have all been shown to alter drug metabolism when administered in patients or in animal models (4,20).
Additionally, viral double stranded RNA and polyI:C are sensed by the endosomal receptor, Toll-like Receptor (TLR3), as well as recently discovered cytoplasmic receptors, such as RNA helicase retinoic acid-inducible gene-I (RIG-I) (21). These receptors have cell-type and tissue-specific roles in sensing polyI:C; however, it has not been characterized which receptors are involved in mediating the effects of polyI:C on hepatic drug metabolism (22).
Here, we use polyI:C and Vesicular Somatitis Virus (VSV), a double stranded RNA virus, to study how activation of anti-viral responses can modulate APAP metabolism and hepatotoxicity. We provide a mechanism by which in vivo administration of polyI:C suppresses APAP-induced hepatotoxicity independent of IFN production or in the absence of TLR3 through transcriptional downregulation of RXRα and PXR and their downstream CYPs.
Materials and Methods
Animal Experiments
Age matched 6–9 week old male mice were used for all experiments. Sources for the different strains of mice can be found in the supplementary material. All experiments were performed in accordance with guidelines from the University of California, Los Angeles Institutional Animal Care and Use Committee. For ASA-induced toxicities, mice were given ASA (180mg/L, Sigma-Aldrich) in drinking water for 5 days. For APAP hepatotoxicity studies, mice were fasted for 18 hours and then administered Vehicle (normal Saline, 0.9% NaCl) or APAP (175–600 mg/kg, Sigma-Aldrich) by intraperitoneal injections (i.p.). For serum and histological studies, mice were sacrificed at 6–7 hours post APAP administration and serum and liver samples were retrieved. For polyI:C and VSV studies, mice were injected with saline or polyI:C (100 µg, Invivogen) or VSV (2.5e7 pfu) i.p. 24 hours prior to APAP treatment. GFP-tagged VSV was a kind gift from G. Barber (University of Miami, Miami, FL).
To study the effects of PCN on APAP treatment, mice were injected (i.p.) with PCN (75 mg/kg, Sigma-Aldrich) or control (1% DMSO, corn oil) 24 hours prior to APAP treatment. To study the effects of ethanol (EtOH) on APAP treatment, mice were given 20% EtOH (Gold Shield Chemical Co) in water ad libitum for 5 days prior to APAP administration. PolyI:C treatment for these experiments occurred at Day 3 and Day 5. Serum alanine aminotransferase (ALT) levels were determined using manufacturer’s protocol (TECO Diagnostics).
Histology and Immunohistochemistry Analysis
For H&E staining, liver samples were fixed in formalin for 48 hours. H&E straining was performed by UCLA Tissue Procurement Core Laboratory (TPCL). APAP-protein adduct staining was done using anti-APAP rabbit antibodies (Biogenesis/AbDserotec) as previously described (23).
Reverse-Transcription Polymerase Chain Reaction Analysis
For quantitative real-time PCR (Q-PCR), total liver RNA was isolated and cDNA was synthesized according to manufacturer’s protocol: Trizol (RNA) and Bio-Rad iScript (cDNA). PCR reactions were set up using Quantise Master Mix (2X SensiMix SYBR and Fluorescein).
Results
VSV infection suppresses APAP-induced hepatotoxicity
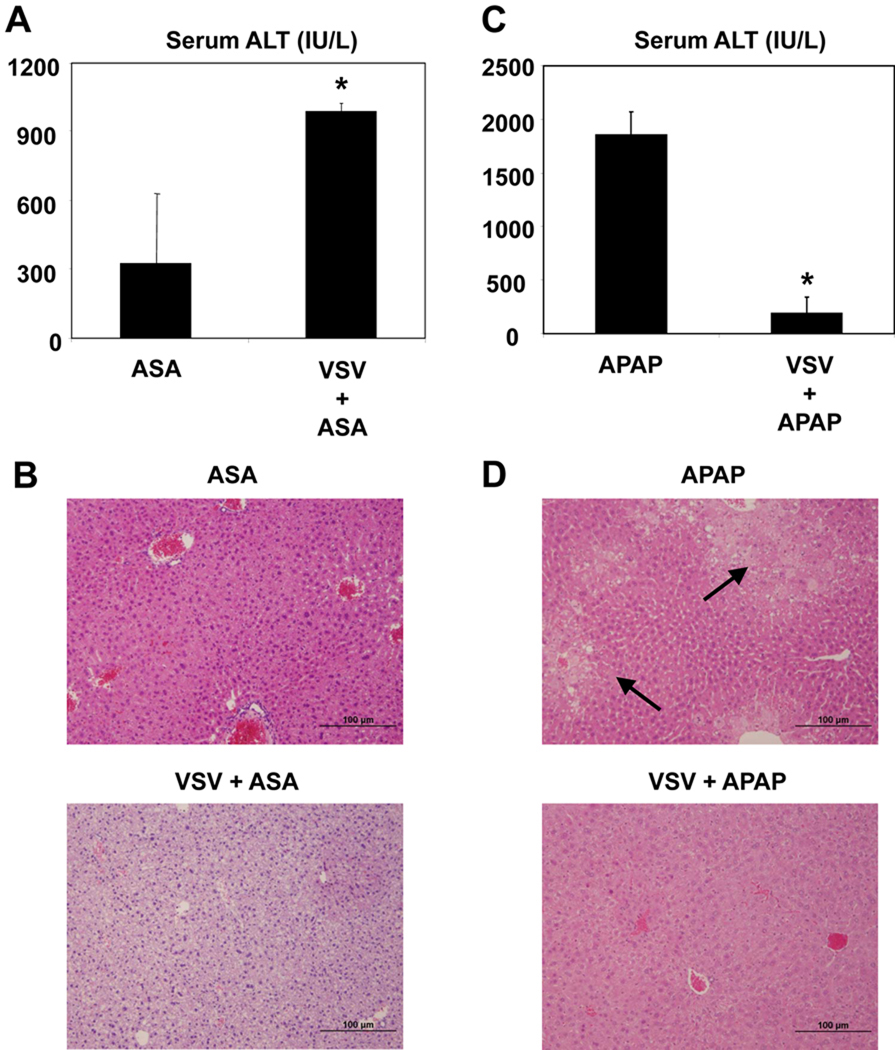
Mice were given ASA (180 mg/L) in their drinking water for 5 days. Mice that were infected with VSV (2.5e7 pfu) at day 1 experienced higher ASA-induced hepatotoxicity compared to uninfected mice. This is evidenced by higher levels of serum ALT in mice treated with VSV and ASA compared to the ASA only group (Figure 1A). Histological analysis of livers from VSV infected mice further evidenced hepatic injury as indicated by increased fat (steatosis) on oil-red staining (data not shown) as well as H&E staining sections (Figure 1B).
