SUMMARY
CD28 and CTLA-4 are cell surface cosignaling molecules essential for the control of T cell activation upon the engagement of their ligands, B7-1 and B7-2 from antigen-presenting cells. By employing a newly developed receptor array, we have demonstrated that B7-H2, best known as the ligand of Inducible Costimulator, was a ligand for CD28 and CTLA-4 in human, whereas these interactions were not conserved in mouse. B7-H2 interacted with CD28 in a non-overlapping domain as B7-1 and B7-2, and was essential for the costimulation of human T cells’ primary responses to allogeneic antigens and memory recall responses. Similar to B7-1 and B7-2, B7-H2 costimulation via CD28 induced survival factor Bcl-xL, downregulated cell cycle inhibitor p27kip1 and triggered signaling cascade of ERK and AKT kinases-dependent pathways. Our findings warrant re-evaluation of CD28 and CTLA-4’s functions previously attributed exclusively to B7-1 and B7-2, and has important implications in therapeutic interventions against human diseases.
INTRODUCTION
The B7-CD28 family of costimulatory molecules modulate T cell receptor signals and play essential roles in the control of T cell-mediated immune responses (Carreno and Collins, 2002; Chen, 2004). CD28, the most extensively studied cosignaling receptor, accepts a costimulatory signal from B7-1 (CD80) or B7-2 (CD86, B70) and promotes activation of naive T cells in the presence of a T cell receptor signal (Linsley et al., 1990). On the other hand, CTLA-4, a CD28 homolog expressed on activated T cells, serves as a checkpoint to attenuate T cell responses upon ligation of B7-1 and/or B7-2 (Krummel and Allison, 1995; Walunas et al., 1994). Inducible Costimulator (ICOS), another CD28 homolog in the same gene cluster with CD28 and CTLA4, is expressed on activated T cells and costimulates T cell activation upon binding of a distinct ligand B7-H2 (ICOSLG, GL50, B7RP1, CD275, ICOSL, LICOS) (Hutloff et al., 1999; Swallow et al., 1999; Wang et al., 2000; Yoshinaga et al., 1999).
Although CD28 and ICOS have distinct intracellular domains, they share a great functional redundancy, including their capacity to costimulate growth, survival and differentiation of T cells, as well as the requirement for antibody response (Dong et al., 2001; Linterman et al., 2009; McAdam et al., 2001; Tafuri et al., 2001). Both CD28 and ICOS signals are shown to have similar capacity in costimulating an array of cytokines, including interleukin-4 (IL-4), interleukin-5 (IL-5), interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) (Hutloff et al., 1999). The main difference between CD28 and ICOS pathways is that CD28 induces high amounts of IL-2 and upregulates survival factor Bcl-xL (Boise et al., 1995; Parry et al., 2003), whereas ICOS preferentially costimulates IL-10 (Hutloff et al., 1999). These findings are consistent with observations in the microarray analysis of T cell transcription profiles, which show highly similar patterns upon costimulation by both CD28 and ICOS, especially in human T cells (Riley et al., 2002).
A possible explanation for the functional redundancy of these two distinct costimulatory pathways is the presence of a shared ligand. Even though the interaction between the putative ligand and CD28 may be well below the detectable level by conventional binding technology, it is still sufficient to trigger T cell functions. In order to detect these interactions of cell surface proteins, we established a highly sensitive, comprehensive receptor array coupled with a high-throughput screening system. Employing this new methodology, we re-evaluated possible receptor-ligand interactions in the CD28 and ICOS molecular pathways.
RESULTS
Identification of B7-H2-CD28 interaction by a receptor array
We selected more than 2,000 full length human transmembrane genes based on their immune and hematopoietic cell surface expression (Table S1 available online). All of these genes were cloned into mammalian expression vectors. Each individual plasmid was introduced into 293T cells in a 384-well plate format using an optimized transfection protocol. Over 95% of the genes from randomly selected plasmids in our collection expressed highly on cell surface, which was confirmed by flow cytometry analysis (data not shown). For screening unknown counter-receptors, the target gene (encoding a secreted protein) or the extracellular domain of the target gene (encoding a transmembrane protein) was genetically fused to a tag gene (mouse IgG2a Fc, human IgG1 Fc, FLAG or 6xHIS), and the purified recombinant fusion protein was used to bind the receptor array. A fluorescence-labeled secondary antibody against the tag was applied to detect the binding of the target protein to the transfected 293T cells, and was screened by the Applied Biosystems 8200 Cellular Detection System (CDS).
Since our receptor array approach has better sensitivity for detection of molecular interactions than other methods, we first screened recombinant human CD28-immunoglobulin (CD28Ig) fusion proteins for system validation and for additional ligands (Figure 1a). As expected, CD28Ig bound 293T cells expressing B7-1 or B7-2 genes in the array. The cells expressing Fc receptors, used as internal controls, also stained positive due to the binding of the human Fc tag on CD28Ig. To our surprise, CD28Ig was found to bind cells expressing B7-H2, albeit with a lower affinity than B7-1 and B7-2 transfectants (Figure 1a and 1b). The binding activity to tight junction adhesion molecule occludin (OCLN) was also found but this interaction was demonstrated to be non-specific (data not shown). There was no additional binding activity by CD28Ig in our receptor array. Therefore, CD28 and ICOS may share a common ligand, B7-H2.
Figure 1. Identification of B7-H2-CD28 interaction by high throughput screening of a receptor array.
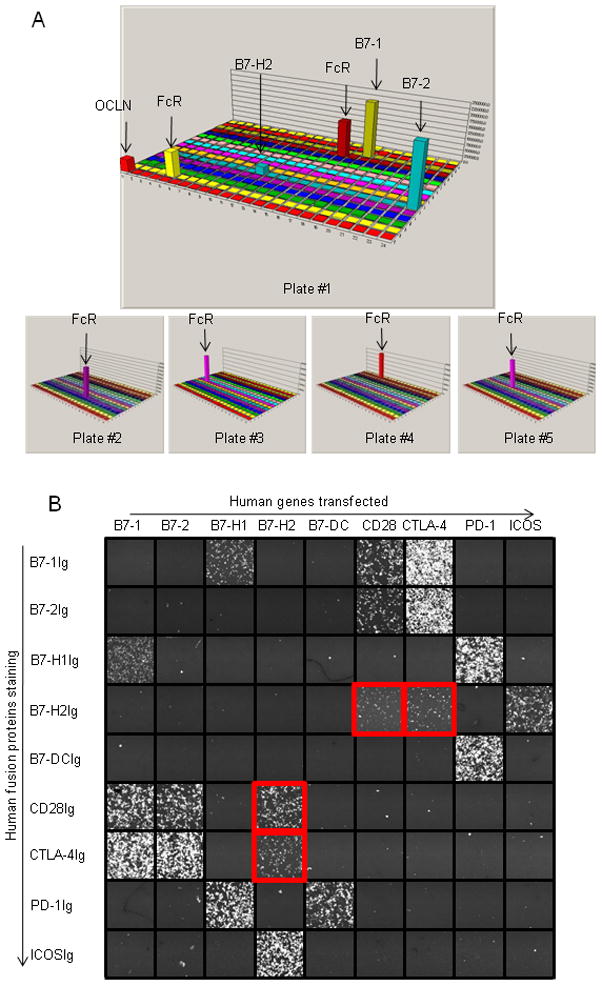
(a) Screening of additional ligands for CD28 in a receptor array. CD28Ig was used as bait in the screening system. The 3-D illustration represents the result of a set of five 384-well plates. Each bar represents the total fluorescence intensity in the FL1 gate in each well of a 384-well plate. The Fc Receptors transfectants are used as positive controls.
(b) Interactions among B7-CD28 family molecules. 293T cells were transfected with full length human B7-CD28 family genes as indicated on the x-axis. Fusion proteins were added to the culture as indicated on the y-axis to evaluate their bindings to the transfectants by CDS. Graphic view of individual wells is captured by 8200 CDS.
Specificity of B7-H2 interactions with CD28 and CTLA-4
We first validated the B7-H2-CD28 interaction by staining of CD28Ig to B7-H2 transiently transfected 293T cells using flow cytometry analysis. High B7-H2 expression was verified by positive staining of B7-H2 monoclonal antibody (mAb) and negative staining of B7-1 and B7-2 mAbs (Figure 2a). The majority of cells also stained positively by ICOSIg, demonstrating the specific expression of B7-H2 on 293T cells. Importantly, CD28Ig showed strong binding to B7-H2+ 293T cells, and this binding was completely blocked by the inclusion of a mAb against CD28 (clone CD28.6). Since both B7-1 and B7-2 bind CTLA-4 in addition to CD28, we subsequently tested whether B7-H2 could interact with CTLA-4. CTLA-4Ig also bound B7-H2+ 293T cells (Figure 1c) and the binding was largely abolished by a mAb against CTLA-4 (clone 14D3) (Figure 2a). To further confirm these new interactions, we transiently transfected 293T cells to express high amounts of CD28 or CTLA-4, as shown by respective mAb staining (Figure 2b and 2c). B7-H2Ig bound to both transfectants, albeit weaker than B7-1 and B7-2 fusion proteins. Inclusion of a mAb against B7-H2 (Clone MIH12) or ICOSIg completely abrogated these interactions (Figure 2b and 2c). The binding of dimeric CD28 and CTLA4 Ig fusion protein to immobilized B7-H2 was also validated by surface plasmon resonance (SPR) analysis at both 25 and 37 °C (Figure S1). Altogether, our data support the specificity of B7-H2-CD28 and B7-H2-CTLA-4 interactions and indicate an overlapping binding site on B7-H2 for interacting with ICOS, CD28 and CTLA-4.
Figure 2. B7-H2 bound both CD28 and CTLA-4.
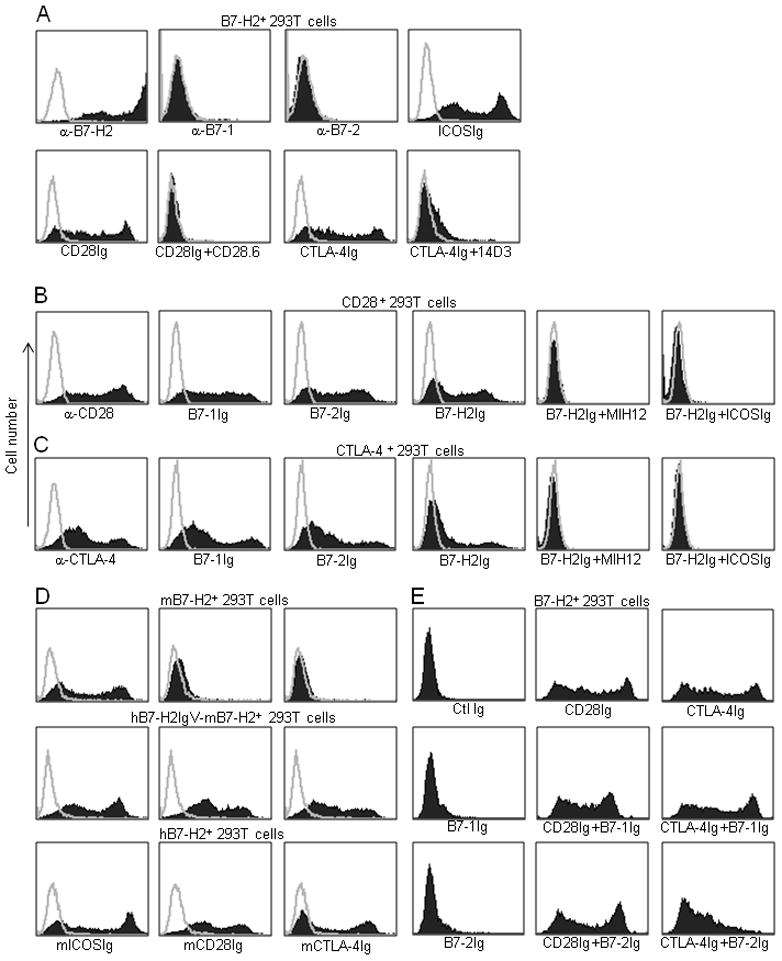
(a–c) Specificity of B7-H2 bindings to CD28 and CTLA-4. 293T cells were transiently transfected with human full length B7-H2 (a), CD28 (b) or CTLA-4 (c) plasmids, and were subsequently stained by the indicated specific mAb or fusion proteins. In some experiments, additional mAb or fusion proteins were also included to examine the specificity of the binding. (d) Murine B7-H2 did not interact with murine CD28 and CTLA-4, while human B7-H2 interacts with murine ICOS, CD28 and CTLA-4. 293T cells were transiently transfected with mouse full length B7-H2, a hB7-H2IgV-mB7-H2 chimeric construct or human full length B7-H2 plasmids and were stained by the indicated fusion proteins. (e) Interactions of CD28 and CTLA-4 with their three ligands. To examine the competition between B7-H2 and B7-1 or B7-2 for binding CD28 and CTLA-4, B7-H2+ 293T cell transfectants were stained by CD28Ig and CTLA-4Ig, which were preincubated with excessive amount of B7-1Ig or B7-2Ig. Isotype-matched human Ig (Ctl Ig) was included as controls.
We next examined whether these newly discovered interactions were also conserved in mice. Both murine CD28Ig and CTLA-4Ig did not stain murine B7-H2 transfected 293T cells by flow cytometry analysis, whereas ICOSIg bound the transfectant as expected (Figure 2d). When the murine B7-H2 IgV region (Met1-Val149) was replaced with its corresponding human counterpart (Met1-Gln123), this chimeric B7-H2 was capable of binding murine CD28 and CTLA-4 (Figure 2d), indicating that interactions of B7-H2 with CD28 and CTLA-4 are not conserved in mice. Similar to the chimeric protein, wild-type human B7-H2Ig, could also bind the cells transfected to express murine CD28 and CTLA-4 (Figure 2d).
We next addressed the question whether B7-H2 could compete with B7-1 and B7-2 for binding CD28. Flow cytometry analysis showed saturated doses of B7-1Ig and B7-2Ig had only minimal effect on the binding of CD28Ig to B7-H2+ 293T cells (Figure 2e). Therefore, CD28 binds B7-H2 and B7-1 or B7-2 through different interfaces, suggesting that B7-H2 and B7-1 or B7-2 could bind CD28 simultaneously. A similar test examined the interactions of the three ligands to CTLA-4. Interestingly, saturated B7-2Ig but not B7-1Ig partially blocked the binding of CTLA-4Ig to B7-H2+ 293T cells (Figure 2e), indicating the binding sites on CTLA-4 for B7-H2 and B7-2 may overlap.