Figure 1. Opposite effects of VSV infection on ASA and APAP induced hepatotoxicity.
(A, B) Mice were infected with VSV (2.5 × 107 pfu, i.p.) at day 1 and given ASA (180 mg/L) in drinking water for 5 days. At Day 5, animals were euthanized and serum samples were collected and analyzed for serum ALT (A), and liver samples were fixed and stained with H&E (B). (C, D) For the APAP studies, mice were infected with VSV (2.5 × 107 pfu, i.p.) 24 hours prior to drug treatment. Animals were fasted for 18 hours before administration of APAP (350 mg/kg, i.p). Serum samples were collected 6 hours after APAP administration for ALT analysis (C) and liver samples were fixed and stained with H&E (D). (n=4, mean +/− Standard Deviation (SD), *P<0.05 compared to uninfected)
Mice were infected with VSV (2.5e7 pfu) and toxic doses of APAP (350mg/kg) were administered 24 hours later to VSV infected mice and uninfected controls, and serum ALT and histological evaluation of liver tissue were used as markers for hepatotoxicity. Mice which were infected with VSV (2.5e7 pfu) 24 hours prior to receiving APAP (350 mg/kg) had lower levels of serum ALT compared to uninfected controls 6 hours after APAP administration (Figure 1C). Furthermore, histological evaluation of livers from VSV infected mice did not reveal necrosis in contrast to mice treated with APAP alone (Figure 1D). These data demonstrate that concomitant VSV infection suppressed APAP-induced hepatotoxicity, which is opposite to what we observed with ASA.
PolyI:C pretreatment suppresses APAP-induced hepatotoxicity
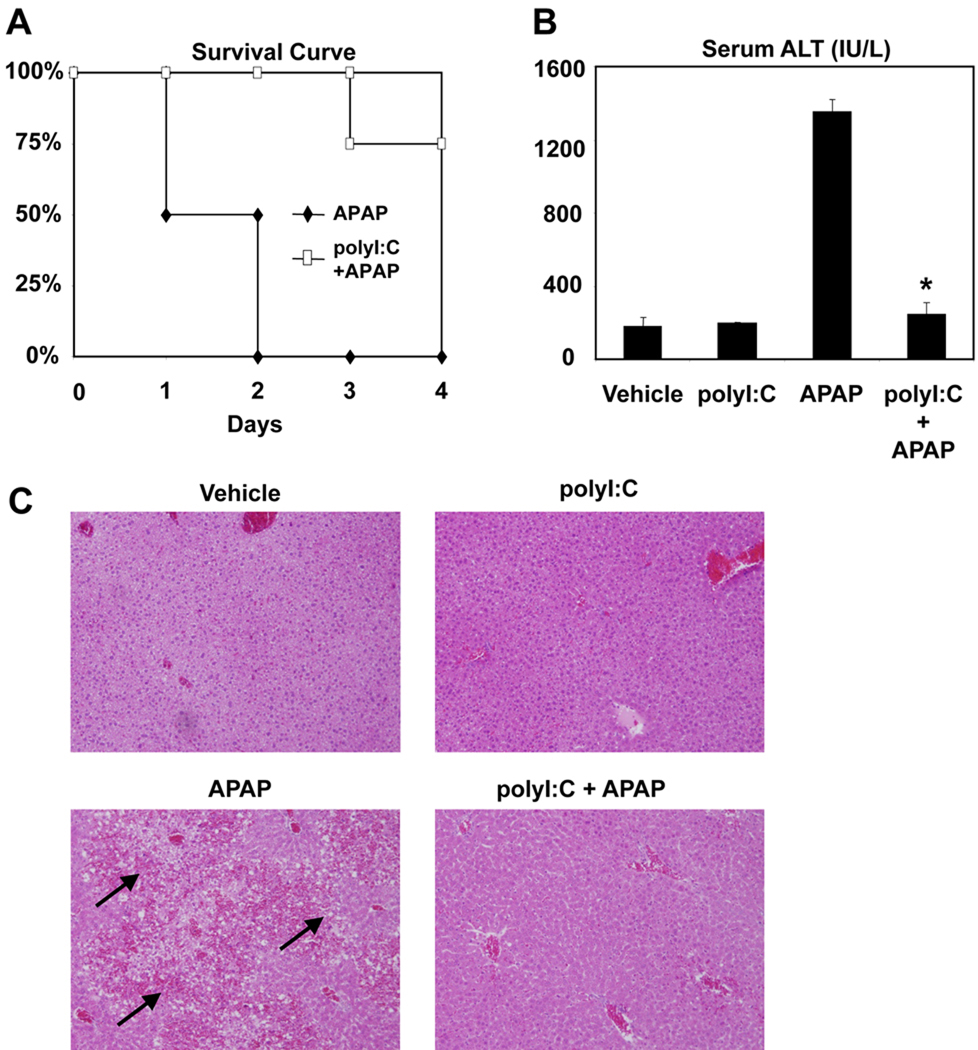
In order to determine whether our observations are applicable to viruses other than VSV, mice were pretreated with polyI:C for 24 hours prior to APAP administration. APAP (600 mg/kg) was administered with or without 24-hour polyI:C pretreatment and the weight and body temperature of animals were monitored for 5 days. As seen in Figure 2A, mice which received polyI:C had a higher survival rate than those given APAP alone. Mice pretreated with polyI:C exhibited lower serum ALT levels compared to un-treated controls in response to 6 hours of APAP (350 mg/kg) treatment (Figure 2B) and evidenced fewer necrotic foci on histological analysis (Figure 2C). Administration of polyI:C one hour before APAP treatment did not have any effects on APAP-induced injury (Data not shown).
Figure 2. PolyI:C protection against APAP-induced hepatotoxicity.
(A) Wild-type (n=8) were treated with saline or polyI:C (100 µg, i.p.) 24 hours prior to treatment with APAP (600 mg/kg, i.p.). Mice were followed for 5 days post treatment. (B) Wild-type mice (n=4) were treated with saline or polyI:C (100µg, i.p.) 24 hours prior to treatment with APAP (350mg/kg, ip). 6 hours post-APAP treatment, mice were sacrificed and serum samples were collected and analyzed for serum ALT levels (TECO Diagnostic). (C) Liver samples were formalin fixed and stained with H&E. Arrows indicate foci of necrosis. (n=4, mean +/− SD, *P<0.05 compared to polyI:C untreated)
PolyI:C-mediated repression of nuclear hormone receptors and downstream APAP metabolizing genes
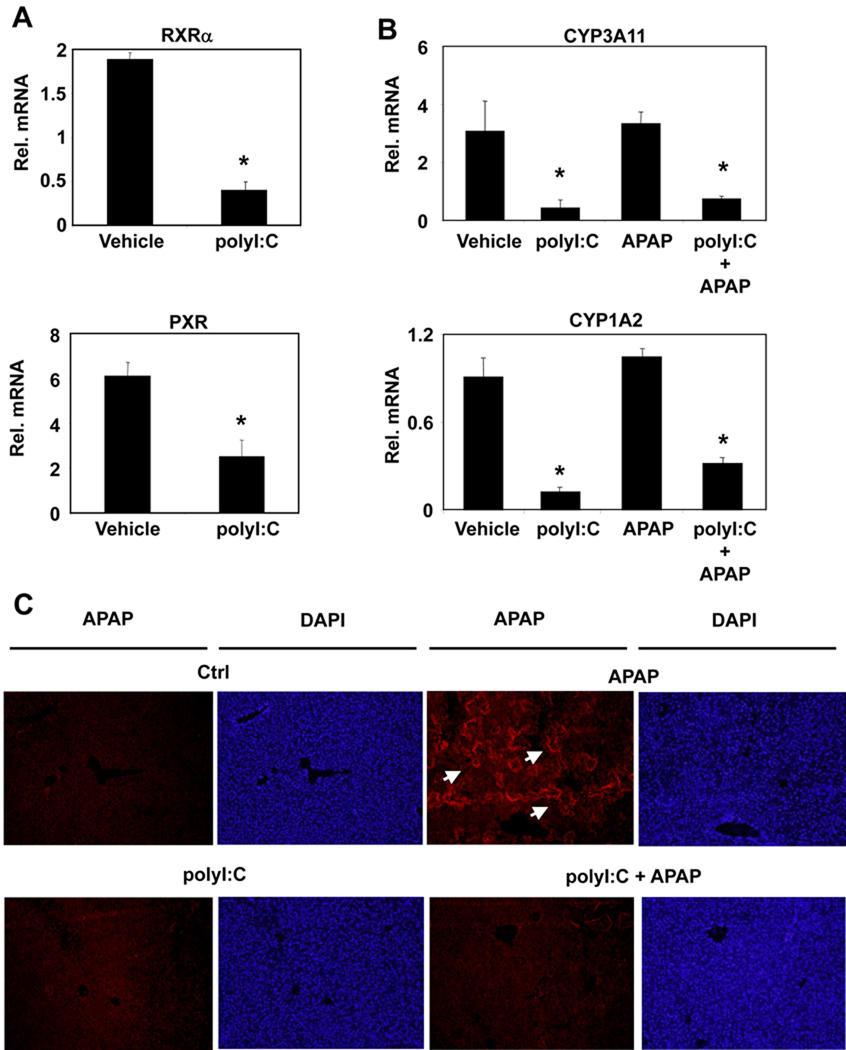
CYP enzymes that contribute to the metabolism of APAP to NAPQI, such as CYP3A11 and CYP1A2, have been identified as targets of the nuclear hormone receptors RXRα, PXR (25,24,15). Since we previously demonstrated that the innate immune response to dsRNA inhibits RXRα expression, we assessed the effects of polyI:C treatment on these nuclear hormone receptors as well as their downstream CYPs involved in APAP-mediated toxicity. Following i.p. injections of polyI:C, both hepatic RXRα and PXR expressions were downregulated at 24 hours (Figure 3A). Similarly, the mRNA expression levels of CYP3A11 and CYP1A2 were also suppressed after polyI:C treatment while CYP2E1 mRNA levels were not altered significantly (Figure 3B, Supplementary Figure 1).
Figure 3. PolyI:C decreases liver nuclear hormone receptors and their downstream genes and reduces APAP adduct protein formation.
(A) Wild-type mice were treated with saline or polyI:C (100µg, i.p.). Twenty four hours post-treatment, liver RNA was isolated and analyzed by Q-PCR. (B, C) Wild-type mice were treated with saline or polyI:C (100µg, i.p.) 24 hours prior to treatment with APAP (350mg/kg, ip). 6 hours post-APAP treatment, mice were sacrificed and liver samples were collected. Liver RNA was isolated and analyzed by Q-PCR (B) and formalin fixed liver samples were indirectly stained for APAP-bound proteins and the nucleus was identified using DAPI stain (C). (n=4, mean +/− SD, *P<0.05 compared to polyI:C untreated)
One mechanism by which NAPQI mediates hepatotoxicity is through covalent binding with cysteine groups on proteins to form APAP-protein adducts (26). Immunofluorescent analysis demonstrated that APAP induced hepatotoxicity correlated with increased formation of APAP-protein adducts and NAPQI generation as indicated by the representative images (Figure 3C) and the ImageJ analysis of different liver sections in each group (Supplementary Figure 2) (23). Liver sections of polyI:C pretreated mice did not exhibit APAP-protein adduct formation, suggesting decreased APAP metabolism. Decreased levels of APAP-protein adduct formation can potentially correlate with decreased NAPQI generation due to transcriptional suppression of CYP3A11 and CYP1A2 in response to polyI:C treatment. These findings suggest that the protective effect of polyI:C against APAP-mediated hepatotoxicity could result from the repression of nuclear hormone receptors and their target genes.