B7-H2 utilized overlapping but different binding sites to interact with ICOS, CD28 and CTLA-4
To understand the architecture of the interactions between B7-H2 and its three receptors, we conducted site-directed mutagenesis on B7-H2 to identify critical residues for the three interactions. Selection of residues for mutation was based on their locations in the binding interfaces of B7-H2 and three receptors predicted from the crystal structure of B7-2-CTLA-4 (Chattopadhyay et al., 2006; Schwartz et al., 2001) (Figure 3a left panel). These residues were all converted to alanine, a small amino acid with neutral charge, to avoid a large overall structure modulation (Chattopadhyay et al., 2006). Wild-type and mutated B7-H2 full length genes were expressed on the surface of 293T cells and confirmed by anti-B7-H2 staining. The binding of these cells by CD28Ig, CTLA-4Ig and ICOSIg were determined by flow cytometry analysis. The relative binding capacity of these recombinant fusion proteins to B7-H2 mutants were compared with wild-type B7-H2 (Figure 3a right panel). All mutants, which lost their binding to CD28 (Y51A, Y53A, L116A and F122A), also did not interact with CTLA-4, indicating that B7-H2’s binding sites for CD28 and CTLA-4 might largely overlap, i.e. CD28 and CTLA-4 should compete for the binding to B7-H2. Indeed, CTLA-4Ig (Abatacept) bound B7-H2+ 293T cells (Figure 3b) and completely blocked B7-H2Ig binding to CD28+ 293T cells (Figure 3c).
Figure 3. Interactions of B7-H2 with CD28, CTLA-4 and ICOS.
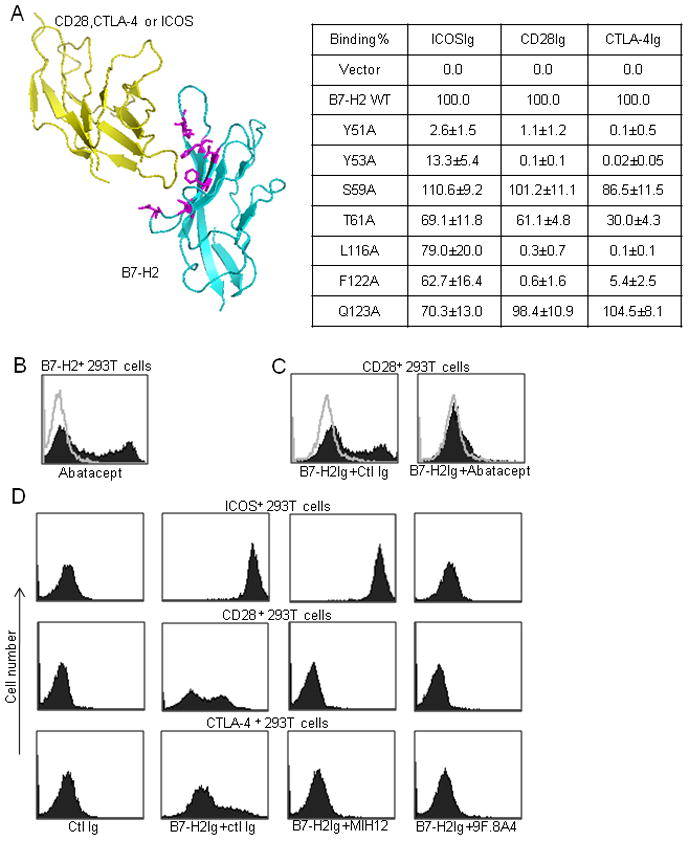
(a) Left panel: Ribbon diagram of interacting model between B7-H2 and ICOS, CD28 or CTLA-4. The model was built by Pymol software (DeLano Scientific LLC.) based on crystal structure of B7-2-CTLA-4 (RCSB PDB 1i85). Right panel: binding site analysis of B7-H2 with its receptors by site-directed mutagenesis. Single point mutations were introduced into B7-H2 residues buried at the ligand-receptor interacting interface (shown in purple in ribbon diagram). B7-H2 plasmids (wild-type and mutants) were then transiently transfected into 293T cells and the expression of B7-H2 was confirmed by B7-H2 antibody staining. Binding of human ICOSIg, CD28Ig and CTLA-4Ig to B7-H2 mutants in transfected cells were measured by flow cytometry and normalized against 293T cells expressing wild-type B7-H2 (100%) and vector control (0%). The results were expressed as the percentage of binding to wild-type B7-H2. In addition to mean (n=3), 95% confidence intervals were given for each binding.
(b–c) CTLA-4 competes with CD28 for B7-H2 binding. (b) CTLA-4Ig (Abatacept) was used to stain B7-H2+ 293T cells. (c) B7-H2Ig was pre-incubated with an excessive amount of Abatacept or control Ig for 15 min before staining CD28+ 293T cells.
(d) Identification of mAbs that differentially abrogate B7-H2-CD28 and B7-H2-ICOS interactions. ICOS+, CD28+ or CTLA-4+ 293T cell transfectants were stained by B7-H2Ig or control (ctl) Ig. B7-H2 mAbs MIH12, 9F.8A4 or control mouse Ig, were included in the cultures to examine their blocking effects on B7-H2Ig binding.
Two mutants (Y51A, Y53A) that lost their binding to ICOS, also showed no binding to CD28 and CTLA-4. Interestingly, two mutants (L116A and F122A), which largely retained the ability to bind ICOS, had only minimal binding capacity to CD28 and CTLA-4 (Figure 3a right panel). These results support that B7-H2 uses overlapping but different domains to interact with ICOS vs. CD28 and CTLA-4, whereas its binding sites for CD28 and CTLA-4 are very similar.
The finding that B7-H2 utilizes overlapping but differential binding sites to interact with ICOS vs. CD28 and CTLA-4 encouraged us to seek B7-H2 mAbs that selectively block individual interactions to facilitate functional analysis. As shown in Figure 3d, mAb clone MIH12 specifically blocked B7-H2Ig binding to CD28+ and CTLA-4+ 293T cells but had no effect on B7-H2Ig binding to ICOS+ 293T cells, whereas mAb clone 9F.8A4 abrogated B7-H2Ig binding to all three receptors by flow cytometry analysis (Figure 3d). We have not yet identified a mAb that specifically blocks B7-H2 binding to CD28 but not to CTLA-4 and vice versa (data not shown) and this may be due to the fact that B7-H2 binding sites for CD28 and CTLA-4 are largely overlapping (Figure 3a).
B7-H2 costimulated human T cell proliferation and cytokine production via CD28
In an initial attempt to demonstrate a functional interaction of B7-H2 and CD28, we utilized an in vitro costimulation assay in which suboptimal concentrations of human CD3 mAb were immobilized in the wells of a 96-well plate as a mimicry of T cell receptor (TCR) signaling. B7-H2Ig was co-immobilized in the same well to provide a costimulatory signal for purified human T cells. While suboptimal concentrations of CD3 mAb (up to 0.1 μg/ml) led to minimal proliferation of T cells, inclusion of B7-H2Ig in the wells induced strong T cell proliferation as evidenced by increased incorporation of tritiated thymidine (Figure 4a). The effect of B7-H2 was dependent on TCR signaling because B7-H2Ig alone, in the absence of CD3 mAb, did not stimulate any detectable proliferation (data not shown). Inclusion of mAb MIH12, which specifically blocks B7-H2 binding to CD28 and CTLA-4 but not to ICOS, suppressed about 50% of B7-H2Ig-mediated costimulation. The mAb 9F.8A4, which blocks B7-H2 binding to all three receptors, completely abolished the effect of B7-H2Ig (Figure 4a). Similar results were acquired from different donors (Figure S2). Accordingly, CD28 mAb (clone CD28.6) partially inhibited B7-H2 mediated T proliferation, whereas ICOS mAb (C398.4A) suppressed the majority of the B7-H2 costimulatory effect. The complete inhibition was achieved by the combination of CD28 and ICOS mAbs (Figure 4b).
Figure 4. B7-H2 costimulation of human T cells through CD28.