PolyI:C protects against synergistic xenobiotic/APAP induced hepatotoxicity
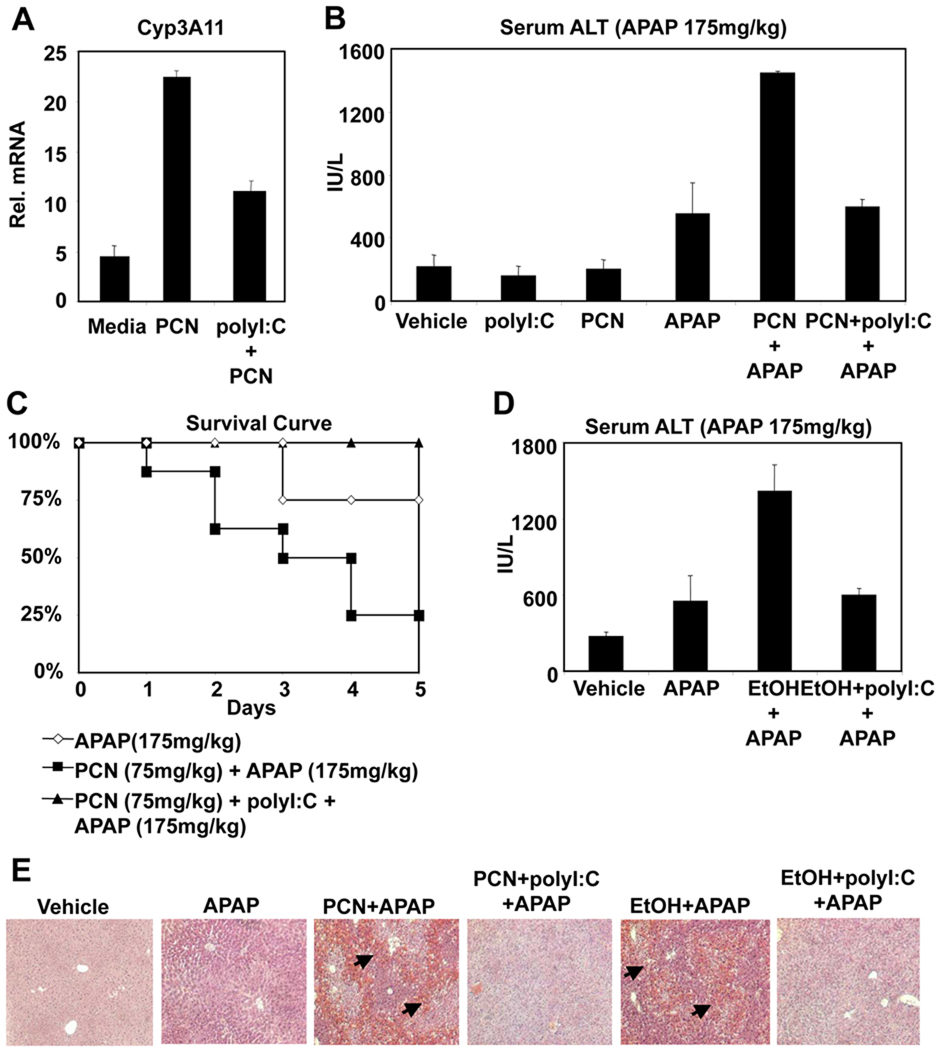
Previous studies have demonstrated that the PXR/RXRα activator pregnenolone 16α-carbonitrile (PCN), can increase APAP-hepatotoxicity through induction of CYP3A11 and CYP1A2 in mice (27). If polyI:C-mediated protection against APAP-hepatotoxicity is caused through the repression of nuclear hormone receptors and their target CYP genes, then polyI:C should also be effective at protecting against nuclear hormone receptor enhanced APAP-hepatotoxicity. Pretreatment of mice with PCN led to induction of CYP3A11, an effect which was suppressed in the presence of polyI:C (Figure 4A). Consequently, PCN pretreatment greatly enhanced serum ALT levels following treatment with normally non-toxic levels of APAP (Figure 4B). Administration of polyI:C abrogated APAP-induced hepatotoxicity which was enhanced by PCN. This was seen by both serum ALT measurement and histology (Figure 4B and 4E). Additionally, polyI:C administration protected mice against PCN enhanced APAP lethality, further supporting the mechanism where polyI:C protection occurs through repression of nuclear hormone receptors and downstream CYPs (Figure 4C).
Figure 4. PolyI:C protects against APAP-mediated hepatotoxicity promoted by PCN or EtOH.
(A) Mice were treated with saline, PCN (75mg/kg, i.p.) or PCN and polyI:C (100µg, i.p.). After 24 hours, liver RNA was isolated and analyzed by Q-PCR. (B) Mice were treated with saline, polyI:C (100 µg, i.p.) or PCN (75mg/kg, i.p.) 24 hours prior to treatment with APAP (175 mg/kg, i.p.). (n=4, mean +/− SD, *P<0.05 compared to polyI:C untreated) (C) Mice (n=8) were treated with saline or polyI:C (100 µg, i.p.) and PCN (75 mg/kg, i.p.) 24 hours prior to administration of APAP (175 mg/kg, i.p.). Mice were followed for 5 days following treatment. (D) Alternatively, wild-type mice were given 20% EtOH ad libidum for 5 days, and saline or polyI:C were injected on Day 3 and Day 5. Mice were fasted 18 hours prior to APAP treatment (175 mg/kg) on Day 6. Six hours after APAP treatment, serum samples were isolated and analyzed for ALT levels. (E) Mice were treated as in B and D. Six hours post APAP treatment, mice were sacrificed and liver samples were isolated. Liver samples were formalin fixed and stained with H&E. Arrows indicate foci of necrosis.
Another example of hepatotoxicity from APAP in combination with CYP-inducing substances is APAP therapy following regular alcohol ingestion, which induces expression of CYP2E1 and CYP3A isoforms and enhances sensitivity to APAP (28,29). Indeed, polyI:C was effective at preventing ethanol from potentiating APAP induction of serum ALT levels and hepatotoxicity (Figure 4D and 4E).
PolyI:C-mediated suppression of APAP-induced hepatotoxicity is independent of Type I IFN
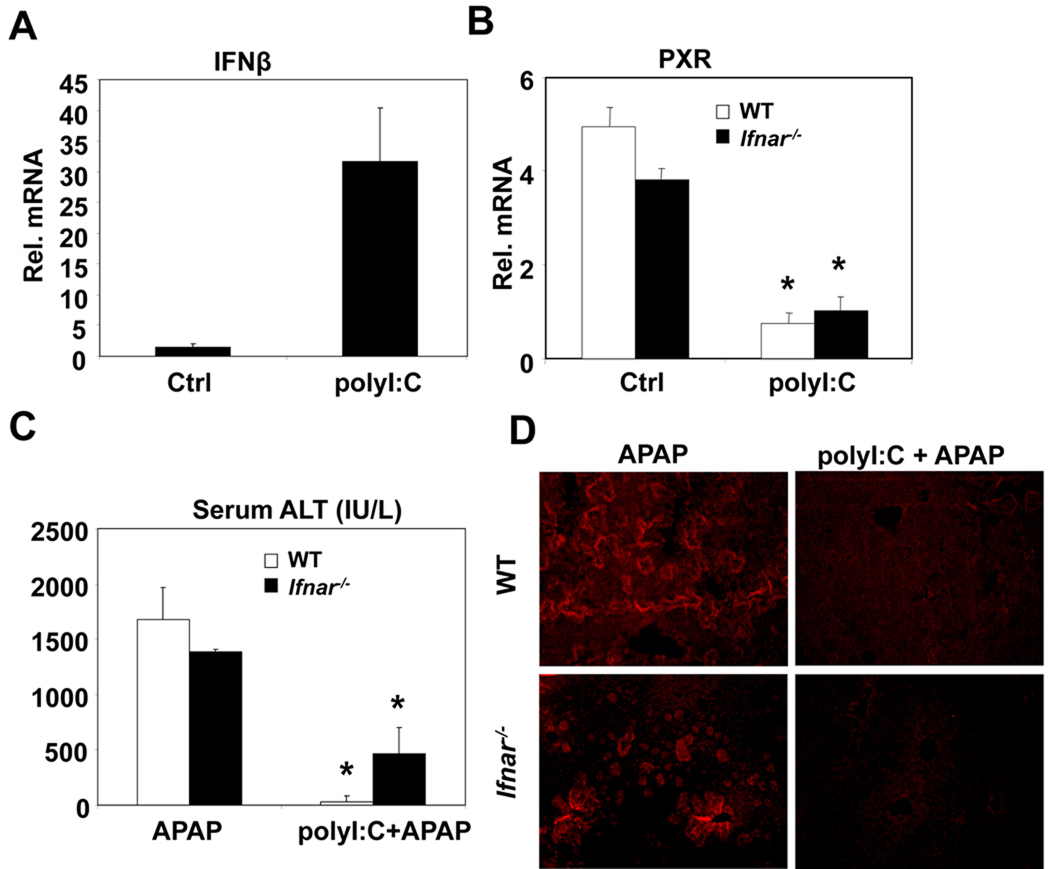