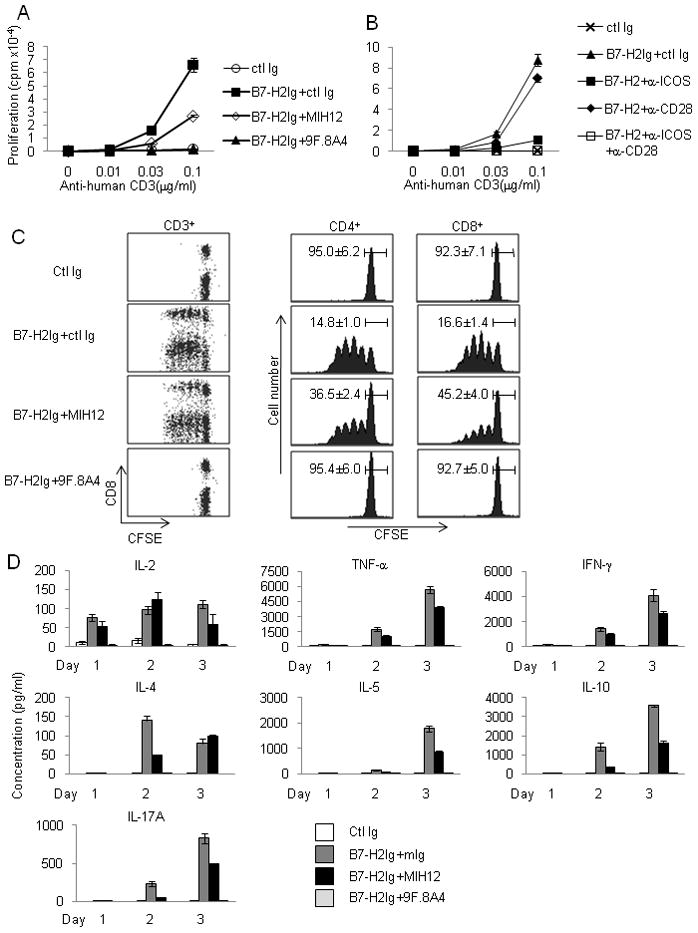
(a–b) B7-H2-CD28 interaction costimulates human T cell proliferation. Purified PBMC CD3+ T cells at 2.5x105/wells were stimulated with immobilized human CD3 mAb (OKT3) and B7-H2Ig or control Ig (ctl Ig). (a) B7-H2 mAb (clone MIH12 or 9F.8A4) or control mouse Ig (ctl Ig) was added to block immobilized B7-H2Ig. (b) CD28 mAb (CD28.6) and/or ICOS mAb (C398.4A) or control Ig was added to the culture to block B7-H2 mediated costimulation. 3HTdR was added during the final six hours of culture. Similar results were also obtained in two additional experiments using different donors.
(c) B7-H2-CD28 interaction promotes division of human T cells. Using the same condition as in (a), CFSE labeled CD3+ T cells were stimulated with immobilized anti-human CD3 and B7-H2Ig. B7-H2 mAb (clone MIH12 or 9F.8A4) was used to block coated B7-H2Ig. T cells were collected on day 4 and analyzed by a FACSCalibur flow cytometer. Average percentage of undivided T cell (n=3) and 95% confidence intervals are given.
(d) B7-H2 costimulates cytokine production from human T cells through CD28. Purified CD4+ T cells at 2.5x105/wells were stimulated by immobilized CD3 mAb and B7-H2Ig as described in (a). MIH12, 9F.8A4 or control mouse Ig (ctl Ig) was added to block B7-H2Ig. Supernatants were collected daily up to three days. Cytokine concentrations were measured by BD Cytometric Bead Array (CBA) human T helper-1 cell and T helper-2 cell (Th1-Th2 cell) cytokine kit and CBA IL-17A Flex set, and analyzed by FCAP Array software. Each data point is an average of three samples with standard deviation. Similar results were also obtained in two additional experiments using different donors.
Next we assessed the costimulatory capacity of B7-H2 mutant fusion proteins. B7-H2 L116A, and F122A mutants largely maintained binding to ICOS, but not to CD28 and CTLA-4, whereas the Y51A mutant lost binding capacity to all three receptors (Figure 3a). L116A and Y51A could no longer costimulate T cell proliferation as wild-type B7-H2 and Q123A did (Figure S3). Meanwhile, F122A also lost the majority of its costimulatory capacity, indicating the B7-H2 interface for CD28 interaction is important for B7-H2 function.
We also used carboxyfluorescein succinimidyl ester (CFSE)-labeled human T cells to monitor cell division. The blockade of the B7-H2-CD28 interaction with MIH12 had a partial inhibitory effect on the division of both CD4+ and CD8+ T cells, whereas 9F.8A4 completely suppressed T cell division (Figure 4c), a result consistent with the thymidine incorporation assay. Activated T cells in this setting expressed high amounts of ICOS in addition to CD28 (data not shown). Because CTLA-4 did not play a dominant role in this assay, we conclude that ligations of B7-H2 to both CD28 and ICOS on T cells independently contribute to the costimulatory function of B7-H2 in this assay. Therefore, B7-H2 could promote T cell growth via engaging CD28.
One characteristic feature of CD4+ T cell costimulation through both CD28 and ICOS is to induce large amounts of cytokines, in contrast to TCR engagement alone, which produces minimal cytokines (Hutloff et al., 1999). We first compared cytokine profiles induced by B7-1Ig vs. B7-H2Ig in the same concentration employing a cytokine array (Figure S4). Consistent with previous finding using CD28 and ICOS agonist mAbs (Riley et al., 2002) , costimulation through B7-1 and B7-H2 induced an overlapping spectrum of cytokines including TNF-α, IFN-γ, IL-4, IL-5, IL-10 and IL-17A. Only B7-1Ig stimulated a large amount of IL-2 (100 fold higher than B7-H2Ig). B7-1 also elicited higher amounts of TNF-α and IFN-γ, while B7-H2 appeared to induce more IL-17A (Figure S4). We did not observe a superior ability of B7-H2Ig in the stimulation of IL-10 production in our assay over 3 days, as shown previously by an ICOS agonist mAb (Hutloff et al., 1999).
In the context of the overlapping cytokine profile induced by B7-1 and B7-H2, we evaluated the relative contribution of B7-H2-CD28 vs. B7-H2-ICOS interaction to the overall B7-H2-mediated cytokine production by antibody blockade (Figure 4d). MIH12 partially decreased production of the majority of cytokines, with more profound effects on TNF-α, IFN-γ, IL-5, IL-10 and IL-17A, but less effective for IL-2 and IL-4. In contrast, blockade by 9F.8A4 reduced all cytokine production to background (Figure 4d), indicating that B7-H2-CD28 costimulation contributes to the cytokine production, which were exclusively attributed to the B7-H2-ICOS interaction in the past.
Blockade of B7-H2-CD28 interaction inhibited human T cell activation
We sought to evaluate the role of the B7-H2-CD28 interaction in more physiologically relevant models in vitro, including antigen-specific memory T cell recall response and primary T cell response to allogeneic antigens. In the first model, monocyte-derived dendritic cells (DC) were incubated with tetanus toxoid (TT) to stimulate autologous peripheral T cells from adult individuals who were previously immunized with tetanus vaccine. The proliferation and IFN-γ production of polyclonal memory T cells in response to TT antigen were examined in the presence of selected mAbs. First, we demonstrated that B7-H2 and B7-2 were highly expressed on DCs in these experiments (data not shown). While T cells proliferated vigorously to TT-DC stimulation, inclusion of B7-H2 mAb MIH12 reduced TT-elicited proliferation by approximately 20%, a small but significant inhibition (P<0.05) (Figure 5a). However, this treatment had a profound effect on IFN-γ production: over 80% IFNγ production in the culture was suppressed (Figure 5b). Addition of CD28 blocking mAb (clone CD28.6, non-costimulatory), ICOS mAb C398.4A or B7-H2 mAb 9F.8A4 inhibited more than 50% of proliferation and more than 90% of IFN-γ production (Figure 5a and 5b). These findings indicate that both CD28 and ICOS pathways are required for the proliferation and IFN-γ production of memory T cells in this setting. B7-H2-CD28 interaction, albeit playing a minor role in the proliferation of memory T cells, is critical for TT-induced IFN-γ production from memory T cells.