PolyI:C was first utilized to study the effects of viral infections on drug metabolism as an interferon inducing agent (19). However, there has not been a conclusive study which addresses whether the effects of polyI:C on drug metabolism are truly dependent on IFN induction. In our model, polyI:C administration induced transcription of Type I IFNs such as IFNβ in the liver after 24 hours (Figure 5A). Thus, we evaluated the contribution of IFN in polyI:C-mediated protection against APAP-induced hepatotoxicity in mice deficient in IFN signaling. Since IFN receptor-1 and IFN receptor-2 need to heterodimerize for effective IFN signaling, IFN signaling is absent in Type I Interferon Receptor-1 (IFNAR) deficient mice (30). In our model, polyI:C was able to reduce RXRα and PXR mRNA levels and their downstream CYPs in IFNAR-deficient mice similar to wild type mice after 24 hours (Figure 5B, Supplementary Figure 3). Furthermore, in mice deficient for IFNAR, polyI:C was still able to attenuate APAP metabolism and toxicity (Figure 5C). In order to confirm that polyI:C’s protective effect against APAP toxicity in IFNAR deficient mice were through decreased metabolism, APAP adduct protein levels were measured. Liver sections of polyI:C pretreated wildtype and IFNAR deficient mice did not exhibit APAP-protein adduct formation, suggesting decreased APAP metabolism (Figure 5D).
Figure 5. Involvement of Interferon and cytokines in PolyI:C protection against APAP-induced hepatotoxicity.
(A, B) Mice were treated with saline or polyI:C (100µg, i.p.). Twenty four hours post-treatment, liver RNA was isolated and analyzed by Q-PCR. (n=4, mean +/− SD, *P<0.05 compared to polyI:C untreated) (C, D) Wildtype and Ifnar−/− mice were treated with polyI:C and APAP as described above in figure 2B. Six hours after APAP administration, blood and liver samples were collected. Serum samples were analyzed for ALT levels (C) and Formalin fixed livers were indirectly stained for APAP-bound proteins (D). (n=4, mean +/− SD, *P<0.05 compared to polyI:C untreated)
We then analyzed two additional cytokines induced by polyI:C, TNFα and IL-1, which have been shown to modulate CYP expression when administered in patients or animals (31). However, both TNFα-deficient mice (Tnfa−/−) and IL-1 receptor-deficient mice (IL-1R−/−) were protected by polyI:C against APAP-induced hepatotoxicity (Supplementary Figure 4).
PolyI:C can attenuate APAP-induced hepatotoxicity through both its extracellular and cytoplasmic receptors
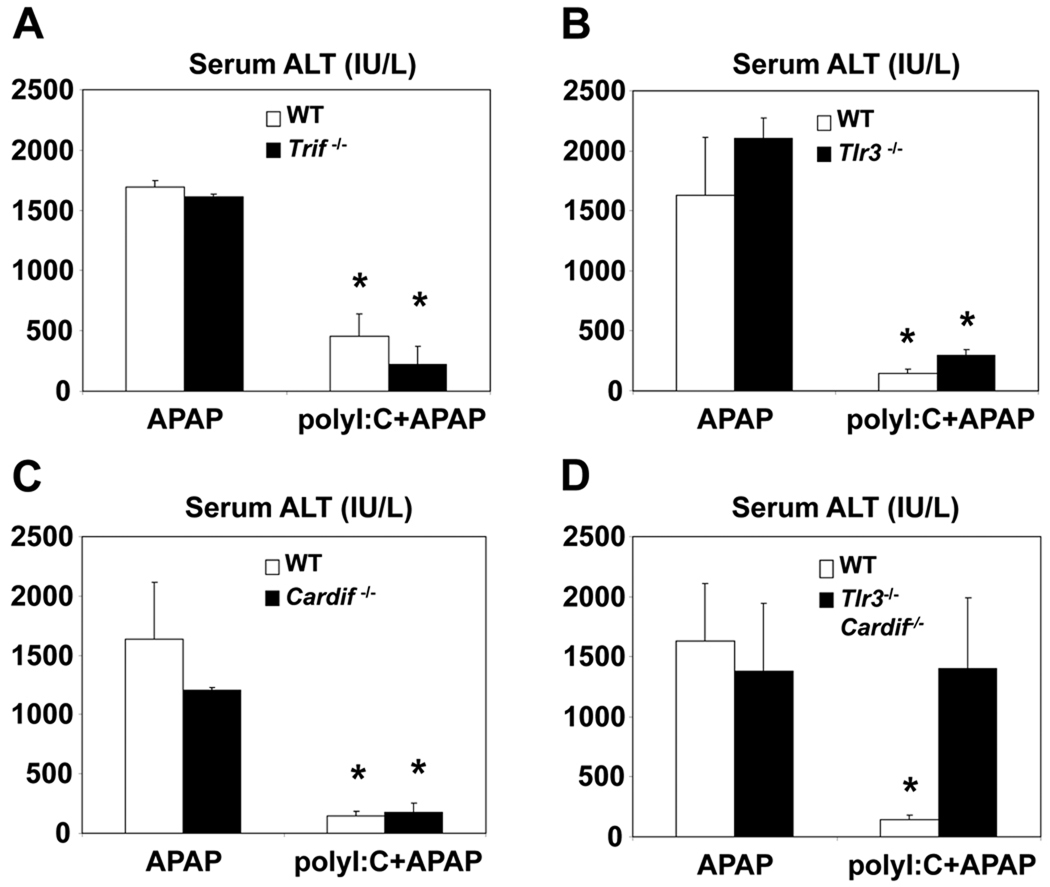
TLR3 is the primary membrane-bound receptor for mediating the innate immune response to polyI:C (21). In the absence of TLR3, APAP-induced hepatotoxicity was suppressed when mice were pretreated with polyI:C (Figure 6B). This finding was confirmed using mice deficient in TRIF, the adaptor protein required for TLR3 signaling (18) (Figure 6A). Moreover, mice lacking Cardif, the adaptor protein for cytoplasmic receptors of polyI:C, were also protected against APAP induced hepatic injury (18) (Figure 6C). However, polyI:C pretreatment in double knockout mice deficient in both Cardif and TLR3 failed to protect against APAP induced hepatotoxicity (Figure 6D). These findings suggest that membrane-bound and cytosolic receptors of polyI:C play complementary roles in this animal model.
Figure 6. PolyI:C can decrease APAP hepatotoxicity through either TLR3 or Cardif pathways.
Wildtype, Trif−/−, Tlr3−/−, Cardif−/− and Tlr3/Cardif double knockout mice were treated with polyI:C and APAP as described above. Six hours after APAP treatment, mice were sacrificed and serum samples were collected and analyzed for ALT levels. (n=4, mean +/− SD, *P<0.05 compared to polyI:C untreated)
Discussion
There are many documented examples of impaired drug metabolism in patients with viral infections (1,2). These effects have been attributed to modulation of CYP enzymes in response to activation of the innate immune system (4). While the activity and expression levels of CYPs have been shown to be altered during viral infection or inflammatory states, the underlying molecular mechanisms are not well-characterized.
Our previous work identified a potential mechanism of how innate immune activation can lead to enhanced ASA-induced hepatotoxicy through downregulation of CYP3A11, the CYP enzyme required for the clearance of the toxic intermediate of ASA (5). PolyI:C stimulation can lead to transcriptional downregulation of RXRα and subsequently decreasing the presence of RXRα on the PXR/RXR ER6 binding region on the promoter of CYP3A4 (human homolog of Cyp3a11) in Huh7 cells (5). Here, we study the effects of such crosstalk between anti-viral responses and nuclear hormone receptors on the transcriptional regulation of CYPs involved in the metabolism and toxicity of another commonly used analgesic, APAP.
In this study, we report that VSV infection as well as polyI:C pretreatment results in attenuated APAP-induced hepatotoxicity in mice. Early studies have also reported similar phenomena; however, the molecular mechanism by which such protection is mediated was never studied in detail (32). Our findings suggest that this protection against APAP-induced toxicity can potentially be due to inhibition of nuclear hormone receptor-regulated metabolism as we have shown that polyI:C suppresses expression of PXR, RXRα and their target genes, Cyp3A11 and CYP1A2. The transcription of the other CYP involved in APAP metabolism, CYP2E1, however, was not altered as this gene is not downstream of any known nuclear hormone receptors (33). As the result of these modulations, polyI:C pretreatment can potentially decrease generation of NAPQI, the toxic metabolite of APAP.
Given the key role of IFN in proper anti-viral responses, we then set out to assess the involvement of IFN production in the suppression of APAP metabolism observed with polyI:C. The reported effects of polyI:C on drug metabolism were previously attributed to its ability to induce IFN (19). Here, we report that in IFNAR-deficient mice, polyI:C administration is still able to suppress expression of RXRα, PXR and downstream CYPs. It is important to note that IFNAR-deficient mice were equally sensitive to APAP-induced hepatotoxicity as wild type mice in our APAP model in contrast to mice deficient in the Type II IFN receptor which are protected against APAP induced toxicity (34). In other liver injury models, such as ischemia reperfusion injury, IFNAR-deficient mice are less susceptible to hepatic injuries (35). This observation suggests that different innate immune pathways are activated during hepatic injuries induced by drugs (e.g. APAP) or ischemia reperfusion that could enhance tissue damage.
A recent study that can complement our findings also demonstrates suppressed APAP toxicity in mice infected with recombinant deficient adenoviruses, DNA viruses (36). They suggest that polyI:C’s protective effects are due to downregulation of CYP2E1 and decreased generation of NAPQI. In our model, CYP2E1 mRNA levels are not altered after polyI:C treatment. One possible explanation is that replication deficient adenoviral infections can induce type II interferons which have been shown to suppress CYP2E1 expression and activity in mice (37,38). However, here we study the effects of activation of anti-viral pathways in response to double stranded RNA stimulants such as VSV and polyI:C, which do not lead to type II interferon induction.
Additionally, we evaluated the involvement of inflammatory cytokines induced by polyI:C in the metabolism and toxicity of APAP. Activation of innate immune cells during viral infections can lead to the release of TNFα and IL-1 (39). Previous studies have demonstrated the effects of TNFα or IL-1 treatment on CYPs, with activity and expression of different CYPs being suppressed or enhanced by either TNFα or IL-1 (4). Thus, induction of these cytokines during viral infections could potentially explain the mechanism by which polyI:C pretreatment suppresses APAP-induced toxicity. However, our results illustrate that mice deficient in TNFα or IL-1 receptors are still protected against APAP-induced hepatotoxicity after polyI:C pretreatment. There are other potential factors activated by polyI:C which may contribute to this protective phenotype that we did not explore. It has been suggested that activation of the p65 NF-κB subunit can result in the direct inhibition of RXRα DNA binding capabilities and thus repression of RXRα-regulated genes (40). While this proposed mechanism is interesting, the experimental support for this hypothesis is primarily found in vitro. Future in vivo experiments will provide greater insight into the role that NF-κB may play in repression of genes downstream of nuclear hormone receptors and innate immune response-mediated protection against APAP hepatotoxicity.
We also examined the induction of known hepatoprotective genes against APAP-induced hepatotoxicity. HO-1 and Metallothinein have been shown to play protective roles against APAP toxicity; however, the role of iNOS remains controversial (41–43). We found that polyI:C treatment of mice for 24 hours increased liver mRNA levels of Heme Oxygenase-1 (HO-1), inducible Nitric Oxide Synthase (iNOS), and Metallothinein-2 (Mt-2) (Supplementary Figure 5). Even though decreased NAPQI formation can explain the protective effects of polyI:C against APAP toxicity, induction of these genes by polyI:C can also contribute to this phenotype.