Figure 5. B7-H2-CD28 interaction is important for T cell activation.
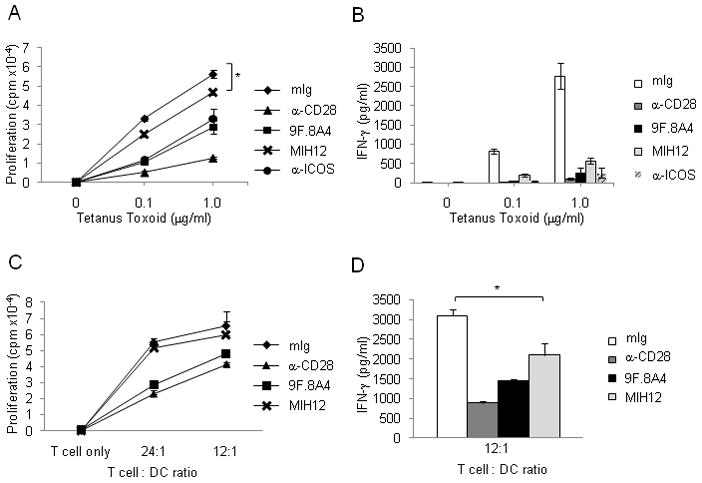
(a–b) B7-H2-CD28 interaction enhances response of tetanus toxoid-specific memory T cells. (a) Monocyte-derived dendritic cells at 2.5x104/well and purified autologous T cells at 3x105/well were mixed together and incubated with tetanus toxoid at indicated concentration for five days. Blocking mAbs against CD28 (clone CD28.6), B7-H2 (clone MIH12 and 9F.8A4) or control mouse Ig at 5μg/ml was included in soluble form at the beginning of the culture. 3HTdR was added during the final sixteen hours of culture. * p<0.05. (b) Interferon gamma concentration was measured in day 5 supernatants by BD (CBA) Human Th1-Th2 cell cytokine kit and analyzed by FCAP Array software. Each data point is an average of samples with standard deviation. Similar results were also obtained in two additional experiments using different donors.
(c–d) B7-H2-CD28 interaction promotes IFN-γ release in allogeneic T cell response. (c) Monocyte derived dendritic cells at 0, 1.25 x104, 2.5x104/well and purified allogeneic T cells at 3x105/well were mixed together. Blocking mAbs against CD28 (clone CD28.6), B7-H2 (clone MIH12 and 9F.8A4) or control mouse Ig at 5μg/ml was included in soluble form at the beginning of the culture for five days. 3HTdR was added during the final sixteen hrs of culture.
(d) Interferon gamma concentration was measured in day 5 supernatants by BD (CBA) Human Th1-Th2 cell cytokine kit and analyzed by FCAP Array software. Each data point is an average of three samples with standard deviation. Similar results were also obtained in two additional experiments using different donors.
In the second model, DCs were used for stimulation of allogeneic T cells purified from human peripheral blood. In this culture system, MIH12 also significantly suppressed IFN-γ production from allogeneic T cells (P<0.05) (Figure 5d). Similar to TT antigen-elicited memory T cell response, MIH12 was mainly effective on control of IFN-γ production but only had a small effect on T cell growth (Figure 5c), perhaps due to the weak IL-2 inducing capacity of B7-H2. Our results thus demonstrated that endogenous B7-H2-CD28 interactions play a critical role in promoting activation and cytokine production in human T cells.
B7-H2-CD28 interaction upregulated Bcl-xL expression and downregulated p27kip1
In addition to IL-2 production, another major difference between CD28 and ICOS costimulation is that only CD28 signaling induces increased production of the anti-apoptotic molecule Bcl-xL through its intracellular Src homology 2 binding domain (SH2)(Boise et al., 1995; Parry et al., 2003). We next investigated whether B7-H2-CD28 interaction could also stimulate Bcl-xL protein expression. With suboptimal amounts of CD3 mAb, B7-1g costimulated the expression of Bcl-xL in activated CD4+ T cells, whereas immobilized ICOS agonist mAb was unable to upregulate Bcl-xL, as expected (Figure 6a). B7-H2Ig also enhanced the expression of Bcl-xL, albeit to a lower degree than B7-1Ig on day2. Blockade of the B7-H2-CD28 interaction by MIH12 partially suppressed Bcl-xL upregulation, whereas 9F.8A4 completely inhibited Bcl-xL expression (Figure 6b). Similar to its effect in blocking T cell proliferation, CD28 mAb CD28.6 also partially reduced B7-H2 induced Bcl-xL upregulation, whereas soluble CTLA4 mAb 14D3 had no effect. Meanwhile, ICOS mAb C398.4A in soluble form inhibited the Bcl-xL upregulation, suggesting ICOS is important but not sufficient for Bcl-xL expression. The complete inhibition was achieved only when both B7-H2-CD28 and B7-H2-ICOS interactions were blocked by 9F.8A4. Optimal Bcl-xL expression induced by B7-H2 thus might require a cooperation of both CD28 and ICOS signals. In summary, our results indicate that, in addition to the induction of cytokines and T cell activation, B7-H2-CD28 interaction provides a crucial signal to enhance Bcl-xL expression.
Figure 6. B7-H2-CD28 interaction promotes Bcl-xL expression and downregulates p27kip1 in activated CD4+ T cells.
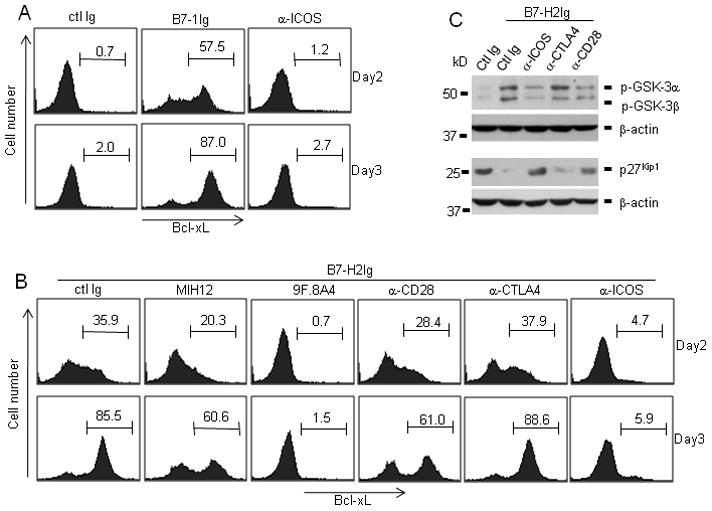
Purified CD4+ T cells at 3.5x105/well were stimulated with immobilized human CD3 mAb (0.1μg/ml) and 5μg/ml of immobilized (a) control Ig (ctl Ig), B7-1Ig, ICOS agonistic mAb (C398.4A), or (b and c) B7-H2Ig. In some wells, B7-H2 mAb (MIH12 or 9F.8A4), CD28 mAb (CD28.6), CTLA-4 mAb (14D3), ICOS mAb (C398.4A) or control mouse Ig was used to block B7-H2Ig mediated response. (b) Activated T cells were harvested on day 2 and day3, intracellularly stained by Bcl-xL mAb and analyzed by flow cytometry. (c) Activated T cells were harvested at 4 hours and 48 hours after activation. Cell lysates were prepared. 100μg total protein per sample was loaded and separated by 12% SDS-PAGE. The amount of phosphorylated GSK-3 (harvested at 4 hours) and p27kip1 (harvested at 48 hours) and beta-actin were determined by immunoblotting with respective antibodies. Beta actin was used as loading control.
It has been previously shown that CD28 promotes T cell proliferation through downregulation of cyclin-dependent kinase (CDK) inhibitor 1B (CDKN1B, p27kip1)(Appleman et al., 2002). p27kip1 binds to and inhibits the activation of Cyclin E-CDK2 or Cyclin D-CDK4 complex, resulting in cell cycle arrest (Sherr and Roberts, 1999). As a result, the degradation of p27kip1 is crucial for cell cycle progression. With a suboptimal amount of CD3 mAb, immobilized B7-H2 downregulated p27kip1 protein in human T cells on day2 (Figure 6c), which correlated with increased cellular proliferation (Fig. 4a) and upregulation of Bcl-xL (Fig. 6b). Since the degradation of p27kip1 is modulated by phosphatidylinositol 3-kinase (PI3K) and serine-threonine kinase AKT(PKB) dependent signaling pathways (Medema et al., 2000), we further investigated the inactivation phosphorylation of glycogen synthase kinase-3 (GSK-3), one of AKT’s downstream targets. Compared with control Ig group, GSK-3 phosphorylation was markedly increased in B7-H2 costimulated T cells four hours after activation (Fig. 6c). More importantly, ICOS and CD28 blockade with mAbs partially suppressed GSK-3 phosphorylation and increased the expression of p27kip1, whereas CTLA4 mAb had no effect. Combined, these results indicate increased expression of Bcl-xL and downregulation of p27kip1 might integrate TCR signal and B7-H2-CD28 mediated costimulatory signal to promote survival and cell cycle progression in human T cells.
Human B7-H2 costimulated the activation of murine ICOS-deficient T cells via CD28
Blockade of ICOS or CD28 by mAbs are often incomplete and this may jeopardize our further study of intracellular signaling events upon B7-H2-CD28 interaction. To exclude the effect of residual ICOS or CD28, we took advantage of the cross-species interactions of human B7-H2Ig with mouse ICOS and CD28 (Fig. 2f) and employed ICOS genetically ablated T cells to investigate whether B7-H2 could costimulate Icos-/- T cell via CD28. As shown in Figure 7a, human B7-H2, but not mouse B7-H2 costimulated T cells from ICOS deficient mice to proliferate. Inclusion of a mouse CD28 mAb completely eliminated the B7-H2 mediated effect (Figure 7b). In wild-type T cells, human B7-H2 was also a much stronger costimulator than mouse B7-H2 to promote wild-type mouse T cell to proliferate. These findings validated the lack of B7-H2-CD28 interaction in mouse system. Furthermore, B7-H2 could costimulate purified CD4+ Foxp3- naive T cells and CD4+ Foxp3+ T regulatory cells from ICOS deficient mice in an IL-2 dependent fashion, since addition of IL-2 neutralizing mAb greatly inhibited the growth of both cells (Figure S5).
Figure 7. B7-H2-CD28 interaction induces early activation of ERK and PI3K-AKT dependent pathways.
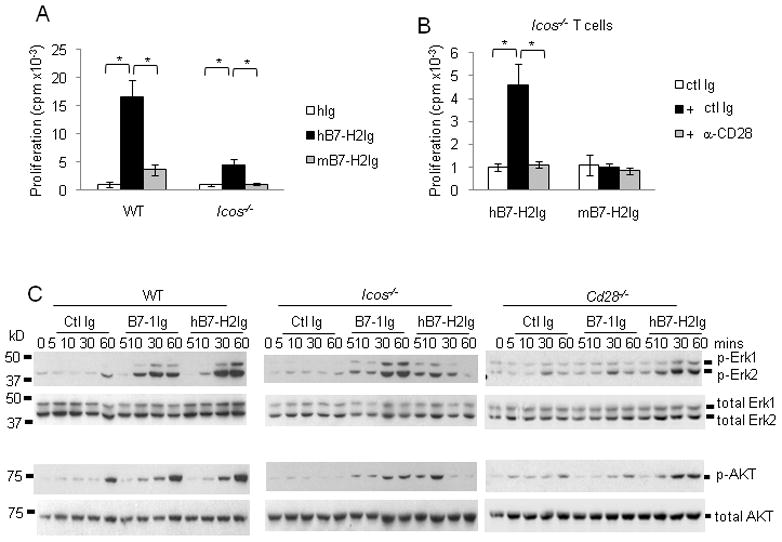
(a) Purified native CD3+ T cells from wild-type or ICOS deficient mice at 3x105/well were stimulated with immobilized 0.8μg/ml mouse CD3 mAb and 5μg/ml human or mouse B7-H2Ig or control Ig (ctl Ig). (b) In some wells, mouse T cells were first incubated with 5μg/ml mouse CD28 mAb (37.51) or control Ig for 15 minutes. After washing away unbound mAb, T cells were added to 96 well plates. 3HTdR was added during the final six hours of culture. Similar results were also obtained in two additional experiments. * p<0.05. (c) Purified mouse CD4+ T cells from wild-type, ICOS deficient or CD28 deficient mice were activated with 5μg/ml immobilized anti-CD3 for 3 days, and then rested for 3 days at the presence of 50IU/ml rmIL-2. T cells were subsequently harvested and added into 96 well plates pre-coated with 0.1μg/ml anti-CD3 plus 5μg/ml human B7-H2, mouse B7-1 or control Ig proteins. Cells were harvested at different time points as indicated. Cell lysates were prepared. 100μg total protein per sample was loaded and separated by 12% SDS-PAGE. The amount of phosphorylated ERK and AKT protein were determined by immunoblotting with phospho-specific antibodies (Thr202 and Tyr204 phospho-Erk1 and Erk 2 or Ser473 phospho-Akt). Membranes were subsequently striped and reprobed with antibodies against total ERK and AKT.
Both CD28 and ICOS costimulation are known to activate extracellular signal-regulated kinase (ERK) and PI3K- AKT dependent signaling cascades (Parry et al., 2003; Prasad et al., 1994). Purified, wild-type mouse CD4+ T cells were used to determine the relative contribution of B7-H2-CD28 and B7-H2-ICOS interactions in triggering ERK and AKT activation. ICOS-deficient or CD28-deficient mice were first activated with anti-CD3 for 3 days. After resting for another 3 days in the presence of IL-2, T cells were harvested and activated with immobilized CD3 mAb and human B7-H2, mouse B7-1, or control Ig proteins. As shown in Figure 7c, cell lysates were prepared 5, 10, 30 and 60 min after incubation and the activation of ERK1, ERK2 and AKT was determined by immunoblots with phosphorylation specific mAbs. In wild-type T cells, simultaneous cross-linking of TCR and B7-1 or B7-H2 enhanced both ERK and AKT phosphorylation as early as 10 min after cross-linking, while suboptimal CD3 cross-linking alone induced minimal ERK and AKT phosphorylation. B7-1 and B7-H2 appear to have comparable abilities to induce ERK and AKT activation in wild type T cells. However, in ICOS deficient T cells, the kinetic responses were quite different. While B7-1 costimulation induced patterns of ERK and AKT activation similar to that of wild-type T cells, B7-H2 induced an earlier (5 to10 min) ERK and AKT phosphorylation followed by a quick reduction to background after 30 min. This observation indicates that B7-H2-CD28 interaction induces a transient activation of the ERK and AKT pathways at very early time points during T cell activation, and sustained phosphorylation of ERK and AKT may require B7-H2-ICOS interaction. In contrast, we did not observe an earlier (5 to10 min) response of ERK and AKT phosphorylation in wild-type T cells. This may be due to the competition of ICOS and CD28 for B7-H2 binding since the B7-H2-ICOS interaction has a much higher affinity. In Cd28−/− T cells, B7-1 dependent AKT and ERK phosphorylations were completely abolished. Meanwhile, B7-H2 mediated activation of AKT and ERK showed patterns similar to wild-type T cells, indicating that the B7-H2-ICOS interaction dictates AKT and ERK activation in wild-type T cells.