Finally, we sought to identify which receptors were necessary to sense polyI:C in our animal model. Prior to 2005, the only known receptor class for polyI:C was TLR3 (21). We now know of another family of polyI:C receptors, retinoic acid-inducible gene I-like helicases (including RIG-I and melanoma-differentiation-associated gene 5 (MDA5)). Several studies have suggested that these receptors may function in a cell-type specific manner to sense polyI:C or viral dsRNA. TLR3 has been shown to play an important role in sensing polyI:C in epithelial cells, while only playing a minor role in dendritic cells (44). In contrast, RIG-I and MDA5 play more important roles in sensing polyI:C in fibroblasts and dendritic cells in comparison to TLR3 (45). However, it is not clear whether these two families of receptors play redundant roles in sensing polyI:C in the liver (46). Our data illustrate that polyI:C, when administered i.p., can suppress APAP-induced hepatotoxicity in the absence of TRIF or Cardif, the adaptor proteins required for signal transduction of TLR3 or RIG-I/MDA5, respectively (46). This is the first study to report that polyI:C administration in vivo can exert physiological effects in the absence of TLR3 through Cardif-dependent receptors in the liver.
In summary, the results of this study suggest that activation of anti-viral responses can alter drug metabolism through transcriptional downregulation of CYP3A11 and CYP1A2 independent of IFN production. Understanding the factors that contribute to or alleviate drug toxicity is important for the proper use of drugs under various clinical cases, including the use of common analgesics to relieve pain or fever during viral infections. This study, in conjunction with our previous work, provides further evidence that the use of APAP may be safer in the context of a viral infection than ASA therapy. Furthermore, PolyI:C is now an FDA approved drug that is being evaluated as an anti-cancer therapeutic agent (e.g. ovarian and renal cancer) as well as for chronic fatigue syndrome and AZT-resistant HIV (47–49). Thus, it is important to study potential uncharacterized adverse effects that could occur in virally infected patients or individuals receiving polyI:C, a function for which the animal model we have established can be used. Identification of molecular mechanisms of the crosstalk between innate immune responses and nuclear hormone receptor-regulated metabolism can provide insight into the biological consequences of various drug treatments during viral infections, allowing for safer and more accurate assessment of proper drug therapy.
Supplementary Material
Acknowledgments
We would like to thank Dr. Peter Edwards for reviewing the manuscript.
Financial Support
A.A. Ghaffari was supported by Microbial Pathogenesis Training Grant T32-AI07323. E.K. Chow was supported by the Ruth L. Kirschstein National Research Award GM07185. Part of this work was also supported by National Institutes of Health research grants R01 AI078389, AI056154 and AI069120.
List of Abbreviations
- CYP
Cytochrome P450
- RXR α
Retinoid X Receptor α
- ASA
Aspirin
- APAP
Acetaminophen
- NAPQI
N-acetyl-p-benzoquinone imine
- PXR
Pregnane X Receptor
- SXR
Steroid Xenobiotic Receptor
- CAR
Constitutive Androstane Receptor
- IFN
Type I Interferons
- VSV
Vesicular Somatitis Virus
- TNFα
Tumor Necrosis Factor α
- IL-1
Interleukin-1
- TLR3
Toll-like Receptor 3
- (RIG-I)
Retinoic acid-inducible gene-I
- ALT
alanine transaminase
- PCN
pregnenolone 16α-carbonitrile
- IFNAR
Type I Interferon Receptor-1
- MDA5
melanoma-differentiation-associated gene 5
Contributor Information
Amir A. Ghaffari, Email: amirghaffari@ucla.edu.
Edward K. Chow, Email: echow310@gmail.com.
Shankar S. Iyer, Email: shankar.iyer@ucla.edu.
Jane C. Deng, Email: jdeng@mednet.ucla.edu.
Genhong Cheng, Email: GCheng@mednet.ucla.edu.
References
- 1.Chang KC, Bell TD, Lauer BA, Chai H. Altered theophylline pharmacokinetics during acute respiratory viral illness. Lancet. 1978;1:1132–1133. doi: 10.1016/S0140-6736(78)90305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenzie H, Parratt D, White RG. IgM and IgG antibody levels to ampicillin in patients with infectious mononucleosis. Clin. Exp. Immunol. 1976;26:214–221. [PMC free article] [PubMed] [Google Scholar]
- 3.Kokwaro GO, Ismail S, Glazier AP, Ward SA, Edwards G. Effect of malaria infection and endotoxin-induced fever on the metabolism of antipyrine and metronidazole in the rat. Biochem. Pharmacol. 1993;45:1243–1249. doi: 10.1016/0006-2952(93)90276-3. [DOI] [PubMed] [Google Scholar]
- 4.Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab. Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 5.Chow EK, Castrillo A, Shahangian A, Pei L, O'Connell RM, Modlin RL, et al. A role for IRF3-dependent RXRalpha repression in hepatotoxicity associated with viral infections. J. Exp. Med. 2006;203:2589–2602. doi: 10.1084/jem.20060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugliese A, Beltramo T, Torre D. Reye's and Reye's-like syndromes. Cell Biochem. Funct. 2008;26:741–746. doi: 10.1002/cbf.1465. [DOI] [PubMed] [Google Scholar]
- 7.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N. Engl. J. Med. 2002;347:1094–1103. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- 8.Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J. Clin. Gastroenterol. 2009;43:342–349. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 9.Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, et al. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol. Appl. Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
- 10.Thummel KE, Lee CA, Kunze KL, Nelson SD, Slattery JT. Oxidation of acetaminophen to N-acetyl-p-aminobenzoquinone imine by human CYP3A4. Biochem. Pharmacol. 1993;45:1563–1569. doi: 10.1016/0006-2952(93)90295-8. [DOI] [PubMed] [Google Scholar]
- 11.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streeter AJ, Dahlin DC, Nelson SD, Baillie TA. The covalent binding of acetaminophen to protein. Evidence for cysteine residues as major sites of arylation in vitro. Chem. Biol. Interact. 1984;48:349–366. doi: 10.1016/0009-2797(84)90145-5. [DOI] [PubMed] [Google Scholar]
- 13.Honkakoski P, Negishi M. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;347:321–337. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem. Biophys. Res. Commun. 2002;293:145–149. doi: 10.1016/S0006-291X(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 15.Wolf KK, Wood SG, Hunt JA, Walton-Strong BW, Yasuda K, Lan L, et al. Role of the nuclear receptor pregnane X receptor in acetaminophen hepatotoxicity. Drug Metab. Dispos. 2005;33:1827–1836. doi: 10.1124/dmd.105.005256. [DOI] [PubMed] [Google Scholar]