Finally, we compared the cytokine profile of CD4 T cells from wild-type and ICOS-deficient mice in response to human B7-H2 costimulation (Figure S6). Consistent with our observations in human T cells, B7-H2 costimulation enhanced the production of TNF-α, IFN-γ, and IL-17A from Icos−/− mouse T cells, albeit in much lower amounts than in wild-type T cells. B7-H2-CD28 interaction also induced a low IL-2 secretion from Icos−/− T cells. On the other hand, mouse T cells produced low basal amounts of IL-4 and IL-10 upon B7-H2 costimulation, which was markedly different from human T cell responses (Figure S4).
In summary, we confirmed the functional interaction of B7-H2-CD28 in mouse ICOS deficient T cells and found B7-H2-CD28 interaction activated ERK and AKT dependent signaling pathways.
DISCUSSION
In this report, we have presented the evidence that B7-H2 is a costimulatory ligand for CD28. The non-competing nature of B7-H2 and B7-1or B7-2 for binding CD28 suggests these ligands could work independently, simultaneously, and even synergistically to provide maximal CD28 costimulation for T cells. Our findings also provide a molecular basis for the crosstalk between CD28, CTLA-4 and ICOS pathways, which may help explain the functional redundancy of CD28 and ICOS costimulation. Due to the lack of this interaction in mouse, our discovery also warrants careful re-evaluation of knowledge previously acquired from murine models.
To identify additional cell surface interactions, we employed a new receptor array system with high throughput capacity. The human genome contains ~5,000 genes predicted to encode cell membrane proteins (Lander et al., 2001; Venter et al., 2001). However, fewer than 1000 proteins have been identified to have counter-receptors, largely due to the lack of sensitive and robust methods. Many cell surface proteins or their mRNA are in low abundance and their expressions vary according to environmental cues. In addition, the majority of cell surface molecular interactions are fast-on-and-fast-off with low affinities. While this feature may allow maximal control of signaling and effector function in immune responses, it increases the difficulty to identify cell surface interactions by conventional methods, including expression cloning and protein micro-sequencing. The Cellular Detection System allows us to scan only the bottom of a microplate well at a depth of 100 μm, avoiding background from non-binding secondary antibody in solution. This system yields a high signal-to-noise ratio and eliminates all washing steps. High expression of individual protein on cell surface by 293T cell transient transfection also vastly increases the avidity of its interaction with counter-receptor. An overnight incubation without any disturbing wash procedure further helps to stabilize weak interactions. Combining increased avidity and a highly sensitive detection system, this receptor array enabled us to identify receptor-ligand interactions which are otherwise undetectable on primary cells by other conventional methods, including flow cytometry. For example, the recently published B7-1-B7-H1 interaction with the binding affinity around 1μM (Butte et al., 2007) could be easily detected in our screening system. Therefore, our receptor array screening system represents a promising approach to discover new molecular interactions on the cell surface. With the expansion of this array to include all plasma membrane proteins, this system could be utilized to identify virtually any protein interactions on cell surface.
It was reported that monomeric B7-H2 bound to ICOS but not CD28 and CTLA4, whereas tetrameric B7-H2 bound CD28 and CTLA-4 at 25°C but not 37°C by surface plasmon resonance (SPR) (Brodie et al., 2000). Here we identified B7-H2-CD28 interactions in a cell-base system at 37 °C. We can confirm these interactions by dimeric B7-H2, CD28 and CTLA-4 fusion proteins at both 25 and 37°C in SPR. Flow cytometry analysis using 293T cell transfectants and fusion proteins performed at room temperature and 37°C (data not shown) further validated B7-H2 interactions with CD28 and CTLA-4. The discrepancy of our results and those of Brodie (Brodie et al., 2000) may be due to the different forms of fusion proteins (monomeric vs. dimeric) used in respective experiments. B7-H2 is expressed constitutively by hematopoietic and non-hematopoietic cells in peripheral organs, whereas B7-1 and B7-2 are largely inducible molecules expressed on selective antigen-presenting cells. This distinct expression pattern of three ligands may enable them to regulate T cell responses through CD28 in spatially and temporally divergent settings.
B7-H2-CD28 interaction may be particularly important when T cells migrate to peripheral tissues where B7-1 and B7-2 expression is limited. CD28 is constitutively expressed on naive T cells whereas ICOS and CTLA-4 are inducible upon antigen stimulation (Hutloff et al., 1999). Therefore, the role of B7-H2 on naive T cells should be primarily promoting T cell activation through CD28. B7-H2’s role on activated T cells, however, should be predominantly costimulatory mediated via ICOS, since ICOS has a higher affinity for B7-H2 than CD28 and CTLA-4. The role of B7-H2-CTLA4 interaction remains to be explored.
Competitive binding experiments suggest CD28 utilizes different interfaces to interact with B7-H2 compared with B7-1 and B7-2. Previous studies show CD28 and CTLA-4 interact with B7-1 and B7-2 through a highly conserved MYPPPY motif in the CDR3-like loop (Harper et al., 1991; Peach et al., 1994). Therefore, it is highly likely that B7-H2 is a non-redundant ligand and could work in the presence of B7-1 and B7-2 to deliver synergistic or complementary costimulatory signals. Our findings thus reveal a new feature of CD28 costimulation: more than one interface on a costimulatory receptor is available for different ligands to engage. This mechanism may be particularly important when one of these receptors is deficient. Human ICOS deficiency due to deletion mutations causes no major impairment on T cell functions (Bossaller et al., 2006; Grimbacher et al., 2003; Warnatz et al., 2006) with few exceptions (Takahashi et al., 2009). In contrast, mice with a genetic ablation of Icos have reduced T cell activation, lower IL-4 and IL-10 production, and a decrease in the memory T cell population (Burmeister et al., 2008; Dong et al., 2001). Since B7-H2-CD28 interaction is not conserved in mouse, our results imply that B7-H2-CD28 interaction may compensate T cell function in the absence of ICOS in human.
Our mutagenesis study indicates that B7-H2 uses similar, but not identical binding sites to interact with CD28 and CTLA-4. This finding laid a theoretic basis for differential blockade of these interactions with antibody. MIH12, a mAb selectively blocking B7-H2 interactions with CD28 and CTLA-4 but not ICOS, suppressed T cell proliferation in the B7-H2 mediated costimulation in vitro, and had a strong inhibitory effect on TNF-α, IFN-γ, IL-5, IL-10 and IL-17A production. B7-H2-CD28 blockade also significantly suppressed IFN-γ production but was less effective on TT antigen-elicited memory and allogeneic T cell responses. These findings may be due to the weak ability of B7-H2 to costimulate IL-2 production.
The mechanism underlying B7-H2 as a weak stimulator of IL-2 upon engaging CD28 remains to be elucidated. It is possible that upregulated ICOS after activation competes with CD28 for B7-H2 binding, because the ICOS-B7-H2 interaction has a higher affinity than CD28-B7-H2. In addition, B7-H2 engages CD28 at a more discrete domain than B7-1 does, and with a lower affinity.
Besides cytokine production, both B7-H2 and B7-1 promote Bcl-xL expression in activated CD4+ T cells. This is in sharp contrast to the observation that only CD28 but not ICOS agonist mAb induces Bcl-xL. Inclusion of MIH12 or CD28 mAbs reduced Bcl-xL expression, indicating B7-H2-CD28 interaction is important for B7-H2 mediated Bcl-xL induction. These results also implicate that different extracellular domains on CD28 govern distinctive cellular functions, including IL-2 production, Bcl-xL induction, and the generation of other cytokines. In addition, we found that B7-H2 costimulation also leads to the downregulation of the cell cycle inhibitor, p27kip1, possibly mediated through the PI3K-AKT signaling cascades. CD28 blockade by mAb partially inhibited GSK-3 phosphorylation and p27kip1 degradation, implicating an active role of the B7-H2-CD28 interaction in these pathways. Thus, Bcl-xL and p27kip1 might be important links integrating TCR and B7-H2-CD28 mediated signals to survival and cellular proliferation. This notion is supported by the study using ICOS deficient mouse T cells in which B7-H2 induced an early ERK and AKT phosphorylation in the absence of ICOS, whereas B7-H2 promoted and sustained ERK and AKT phosphorylation in wild-type T cell.
Our findings also warrant re-evaluation of CD28 and ICOS pathways as targets for immunotherapeutic manipulation. For example, the immunosuppressive effect of CTLA-4Ig (Abatacept) could be interpreted, at least partially, as interference of B7-H2-CD28 interaction, since high dose of CTLA-4Ig will compete with CD28 for B7-H2 binding. All together, our findings provide important information for the current effort in manipulating these pathways for treating human diseases.
EXPERIMENTAL PROCEDURES
Plasmids, fusion proteins and monoclonal antibodies
Human full length plasma membrane genes (>2000) were purchased from Open Biosystems (Huntsville, AL) or cloned into mammalian expression vector individually. Human and mouse fusion proteins were purchased from R&D systems (Minneapolis, MN) or expressed in 293T cell and purified by Protein A columns. Antibodies used in flow cytometry and in vitro studies were purchased from BD Biosciences (San Jose, CA), BioLegend (San Diego, CA) or eBioscience (San Diego, CA). Anti-human Ig FMAT blue was purchased from Applied Biosystems (Foster City, CA). Western blot antibodies were all purchased from Cell Signaling (Danvers, MA). Detailed information of each antibody can be found in supplemental experimental procedures online.
Animals
C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD). Cd28−/− and Icos−/− mice in B6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). Aged matched 6–8 week-old mice were used for all experiments. All mouse protocols were in accordance with NIH guidelines and were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine and Yale University School of Medicine.
High-throughput transfection and screening
Plasmids with human transmembrane genes were diluted by OPTI-MEM media and placed individually into five 384-well plates at 60ng/well. 0.15μl Lipofectamine 2000 was added to each well and mixed with plasmids for 30 min. Subsequently, ten thousand 293T cells were added to each well to perform transient transfection. Eight hours after transfection, 50ng human CD28Ig and 50ng anti-human Ig FMAT blue secondary antibody were added into each well. The plates were read twenty-four hours after transfection by the Applied Biosystems 8200 cellular detection system and analyzed by CDS 8200 software.
T cell costimulation assay
Human peripheral mononuclear cells (PBMCs) from healthy donors were purchased from Research Blood Components (Brighton, MA). T cells were negatively selected from PBMCs and purified using a human pan-T cell selection kit or a human CD4 T cell selection kit (Miltenyi Biotec, Auburn, CA). Human CD3 mAb (OKT3) was pre-coated in 96-well plates at the indicated concentrations. B7-H2Ig or isotype-matched control Ig at 5μg/ml was also immobilized in the wells. Purified human T cells were added into each well at 2.5x105/well and cultured for three days. In some experiments, B7-H2 mAb, MIH12 or 9F.8A4 was added at 10μg/ml at the beginning of culture, and unbound mAbs were washed away by media before addition of T cells. To block CD28, T cells were first incubated with 5μg/ml human CD28 mAb (CD28.6) for 15 minutes. T cells were then added to 96 well plates after washing away unbound CD28.6. For ICOS blockade, anti-human ICOS (C398.4A) was added to the culture at 10μg/ml in soluble form. 3HTdR was added during the final six hours of culture. 3H-TdR incorporation was counted with a MicroBeta Trilux liquid scintillation counter (PerkinElmer, Waltham, MA). T cell costimulation assays were repeated using three different donors.
Bcl-xL intracellular staining
Mouse anti-human Bcl-xL PE (clone 7B2.5) was purchased from Southern Biotech (Birmingham, AL). Activated human CD4 T cells were harvested and washed with cold PBS. Intracellular staining for Bcl-xL was preformed according to manufacturer’s protocol (Cytofix/Cytoperm, BD).
T cell lysate preparation and Western Blot
T cells were harvested at indicated time after activation. Western blot detection was performed using SuperSignal West femto kit from Thermo Scientific.
Statistics
T cell proliferation were measured by thymidine incorporation and the amount of IFNγ in the supernatant were compared between control Ig and blocking antibody groups using 2-tailed student T test. P values less than 0.05 were considered statistically significant. The error bars in figures represent Standard Deviation (SD).
Supplementary Material
Acknowledgments
We thank Dr. Nancy Xu and Beth Cadugan for editing, and Dr. Pierre Coulombe and Dr. Brian Learn for their assistance with the BIAcore 3000 instrument. This work was partially supported by grants CA97085 and CA113341 from the National Institutes of Health. All authors declare no competing financial interests.
Footnotes
Supplemental Information. Six supplemental figures and supplemental experimental procedures can be found online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- Brodie D, Collins AV, Iaboni A, Fennelly JA, Sparks LM, Xu XN, van der Merwe PA, Davis SJ. LICOS, a primordial costimulatory ligand? Curr Biol. 2000;10:333–336. doi: 10.1016/s0960-9822(00)00383-3. [DOI] [PubMed] [Google Scholar]
- Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay K, Bhatia S, Fiser A, Almo SC, Nathenson SG. Structural basis of inducible costimulator ligand costimulatory function: determination of the cell surface oligomeric state and functional mapping of the receptor binding site of the protein. J Immunol. 2006;177:3920–3929. doi: 10.4049/jimmunol.177.6.3920. [DOI] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- Harper K, Balzano C, Rouvier E, Mattei MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147:1037–1044. [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong R, Silva D, Withers D, Anderson G, Verma NK, Brink R, Hutloff A, Goodnow CC, Vinuesa CG. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–241. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- Peach RJ, Bajorath J, Brady W, Leytze G, Greene J, Naemura J, Linsley PS. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J Exp Med. 1994;180:2049–2058. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KV, Cai YC, Raab M, Duckworth B, Cantley L, Shoelson SE, Rudd CE. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc Natl Acad Sci U S A. 1994;91:2834–2838. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Matsumoto K, Saito H, Nanki T, Miyasaka N, Kobata T, Azuma M, Lee SK, Mizutani S, Morio T. Impaired CD4 and CD8 effector function and decreased memory T cell populations in ICOS-deficient patients. J Immunol. 2009;182:5515–5527. doi: 10.4049/jimmunol.0803256. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–2813. [PubMed] [Google Scholar]
- Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, van Dongen JJ, Orlowska-Volk M, Knoth R, Durandy A, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107:3045–3052. doi: 10.1182/blood-2005-07-2955. [DOI] [PubMed] [Google Scholar]
- Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