- 16.Anapolsky A, Teng S, Dixit S, Piquette-Miller M. The role of pregnane X receptor in 2-acetylaminofluorene-mediated induction of drug transport and -metabolizing enzymes in mice. Drug Metab. Dispos. 2006;34:405–409. doi: 10.1124/dmd.105.006197. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Zhang X, Bardag-Gorce F, Robel RCV, Aguilo J, Chen L, et al. Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol. Pharmacol. 2004;65:550–557. doi: 10.1124/mol.65.3.550. [DOI] [PubMed] [Google Scholar]
- 18.Pichlmair A. Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Sakai H, Okamoto T, Kikkawa Y. Suppression of hepatic drug metabolism by the interferon inducer, polyriboinosinic acid:polyribocitidylic acid. J. Pharmacol. Exp. Ther. 1992;263:381–386. [PubMed] [Google Scholar]
- 20.Bleau A, Maurel P, Pichette V, Leblond F, du Souich P. Interleukin-1beta, interleukin-6, tumour necrosis factor-alpha and interferon-gamma released by a viral infection and an aseptic inflammation reduce CYP1A1, 1A2 and 3A6 expression in rabbit hepatocytes. Eur. J. Pharmacol. 2003;473:197–206. doi: 10.1016/s0014-2999(03)01968-x. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol. Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 22.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Bartolone JB, Cohen SD, Khairallah EA. Immunohistochemical localization of acetaminophen-bound liver proteins. Fundam Appl Toxicol. 1989;13:859–862. doi: 10.1016/0272-0590(89)90339-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- 25.Dai G, Chou N, He L, Gyamfi MA, Mendy AJ, Slitt AL, et al. Retinoid X receptor alpha Regulates the expression of glutathione s-transferase genes and modulates acetaminophen-glutathione conjugation in mouse liver. Mol. Pharmacol. 2005;68:1590–1596. doi: 10.1124/mol.105.013680. [DOI] [PubMed] [Google Scholar]
- 26.Albano E, Rundgren M, Harvison PJ, Nelson SD, Moldéus P. Mechanisms of N-acetyl-p-benzoquinone imine cytotoxicity. Mol. Pharmacol. 1985;28:306–311. [PubMed] [Google Scholar]
- 27.Guo GL, Moffit JS, Nicol CJ, Ward JM, Aleksunes LA, Slitt AL, et al. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol. Sci. 2004;82:374–380. doi: 10.1093/toxsci/kfh286. [DOI] [PubMed] [Google Scholar]
- 28.Teschke R, Stutz G, Strohmeyer G. Increased paracetamol-induced hepatotoxicity after chronic alcohol consumption. Biochem. Biophys Res. Commun. 1979;91:368–374. doi: 10.1016/0006-291x(79)90628-4. [DOI] [PubMed] [Google Scholar]
- 29.Feierman DE, Melinkov Z, Nanji AA. Induction of CYP3A by ethanol in multiple in vitro and in vivo models. Alcohol. Clin. Exp. Res. 2003;27:981–988. doi: 10.1097/01.ALC.0000071738.53337.F4. [DOI] [PubMed] [Google Scholar]
- 30.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prandota J. Important role of proinflammatory cytokines/other endogenous substances in drug-induced hepatotoxicity: depression of drug metabolism during infections/inflammation states, and genetic polymorphisms of drug-metabolizing enzymes/cytokines may markedly contribute to this pathology. Am J Ther. 2005;12:254–261. [PubMed] [Google Scholar]
- 32.Renton KW, Dickson G. The prevention of acetaminophen-induced hepatotoxicity by the interferon inducer poly(rI. rC) Toxicol. Appl. Pharmacol. 1984;72:40–45. doi: 10.1016/0041-008x(84)90247-3. [DOI] [PubMed] [Google Scholar]
- 33.Shadley JD, Divakaran K, Munson K, Hines RN, Douglas K, McCarver DG. Identification and Functional Analysis of a Novel Human CYP2E1 Far Upstream Enhancer. Molecular Pharmacology. 2007;71:1630–1639. doi: 10.1124/mol.106.031302. [DOI] [PubMed] [Google Scholar]
- 34.Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- 35.Zhai Y, Shen X, O'Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J. Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 36.Getachew Y, James L, Lee WM, Thiele DL, Miller BC. Susceptibility to acetaminophen (APAP) toxicity unexpectedly is decreased during acute viral hepatitis in mice. Biochem. Pharmacol. 2010;79:1363–1371. doi: 10.1016/j.bcp.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung RS, Qin L, Bromberg JS. TNFalpha and IFNgamma induced by innate anti-adenoviral immune responses inhibit adenovirus-mediated transgene expression. Mol. Ther. 2001;3:757–767. doi: 10.1006/mthe.2001.0318. [DOI] [PubMed] [Google Scholar]
- 38.Qiu LO, Linder MW, Antonino-Green DM, Valdes R. Suppression of cytochrome P450 2E1 promoter activity by interferon-gamma and loss of response due to the -71G>T nucleotide polymorphism of the CYP2E1*7B allele. J. Pharmacol. Exp. Ther. 2004;308:284–288. doi: 10.1124/jpet.103.057208. [DOI] [PubMed] [Google Scholar]
- 39.Dufour J, Clavien P. Signaling Pathways in Liver Diseases. Springer; 2009. [Google Scholar]
- 40.Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J. Biol. Chem. 2006;281:17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 41.Chiu H, Brittingham JA, Laskin DL. Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicol. Appl. Pharmacol. 2002;181:106–115. doi: 10.1006/taap.2002.9409. [DOI] [PubMed] [Google Scholar]
- 42.Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur. J. Immunol. 2006;36:1028–1038. doi: 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- 43.Saito C, Yan H, Artigues A, Villar MT, Farhood A, Jaeschke H. Mechanism of protection by metallothionein against acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010;242:182–190. doi: 10.1016/j.taap.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, et al. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 45.Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Ieki K, Kuga H, et al. Role of RIG-I, MDA-5, and PKR on the expression of inflammatory chemokines induced by synthetic dsRNA in airway epithelial cells. Int. Arch. Allergy Immunol. 2007;143 Suppl 1:80–83. doi: 10.1159/000101411. [DOI] [PubMed] [Google Scholar]
- 46.Akira S. Pathogen recognition by innate immunity and its signaling. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 2009;85:143–156. doi: 10.2183/pjab.85.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giantonio BJ, Hochster H, Blum R, Wiernik PH, Hudes GR, Kirkwood J, et al. Toxicity and response evaluation of the interferon inducer poly ICLC administered at low dose in advanced renal carcinoma and relapsed or refractory lymphoma: a report of two clinical trials of the Eastern Cooperative Oncology Group. Invest New Drugs. 2001;19:89–92. doi: 10.1023/a:1006458232384. [DOI] [PubMed] [Google Scholar]
- 48.Gillespie D, Hubbell HR, Carter WA, Midgette P, Elsasser W, Mullaney R, et al. Synergistic inhibition of AZT-resistant HIV by AZT combined with poly(I):poly(C12U), without synergistic toxicity to bone marrow progenitor cell elements. In Vivo. 1994;8:375–381. [PubMed] [Google Scholar]
- 49.Cheng Y, Xu F. Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol. Ther. 2011;10:1219–1223. doi: 10.4161/cbt.10.12.13450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.